Structural intervention
REC Interv Cardiol. 2021;3:112-118
A decade of left atrial appendage closure: from procedural data to long-term clinical benefit
Una década de cierre percutáneo de la orejuela izquierda: desde el procedimiento hasta el beneficio a largo plazo
aServicio Endovascular, Hospital Virgen Macarena, Seville, Spain
bDepartamento de Medicina Preventiva y Salud Pública, Facultad de Medicina, Seville, Spain
ABSTRACT
Introduction and objectives: Although early discharge protocols after transcatheter aortic valve implantation (TAVI) have demonstrated to be safe in various studies, they are usually applied in high-experience centers. This study analyzes the length of stay of the first 100 patients undergoing TAVI in a center without on-site cardiac surgery, differentiating between very early (< 24 hours), early (24-48 hours), and late discharge (> 48 hours). Furthermore, the study evaluates the feasibility of an early discharge protocol during the team’s learning curve.
Methods: We conducted a prospective observational study from April 2022 through January 2024. A preand postoperative management protocol was implemented, including assessments in the Valvular Heart Disease Clinic, admission to the cardiac surgery intensive care unit with electrocardiographic monitoring, and specific discharge criteria in full compliance with an established protocol for the management of conduction disorders. Early follow-up evaluations were performed in the outpatiently after discharge.
Results: A total of 100 patients (50% women) were included, with a mean age of 82.4 ± 5.3 years and a EuroSCORE II score of 4.38 ± 5.1%. The median length of stay was 2 days (range, 1-19). A total of 27.27% of patients were discharged in < 24 hours, 48.49% within the 24-48 hours following implantation, and 24.24% 48 hours later. The 30-day cardiovascular mortality rate was 1%. A total of 6 patients were readmitted with procedural complications within the first 30 days.
Conclusions: The implementation of a standardized care protocol allows for early and safe discharge in most patients, even during the team’s learning cuve.
Keywords: TAVI. Transcatheter aortic valve implantation. Length of stay. Early discharge. Learning curve.
RESUMEN
Introducción y objetivos: Los protocolos de alta precoz tras el implante percutáneo de válvula aórtica (TAVI) han demostrado ser seguros en diversos estudios, aunque solo se aplican en centros con amplia experiencia. Este estudio analiza la duración de la estancia hospitalaria de los primeros 100 pacientes receptores de TAVI en un centro sin cirugía cardiaca in situ, diferenciando entre alta muy temprana (< 24 horas), temprana (24-48 horas) y tardía (> 48 horas), y evalúa la viabilidad de un protocolo de alta temprana durante la fase de aprendizaje del equipo.
Métodos: Estudio observacional prospectivo realizado entre abril de 2022 y enero de 2024. Se implementó un protocolo de cuidados prey posprocedimiento, que incluye valoración en la consulta de patología valvular, ingreso en la unidad de cuidados agudos cardiológicos con monitorización electrocardiográfica y criterios específicos para el alta según un protocolo establecido para el tratamiento de los trastornos de la conducción. Se realizó una evaluación precoz en la consulta tras el alta.
Resultados: Se incluyó a 100 pacientes (el 50% mujeres), con una edad media de 82,4 ± 5,3 años y EuroSCORE II de 4,38 ± 5,1%. La mediana de estancia hospitalaria fue de 2 días (rango: 1-19). Se dio de alta al 27,27% de los pacientes en < 24 horas, al 48,49% en las 24-48 horas posteriores al implante y al 24,24% después de 48 horas. La mortalidad de causa cardiovascular a 30 días fue del 1%. En los primeros 30 días, 6 pacientes reingresaron por motivos relacionados con el procedimiento.
Conclusiones: La aplicación de un protocolo de cuidados estandarizado permite un alta temprana y segura en la mayoría de los pacientes, incluso durante la fase de aprendizaje del equipo.
Palabras clave: TAVI. Implante percutáneo de válvula aórtica. Estancia hospitalaria. Alta temprana. Curva de aprendizaje.
Abbreviations
CLBBB: complete left bundle branch block. CRBBB: complete right bundle branch block. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
In our setting, transcatheter aortic valve implantation (TAVI) has become the treatment of choice for patients older than 75 years or with high surgical risk.1 Despite the good results documented in various studies, the stay after the procedure remains considerably long. According to data from the Spanish registry2, the mean length of stay is approximately 8 days. Given the increasing volume of patients, it is essential to implement protocols that optimize the length of stay and facilitate early discharge.
Experiences documented to this date on early discharge protocols after TAVI have demonstrated their safety profile.3-13 However, there is no uniform definition of the term “early discharge,” as it can range from 24 to 72 hours after the procedure.3-13
Most studies share common characteristics. On the one hand, they focus on procedures with a minimalist approach that favors faster patient recovery.14,15 On the other hand, many of them exclusively include patients with favorable pre-implant conditions,3-5,10,12 such as absence of frailty, adequate femoral access for transcatheter closure, absence of advanced conduction disorders, low-risk aortic annulus anatomy, body mass index < 35, left ventricular ejection fraction > 30%, and adequate family support. Consequently, these protocols only cover 22-55% of patients treated with TAVI.
A study conducted by a Spanish group has shown that early discharge, combined with artificial intelligence-based follow-up, is a safe strategy comparable to prolonged hospitalization in an unselected population after TAVI.13
Another important aspect is the type of valves used in the studies. Although the safety profile of early discharge after balloon-expandable valve implantation has been demonstrated,6,7 evidence on self-expanding valves is scarcer, due to doubts about the occurrence of conduction disturbances in the following days. However, in recent years, experiences have been published indicating that early discharge after the implantation of this type of valve is also safe.4,5,8,13,14
Finally, another relevant issue in these studies is that they have been conducted in highly experienced centers.3-13 Several analyses show that centers with a higher volume of procedures and more accumulated experience have lower complication rates and better overall results,16,17 which may translate into greater confidence in adopting early discharge practices.
It seems clear that reducing the length of stay through the implementation of early discharge protocols is a strategy that has demonstrated its feasibility in experienced centers. However, its application in those starting TAVI programs requires additional studies that ensure comparable results in terms of safety. Therefore, the objective of our study is to evaluate the length of stay of the first 100 patients treated with TAVI in our hospital (very early discharge: < 24 hours; early discharge: 24-48 hours; late discharge: > 48 hours) and determine the feasibility of establishing an early discharge protocol during the team’s learning curve.
METHODS
Patient selection and follow-up
We conducted a prospective, single-center registry that consecutively included all patients with severe symptomatic aortic stenosis who underwent TAVI in a center without on-site cardiac surgery, from the beginning of the program. The reference cardiac surgery department is located in a different center 2 km away.
The patients’ baseline characteristics, pre- and postoperative electrocardiographic and echocardiographic findings, the procedural characteristics, and the 30-day and 1-year clinical outcomes were recorded. The registry has been approved by Hospital Universitario Nuestra Señora de Candelaria ethics committee. Relevant informed consents were obtained.
Pre- and postoperative care protocol
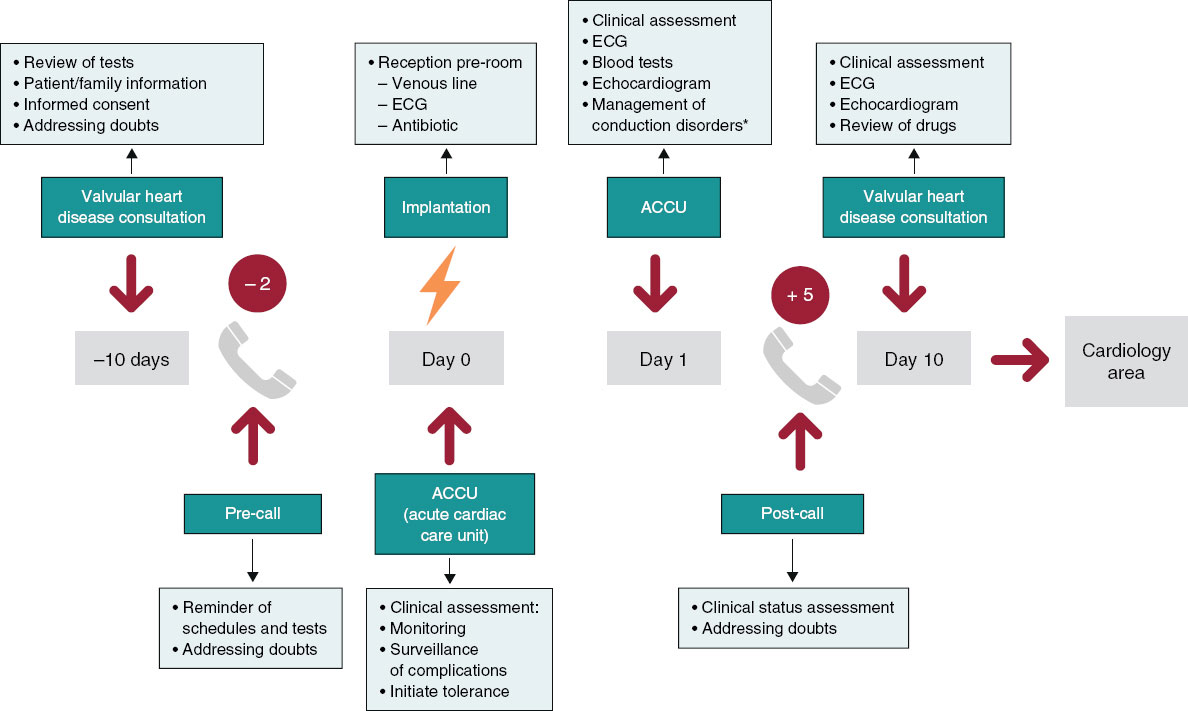
We developed a pre- and postoperative care protocol to standardize patient management (figure 1), in such a way that during the week prior to implantation, a cardiologist and a nurse specialized in TAVI jointly assess patients in the monographic valvular heart disease clinic. During this visit, additional tests are reviewed, the patient and their family are briefed on the procedure and possible complications, the informed consent form and a leaflet with relevant information are provided (drug management, how to proceed on implantation day, contact telephone number, etc.). Patients receive a call from nursing staff 48 hours prior to the intervention to remind them of the instructions.

