ABSTRACT
For many years, left main coronary artery disease has remained as the last frontier resisting percutaneous coronary intervention. Until recently, the most relevant clinical studies in this regard as well as clinical practice guidelines favored surgical revascularization almost as the only treatment pathway for the management of this condition. The changes that have occurred over the last 10 to 15 years since the appearance of drug-eluting stents and their technological advances have been extraordinary. This, added to the publication of randomized clinical trials that compared both revascularization modalities, has placed percutaneous coronary interventions at a similar level to surgery in a large number of patients. The anatomical, technical, and strategic aspects are essential for the percutaneous management of left main coronary artery lesions given their tremendous clinical variability. In this article we will be reviewing their anatomy, angiography, intracoronary diagnostic techniques, and different percutaneous revascularization strategies. As long as future clinical studies do not definitively favor percutaneous over surgical revascularization or vice versa, individual discussions on each particular case by the heart team and our patients’ preferences should guide our clinical decision-making process.
Keywords: Coronary artery disease. Left main coronary artery. Percutaneous coronary intervention. Coronary artery bypass graft.
RESUMEN
La enfermedad del tronco coronario izquierdo ha permanecido muchos años como la última frontera que se resistía al intervencionismo coronario percutáneo. Hasta hace poco tiempo, los estudios clínicos más relevantes en este campo, así como las guías clínicas, han sido favorables a la revascularización quirúrgica casi como forma exclusiva de tratamiento de esta patología. Los cambios ocurridos en los últimos 10-15 años, desde la aparición de los stents farmacoactivos y su mejora tecnológica, han sido vertiginosos. La realización de estudios aleatorizados que han comparado ambas modalidades de revascularización ha llevado al intervencionismo percutáneo a la altura de la cirugía en un alto porcentaje de pacientes. Los aspectos anatómicos, técnicos y de estrategia son fundamentales en el tratamiento percutáneo de estas lesiones, dada su enorme variabilidad clínica. En tanto los estudios clínicos futuros no se decanten definitivamente a favor de la revascularización percutánea o de la quirúrgica, la discusión individualizada de cada caso en un equipo multidisciplinario y las preferencias de los pacientes deberían guiar la decisión clínica.
Palabras clave: Enfermedad coronaria. Tronco coronario izquierdo. Intervencionismo coronario percutáneo. Cirugía de revascularización coronaria.
Abbreviations:
LAD: left anterior descending coronary artery. CABG: coronary artery bypass graft. LCx: left circumflex artery. FFR: fractional flow reserve. IVUS: intravascular ultrasound. LMCA: left main coronary artery. PCI: percutaneous coronary intervention.
INTRODUCTION
Significant left main coronary artery (LMCA) disease is present in 4% to 5% of all coronary angiographies.1 Since the LMCA supplies over 75% of all the myocardial blood flow, the risk associated with its lesions is the highest of all possible coronary lesions. Without revascularization, its prognosis is poor and mortality rate can be up to 37% at 3-year follow-up.2 Revascularization can be surgical or percutaneous, each one with its corresponding advantages and limitations. Assessing anatomic spread correctly, the complexity of coronary artery disease, the patient’s comorbidities, and the operator’s expertise in complex percutaneous coronary interventions (PCI) are key factors when choosing the right revascularization strategy. There are different models and scales to guide the selection of patients. However, none of them has become the leading model yet.3,4
HISTORIC PERSPECTIVE
Coronary artery bypass graft (CABG) has been the standard of care for the management of patients with LMCA disease based on early clinical trials that proved its prognostic benefit in patients assigned to surgery compared to medical therapy.5 Patients with severe LMCA disease were excluded from most of the early clinical trials and, until recently, no specific trial compared the results of surgery vs PCI as one of its endpoints.6,7 Currently, there are randomized clinical trials that have confirmed the utility of the PCI to treat LMCA disease; actually, the American and European clinical guidelines consider it the recommended strategy in certain settings.8,9 Approximately, 50% of this type of lesions are revascularized percutaneously in our setting with an annual 5% increase.10
ANATOMIC CONSIDERATIONS
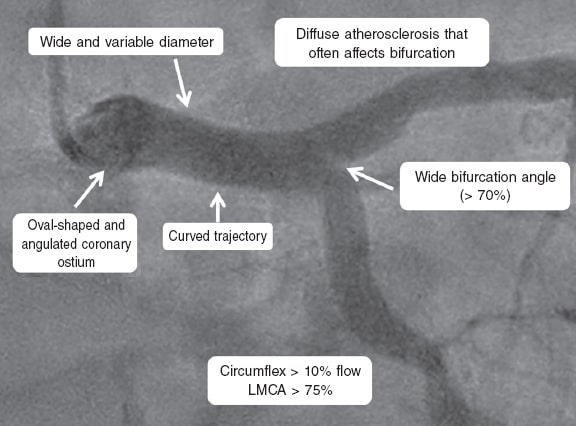
Anatomically speaking, the LMCA can be divided into 3 portions: ostial portion, mid-portion, and distal portion; the latter is a bifurcation with an angle that is typically wider compared to other coronary bifurcations (> 70°). It supplies at least 75% of the total coronary flow. The LMCA caliber is often 5 mm ± 0.5 mm11 and its mean length is 10.5 mm ± 5.3 mm.12 In up to 30% of the cases it originates a third branch, the ramus intermedius or bisector branch (figure 1).
Figure 1. Main anatomical characteristics of the left main coronary artery (LMCA).
LMCA atherosclerotic disease is often diffuse. When the bifurcation is affected (in 70% of cases) there is also often presence of plaque at the beginning of the left anterior descending coronary artery (LAD) and left circumflex artery (LCx).11 At times, the origin of both the LAD and the LCx is independent from the left coronary sinus without LMCA per se (0.41% to 0.67% of cases).13,14 In 0.03% of patients, the origin of the LMCA is anomalous describing its trajectory between the aorta and the pulmonary artery, a pattern associated with a high risk of sudden death.14,15
LEFT MAIN CORONARY ARTERY ASSESSMENT
Angiography
The clinical practice guidelines of the European Society of Cardiology establish that the revascularization of the LMCA is indicated for patients with angiographic stenoses > 50% and documented myocardial ischemia.16 The practical problem here is that coronary angiography has limitations when evaluating LMCA disease with great intra and interobserver variability.17,18
Some ostial lesions can be overestimated due to catheter-induced overlapping and artifact or the presence of an associated spasm. Consequently, distal lesions may be difficult to assess due to the often diffuse affectation of the bifurcation and lack of a healthy reference vessel. Damping and/or ventricularization of the pressure curve are indirect data of LMCA disease.19
The correct assessment of the severity of LMCA disease is essential given the evidence that functionally nonsignificant lesions have a favorable prognosis without revascularization,20 and the early graft failure seen in nonsignificant lesions.21 In this regard, clinical practice guidelines accept the value of diagnostic imaging modalities like intravascular ultrasound (IVUS) and the pressure guidewire to estimate the severity of LMCA disease.