Figure 1. Pre- and postoperative care protocol for transcatheter aortic valve implantation. ECG, electrocardiogram.
On the morning of the procedure, patients go to the interventional cardiology unit of our center, where a venous line is established, an electrocardiogram is performed, and prophylactic antibiotics are administered. After implantation, they are admitted to the acute cardiac care unit with electrocardiographic monitoring. The next day, the absence of complications is ruled out, an electrocardiogram and a transthoracic echocardiogram are performed, and the discharge decision is made according to the protocol for the approach and treatment of conduction disorders by Rodés-Cabau J et al.18 adapted to our center (figure 2).
During follow-up, a telephone consultation is conducted 48 hours after discharge to rule out any complications, and a face-to-face consultation with electrocardiogram and transthoracic echocardiogram is performed 10 days later. If progress is adequate, follow-up continues in general cardiology clinics. The care protocol and the algorithm for the treatment of conduction disorders are showin in figure 1 and figure 2, respectively.

Figure 2. Protocol for the management of conduction disorders after transcatheter aortic valve implantation. Very early discharge: < 24 hours; early discharge: 24-48 hours. AVB, atrioventricular block; ECG, electrocardiogram; EPS, electrophysiological study; LBBB: left bundle branch block; RBBB, right bundle branch block.
Procedural characteristics
During the team’s learning curve, a “mixed” approach was selected. Procedures were performed under general anesthesia. Regarding vascular access, transcatheter transfemoral primary access and closure with double Prostyle (Abbott Vascular, United States) and AngioSeal (Terumo) were prioritized; the radial route was used as secondary access. Pacing was performed with a balloon-tipped electrocatheter via jugular venous access. Urinary catheterization was omitted. The self-expanding Evolut R/PRO+ (Medtronic, United States and Ireland) and ACURATE neo2 (Boston Scientific, United States) valve were implanted. Transthoracic echocardiography was used for postoperative monitoring.
Endpoints
The endpoint of this study is to analyze the length of stay of the first 100 patients undergoing TAVI in our center, differentiating between very early (< 24 hours), early (24-48 hours), and late discharge (> 48 hours) and evaluate the possibility of establishing an early discharge protocol during the team’s learning curve.
In addition, we aim to evaluate clinical outcomes according to the VARC-3 standardized definitions,19 including cardiovascular and non-cardiovascular mortality at 30 days and between 30 days and 1 year, procedural or cardiovascular-related rehospitalizations at 30 days, need for pacemaker implantation in the same period, and rate of neurological events, bleeding complications > BARC 3a, major vascular complications, and cardiac structural complications.
Statistical analysis
Qualitative variables are expressed as absolute frequency and percentage, and the continuous ones as mean and standard deviation.
RESULTS
The first 100 patients treated with TAVI in a tertiary referral center without an on-site cardiac surgery department were prospectively included from April 2022 through January 2024.
The patients’ baseline characteristics
The patients’ baseline characteristics are shown in table 1. Of note, 50% were men, with a mean age of 82.4 ± 5.3 years. The STS score was 4.3 ± 5.1% and the EuroSCORE II score, 4.38 ± 5.1%. The main indication for implantation was age older than 75 years in 96% and high surgical risk in patients younger than 75 years in 4%. Regarding baseline conduction disorders, 10% of patients had complete left bundle branch block, 11%, complete right bundle branch block, and 12% a previously implanted pacemaker.
Table 1. Patient characteristics and indication for transcatheter aortic valve implantation
| Variables | Values |
|---|---|
| Baseline characteristics | |
| Age, years | 82.4 ± 5.36 |
| Sex (male/female) | 50/50 |
| Cardiovascular risk factors | |
| Hypertension | 73 (73) |
| Diabetes mellitus | 43 (43) |
| Dyslipidemia | 61 (61) |
| Active smoking | 16 (16) |
| Past medical history | |
| Coronary artery disease | 37 (37) |
| Previous cardiac surgery | 13 (13) |
| Atrial fibrillation | 34 (34) |
| Heart failure | 31 (31) |
| Chronic kidney disease | 44 (44) |
| Previous permanent pacemaker | 12 (12) |
| Previous LBBB | 10 (10) |
| Previous RBBB | 11 (11) |
| Baseline echocardiogram | |
| LVEF, % | 58.8 ± 10.1 |
| Peak gradient, mmHg | 71.4 ± 15.8 |
| Mean gradient, mmHg | 44.8 ± 10.8 |
| Aortic valve area, cm2 | 0.75 ± 0.139 |
| Aortic regurgitation | 36 (36) |
| Bicuspid aortic valve | 3 (3) |
| Surgical risk | |
| EuroSCORE II | 4.32 ± 5.15 |
| STS score | 4.38 ± 3.34 |
| Indication for implantation | |
| Age > 75 years | 96 (96) |
| High surgical risk in patients < 75 years | 4 (4) |
|
LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons. Unless otherwise indicated, data are expressed as frequency and percentage (n, %) or mean ± standard deviation. |
|
Procedural characteristics and perioperative results
Procedural characteristics and perioperative results are summarized in table 2. Procedures were performed under general anesthesia, and all patients were extubated in the operating room. Access was transcatheter transfemoral in 95% of patients, with closure using double Prostyle (Abbott Vascular, United States) and AngioSeal (Terumo, Japan). A total of 5% of these patients required surgical access by the vascular surgery service (2%, femoral; 3%, axillary). Second access was radial in 98% of cases.
Table 2. Procedural characteristics and perioperative outcomes
| Procedural characteristics and outcomes | Values |
|---|---|
| Characteristics | |
| Proctored (yes/no) | 24/76 |
| Vascular access | |
| Transcatheter femoral | 95 (95) |
| Surgical femoral | 2 (2) |
| Surgical axillary | 3 (3) |
| Native valve | 98 (98) |
| Valve-in-valve | 2 (2) |
| Predilation | 88 (88) |
| Postdilation | 22 (22) |
| Type of valve | |
| Evolut R/PRO+, Medtronic | 87 (87) |
| ACURATE neo2, Boston Scientific | 13 (13) |
| Perioperative outcomes | |
| Intraoperative mortality | 0 (0) |
| Post-implantation gradient > 20 mmHg | 0 (0) |
| Aortic regurgitation > grade II | 2 (2) |
| Aortic annulus rupture | 0 (0) |
| Aortic dissection | 0 (0) |
| Coronary artery occlusion | 0 (0) |
| Device embolization | 0 (0) |
| Conversion to surgery | 0 (0) |
|
Data are expressed as frequency and percentage (n, %). |
|
A total of 24 proctored cases were performed. Valve implantation was successful in 100% of cases. A total of 98 procedures were performed on native aortic valves (95, trileaflet; 3, bicuspid) and 2 on degenerated surgical bioprostheses using the chimney stent technique. Self-expanding valves were implanted (87%, Evolut R/PRO+; 13%, ACURATE neo2).
The immediate outcome was monitored with transthoracic echocardiography. More than moderate residual aortic regurgitation occurred in only 2 patients. There were no annular ruptures, aortic complications, coronary artery occlusions, device embolizations, or need for conversion to surgery. No patients died during the procedure.
Mortality and complications after TAVI
Mortality and complications after TAVI are shown in table 3. The 30-day cardiovascular mortality rate was 1% (1 patient who died during hospitalization due to heart failure complicated by a respiratory sepsis). In the follow-up after discharge, 2 deaths due to non-cardiovascular causes were recorded: 1 patient died from aspiration pneumonia at 6 months and another due to complications derived from colon cancer 9 months after implantation.
Table 3. Complications and mortality after transcatheter aortic valve implantation
| Complications and Mortality | n (%) |
|---|---|
| Transient LBBB | 26 (33) |
| Persistent LBBB at discharge | 6 (7.6) |
| Pacemaker implantation at 30 days | 11 (12.5) |
| Stroke | 1 (1) |
| Bleeding complications > BARC 3a | 1 (1) |
| Major vascular complications | 4 (4) |
| Cardiovascular rehospitalization at 30 days | 6 (6) |
| Cardiovascular mortality at 30 days | 1 (1) |
| Non-cardiovascular mortality at 30 days | 0 (0) |
| Cardiovascular mortality from 30 days to 1 year | 0 (0) |
| Non-cardiovascular mortality from 30 days to 1 year | 2 (2) |
|
BARC: Bleeding Academy Research Consortium scale; LBBB: left bundle branch block. a Data are expressed in numbers and percentages (n, %). |
|
Within the first 30 days, 6 patients required admission for procedural or cardiovascular-related causes. The reason for admission was infection in 3 patients: a wound infection in a patient who underwent surgical femoral access, an early infective endocarditis that had a good outcome with optimal medical therapy, and a pacemaker pocket infection that required device explantation and contralateral implantation. One patient was admitted with heart failure for developing rapid atrial fibrillation. Two patients were admitted for syncope; one had a PR interval of 350 ms and the other complete atrioventricular block. Both underwent permanent pacemaker implantation.
The rate of major vascular complications was 4%: 3 patients presented stenosis or dissection of the common femoral artery requiring stent implantation during the same procedure; furthermore, a femoral pseudoaneurysm was detected in 1 patient and was surgically repaired. One case of BARC > 3a bleeding complication was detected; the patient required transfusion of 2 packed red blood cell concentrates due to lower GI bleeding.
One patient had a stroke at 24 hours. The need for permanent pacemaker implantation was 12.5% within the first 30 days. The delay for permanent pacemaker implantation once the indication was established was < 48 hours.
Length of stay
The pre-specified care protocol was implemented in all patients (figure 1 and figure 2). As a result, the median length of stay was 2 days (range, 1-19) for all patients (table 4). Regarding time to discharge (figure 3), 27 patients (27.27%) were discharged within the first 24 hours (very early discharge), 48 (48.49%) between 24 and 48 hours after implantation (early discharge), while 24 patients (24.24%) had to be hospitalized for more than 48 hours (late discharge). Late discharges corresponded to 8 patients whose TAVI was performed during admission for heart failure or cardiogenic shock, 10 patients who had pre-existing conduction disorders (mainly first-degree atrioventricular block or complete right bundle branch block) or who developed them after implantation and required prolonged electrocardiographic monitoring, 2 patients who underwent surgical femoral access, 2 patients with major vascular complications, 1 patient who had a stroke at 24 hours, and 1 patient who developed bacteremia due to Streptococcus mitis.

Figure 3. Length of stay of the first 100 TAVI patients. TAVI, transcatheter aortic valve implantation.
Table 4. Length of stay
| Length of stay | Time |
|---|---|
| Length of stay, days | 2 (1-19) |
| Very early discharge < 24 hours | 27 (27.27) |
| Early discharge 24-48 hours | 48 (48.49) |
| Late discharge > 48 hours | 24 (24.24) |
|
ME: median; n: number. Data are expressed in days and median (interquartile range) or in number and percentage (n, %). |
|
DISCUSSION
The main findings of our study are a) it is feasible to establish a protocol that favors the early discharge of patients during the medical team’s learning curve; b) approximately 75% of patients achieve early discharge (< 48 hours); and c) it is a safe strategy associated with a low rate of adverse events at 30 days.
The progressive increase in the number of patients we will be facing in the coming years makes it essential to establish protocols that optimize the length of stay and allow for efficient use of resources.
Several experiences can be found in the literature confirming that early discharge protocols after TAVI are safe. The main problems they present are the lack of a standardized definition of early discharge, which can vary from 24 to 72 hours,3-13 and that most studies on early discharge agree on including patients with favorable anatomical characteristics,3-5,10,12 such as adequate femoral access for transcatheter closure, absence of advanced conduction disorders, low-risk aortic annulus anatomy, body mass index < 35, and left ventricular ejection fraction > 30%; in addition, they exclude patients with factors that could prolong the length of stay, such as frailty or lack of family support. Following these criteria, only 59% of our patients would have been eligible for an early discharge program, yet we were able to discharge 75% of the patients within the first 48 hours. In our series, the median length of stay for outpatients with the favorable characteristics described above is 2 days (1-10), and that of outpatients who did not meet favorable criteria is 2 days (1-18), while the length of stay of the 8 patients undergoing TAVI during hospitalization for decompensated heart failure or cardiogenic shock is 10.5 days (1-30). In this regard, the experience of Herrero et al.13 is noteworthy, who demonstrated in their study that most patients (73%) in an unselected population can be safely discharged within the first 24-48 hours.
The minimalist approach14,15 is another key aspect that has been shown to favor early discharge. This approach is especially suitable when conducted by experienced teams and applied to collaborative, hemodynamically stable patients without anatomical characteristics involving a higher risk of complications. Its implementation should be progressive, so that the safety profile of the procedure is guaranteed as the fundamental priority. We opted to start the TAVI program with a “mixed” approach with general anesthesia, mainly femoral or radial access, pacing with a transvenous pacemaker, and monitoring with transthoracic echocardiography until the team overcame the learning curve. All patients were successfully extubated in the operating room. Therefore, we believe that this type of approach did not cause any delays in patient discharge. Once the first 100 cases have been completed, we are transitioning towards a fully minimalist procedure due to the benefits this entails for the patient.
The safety profile of early discharge after the implantation of self-expanding valves has been widely discussed4,5,8,13,14, due to the increased risk of developing cardiac conduction disorders. In our study, all patients received a self-expanding valve. The algorithm by Rodés-Cabau et al.18 was applied to determine the duration of electrocardiographic monitoring and manage conduction disorders. The need for permanent pacemaker implantation was confirmed in 12.5% of cases within the first 30 days. Only 2 patients required readmission after discharge due to advanced conduction disorders requiring pacemaker implantation.
In addition, it is important to have a close follow-up system that allows for the early detection of post-discharge complications and ensures continuity of care. To this end, our patients receive a telephone consultation 48 hours after discharge, and a face-to-face consultation with electrocardiograms and echocardiograms being performed at 10 days. They also have a telephone number to contact the team during working hours. Undoubtedly, this is a system that takes human and economic resources. Experiences have been reported in which the implementation of a virtual voice assistant facilitates the detection of complications, thus demonstrating the effectiveness of this system,13 which should be considered the standard we should look for in clinical practice.
Reducing the length of stay after TAVI requires a real commitment from the heart team to the implementation and adherence to an early discharge protocol. The BENCHMARK study20 showed that adherence to an 8-point structured protocol allows for shorter lengths of stay of approximately 2 days. The key aspects identified in this protocol include educating the health care team, a clear definition of discharge criteria, a structured decision algorithm to assess the need for pacemaker implantation, echocardiographic or angiographic follow-up of the puncture site, early patient mobilization, patient and family education, and anticipated discharge planning from admission. In our own experience, the role of a cardiologist coordinating the TAVI program, along with close collaboration with key units and services, such as acute cardiac care, imaging, electrophysiology, anesthesiology, vascular surgery, and radiology, has enabled the effective implementation of these strategies. This approach has facilitated the consolidation of an early discharge protocol from the start of the program.
Limitations
Our study has multiple limitations that may affect result extrapolation. On the one hand, this was a study of observational design conducted in a single center and with a small sample size; additionally, a total of 24 cases were performed under supervision. On the other hand, only self-expanding valves were used, meaning that results cannot be generalized to other types of valves.
CONCLUSIONS
During the team’s learning curve, the application of a standardized care protocol allows for early and safe discharge after TAVI in most patients in an unselected population.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Approval was obtained from Hospital Universitario Nuestra Señora de Candelaria ethics committee. Informed consents are available. The SAGER guidelines regarding potential sex and gender biases have been followed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used for the development of the study.
AUTHORS’ CONTRIBUTIONS
R. Pimienta González and A. Quijada Fumero participated developing the protocol, collecting data, and drafting the manuscript. All authors participated in the study design, critically reviewed the text, and approved its final version.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- The constant increase in the number of TAVIs being performed has created the need to establish protocols that reduce the length of stay.
- Several studies have shown that early discharge protocols are safe, as long as they are implemented in experienced centers.
- However, it is still unknown whether it is feasible to apply an early discharge protocol from the beginning of a TAVI program.
WHAT DOES THIS STUDY ADD?
- The implementation of a care protocol adapted to the specific characteristics of each center allows for early and safe discharge of most patients after TAVI, even during the team’s learning curve.
REFERENCES
1. Vahanian A, Beyersdorf F, Praz F, et al.;ESC Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
2. Jimenez-Quevedo P, Munoz-Garcia A, Trillo-Nouche R, et al. Time trend in transcatheter aortic valve implantation:an analysis of the Spanish TAVI registry. REC Interv Cardiol. 2020;2:98-105.
3. Wood DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers:the 3M TAVR study. JACC Cardiovasc Interv. 2019;12:459-469.
4. Asmarats L, Millán X, Cubero-Gallego H, et al. Implementing a fast-track TAVI pathway in times of COVID-19:necessity or opportunity?REC Interv Cardiol. 2022;4:150-152.
5. García Carreño J, Zatarain E, Tamargo M, et al. Feasibility and safety of early discharge after transcatheter aortic valve implantation. Rev Esp Cardiol. 2023;76:655-663.
6. Barbanti M, Van Mourik M, Spence M, et al. Optimising patient discharge management after transfemoral aortic valve implantation:the multicentre European FAST-TAVI trial. EuroIntervention. 2019;15:147-154.
7. Kamioka N, Wells J, Keegan P, et al. Predictors and clinical outcomes of next-day discharge after minimalist transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11:107-115.
8. Moriyama N, Vento A, Laine M, et al. Safety of next-day discharge after transfemoral transcatheter aortic valve replacement with a self-expandable versus balloon-expandable valve prosthesis. Circ Cardiovasc Interv. 2019;12:1-9.
9. Baekke PS, Jørgensen TH, Søndergaard L. Impact of early hospital discharge on clinical outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2021;98:E282-E290.
10. Krishnaswamy A, Isogai T, Agrawal A, et al. Feasibility and safety of same-day discharge following transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2022;15:575-589.
11. Hanna G, Macdonald D, Bittira B, et al. The safety of early discharge following transcatheter aortic valve implantation among patients in Northern Ontario and rural areas utilizing the Vancouver 3M TAVR study clinical pathway. CJC Open. 2022;4:1053-1059.
12. Barker M, Sathananthan J, Perdoncin E, et al. Same-day discharge post-transcatheter aortic valve replacement during the COVID-19 pandemic:the multicenter PROTECT TAVR study. JACC Cardiovasc Interv. 2022;15:590-598.
13. Herrero Brocal M, Samper R, Riquelme J, et al. Early discharge programme after transcatheter aortic valve implantation based on close follow-up supported by telemonitoring using artificial intelligence:the TeleTAVI study. Eur Heart J Digit Health. 2024;6:73-81.
14. Lauck SB, Wood DA, Baumbusch J, et al. Vancouver transcatheter aortic valve replacement clinical pathway:minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9:312-321.
15. Pinar Bermúdez E. Debate:Minimalist approach to TAVI as a default strategy. REC Interv Cardiol. 2021;3:304-306.
16. Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380:2541-2550.
17. Núñez-Gil IJ, Elola J, García-Márquez M, et al. Percutaneous and surgical aortic valve replacement. Impact of volume and type of center on results. REC Interv Cardiol. 2021;3:103-111.
18. Rodes-Cabau J, Ellenbogen KA, Krahn AD, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement:JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:1086-1106.
19. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3:Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717-2746.
20. Frank D, Durand E, Lauck S, et al. A streamlined pathway for transcatheter aortic valve implantation:the BENCHMARK study. Eur Heart J. 2024;45:1904-1916.
*Corresponding author.
E-mail address: raquelpimientagonzalez@gmail.com (R. Pimienta González).