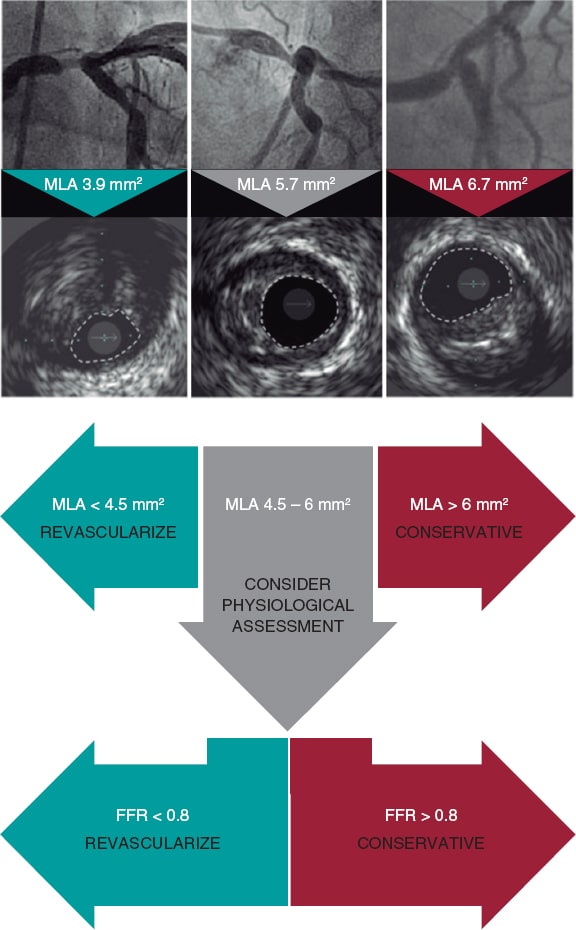
Intracoronary imaging modalities
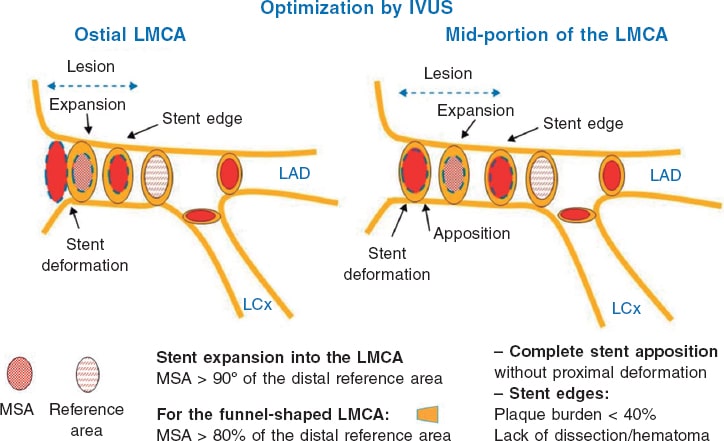
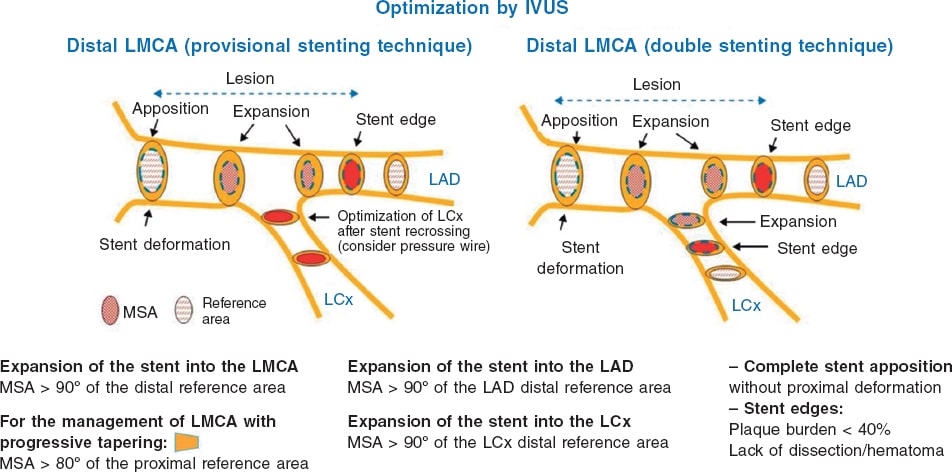
The IVUS provides information on the structure and anatomy of the LMCA as well as on the presence of plaque, its spread, composition, and classification. Several studies have determined a minimum lumen area (MLA) > 6 mm² as the cut-off value to establish severity.22,23 The Spanish multicenter, prospective clinical trial LITRO proved that it was safe to delay the revascularizations of intermediate LMCA lesions with MLAs > 6 mm² with favorable results at the 2-year follow-up.24 Also, the IVUS helps us determine whether the coronary ostia of LAD and LCx have significant disease. When revascularization is indicated, the IVUS provides information on the right size of the stent and the best strategy should be based on the anatomy and calcium load of the LMCA and proximal LAD/LCx; in lesions due to previous in-stent restenosis, the IVUS characterizes their etiology and the possible damage to the borders of the stent. The IVUS-guided PCI of the LMCA is beneficial compared to the angiography-guided PCI.25 The need for stent postdilatation and the existence of distal dissection can be assessed too. Also, it can help us determine the need for stent implantation into the lateral branch or exclude the compromise of this branch after implanting a provisional stent.26 Several parameters have been described for the optimization of IVUS-guided PCIs to treat LMCA disease (figure 2 and figure 3). A large metanalysis of patients from several Spanish registries confirmed that the use of IVUS is associated with better clinical progression, fewer deaths and infarctions, and particularly stent thrombosis. These findings are especially significant in LMCA distal lesions.27 Other registries, a few minor clinical trials, and a combined analysis of them all confirm significant clinical benefit from IVUS-guided PCIs performed on the LMCA with fewer deaths, infarctions, and thrombosis.28 The clinical practice guidelines of the European Society of Cardiology consider the use of IVUS to stratify the severity of all LMCA lesions as an indication type IIa B.16
Figure 2. Key points to optimize the percutaneous coronary interventions performed on the ostial and mid-portions of the left main coronary artery through intravascular ultrasound. IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; MSA, minimum stent area. (Modified with permission from de la Torre Hernández et al.25.)
Figure 3. Key points to optimize the percutaneous coronary interventions performed on the distal left main coronary artery through intravascular ultrasound. IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery; MSA, minimum stent area. (Modified with permission from de la Torre Hernández et al.25.)
The utility of the optical coherence tomography (OCT) for the management of the LMCA is somehow more limited, mainly because of the technical difficulty involved in completing contrast filling and the native area of ostial segments. Another downside of the OCT for the management of the LMCA is its limited penetration depth (2 mm to 3 mm) compared to IVUS (4 mm to 8 mm), and since the LMCA often has diameters between 3.5 mm and 4.5 mm the assessment can be wrong. The MLA cut-off value for the management of LMCA lesions with the OCT is still unknown. On the other hand, due to the different image acquisition of both imaging modalities, the thresholds established as cut-off values with the IVUS don’t work with the OCT.
Pressure wire
The pressure guidewire provides valuable information to stratify the severity of LMCA disease.16,29 In order to stop a presumably ostial disease from impacting measurement, pressures need to be equalized and measured using a guide catheter partially «desintubated» from the LMCA. Obtaining hyperemic indices from the LAD and the LCx leads to better overall assessments of the severity of LMCA disease. Also, it secures the decision-making process on the best therapeutic approach. Some authors suggest that IV adenosine is better than intracoronary adenosine to secure the condition of maximum hyperemia.4
Another important aspect when assessing the LMCA with the pressure guidewire is the physiological interdependence of the coronary tree that may change the values of fractional flow reserve (FFR). In particular, the FFR has been reportedly overestimated in the presence of diffuse disease of the LAD and the LCx and underestimated in cases of significant lateral branch disease.30 Therefore, in the presence of concomitant distal branch disease, measuring the FFR during controlled retrieval can be useful.30
Regarding the pathological threshold, it seems that delaying the PCI with FFR values > 0.8 is safe.31 Although the value of other pressure guidewire indices that don’t require hyperemia like the instantaneous wave-free ratio (iFR) has not been fully assessed in the LMCA, a study proved that using the iFR to delay the revascularization of the LMCA is safe.32 Currently, the multicenter clinical trial iLITRO (NCT03767621) is being conducted in Spain. This trial will probably shed light on the utility of the iFR and its pathological threshold in the management of LMCA lesions.
Integrating different techniques
Integrating the IVUS and the pressure guidewire in the assessment of the LMCA of angiographically dubious severity is advised as stated by an international consensus document from the European Association of Percutaneous Cardiovascular Interventions33 (figure 4). Therefore, in ambiguous LMCA lesions, MLAs > 6 mm2 would be indicative of no revascularization, MLAs < 4.5 mm2 to 5 mm2 would be indicative of revascularization, and MLAs between 4.5 mm-5 mm to 6 mm2 would recommend the use of the FFR/iFR indices before making any decision.
Figure 4. Criteria for significant left main coronary artery disease. FFR, fractional flow reserve; MLA, minimum lumen area. (Modified with permission from Johnson et al.33.)
REVASCULARIZATION OF THE LEFT MAIN CORONARY ARTERY
Surgical revascularization
CABG has been the standard of care for patients with LMCA disease since traditional clinical trials confirmed its prognostic benefit in patients randomized to surgery vs medical therapy.5 The CASS registry reported a 4-year survival rate in 88% of operated patients compared to 63% in non-revascularized patients.34 Other studies confirmed that the mortality rate dropped to 65% with surgery.35 This allows a complete revascularization regardless of the characteristics of proximal lesion and technical advances facilitate faster procedures without having to use extracorporeal blood pumps. The main setback is still the non-negligible peri and postoperative morbidity and mortality. Some studies have reported a mortality rate of between 5.5% to 8.5%, a need for ischemia-guided revascularization of 7.1% to 9.4%, and a rate of stroke of 3.1% to 5.1% at the 3-year follow-up.36
Percutaneous revascularization
The arrival of stents improved the results of PCI on the LMCA significantly. However, at the beginning, conventional stents fared worse compared to surgery with mortality rates of 14%, a left ventricular ejection fraction (LVEF) > 40% and 78%, and a LVEF < 40% at the 9-month follow-up.37 With the arrival of drug-eluting stents, the rates of restenosis and adverse events dropped low enough to be able to compare PCI to CABG,38-41 with event-free survival rates at the 1-year follow-up of 98% in patients with LVEF < 40%.38 In patients considered non-eligible for surgery (EuroSCORE > 6 or Parsonnet > 15), the mortality and survival rates without major adverse cardiovascular events were 3.5% and 75.3%, respectively, at the 6-month follow-up.42 These studies already showed that the PCIs performed on the ostial and mid-portions of the LMCA seemed to have a better prognosis compared to those performed on the distal LMCA or that involved bifurcation. The arrival of new antiproliferative drugs, the development of better devices, and the use of new techniques and strategies to treat bifurcation improved results, efficacy, and the good prognosis of the PCIs performed on the LMCA in experienced centers.
Surgical vs percutaneous revascularization
Six landmark randomized clinical trials have compared percutaneous and surgical strategies (table 1). The first ones (LE MANS,43 SYNTAX,44 Boudriot et al.,45 and PRECOMBAT46) were conducted with first-generation drug-eluting stents and reported similar rates of a composite of death, infarction, and stroke for both strategies. The main differences were a higher rate of strokes in the CABG group and a higher rate of new revascularizations after the PCI. The two most recent clinical trials conducted so far, the EXCEL and NOBLE, used second-generation drug-eluting stents and included large cohorts of patients with less complex atherosclerotic disease, which may be indicative of the actual clinical practice.16,47 The difference in results obtained by these studies was very controversial; differences were reported in the definition of endpoint and periprocedural infarction as possible determinants. Actually, unlike the EXCEL, the NOBLE trial excluded periprocedural infarction from the composite of primary events although its inclusion is recommended by the Academic Research Consortium and is part of the universal definition of myocardial infarction. It has been confirmed that periprocedural infarction is associated with a worse prognosis.16 Also, the large difference seen in the rate of stent thrombosis (0.7% in the EXCEL trial vs 3% in the NOBLE) is indicative of the possible influence of the different type of stent used in each of these studies.