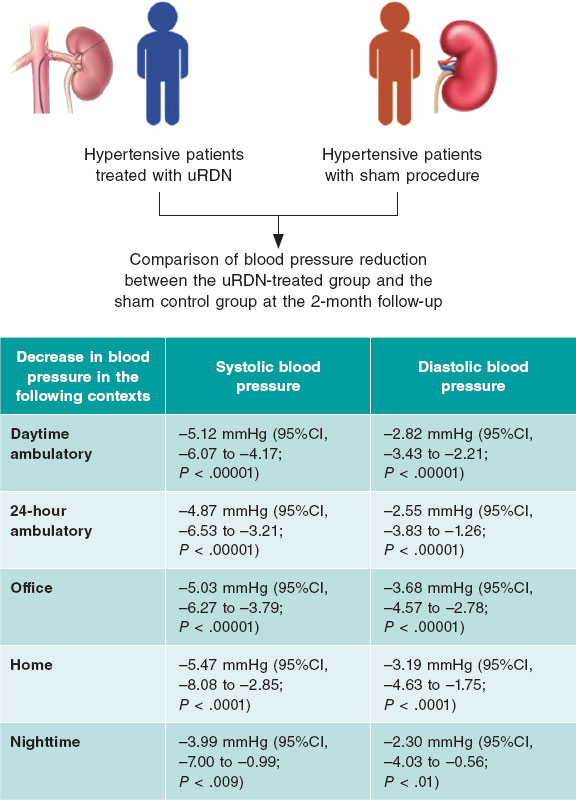
ABSTRACT
Introduction and objectives: Ultrasound renal denervation (uRDN) has emerged as an innovative therapeutic approach for the treatment of hypertension. However, its efficacy compared to medication remains uncertain. We aimed to assess the efficacy profile of uRDN vs sham groups focusing on its impact on daytime ambulatory blood pressure, 24-hour blood pressure, home blood pressure and office blood pressure.
Methods: We conducted a systematic search across Embase, PubMed, and Cochrane Library databases from their inception up 1 November 2024 to identify randomized controlled trials evaluating the efficacy of uRDN. Statistical analyses were performed using RevMan 6.3 software, utilizing the mean and standard deviation method to calculate mean differences with a 95% confidence interval (95%CI).
Results: A total of 4 studies were included in the final analysis with 642 patients. uRDN significantly reduced daytime ambulatory systolic blood pressure (SBP) (−5.12 mmHg; 95%CI, −6.07 to −4.16; P ≤ .00001), 24-h SBP (−4.87 mmHg; 95%CI, −6.53 to −3.21]; P ≤ .00001), office SBP (−5.03 mmHg; 95%CI, −6.27 to −3.79; P ≤ .00001) and showed a decrease in patient medication 6 months after the procedure.
Conclusions: Using uRDN leads to a lower blood pressure in patients within 2 months following the procedure. Additionally, after 6 months a significant decrease in drug use is observed.
This meta-analysis protocol was registered on PROSPERO on 7 July 2024 (CRD42024562852).
Keywords: Resistant hypertension. Ultrasound renal denervation. Systolic blood pressure. Diastolic blood pressure. Antihypertensive treatments.
RESUMEN
Introducción y objetivos: La denervación renal por ultrasonido (DRU) ha surgido como un enfoque terapéutico innovador para la hipertensión arterial resistente. Sin embargo, su eficacia en comparación con la medicación sigue siendo incierta. Nuestro objetivo fue evaluar la eficacia de la DRU frente a grupos simulados, con especial atención a su impacto sobre la presión arterial ambulatoria diurna, la presión arterial de 24 h, la presión arterial domiciliaria y la presión arterial en el consultorio.
Métodos: Se realizó una búsqueda sistemática en las bases de datos Embase, PubMed y Cochrane Library hasta el 1 de noviembre de 2024, para identificar ensayos controlados aleatorizados que evaluaran la efectividad de la DRU. Los análisis estadísticos se realizaron con el programa informático RevMan 6.3, utilizando la media y la desviación estándar para calcular las diferencias de medias con un intervalo de confianza del 95% (IC95%).
Resultados: En el análisis final se incluyeron cuatro estudios con 642 pacientes. La DRU redujo de manera significativa la presión arterial sistólica (PAS) ambulatoria diurna (−5,12 mmHg; IC95%, −6,07 a −4,16; p ≤ 0,00001), la PAS de 24 h (−4,87 mmHg; IC95%, −6,53 a −3,21; p ≤ 0,00001) y la PAS en la consulta (−5,03 mmHg; IC95%, −6,27 a −3,79; p ≤ 0,00001), y logró una disminución de la medicación de los pacientes a los 6 meses del procedimiento.
Conclusiones: El uso de DRU conlleva una reducción de la presión arterial a los 2 meses del procedimiento. Adicionalmente, transcurridos 6 meses se observó una disminución significativa del uso de medicación.
El protocolo de este metanálisis fue registrado en PROSPERO el 7 de julio de 2024 (CRD42024562852).
Palabras clave: Hipertensión resistente. Denervación renal por ultrasonido. Presión arterial sistólica. Presión arterial diastólica. Tratamiento antihipertensivo.
Abbreviations
BP: blood pressure. DBP: diastolic blood pressure. SBP: systolic blood pressure. RCT: randomized controlled trial. uRDN: ultrasound renal denervation.
INTRODUCTION
Hypertension is highly prevalent worldwide and well recognized as a major risk factor for cardiovascular, cerebrovascular, and renal complications.1 Despite the availability of numerous antihypertensive drugs that effectively mitigate hypertension-related organ damage,1,2 a substantial proportion of patients fail to attain adequate blood pressure (BP) control,3 which may be attributed to factors such as medication non-adherence or the presence of resistant hypertension,4,5 which is defined as the presence of uncontrolled BP of, at least, 130/80 mmHg despite the simultaneous prescription of, at least, 3 or 4 antihypertensive drugs of different classes, or controlled BP despite the prescription of, at least, 4 drugs, at the maximum tolerated doses, including a diuretic.6 The pathophysiology of hypertension is intricate and includes a diverse array of mechanisms, with sympathetic overdrive emerging as a pertinent factor in all forms of hypertension.7 Consequently, novel therapeutic approaches have emerged, including renal denervation (RDN), which aims to decrease renal sympathetic activity thereby reducing BP. RDN has drawn considerable attention as a guideline-recommended BP lowering treatment along with lifestyle changes and pharmacotherapy for patients with resistant hypertension.8,9 Recently, there has been growing consensus that RDN should also be considered for individuals whose hypertension is due to no therapeutic adherence.10-12 Early randomized controlled clinical trials yielded inconsistent findings on the efficacy profile of the intervention, with a substantial proportion of patients failing to respond across the trials.13,14 Potential explanations for the heterogeneous results include insufficient operator experience using the Symplicity Flex catheter (Medtronic, United States), the study participants’ baseline characteristics, and changes of antihypertensive medication.15 Subsequently, sham-controlled trials with better study designs, catheter technologies and procedural techniques have improved the BP-lowering safety and efficacy profile of RDN.16-18 Currently, various catheter systems are used for RDN, utilizing different technologies, such as radiofrequency-based systems like Symplicity Spyral (Medtronic, United States). Ultrasound-based catheters have also been developed, such as the Paradise (Recor Medical, United States), whose efficacy has been evaluated in multiple studies. Finally, there is a system based on alcohol-mediated RDN.19 Recently, the U.S. Food and Drug Administration (FDA) approved Medtronic Symplicity Spyral and Recor Paradise system as adjuvant therapies for the treatment of hypertension.20
The efficacy of the latter was evaluated in a multicenter, randomized, blinded and sham-controlled trial. Subsequently, it was determined in the REQUIRE RADIANCE-HTN SOLO,16 RADIANCE HTN TRIO17 and RADIANCE II,18 and REQUIRE21 trials. Results were heterogeneous between the RADIANCE and REQUIRE trials, which had limitations that may account for the varied results.10 Finally, uRDN was concluded to be safe for the treatment of hypertension, even in patients with resistant hypertension and poor medical adherence.19 The aim of this study is to conduct a systematic review and a meta-analysis to examine the antihypertensive efficacy of uRDN in patients with hypertension vs a sham group treatment.
METHODS
We conducted a systematic review and meta-analysis which strictly followed the clinical practice guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22 Methodological procedures were conducted in full compliance with the Cochrane Handbook of Systematic Reviews and Meta-Analysis of Interventions. This meta-analysis protocol was registered on PROSPERO 7 July 2024, under protocol ID: CRD42024562852.
Criteria of the included studies
Inclusion criteria were established to identify relevant studies: patients with resistant hypertension and randomized controlled trials (RCTs) comparing uRDN with sham groups, which did not undergo uRDN; RCTs reporting office, daytime ambulatory, home and 24-h ambulatory BP changes from baseline were included. We excluded those underreporting, at least, 1 of the following outcomes of interest: changes in BP between baseline and, at least, a 2-month follow up. In addition, we excluded non-English publications, case-control studies, case reports, single arm studies, letters to the editors, basic science research, meta-analyses, and review articles.
Literature search strategy
We conducted a comprehensive search across PubMed, EMBASE, and COCHRANE, from their inception until 1 November 2024. Keywords and free-text terms were used to explore literature on hypertension, renal denervation, and ultrasound ablation. Detailed search information for each database is provided in the Search strategy section of the supplementary data.
Screening of literature search strategy
Initially, a comprehensive database search was conducted to compile all relevant records. Duplicate entries were, then, manually removed using Zotero software. Afterwards, references were screened by title and abstract. When necessary, a full-text review was performed to ensure relevance and accuracy. Two authors (C. J. Palomino-Ojeda and L. H. García-Mena) independently assessed each the inclusion and quality of each article. Discrepancies were resolved by a third author (J. M. Guerrero-Hernández). Additionally, references cited in the included studies were scrutinized and included if they fulfilled the eligibility criteria.
Data extraction
Data extraction was conducted using Excel spreadsheets to record the following information: a) baseline characteristics of the study population, b) summaries detailing the characteristics of the included studies, c) outcome measures, and d) domains evaluated for quality assessment.
Assessing the risk of bias
Randomized controlled trials were assessed using Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2)22,23 from the Cochrane Handbook of Systematic Reviews of Interventions. Our analysis included a funnel plot for the primary endpoint daytime ambulatory systolic blood pressure (SBP) shown in figure 1 of the supplementary data.

Figure 1. PRISMA flow diagram. RDN, renal denervation.
Outcome measures
BP changes were assessed by comparing baseline values and follow-up measurements taken, at least, 2 months later. The mean difference was analyzed using the mean and standard deviation.
Data analysis
The efficacy profile of uRDN vs the sham control was analyzed using continuous data to calculate the mean difference with its corresponding 95% confidence interval (95%CI).24 This analysis assessed BP changes across groups with an, at least, 2-month follow-up while evaluating their mean difference.