Table 1. Main comparative studies between percutaneous and surgical revascularization
| Study | Year | n | Mean SYNTAX score | Distal LMCA lesions | Type of stent | Endpoint(PCI vs CABG) | Secondary endpoints(PCI vs CABG) |
|---|---|---|---|---|---|---|---|
| LE MANS43 | 2008 | 105 | n/a | 58% | Conventional, first-generation covered stents | Change of LVEF at the 1-year follow-up: 3.3% ± 6.7% vs 0.5% ± 0.8%P = .047 |
|
| SYNTAX LM44 | 2010 | 705 | 30 | 61% | Taxus | Death, stroke, AMI or need for revascularization at the 1-year follow-up: 15.8% vs 13.6%; P = .44 |
|
| Boudriot et al.7 | 2011 | 201 | 23 | 72% | Cypher | Death, AMI or need for revascularization at the 1-year follow-up: 19.0% vs 13.9%; P for non-inferiority = .19 |
|
| PRECOMBAT46 | 2011 | 600 | 25 | 64% | Cypher | Death, stroke, AMI, ID-TLR at the 1-year follow-up: 8.7% vs 6.7%; P for non-inferiority = .01 |
|
| EXCEL16 | 2017 | 1905 | 21 | 81% | Xience | Death, stroke or AMI at the 3-year follow-up: 15.4% vs 14.7%:P for non-inferiority = .02; P for superiority = .98. |
|
| NOBLE38 | 2017 | 1201 | 22 | 81% | BioMatrix Other drug-eluting stents | Death, stroke, periprocedural AMI or need for revascularization at the 5-year follow-up: 29% vs 19%; P = .0066. |
|
|
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; ID-TLR, ischemia-driven target lesion revascularization; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; N/A, not applicable; PCI, percutaneous coronary intervention. |
|||||||
In general, the results of these studies suggest that when complete revascularization is achieved, both surgery and the PCI achieve similar results for the composite of death, infarction, and stroke at the 5-year follow-up.48 However, there is an early benefit for the PCI in terms of periprocedural infarction and stroke that is compensated by the higher risk of infarction at the long-term follow-up. The risk of requiring a new revascularization is evenly higher in patients treated with PCI compared to surgical patients.
Another issue that should be taken into consideration is the correlation between the results of the PCI and the SYNTAX score. The first clinical trials conducted on this topic already suggested that higher scores probably led to a better prognosis with CABG. Some metanalyses have described that, overall, long-term cardiovascular mortality seems to be directly proportional to the angiographic complexity of LMCA disease. Therefore, patients with low SYNTAX scores had a better prognosis with PCI compared to patients with higher scores. Also, patients with high SYNTAX scores showed a non-significant tendency towards a higher 10-year survival rate with surgery compared to PCI.49,50 One of the main setbacks of this score is that it only includes anatomical variables. Currently, there are other scales including angiographical, clinical, and even functional variables, but their utility as long-term prognostic markers of LMCA disease has not been properly studied yet.51
The current clinical practice guidelines on coronary revascularization16 establish the indication for CABG or PCI based on the SYNTAX score (table 2). If complexity is low, the PCIs performed on the LMCA have the same indication as surgery (IA). The PCI is an alternative to surgery in patients with intermediate SYNTAX scores (IIa A) and greater evidence is needed in patients with high SYNTAX scores before clearly recommending PCI.
Table 2. Indication, level, and class of evidence of significant left main coronary artery disease according to the clinical practice guidelines established in 2018 by the European Society of Cardiology8
| Left main coronary artery disease | Surgery | PCI | ||
|---|---|---|---|---|
| Low SYNTAX score (0-22) | I | A | I | A |
| Intermediate SYNTAX score (23-32) | I | A | IIa | A |
| High SYNTAX score (≥ 33) | I | A | III | B |
Patient selection
The European clinical practice guidelines highlight the importance of the heart team in the decision-making process on which revascularization strategy should be used in stable patients with LMCA disease. This team should include clinical and interventional cardiologists and cardiac surgeons. However, in emergent procedures, surgery is not often a viable option due to the delay involved and the progressive worsening of prognosis in relation to ischemic time. Pappalardo et al.52 described in-hospital mortality rates of 21% (basically due to multiorgan failure) in patients with acute myocardial infarction and acute occlusion of the LMCA. However, patients who survived hospitalization and were treated with PCI had a good prognosis with a 1-year survival rate of 89.5%.
In the remaining cases it would be desirable to avoid performing interventional procedures ad hoc after the diagnostic procedure. The different revascularization options should be discussed with the clinical cardiologist, the cardiac surgeon, and especially with the patient. The latter should also be objectively informed of the theoretical pros and cons of every technique and the specific results obtained by the treating center making him part of the decision-making process. Other clinical, anatomic and general factors should be taken into consideration too (table 3). Finally, if performing a PCI on the LMCA is considered the best option, the administration of the right premedication, assessment by the heart team, and procedural planning on the technique and materials that will be used are all associated with higher rates of success.
Table 3. Factors impacting the modality of revascularization of the left main coronary artery
| In favor of PCI | In favor of CABG | |
|---|---|---|
| General factors |
|
|
| Clinical factors |
|
|
| Anatomical factors |
|
|
| Patients’ preferences and needs | ||
|
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; LMCA, left main coronary artery; PCI, percutaneous coronary intervention. |
||
Since most clinical trials have been conducted in centers with coronary care units, performing PCIs on the LMCA in centers without these units has been controversial. However, since there is evidence of the good outcome of PCIs in centers without these units,53-55 it is widely accepted that PCIs can be performed on the LMCA in these centers safely as long as an experienced medical team is in charge and the necessary technical equipment used. Also, the patient’s informed consent needs to have been collected, and a previous protocol established for urgent transfers to hospitals with coronary care units in the hypothetical case that the patient may require urgent surgery.