Furthermore, an examination was conducted to discern any variation among office BP, ambulatory daytime BP, 24-h ambulatory BP, nighttime ambulatory BP, and home BP outcomes in trials that reported these results. This was achieved by computing the mean and its associated standard deviation for the difference between the 2 outcomes. The validated Campbell calculator was used to convert the measures of dispersion from the outcomes in the REQUIRE trial for data analysis.24 The level of heterogeneity was assessed using the I2 statistic.
Sensitivity analyses were conducted using a random effects model to account for variability among studies.25 Subgroup analyses were predefined for first and second-generation RDN trials, with tests for interaction for the primary endpoint.
Assessment of heterogeneity
Heterogeneity among the included studies was assessed using Cochran’s Q statistic. Additionally, the I2 statistic was used to quantify the proportion of total variation attributed to heterogeneity, with values > 50% indicating high heterogeneity. All statistical analyses (including the calculation of standardized mean difference, relative risk, and mean difference) were performed using RevMan 6.3. software.22
RESULTS
Study selection
A total of 448 studies were identified across database searches. A total of 392 studies were screened after removing duplicate studies, 388 of which were excluded due to single-arm study (n = 6); publication in a language different than English (n = 1); case-control or case report studies and literature review (n = 140); basic scientific research (n = 24); editorial letter (n = 8); different type of RDN studies (n = 83); studies with ≤ 10 participants (n = 6); and does not compare intervention of interest (n = 120). Finally, 4 studies meet all inclusion criteria and were eligible for analysis. An analysis of 642 patients from the 4 selected articles was conducted as they met the inclusion criteria. The PRISMA flow diagram of the study selection process is shown in figure 1.
Study characteristics
The studies included in our analysis included a total of 4 RCTs published from 2018 through 2023.16-18,21 All studies used uRDN and a sham control group. Two studies were performed in the United States/Europe,16,17 1 study only in the United States18 and the rest in Japan and South Korea.21 The baseline characteristics of the included studies were analyzed and summarized in table 1. Characteristics of the entire patient population are shown in table 2.
Table 1. Baseline characteristics and following intervention of the included studies population
| Reference | ||||||||
|---|---|---|---|---|---|---|---|---|
| RADIANCE HTN SOLO 201816 | RADIANCE-HTN TRIO 202117 | REQUIRE 202221 | RADIANCE II 202318 | |||||
| Group | uRDN | SHAM control | uRDN | SHAM control | uRDN | SHAM control | uRDN | SHAM control |
| N | 74 | 72 | 69 | 67 | 69 | 67 | 150 | 74 |
| Gender, female | 28 | 33 | 13 | 14 | 21 | 14 | 47 | 17 |
| Gender, male | 46 | 39 | 56 | 53 | 48 | 53 | 103 | 57 |
| Age, years, mean (SD) | 54.4 (10·2) | 53.8 (10·0) | 52.3 (7.5) | 52,8 (9.1) | 50.7 (11.4) | 55.6 (12.1) | 55.1 (9.9) | 54.9 (7.9) |
| Body mass index, mean (SD) | 29.9 (5.9) | 29.9 (5.0) | 32.8 (5.7) | 32.6 (5.4) | 29.5 (5.5) | 28.4 (4.5) | 30.1 (5.2) | 30.6 (5.2) |
| Abdominal obesity | 41 | 44 | 54 | 55 | – | – | 90 | 46 |
| GFR mL/min/1.73 m2 | 84.7 (16.2) | 83.2 (16.1) | 86 (25.2) | 82.2 (19.2) | 74.2 (16.2) | 69.6 (17.1) | 81.4 (14.4) | 82.3 (14.9) |
| GFR < 60 mL/min/1.73 m2 | 1 | 3 | 8 | 7 | 15 | 18 | 7 | 3 |
| Type 2 diabetes mellitus | 2 | 5 | 21 | 17 | 18 | 20 | 9 | 5 |
| Cardiovascular disease | – | – | 8 | 9 | 9 | 9 | 1 | – |
| Systolic BP at office screening, mm Hg | 142.6 (14.7) | 144.6 (15.9) | 161.9 (15.5) | 163.6 (16.8) | 157.6 (19.5) | 160.4 (14.9) | 155.8 (11.1) | 154.3 (10.6) |
| Diastolic BP at office screening, mm Hg | 92.3 (10.1) | Mean 93.6 (8.3) | 105.1 (11.6) | 103.3 (12.7) | 97.7 (16.6) | 95.3 (14.2) | 101.3 (6.7) | 99.1 (5.6) |
| HR at office screening, beats/min | 72 (12.1) | 72.6 (12.3) | 74.5 (11) | 77.6 (12.9) | 75.3 (10.8) | 71.5 (12.8) | 74.1 (12.0) | 73.6 (11.9) |
| Number of antihypertensive drugs at screening | 1: 33 2: 28 3: 1 | 1: 28 2: 27 3: 1 | 3: 27 4: 22 5: 20 | 3: 28 4: 24 5: 15 | 3: 32 4: 20 5: 17 | 3: 29 4: 23 5: 15 | 1: 52 2: 44 > 2: 0 | 1: 25 2: 25 > 2: 1 |
| Procedural time | 72.3 (23.3) | 38.2 (12.6) | 83.66 (22.71) | 41.33 (12.87) | 86.7 (54.0) | 40.2 (11.6) | 76.7 (25.2) | 43.9 (16.6) |
| Office systolic blood pressure at 2 months | 143.7 (16.7) | 149.7 (17.4) | 147.1 (20.3) | 152.1 (22) | – | – | 145.8 (15.9) | 151.2 (16.4) |
| Office diastolic blood pressure at 2 months | 94.2 (10.1) | 98 (10) | 96.6 (13.9) | 98.7 (13.8) | – | – | 96.0 (10.2) | 98.1 (11.2) |
| Daytime ambulatory systolic BP at 2 months | 141.9 (11.9) | 147.9 (13.3) | 141.0 (16.1) | 146.3 (18.8) | – | – | 135.6 (13) | 142.9 (10.5) |
| Daytime ambulatory diastolic BP at 2 months | 87.9 (7.1) | 90.9 (7.9) | 88.5 (11.6) | 90.7 (12.2) | – | – | 83.1 (7.6) | 87.0 (6.3) |
| 24-hour systolic BP at 2 months | 135.6 (11.4) | 140.7 (11.8) | 135.2 (16.0) | 140.5 (18.7) | – | – | 135.6 (13.0) | 142.9 (10.5) |
| 24-hour diastolic BP at 2 months | 83 (6.8) | 85.7 (7.1) | 83.6 (10.9) | 85.8 (12) | – | – | 83.1 (7.6) | 87.0 (6.3) |
| Home systolic BP at 2 months | 139.4 (11.7) | 146.6 (15.4) | 144.6 (18.2) | 149.9 (18.9) | – | – | 143.4 (12.3) | 148.8 (12.3) |
| Home diastolic BP at 2 months | 89.9 (7.8) | 93.3 (8.5) | 93.2 (14.7) | 96 (12.8) | – | – | 92.7 (7.4) | 95.5 (8.1) |
| Nighttime ambulatory systolic BP at 2 months | 125.6 (12.8) | 129.4 (13.1) | 126.3 (18.4) | 76.2 (12.2) | – | – | 125.5 (15.0) | 132.4 (12.2) |
| Nighttime ambulatory diastolic BP at 2 months | 74.8 (8.5) | 77.3 (8.5) | 131.9 (20.9) | 78.4 (13.2) | – | – | 75.1 (9.7) | 79.6 (7.5) |
|
BP, blood pressure; GFR, glomerular filtration rate; SD, standard deviation; uRDN, ultrasound renal denervation. |
||||||||
Table 2. Summary of included studies
| Study ID | Country | Study design | Total population | Compare interventions | Key findings |
|---|---|---|---|---|---|
| United States/Europe | RCT | 146 | uRDN vs SHAM control | Renal denervation resulted in a greater reduction in daytime ambulatory systolic blood pressure compared with a sham procedure | |
| United States/Europe | RCT | 136 | uRDN vs SHAM control | Renal denervation reduced daytime ambulatory systolic blood pressure more than the sham procedure | |
| Japan and South Korea | RCT | 136 | uRDN vs SHAM control | Is the first trial of ultrasound renal denervation in Asian patients with hypertension on antihypertensive therapy | |
| The study did not show a significant difference in ambulatory blood pressure reductions in treated patients with resistant hypertension | |||||
| United States | RCT | 224 | uRDN vs SHAM control | The primary efficacy outcome was the mean change in daytime ambulatory SBP at 2 months | |
| No major adverse events were reported in either group | |||||
|
RCT: randomized controlled trial; SBP, systolic blood pressure; uRDN: ultrasound renal denervation. |
|||||
In the analysis of 642 patients, the mean age was 54.15 years ± 9.95, 70.8% were men, and the mean body mass index was estimated at 30 kg/m2 ± 5.3. Regarding comorbidities, 15.1% had type 2 diabetes mellitus, and 5.6%, cardiovascular disease. The mean glomerular filtration rate (GFR) was estimated at 82.25 mL/min/1.73 m2 ± 16.2. In addition, 9.6% of patients had GFR levels < 60 mL/min/1.73 m2. Of note, eligibility criteria in all trials include an estimated GFR > 40 mL/min/1.73 m2. Two studies— the RADIANCE-HTN SOLO and the RADIANCE II—included patients on 1 to 3 antihypertensive drugs and were designed as “Off Med” studies, meaning patients underwent a washout period with no antihypertensive treatment for 4 weeks in the RADIANCE-HTN SOLO and 8 weeks in the RADIANCE II. Additionally, patients who experienced complications such as high BP were given antihypertensive escape therapy.26 On the other hand, the RADIANCE-HTN TRIO and REQUIRE trials included patients on 3 to 5 antihypertensive drugs and evaluated the uRDN in patients on concomitant antihypertensive therapy. However, only the RADIANCE-HTN TRIO trial standardized antihypertensive treatment over a 4-week regimen with a fixed-dose of 3 drugs in a single pill including amlodipine 10 mg; valsartan 160 mg (or olmesartan 40 mg); and hydrochlorothiazide 25 mg. Additionally, treatment adherence was assessed by mass spectrometry.21,27,28 Enrollment criteria were comparable across the analyzed studies. Similarly, exclusion criteria were consistent in all studies; however, the RADIANCE trials additionally excluded patients with anatomical variations or alterations in renal artery anatomy, as detected on renal computed tomography or magnetic resonance angiography.27 In all studies, patients were blinded prior to the uRDN procedure. Furthermore, in all RADIANCE trials, blinding was implemented after the washout period or after the patients completed the fixed-dose treatment.18,26,27
Daytime ambulatory blood pressure
Patients treated with uRDN for up to 2 months experience a significant reduction of −5.12 mmHg (95%CI, −6.07 to −4.17; P < .00001); I2 = 2%) in daytime ambulatory SBP vs the sham group. Similarly, ambulatory diastolic blood pressure (DBP) dropped down to −2.82 mmHg (95%CI, −3.43 to −2.21; P < .00001; I2 = 0%) in patients with uRDN vs the sham group (figure 2A).

Figure 2. Meta-analysis of the effect of uRDN on blood pressure va a sham control. A: difference in daytime ambulatory BP up to 2 months; B: difference in 24-hour BP up to 2 months; C: difference in office BP up to 2 months; and D: difference in home BP up to 2 months. Forest plots showing the mean difference and SD from random assignments between the uRDN and sham control groups. 95%CI, 95% confidence interval; BP, blood pressure; SD, standard deviation; uRDN, ultrasound renal denervation. The bibliographical references mentioned in this figure correspond to Azizi et al.,16-18 and Kario et al.21
24-hour ambulatory blood pressure
24-h BP was evaluated up to 2 months after uRDN. Analysis of SBP showed a significant reduction of −4.87 mmHg (95%CI, −6.53 to −3.21; P < .00001; I2 = 42%). Meanwhile, 24-h DBP dropped down to −2.55 mmHg (95%CI, −3.83 to −1.26; P < .00001; I2 = 62%) in patients on uRDN (figure 2B).
Office blood pressure
SBP dropped down to −5.03 mmHg after 2 months (95%CI, −6.27 to −3.79; P < .00001; I2 = 0%) in uRDN patients. DBP showed a significant decrease of −3.68 mmHg (95%CI, −4.57 to −2.78; P < .00001; I2 = 31%) with the uRDN intervention (figure 2C).
Home blood pressure
Analysis of home BP after 2 months showed a decrease in SBP of −5.47 mmHg (95%CI, −8.08 to −2.85; P < .0001; I2 = 75%), while DBP dropped down to −3.19 mmHg (95%CI, −4.63 to −1.75; P < .0001; I2 = 69%) in patients on uRDN (figure 2D).
Nighttime blood pressure
Nighttime BP was evaluated at the 2-month follow-up. We found that SBP dropped down to −3.99 mmHg (95%CI, −7.00 to −0.99; P = 0.009; I2 = 70%), while DBP dropped down to −2.30 mmHg (95%CI −4.03 to −0.56; P = .01; I2 = 64%) in patients on uRDN (figure 2 of the supplementary data).
Drugs 6 months after uRDN
Patient drugs 6 months after uRDN were only reported in the RADIANCE-HTN SOLO and RADIANCE-HTN TRIO clinical trials. Data analysis revealed that uRDN leads to using fewer antihypertensive drugs by −0.52 (95%CI, −0.91 to −0.13; P = 0.009; I2 = 69%) vs the sham control group (figure 3).

Figure 3. Patients on uRDN used less antihypertensive medication prescribed 6 months after the procedure vs the sham group. 95%CI, 95% confidence interval; BP, blood pressure; SD, standard deviation; uRDN, ultrasound renal denervation. The bibliographical references mentioned in this figure correspond to Azizi et al.16 and Azizi et al.17
Risk of bias assessment
Among the 4 studies included, the risk of bias remained consistent at a moderate level, which was attributed to the inability to blind the interventional cardiologist conducting the uRDN, although outcome assessors were blinded to the interventions performed. Studies were categorized as having moderate risk16-18,21 (table 3). Data on risk of bias can be found in table 1 of the supplementary data. In addition, the funnel plot of daytime ambulatory SBP (figure 1 of the supplementary data) showed a slight asymmetry, as points tend to concentrate towards the left side of the combined effect, which could suggest a possible publication bias. In addition, the points closer to the vertex represent studies with lower standard error due to a larger sample size. The heterogeneity of the funnel plot reflects variations in effects across studies. Plot points are within the funnel lines, but one of them towards the lower right seems further away from the rest, which could indicate a possible outlier or methodological or population differences.29
Table 3. Risk of bias summary for randomized studies (RoB 2)
| Trials | Risk of bias domains | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Overall | |
| RADIANCE HTN SOLO16 | Low | Low | Low | Low | Low | Low |
| RADIANCE HTN TRIO17 | Low | Low | Low | Low | Low | Low |
| REQUIRE21 | Low | Low | Low | Low | Low | Low |
| RADIANCE II18 | Some concerns | Low | Low | Low | Low | Some concerns |
|
D1: bias arising from the randomization process. D2: bias due to deviation from intended intervention. D3: bias due to missing outcome data. D4: bias in outcome measurement. D5: bias in selection of the reported result. |
||||||
DISCUSSION
This meta-analysis includes data from 4 randomized controlled trials that evaluated the efficacy profile of uRDN in patients with true resistant hypertension and off-medication hypertensive patients vs a sham group. Antihypertensive efficacy was evaluated across different clinical settings such as 24-h ambulatory BP, home BP, office BP, and daytime BP. Our results demonstrated significant BP-lowering efficacy at the 2-month follow-up vs the sham procedure. Furthermore, at the 6-month follow-up, fewer antihypertensive drugs were prescribed to patients on uRDN vs those from the sham group. These results support the use of uRDN as an adjuvant therapy for hypertension and as a valuable option for reducing BP as well as the number of antihypertensive drugs.
Previous studies have demonstrated the safety profile of RDN for the treatment of resistant hypertension, such as the first-generation SIMPLICITY HTN trials.30 However, the SIMPLICITY HTN-3 study showed no differences in the 24-h BP reduction vs the sham group, casting doubts on the benefits of RDN.14 Subsequently, new catheters were developed for performing RDN, and standardized criteria were established for conducting RDN trials with a sham group.12,19 Currently, uRDN has emerged as a novel option as an adjuvant therapy treatment of hypertension. It is based on catheter systems, such as the TIVUS and Paradise systems, which utilize ultrasound energy for the thermal ablation of afferent and efferent renal nerves.19,31
Our results demonstrated a reduction in both SBP and DBP at the 2-month follow-up, with a more pronounced effect on SBP in patients on uRDN vs the sham control group. We observed a reduction of −5.12 mmHg in ambulatory SBP, −4.87 mmHg in 24-h SBP, −5.03 mmHg in office SBP, and −5.47 mmHg in home SBP. These findings are particularly relevant since SBP has turned out to be a strong predictor of future cardiovascular events and mortality, regardless of age in adults.32 The CI values for home BPS had the widest range. Furthermore, the RADIANCE HTN-SOLO trial demonstrated a wide CI in both office and home SBP. This observation is an opportunity for future trials to focus on patient training to standardize home BP measurement since day-to-day home BP has been proposed as a potential predictor of cardiovascular disease.33
Although the observed BP reduction may seem minimal and lack significant clinical relevance, it is important to note that these findings reflect the first 2 months of follow-up after uRDN initiation and literature reports that uRDN has a sustained long-term effect on lowering BP values. For example, the HTN RADIANCE-SOLO trial demonstrated that at the 36-month follow-up, office BP decreased 18/11 ± 15/9 mmHg.34 Related to this, previous studies have demonstrated that a 10 mmHg reduction in SBP is associated with a decrease in the relative risk (RR) of major cardiovascular events (RR, 0.80; 95%CI, 0.77-0.83), coronary heart disease (RR, 0.83; 95%CI, 0.78-0.88), stroke (RR, 0.73; 95%CI, 0.68-0.77), heart failure (RR, 0.72; 95%CI, 0.67-0.78), and a 13% reduction in all-cause mortality rate (RR, 0.87; 95%CI 0.84-0.91).2 However, it has recently been reported that even a 5 mmHg decrease is beneficial to reduce the risk of major cardiovascular events, estimating a hazard ratio (HR) of 0.91 (95%CI, 0.89-0.94) for individuals without previous cardiovascular disease and a HR of 0.89 (95%CI, 0.86-0.92) for those with previous cardiovascular disease.1 In addition, reduction of preventable major cardiovascular events by treating hypertension has a positive economic impact in reducing hospitalization expenses due to complications such as heart attack or stroke.35 Hypertension is a prevalent global health concern, and effective BP control is achieved in only 21% of patients.36 In the United States, individuals with hypertension are estimated to incur an additional $2500 to $3000 in annual expenses vs those without hypertension. Maintaining normal BP not only benefits patients but also supports the economic well-being of the entire health care system.37 In fact, studies evaluating the cost-effectiveness of long-term use of radiofrequency RDN have been conducted in the United States and the United Kingdom concluding that this procedure represents a cost-effective option for the treatment of uncontrolled and resistant hypertension, as its sustained BP-lowering effect favors the reduction of cardiovascular morbidity and mortality.38 Similarly, in Spain, an estimate was made of the impact of radiofrequency RDN on quality-adjusted life years, cardiac events, and patient-related lifetime costs. Radiofrequency RDN was found to reduce the risk of stroke (RR, 0.80), myocardial infarction (RR, 0.88), and heart failure (RR, 0.72) throughout a 10-year period, resulting in improved health outcomes and long-term cost savings. Results presented indicate that radiofrequency RDN is a cost-effective therapeutic option that should be taken into consideration in patients with uncontrolled hypertension, including resistant hypertension.39
In addition to the reduction in BP and the positive cost-effectiveness of uRDN, radiofrequency RDN has been demonstrated to be a safe procedure for the patients. The clinical trials that analyzed this meta-analysis found no safety differences between the treated and sham groups. Furthermore, few postoperative adverse events were reported. Most complications were associated with back pain, which was effectively and uneventfully managed.16-18,21 The long-term safety profile of the procedure has been consistently reported, with no adverse effects being reported from uRDN observed at the 1, 3-, and even 8-year follow-up.34,40,41
Our findings also demonstrated that, at the 6-month follow-up, patients on uRDN used fewer prescribed antihypertensive drugs, which suggests that treatment may potentially improve patient outcomes. However, this outcome was only evaluated in the RADIANCE HTN-TRIO and RADIANCE HTN-SOLO trials. In addition, at the 3-year follow-up the RADIANCE HTN-TRIO reported no differences in the number of drugs used by patients initially identified with uncontrolled hypertension, although they decreased office SBP by 10.8 mmHg.34 This is particularly noteworthy as non-adherence to therapy is a significant contributing factor to uncontrolled BP.42 However, the results suggest that the greatest benefit is observed in the maintenance of low BP levels rather than in the decrease in the number of antihypertensive drugs prescribed.