Operators and equipment
The PCIs performed on the LMCA should always be considered high-risk procedures. Actually, the experience of the operators is of paramount importance here. There is evidence that patients treated in high volume centers that perform procedures like this regularly have a better prognosis.56
The equipment should guarantee the proper assessment of the LMCA (IVUS, pressure guidewire). All kinds of materials that may be required to perform the angioplasty and handle all possible complications should be available too. Since it is a high-risk procedure, hemodynamic support devices and resources like the intra-aortic balloon pump and the Impella device (Abiomed, United States) are very important.
ANGIOPLASTY OF THE LEFT MAIN CORONARY ARTERY
Prior to performing the procedure, it is essential to conduct a comprehensive analysis of the case to decide on the strategy, access route (radial or femoral), caliber of the introducer sheath (due to the presumable need for the double stenting technique, 7-Fr catheters via femoral access or “7 in 6-Fr” catheters via radial access are advised), and type of guide catheter. Although radial access has replaced femoral access in many cases, the PCIs performed on the LMCA are probably a niche where femoral access should be considered since obtaining the least support possible can be key here. Also, this access facilitates the use of larger caliber catheters and the possibility of quick hemodynamic support device implantation.
Damage to the distal LMCA or bifurcation complicates the procedure with more chances of needing 2 stents and a worse prognosis. Other factors associated with worse outcomes and prognoses are calcifications, smaller LMCA diameters, and the presence of non-ostial disease in the LAD or LCx.57
Wiring and preparation of the lesion
The use of at least 2 angioplasty guidewires (for the 2 main vessels) will be the standard of use in the PCIs performed on the LMCA with notable exceptions like protected LMCA lesions if rotablation is required or in some cases of isolated and ostial LMCA disease. Using 2 guidewires slightly changes the bifurcation angle, facilitates access to the lateral branch and maintains flow towards it. Using 2 guidewires also helps find this lateral branch in cases of occlusion. Actually, some authors advocate the use of the bailout technique with balloon when flow is compromised after stent implantation into the main vessel.58 Predilatation of the main vessel should be avoided if both vessels have not been protected first due to the high risk of changing and moving the plaque, which could occlude the coronary ostium of a branch complicating further catheterizations.
The use of plaque bulking techniques (rotablation or laser, among other) to change the anatomy and facilitate the angioplasty can be considered. LMCA ostial lesions often consist of abundant calcification and large amounts of elastic muscle fibers, which is associated with a risk of elastic retraction of the lesion both after predilatation and stent implantation. On the other hand, the presence of fibrocalcific plaques can condition the use of cutting balloons as the first step and even rotablation, that has proven beneficial in angioplasties of bifurcated LMCAs prior to the implantation of drug-eluting stents.59,60 When the lateral branch shows severe ostial or heavily calcified disease or access to it becomes complicated, predilatation with a non-compliant small-caliber balloon is advised.
Stent selection
Two different scenarios should be looked into when choosing the right stent: whether only the LMCA or the bifurcation should be treated. Treating the LMCA may be justified only in cases of isolated ostial or mid-portion disease. In this situation, a stent of nominal size should be picked that should reproduce the size of the LMCA as much as possible. Another option would be to implant a stent of a smaller size and overexpand it with a high-pressure balloon of the right dimensions. Several platforms achieve large degrees of expansion without jeopardizing the integrity of its structure.61,62 However, there is no clear evidence that stent overexpansion is a safe practice since it is subject to the suboptimal coverage of the intima layer due to metal-to-artery ratio reduction. Also, it can change the polymer or kinetics of the drug-eluting stent.
When the bifurcation should be treated, the stent implanted into the LMCA should cover the proximal portion of 1 of the 2 main vessels. Also, its size should match the proximal diameter of that main vessel. Another important aspect here is having to use the proximal optimization technique (POT) with a non-compliant balloon to adapt the stent proximal caliber to the LMCA. Recrossing towards the lateral branch or using the double stenting technique can be an option too.
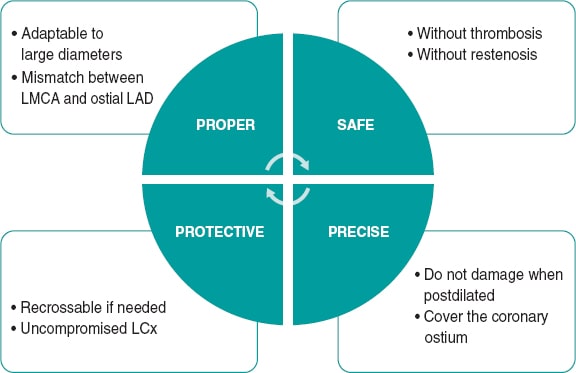
Stents implanted into the LMCA are especially prone to proximal deformation because they are in continuous contact with the guide catheter, due to the need for using the POT, and because they scrape against other devices that come through after implantation.63 Therefore, the resistance of every stent to longitudinal compression is a factor that should be taken into consideration during stent selection. Other fundamental characteristics that should be looked into when choosing the ideal stent to perform PCIs on the LMCA are the safety profile and precision provided by the stent (figure 5).
Figure 5. Technical characteristics of the ideal stent to perform percutaneous coronary interventions on the left main coronary artery. LAD, left anterior descending coronary artery; LCx, left circumflex artery; LMCA, left main coronary artery.
Selection of the bifurcation technique
Non-complex bifurcation
When LMCA disease affects 1 bifurcation branch only or the LCx has a small caliber (< 2.5 mm), the best strategy is the provisional stenting technique with a single stent implanted from the LMCA towards the main vessel. In general, the LAD is the main vessel and only in some cases it would be the LCx. Afterwards, the use of the POT with a non-compliant balloon of the right size is routinely advised.
There are times when it is necessary to fully cover the length of the LMCA. In these cases, it is extremely important to be very precise when implanting the stent to treat the coronary ostium properly and avoid any significant stent protrusions into the aorta.
However, there is still controversy over whether it is necessary to always recross it towards the lateral branch and optimize it with the kissing balloon technique in the bifurcation after using the POT if the provisional stenting technique proves insufficient. The kissing balloon technique should be used with suboptimal final outcomes in the lateral branch, when the main vessel selected is the LCx, and when the future need for a PCI on the lateral branch cannot be discarded.4
Complex bifurcation
When disease affects both bifurcation branches significantly, the use of the double stenting technique should be considered. However, since different registries report that the rates of restenosis and new revascularizations are lower with the single stenting technique,38,64-66 the early approach in many centers and in most complex bifurcations is often using the provisional stenting technique with the possibility of finishing using the double stenting technique, if necessary. With suboptimal results, the expert committee of the European Bifurcation Group recommends using double T stenting, the T and small protrusion (TAP) or the culotte technique as the bailout strategy after provisional stent implantation.67 Once the second stent has been implanted into the lateral branch, individual dilatation in both branches is advised using non-compliant balloons to secure the ostial expansion of the stent of the LAD and the LCx followed by the kissing balloon technique. If it takes over a significant portion of the LMCA, a new proximal dilatation (re-POT) should be performed to optimize the result.
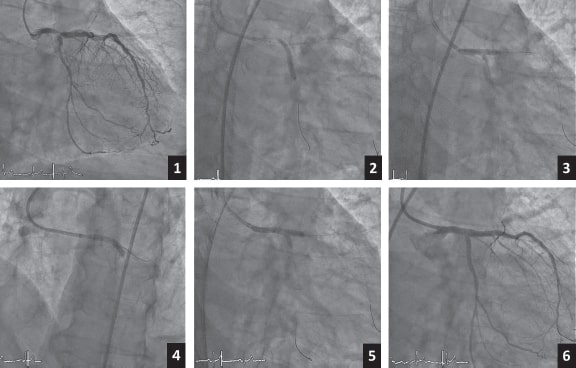
When the double stenting technique is used right away, this selection is often based on different factors: anatomical and angiographic variables, location of the lesion, intracoronary imaging modalities, damage to the LAD and LCx coronary ostia, clinical situation, and even the operator’s skills in each technique. To this day, we still do not have enough evidence to know which is the best technique. Several algorithms and therapeutic strategies have been suggested based on the parameters mentioned above like the ones proposed by Fajadet et al.68 or De Maria and Banning.69 However, none of them has come out victorious maybe due to the huge variability of clinical and angiographic situations and the different experience reported by the different centers. The crush, modified crush, and culotte techniques are still the most widely used today. The double kissing crush technique seems to have good results as it is associated with a lower rate of target lesion failure or stent thrombosis at the 3-year follow-up (figure 6).70
Figure 6. Example of double kissing crush stenting technique. 1: baseline angiography. 2: stent implanted towards the circumflex artery. 3: first kissing balloon inflation. 4: stent implanted towards the left anterior descending coronary artery. 5: second kissing balloon inflation. 6: angiography with final outcome.
Result optimization
The IVUS, the OCT, and the guidewire pressure optimize the results of the angioplasties performed on the LMCA. There is evidence that the suboptimal result of these angioplasties performed on the LMCA is associated with a worse clinical prognosis.71 Although the OCT shows the aforementioned limitations (limited penetration depth compared to the IVUS, possible inadequate filling), the truth is that both imaging modalities can detect significant findings like stent underexpansion, strut malapposition, border dissection, and degree of lateral branch involvement, which could require result optimization.
The imaging modality we have more evidence of in the optimization of angioplasty results of the LMCA is also IVUS that has an associated net clinical benefit.26,72,73 The protocolized use of IVUS for optimization purposes seems to additionally improve the prognosis of these patients.30 However, the ongoing clinical trial OPTIMAL (NCT04111770, Optimization of left main percutaneous coronary intervention with intravascular ultrasound randomized controlled trial), that will be recruiting 800 patients, will shed light on the prognostic effect of using IVUS in PCIs performed on the LMCA compared to angiography alone.
On the other hand, several studies have been conducted on the pressure guidewire and its value as a predictor of events in cases of provisional stent implantation by estimating the flow reserve towards the lateral branch.74
MEDICAL THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION AND FOLLOW-UP
Although angioplasties performed on bifurcations are a predictor of events,54,75 currently, there is no evidence available to recommend a specific antiplatelet therapy in angioplasties performed on the LMCA. Therefore, treatment should be administered based on each patient’s clinical presentation and ischemic and hemorrhagic risk profile. However, we should bear in mind that implanting a stent into the LMCA and performing a PCI on a bifurcation, especially when 2 stents are used, are criteria that add more ischemic risk to the profile of these patients.76-79
The reappearance of suggestive symptoms or documented ischemia justifies an invasive approach. The review coronary angiography performed at the 1-year follow-up in patients with angioplasty on the LMCA has a level IIB C indication according to European clinical practice guidelines,16 and is not justified in all cases. The randomized clinical trial ANGELINE (Angiographic evaluation of left main coronary artery intervention) (NCT04604197) will bring more evidence on the potential advantages of the systematic angiographic review.
CONCLUSIONS
The assessment of LMCA lesions is complex, which is why acquiring different angiographic views and using imaging modalities like IVUS or pressure guidewire is advised.
Currently, the SYNTAX score, the possibility of complete revascularization, and the patient’s comorbidities are the main criteria that should guide the selection of percutaneous or surgical revascularization.
Regarding the PCIs performed on LMCA lesions, there are 2 different categories: isolated ostial or mid-portion LMCA lesions (technically easier to treat and with an excellent prognosis), and bifurcation lesions (with a more complex approach).
Optimizing the PCIs performed on the LMCA is essential using intravascular ultrasound and techniques and stents backed by the highest level of evidence in this setting followed by the proper pharmacological cover.
In conclusion, there is no doubt that PCIs performed on LMCA lesions crossed their own particular Rubicon a long time ago. Alea jacta est (which is Latin for “the die is cast”) and, in the future, new randomized clinical trials on surgical or percutaneous revascularization and technical advances in both modalities will favor one over the other. In the meantime, revascularizations based on every individual patient and in close collaboration with the heart team should guide the routine practice of clinical cardiologists and interventional and cardiac surgeons.
FUNDING
No funding declared.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest regarding the content of this manuscript.
REFERENCES
1. Giannoglou GD, Antoniadis AP, Chatzizisis YS, et al. Prevalence of narrowing >or=50% of the left main coronary artery among 17,300 patients having coronary angiography. Am J Cardiol. 2006;98:1202-1205.
2. Taggart DP, Kaul S, Boden WE, et al. Revascularization for unprotected left main stem coronary artery stenosis stenting or surgery. J Am Coll Cardiol. 2008;51:885-892.
3. Garg S, Stone G, Kappetein AP, et al. Clinical and angio-graphic risk assessment in patients with left main stem stenosis. J Am Coll Cardiol Intv. 2010;3:891-901.
4. Lassen JF, Burzotta F, Banning AP, et al. Percutaneous coronary intervention for the left main stem and other bifurcation lesions:12th consensus document from the European Bifurcation Club. EuroIntervention. 2018;13:15a40-1553.
5. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival:overview of 10-year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
6. Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117-1124.
7. Serruys PW, Onuma Y, Garg S, et al. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010;55:1093-1101.
8. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur Heart J. 2019;40:87-165.
9. Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease:A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212-2241.
10. Cid Álvarez AB, Rodríguez Leor O, Moreno R, Pérez de Prado A. Registro Español de Hemodinámica y Cardiología Intervencionista. XXVIII Informe Oficial de la Sección de Hemodinámica y Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2018). Rev Esp Cardiol. 2019;72:1043-1053.
11. Oviedo C, Maehara A, Mintz GS, et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations:where is the plaque really located?Circ Cardiovasc Interv. 2010;3:105-112.
12. Medrano-Gracia P, Ormiston J, Webster M, et al. A computational atlas of normal coronary artery anatomy. EuroIntervention. 2016;12:845-854.
13. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126.595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28-40.
14. Angelini P. Coronary artery anomalies:an entity in search of an identity. Circulation. 2007;115:1296-305.
15. Cheezum MK, Liberthson RR, Shah NR, et al. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69:1592-1608.
16. Stone GW, Kappetein AP, Sabik JF, et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N Engl J Med. 2019;381:1820-1830.
17. Lindstaedt M, Spiecker M, Perings C, et al. How good are experienced interventional cardiologists at predicting the functional significance of intermediate or equivocal left main coronary artery stenosis?Int J Cardiol. 2007;120:254-261.
18. Fisher LD, Judkins MP, Lesperance J, et al. Reproducibility of coronary arteriographic reading in the coronary artery surgery study (CASS). Cathet Cardiovasc Diagn. 1982;8:565-575.
19. Kern MJ, Lim MJ, Boldstein JA. Hemodynamic Rounds:Interpretation of Cardiac Pathophysiology from Pressure Waveform Analysis. Oxford:John Wiley &Sons;2018. 342-343.
20. Kandzari DE, Colombo A, Park SJ, et al. Revascularization for unprotected left main disease:evolution of the evidence basis to redefine treatment standards. J Am Coll Cardiol. 2009;54:1576-1588.
21. Botman CJ, Schonberger J, Koolen S, et al Does stenosis severity of native vessels influence bypass graft patency?A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83:2093-2097.
22. Mintz GS, Lefèvre T, Lassen JF, et al. Intravascular ultrasound in the evaluation and treatment of left main coronary artery disease:a consensus statement from the European Bifurcation Club. EuroIntervention. 2018;14:e467-e474.
23. Kang SJ, Ahn JM, Song H. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562-569.
24. De la Torre Hernández JM, Hernández F, Alfonso F, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351-358.
25. De la Torre Hernández JM, García Camarero T, Baz Alonso JA, et al. Outcomes of predefined optimisation criteria for intravascular ultrasound guidance of left main stenting. EuroIntervention. 2020;16:210-217.
26. Kang SJ, Ahn JM, Kim WJ. Functional and morphological assessment of side branch after left main coronary artery bifurcation stenting with cross-over technique. Catheter Cardiovasc Interv. 2014;83:545-552.
27. De la Torre Hernández JM, JoséAntonio Baz JA, Gómez Hospital JA, et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease:pooled analysis at patient level of 4 registries. JACC Cardiovasc Interv. 2014;7:244-254.
28. Wang Y, Mintz GS, Gu Z, et al. Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc Disord. 2018;18:115.
29. Ramadan R, Boden WE, Kinlay S. Management of Left Main Coronary Artery Disease. J Am Heart Assoc. 2018;7:e008151.
30. Modi BN, van de Hoef TP, Piek JJ, et al. Physiological assessment of left main coronary artery disease. EuroIntervention. 2017;13:820-827.
31. Hamilos M, Muller O, Cuisset T, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505-1512.
32. Warisawa T, Cook CM, Rajkumar C, et al. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655-1664.
33. Johnson TW, Räber L, di Mario C, et al. Clinical use of intracoronary imaging. Part 2:acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making:an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40:2566-2584.
34. Mock MB, Ringqvist I, Fisher LD, et al. Survival of medically treated patients in the coronary artery surgery study (CASS) registry. Circulation. 1982;66:562-568.
35. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival:overview of 10-year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
36. Modolo R, Chichareon P, Kogame N, et al. Contemporary Outcomes Following Coronary Artery Bypass Graft Surgery for Left Main Disease. J Am Coll Cardiol. 2019;73:1877-1886.
37. Ellis SG, Tamai H, Nobuyoshi M, et al. Contemporary percutaneous treatment of unprotected left main coronary stenosis:Initial results from a multicenter registry analysis 1994-1996. Circulation. 1997;96:3867-3872.
38. Park SJ, Kim YH, Lee BK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis:Comparison with bare metal stent implantation. J Am Coll Cardiol. 2005;45:351-356.
39. Mehilli J, Kastrati A, Byrne R, et al. Paclitaxel- versus Sirolimus-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2009;53:1760-1768.
40. Valgimigli M, Chieffo A, Lefebre T, et al. Revisiting the incidence and temporal distribution of cardiac and sudden death in patients undergoing elective intervention for unprotected left main coronary artery stenosis in the drug eluting stent era. EuroIntervention. 2007;2:435-443.
41. Biondi-Zoccai GG, Lotrionte M, Moretti C, et al. A collaborative systematic review and meta-analysis on 1278 patients undergoing percutaneous drug-eluting stenting for unprotected left main coronary artery disease. Am Heart J. 2008;155:274-283.
42. Chieffo A, Stankovic G, Bonizzoni E, et al. Early and mid-term results of drug-eluting stent implantation in unprotected left main. Circulation. 2005;111:791-795.
43. Buszman PE, Buszman PP, Kiesz RS, et al. Early and long-term results of unprotected left main coronary artery stenting:the LE MANS (Left Main Coronary Artery Stenting) registry. J Am Coll Cardiol. 2009;54:1500-1511.
44. Morice MC, Serruys PW, Kappetein AP, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2010;121:2645-2653.
45. Boudriot E, Thiele H, Walther T, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011;57:538-545.
46. Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718-1727.
47. Holm NR, Mäkikallio T, Lindsay MM, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis:updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. 2020;395:191-199.
48. Giacoppo D, Colleran R, Cassese S, et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis:A systematic review and meta-analysis. JAMA Cardiol. 2017;2:1079-1088.
49. Palmerini T, Serruys P, Kappetein AP, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease:A meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017;190:54-63.
50. Rahouma M, Abouarab A, Di Franco A, et al. Percutaneous coronary intervention versus coronary bypass surgery for unprotected left main disease:a meta-analysis of randomized controlled trials. Ann Cardiothorac Surg. 2018;7:454-462.
51. Raber L, Juni P, Loffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55:1178-1188.
52. Pappalardo A, Mamas MA, Imola F, et al. Percutaneous coronary intervention of unprotected left main coronary artery disease as culprit lesion in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2011;4:618-626.
53. Coughlan JJ, Blake N, Chongprasertpon N, et al. Revascularisation of left main stem disease:a prospective analysis of modern practice and outcomes in a non-surgical centre. Open Heart. 2018;5:e000804.
54. Cheng HY, Wang KT, Lin WH, et al. Percutaneous Coronary Intervention for Left Main Coronary Artery Disease —A Single Hospital Experience without On-Site Cardiac Surgery. Acta Cardiol Sin. 2015;31:267-279.
55. Piqueras Flores J, Sanchez Perez I, Lopez Lluva MT, Lozano F. Clinical results at a long-term follow-up of percutaneous coronary intervention in left main coronary artery disease [abstract]. Eur Heart J Suppl. 2018;7(supplement):S209.
56. Xu B, Redfors B, Yang Y, et al. Impact of Operator Experience and Volume on Outcomes After Left Main Coronary Artery Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2016;9:2086-2093.
57. Teirstein PS. Unprotected left main intervention:patient selection, operator technique, and clinical outcomes. JACC Cardiovasc Interv. 2008;1:5-13.
58. Burzotta F, Trani C. Jailed balloon protection and rescue balloon jailing techniques set the field for safer bifurcation provisional stenting. Int J Cardiol. 2015;201:376-377.
59. Tsuchikane T, Aizawa T, Tamai H, et al. Pre drug-eluting stent debulking of bifurcated coronary lesions. J Am Coll Cardiol. 2007;50:1941-1945.
60. Tanaka N, Terashima M, Kinoshita Y, et al. Unprotected left main coronary artery bifurcation stenosis:Impact of plaque debulking prior to single sirolimus-eluting stent implantation. J Invasive Cardiol. 2008;20:505-510.
61. Foin N, Sen S, Allegria E, et al. Maximal expansion capacity with current DES platforms:a critical factor for stent selection in the treatment of left main bifurcations?EuroIntervention. 2013;8:1315-1325.
62. Ng J, Foin N, Ang HY, et al. Over-expansion capacity and stent design model:An update with contemporary DES platforms. Int J Cardiol. 2016;221:171-179.
63. Rhee TM, Park KW, Lee JM, et al. Predictors and long-term clinical outcome of longitudinal stent deformation:insights from pooled analysis of Korean multicenter drug-eluting stent cohort. Circ Cardiovasc Interv. 2017;10:e005518.
64. Pandya S, Kim Y-H, Meyers S, et al. Drug-eluting versus bare-metal stents in unprotected left main stenosis. JACC Cardiovasc Interv. 2010;3:602-611.
65. Chieffo A, Morici N, Maisano F, et al. Percutaneous treatment with drug-eluting stent implantation versus bypass surgery for unprotected left main stenosis:a single-center experience. Circulation. 2006;113:2542-2547.
66. Carrie D, Lhermusier T, Hmem M, et al. Clinical and angiographic outcome of paclitaxel-eluting stent implantation for unprotected left main coronary artery bifurcation narrowing. Eurolntervention. 2006;1:396-402.
67. Burzotta F, Lassen JF, Banning AP, et al. Percutaneous coronary intervention in left main coronary artery disease:the 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14:112-120.
68. Fajadet J, Capodanno D, Stone GW. Management of Left Main Disease:An Update. Eur Heart J. 2019;40:1454-1466.
69. De Maria GL, Banning AP. Use of Intravascular Ultrasound Imaging in Percutaneous Coronary Intervention to Treat Left Main Coronary Artery Disease. Interv Cardiol. 2017;12:8-12.
70. Chen X, Li X, Zhang JJ, et al. 3-Year Outcomes of the DKCRUSH-V Trial Comparing DK Crush With Provisional Stenting for Left Main Bifurcation Lesions. JACC Cardiovasc Interv. 2019;12:1927-1937.
71. Prati F, Romagnoli E, Gatto L, et al. Clinical Impact of Suboptimal Stenting and Residual Intrastent Plaque/Thrombus Protrusion in Patients With Acute Coronary Syndrome:The CLI-OPCI ACS Substudy (Centro per la Lotta Control L'Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ Cardiovasc Interv. 2016;9:e003726.
72. Kang SJ, Ahn JM, Song H, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562-569.
73. Park SJ, Kim YH, Park DW, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167-177.
74. Nam CW, Hur SH, Koo BK, et al. Fractional flow reserve versus angiography in left circumflex ostial intervention after left main crossover stenting. Korean Circ J. 2011;41:304-307.
75. Serruys PW, Chichareon P, Modolo R, et al. The SYNTAX score on its way out or …towards artificial intelligence:part I. EuroIntervention 2020;16:44-59.
76. Steigen TK, Maeng M, Wiseth R, et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions:the Nordic bifurcation study. Circulation. 2006;114:1955-1961.
77. Colombo A, Bramucci E, Sacca S, et al. Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations:the CACTUS (Coronary Bifurcations:Application of the Crushing Technique Using Sirolimus-Eluting Stents) Study. Circulation. 2009;119:71-78.
78. Hildick-Smith D, De Belder AJ, Cooter N, et al. Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions:the British Bifurcation Coronary Study:old, new, and evolving strategies. Circulation. 2010;121:1235-1243.
79. Maeng M, Holm NR, Erglis A, et al. Long-term results after simple versus complex stenting of coronary artery bifurcation lesions:Nordic Bifurcation Study 5-year follow-up results. J Am Coll Cardiol. 2013;62:30-34.