Of note, the I2 value of the outcomes evaluated showed that daytime SBP and DBP had low heterogeneity, while the 24-h SBP and DBP values had moderate-to-high heterogeneity. On the other hand, office SBP and DBP had low-to-moderate heterogeneity. Finally, home SBP and DBP, as well as nighttime SBP and DBP and drug intake had high heterogeneity. Variations in heterogeneity do not necessarily indicate that the results are not useful;43 possibly, the differences in the heterogeneity of the outcomes assessed is due to differences in the methodology of the studies contemplated in this meta-analysis, which will be discussed below.
Our analysis included the REQUIRE trial, which has certain limitations, such as the absence of a blinded design, a non-standardized uRDN intervention, and dose titration. These factors may have introduced bias, potentially explaining the lack of differences observed between the uRDN and sham groups. In addition, the inclusion criteria of the study did not consider the presence of anatomical variations in the renal arteries vs the RADIANCE trials in which an exclusion criterion is the presence of anatomical variations in renal arteries. This factor, along with therapeutic adherence, could impact the BP reduction results.21,28,44 Based on this perspective, the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) have established the characteristics that must be met by studies evaluating RDN with a sham control group to be considered as high quality: a) multicenter design; b) blinding of patients; c) ambulatory BP change as the primary enpoint; d) use of second-generation RDN systems.10 In this context, RADIANCE clinical trials are characterized by a rigorous methodological protocol, which required a 4- or 8-week stabilization of pharmacological therapy prior to randomization to either uRDN or a sham procedure.45,46 Furthermore, RADIANCE trials monitored therapeutic adherence and were designed to assess the effect of uRDN with and without antihypertensive treatment, minimizing the confounding effects.28,47
A key long-term challenge of the RADIANCE trials is to demonstrate sustained BP-lowering effects vs sham groups. Follow-up studies show that patients from the sham group required higher doses of antihypertensive drugs, while those on uRDN used fewer drugs. Although BP differences across groups decreased throughout time, uRDN patients consistently needed fewer prescriptions.26,27
Results from the RADIANCE trials demonstrate the efficacy profile of uRDN for the treatment of resistant hypertension and patients with poor therapeutic adherence, as observed in the off-study population. Additionally, the REQUIRE trial highlights the potential role of anatomical variations in determining patient suitability for uRDN, underscoring the importance of selecting appropriate criteria for patient selection. In addition, RDN has proven to be a safe procedure with a positive long-term cost-benefit ratio. The key question on uRDN may be: Which patient group would benefit most from uRDN, considering anatomical factors and therapeutic adherence?
Study limitations
To make the most out of the study results, it is important to consider its limitations: a) we only analyzed data from trials that used uRDN, which reduces the size of the population; b) data availability from the studies considered covered a short follow-up period, which limits the ability to determine the long-term antihypertensive efficacy of uRDN; c) differences in supervised drug adherence across the methodological designs limit their applicability to real-world settings; d) Since RCTs included patients with true resistant hypertension and off-med hypertension, the heterogeneous population limits the generalizability of the results to a specific hypertension subtype; and e) The funnel plot showed asymmetry, suggesting a possible publication bias, although results should be interpreted with caution because the funnel plot also indicates that there is a limited amount of data.
CONCLUSIONS
This meta-analysis demonstrates that uRDN treatment effectively reduces both SBP and DBP across various contexts, including 24-h, ambulatory, home and office BP at the initial 2-month follow-up in hypertensive patients (figure 4). Additionally, uRDN was associated with reduced antihypertensive drug use 6 months after the procedure. However, further research is needed to assess its long-term effects and identify the patient groups who may benefit the most.

Figure 4. Central illustration. Summary of the effect on the decrease in systolic and diastolic blood pressure in patients on uRDN compared with patients who received the sham procedure at the 2 months postoperative follow-up. uRDN, ultrasound renal denervation.
FUNDING
This manuscript did not receive financial support from any institution or funding agency for its preparation.
ETHICAL CONSIDERATIONS
The present meta-analysis was conducted based on previously published studies. As the study involved secondary data analysis, no new data was collected from human participants or animals, and the use of SAGER guidelines does not apply to this study. Therefore, ethical approval was deemed unnecessary. All included studies were reviewed in full compliance with ethical guidelines set forth by the respective institutions where the original studies were conducted. All authors state that the data used in this study were obtained exclusively from publicly accessible sources, and no confidential or proprietary information was utilized without appropriate authorization.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
During the preparation of this work the authors used ChatGPT-4o to review the document syntaxis and grammar. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the content of the published article.
AUTHORS’ CONTRIBUTIONS
J.M. Guerrero-Hernández: conceptualization, formal analysis, drafting, review and editing; C. J. Palomino-Ojeda: methodology, investigation, drafting, review and editing; L. H. García-Mena: methodology, formal analysis, writing, review and editing; Ó.Á. Vedia-Cruz: investigation; J. L. Maldonado-García: drafting, review and editing; I. J. Núñez-Gil: investigation, supervision and review; J. A. García-Donaire: review and supervision.
CONFLICTS OF INTEREST
I. J. Núñez-Gil served as a consultant for Medtronic and Recor Medical in the denervation field. J. A. García-Donaire served as consultant for Medtronic and Recor Medical in the denervation field. The rest of the authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- uRDN has emerged as a safe option for the treatment of resistant hypertension, and previous studies have observed greater efficacy in lowering BP vs a sham group.
WHAT DOES THIS STUDY ADD?
- Our results demonstrate that uRDN decreased 24-h, office, daytime and home SBP and DBP within the first 2 months after the procedural follow-up vs a sham group, and a decrease in the number of antihypertensive drugs at the 6-month follow-up. However, further long-term studies are required to confirm the benefit of uRDN.
REFERENCES
1. Adler A, Agodoa L, Algra A, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure:an individual participant-level data meta-analysis. The Lancet. 2021;39:1625-1636.
2. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death:A systematic review and meta-analysis. The Lancet. 2016;387:957-967.
3. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and Control of Hypertension:JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1278-1293.
4. Choudhry NK, Kronish IM, Vongpatanasin W, et al. Medication adherence and blood pressure control:A scientific statement from the american heart association. Hypertension. 2022;79:E1-E14.
5. Egan BM, Zhao Y, Li J, et al. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62:691-697.
6. Flack JM, Buhnerkempe MG, Moore KT. Resistant Hypertension:Disease Burden and Emerging Treatment Options. Curr Hypertens Rep. 2024;26:183-199.
7. Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457-466.
8. Mabin T, Sapoval M, Cabane V, Stemmett J, Iyer M. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57-61.
9. Dasgupta I, Sharp ASP. Renal sympathetic denervation for treatment of hypertension:where are we now in 2019? Curr Opin Nephrol Hypertens. 2019;28:498-506.
10. Barbato E, Azizi M, Schmieder RE, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44:1313-1330.
11. Rodríguez-Leor O, Jaén-Águila F, Segura J, et al. Renal denervation for the management of hypertension. Joint position statement from the SEH-LELHA and the ACI-SEC. REC Interv Cardiol. 2022;4:39-46.
12. Schmieder R, Burnier M, East C, Tsioufis K, Delaney S. Renal Denervation:A Practical Guide for Health Professionals Managing Hypertension. Interv Cardiol. 2023;18:e06.
13. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN):a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957-1965.
14. Bhatt DL, Kandzari DE, O'Neill WW, et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N Engl J Med. 2014;370:1393-1401.
15. Stiermaier T, Okon T, Fengler K, et al. Endovascular ultrasound for renal sympathetic denervation in patients with therapy-resistant hypertension not responding to radiofrequency renal sympathetic denervation. EuroIntervention. 2016;12:e282-e289.
16. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO):a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335-2345.
17. Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO):a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476-2486.
18. Azizi M, Saxena M, Wang Y, et al. Endovascular Ultrasound Renal Denervation to Treat Hypertension:The RADIANCE II Randomized Clinical Trial. JAMA. 2023;329(8):651-661.
19. Lauder L, Kandzari DE, Lüscher TF, Mahfoud F. Renal denervation in the management of hypertension. EuroIntervention. 2024;20:E467-E478.
20. Reuter E. The FDA approved 2 renal denervation devices. There are still questions about who will benefit. MedTech Dive. 13 Dec 2023. Available at:https://www.medtechdive.com/news/renal-denervation-recor-medtronic-evidence/702385/. Accessed 20 Dec 2024.
21. Kario K, Yokoi Y, Okamura K, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension:the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45:221-231.
22. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane;2023. Avalilable at:https://training.cochrane.org/handbook. Accessed 1 Dec 2024.
23. Sterne JAC, Savovic´J, Page MJ, et al. RoB 2:a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366.
24. Wilson DB. Practical meta-analysis effect size calculator (Version 2023.11.27). 2023. Available at:https://www.campbellcollaboration.org/calculator/d-ordinal-freq. Accessed 20 Dec 2024.
25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188.
26. Azizi M, Schmieder RE, Mahfoud F, et al. Six-Month Results of Treatment-Blinded Medication Titration for Hypertension Control After Randomization to Endovascular Ultrasound Renal Denervation or a Sham Procedure in the RADIANCE-HTN SOLO Trial. Circulation. 2019;139:2542-2553.
27. Azizi M, Mahfoud F, Weber MA, et al. Effects of Renal Denervation vs Sham in Resistant Hypertension After Medication Escalation:Prespecified Analysis at 6 Months of the RADIANCE-HTN TRIO Randomized Clinical Trial. JAMA Cardiol. 2022;7:1244.
28. Mauri L, Kario K, Basile J, et al. A multinational clinical approach to assessing the effectiveness of catheter-based ultrasound renal denervation:The RADIANCE-HTN and REQUIRE clinical study designs. Am Heart J. 2018;195:115-129.
29. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
30. Epstein M, De Marchena E. Is the failure of SYMPLICITY HTN-3 trial to meet its efficacy endpoint the “end of the road“for renal denervation? J Am Soc Hypertens. 2015;9:140-149.
31. Haribabu S, Sharif F, Zafar H. Recent trends in renal denervation devices for resistant hypertension treatment. Ir J Med Sci. 2021;190:971-979.
32. McCarthy CP, Natarajan P. Systolic Blood Pressure and Cardiovascular Risk:Straightening the Evidence. Hypertension. 2023;80:577-579.
33. Kario K, Kanegae H, Okawara Y, Tomitani N, Hoshide S. Home Blood Pressure Variability Risk Prediction Score for Cardiovascular Disease Using Data From the J-HOP Study. Hypertension. 2024;81:2173-2180.
34. Rader F, Kirtane AJ, Wang Y, et al. Durability of blood pressure reduction after ultrasound renal denervation:three-year follow-up of the treatment arm of the randomised RADIANCE-HTN SOLO trial. EuroIntervention. 2022;18:E677-E685.
35. Wang G, Grosse SD, Schooley MW. Conducting Research on the Economics of Hypertension to Improve Cardiovascular Health. Am J Prev Med. 2017;53(6 Suppl 2):S115.
36. Kario K, Okura A, Hoshide S, Mogi M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens Res. 2024 47:5. 2024;47:1099-1102.
37. Kumar A, He S, Pollack LM, et al. Hypertension-Associated Expenditures Among Privately Insured US Adults in 2021. Hypertension. 2024;81:2318-2328.
38. Taylor RS, Bentley A, Metcalfe K, et al. Cost Effectiveness of Endovascular Ultrasound Renal Denervation in Patients with Resistant Hypertension. Pharmacoecon Open. 2024;8:525.
39. Rodríguez-Leor O, M. Ryschon A, N. Cao K, et al. Cost-effectiveness analysis of radiofrequency renal denervation for uncontrolled hypertension in Spain. REC Interv Cardiol. 2024;6:305-312.
40. M Zeijen VJ, Völz S, Zeller T, et al. Long-term safety and efficacy of endovascular ultrasound renal denervation in resistant hypertension:8-year results from the ACHIEVE study. Clin Res Cardiol. 2024. https://doi.org/10.1007/s00392-024-02555-7.
41. Azizi M, Daemen J, Lobo MD, et al. 12-Month Results From the Unblinded Phase of the RADIANCE-HTN SOLO Trial of Ultrasound Renal Denervation. JACC Cardiovasc Interv. 2020;13:2922-2933.
42. Hamrahian SM, Maarouf OH, Fülöp T. A Critical Review of Medication Adherence in Hypertension:Barriers and Facilitators Clinicians Should Consider. Patient Prefer Adherence. 2022;16:2749.
43. Borenstein M. How to understand and report heterogeneity in a meta-analysis:The difference between I-squared and prediction intervals. Integr Med Res. 2023;12:101014.
44. Kario K, Kai H, Nanto S, Yokoi H. Anti-hypertensive medication adherence in the REQUIRE trial:post-hoc exploratory evaluation. Hypertens Res. 2023;46:2044-2047.
45. Mahfoud F, Azizi M, Ewen S, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41:1588-1599.
46. Kandzari DE, Mahfoud F, Weber MA, et al. Clinical Trial Design Principles and Outcomes Definitions for Device-Based Therapies for Hypertension:A Consensus Document From the Hypertension Academic Research Consortium. Circulation. 2022;145:847-863.
47. Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding Factors in Renal Denervation Trials:Revisiting Old and Identifying New Challenges in Trial Design of Device Therapies for Hypertension. Hypertension. 2020;76:1410-1417.
*Corresponding authors.
E-mail addresses: lissettegarcia@comunidad.unam.mx (L. H. García-Mena); ibnsky@yahoo.es (I. J. Nuñez-Gil).
ABSTRACT
Introduction and objectives: The prevalence of atrial fibrillation and the number of patients experiencing ischemic strokes despite oral anticoagulation (OAC) are both on the rise, which presents a significant challenge due to the absence of clear and uniform treatment recommendations for these patients. To date, there is no formal combination merging into a high anticoagulant efficacy profile while keeping a low bleeding risk. Transcatheter left atrial appendage occlusion (LAAO) in combination with OAC might provide a balance between safety and efficacy. The objective of this study is to evaluate whether, in ischemic stroke patients, despite anticoagulation, the combination of LAAO plus long-term anticoagulation—direct oral anticoagulants or vitamin K antagonist when indicated—is associated with a lower rate of recurrent cardioembolic events at 12 months vs the optimal medical therapy recommended by the neurologist.
Methods: A total of 380 patients with ischemic stroke despite OAC will be included. Patients will be randomized on a 1:1 ratio to receive the optimal medical therapy (control) or the combination of LAAO plus OAC or OAC. The primary endpoint of the study will be the occurrence of a cardioembolic event—ischemic stroke or arterial peripheral embolism—within the first 12 months after inclusion.
Conclusions: This study is one of the first randomized clinical trials to compare the LAAO plus OAC combination and optimal medical therapy in patients who have experienced ischemic strokes despite being on OAC. If results confirm the superiority of LAAO plus OAC, it could lead to a paradigm shift in treatment guidelines for these patients.
Keywords: Left atrial appendage occlusion. Recurrent stroke. Oral anticoagulation. Direct oral anticoagulation.
RESUMEN
Introducción y objetivos: La prevalencia de la fibrilación auricular y el número de pacientes que sufren ictus isquémicos a pesar de recibir anticoagulación oral (ACO) están aumentando. Este incremento representa un importante desafío debido a la ausencia de recomendaciones claras y uniformes sobre el tratamiento de estos pacientes. Hasta la fecha no existe una combinación que logre una alta eficacia anticoagulante manteniendo un bajo riesgo de hemorragia. El cierre u oclusión percutánea de la orejuela izquierda (OI) añadida a la ACO podría ofrecer un equilibrio entre eficacia y seguridad. El objetivo es evaluar si, en pacientes con ictus isquémico a pesar de recibir ACO, la combinación de cierre de la OI y ACO a largo plazo (anticoagulantes orales de acción directa o bien antagonista de la vitamina K, cuando esté indicado) se asocia con una menor incidencia de eventos cardioembólicos recurrentes a los 12 meses, en comparación con el mejor tratamiento médico propuesto por el neurólogo.
Métodos: Se incluirá a 380 pacientes con ictus isquémico a pesar de recibir ACO. Los pacientes se aleatorizarán en una proporción 1:1 al mejor tratamiento médico (grupo control) o a la combinación de cierre de OI y ACO. El objetivo principal del estudio será la ocurrencia de un evento cardioembólico (ictus isquémico o embolia arterial periférica) dentro de los primeros 12 meses tras la inclusión.
Conclusiones: Este estudio es uno de los primeros ensayos clínicos aleatorizados que compara la combinación de cierre de OI más ACO con el tratamiento médico óptimo en pacientes que han sufrido un ictus isquémico a pesar de estar recibiendo ACO. Si los resultados confirman la superioridad del cierre de OI más ACO, podría significar un cambio de paradigma en las guías de tratamiento para estos pacientes.
Palabras clave: Cierre de la orejuela. Ictus recurrente. Anticoagulación oral. Anticoagulación oral directa.
Abbreviations AF: atrial fibrillation. DOAC: direct oral anticoagulants. LAA: left atrial appendage. LAAO: left atrial appendage occlusion. OAC: oral anticoagulation. VKA: Vitamin K antagonist.
INTRODUCTION
Atrial fibrillation (AF) increases with age and raises the risk of ischemic stroke, systemic embolism, and death, with stroke or transient ischemic attack (TIA) often being the initial presentation. 1,2 Oral anticoagulation (OAC) with direct OAC (DOAC) or vitamin K antagonists (VKA) is effective in reducing these risks.1,2 However, recent randomized clinical trials suggest a 1.0%–1.5% annual stroke rate in AF patients on OAC.3-5 Up to one-third of AF patients who develop ischemic stroke are on OAC at stroke onset, with 8.8% up to 20% being on DOAC,6-9 which poses a significant challenge for secondary prevention purpose. Additionally, these patients tend to be older and have more comorbidities, sometimes requiring off-label low-dose DOAC.7 The use of off-label low-dose DOAC, atrial enlargement, and increased AF burden further raises the risk of stroke despite OAC therapy, suggesting a possible association with advanced cardiac disease or inadequate anticoagulation.10,11
After an ischemic stroke—despite OAC—there are no clear guidelines on the management of OAC, such as switching drugs, targeting a higher international normalized ratio (INR) with AVK, or adding antiplatelet agents. Network meta-analyses show no differences in stroke risk across different DOAC. Observational studies also found no benefits in changing OAC, suggesting additional stroke mechanisms.7 Stroke workups should identify alternative embolisms or sources of thrombosis, such as large-vessel atherosclerosis or small-vessel disease, which may need specific antithrombotic strategies.7,9,10,12 Although adding antiplatelets may reduce platelet activation, it is associated with a higher bleeding risk.13 Therefore, the best secondary prevention for AF patients after stroke despite OAC remains unclear, highlighting the need for new treatments, including novel non-pharmacological approaches such as left atrial appendage occlusion (LAAO).
Recent data from the STR-OAC cohort—an international LAAO registry—showed promising findings in AF patients with stroke despite OAC. In this study, the LAAO cohort had a 2.2% per patient-year stroke rate vs the 9.8% reported in the non-LAAO cohort (HR, 0.33; 95%CI, 0.19-0.59).14 Despite this positive outcome, no RCTs have assessed the feasibility, safety and efficacy profile of using OAC plus LAAO as adjuvant therapy in this complex population. Herein, we propose oral anticoagulation alone vs oral anticoagulation plus left atrial appendage occlusion in stroke patients despite ongoing anticoagulation (ADD-LAAO trial). This is a pragmatic randomized controlled trial (RCT) designed to evaluate the superiority of a hybrid strategy combining transcatheter left atrial appendage occlusion (LAAO) and long-term continued oral anticoagulation (OAC)—either direct oral anticoagulants (DOAC) or VKA when clinically appropriate—vs medical management with OAC alone. The trial aims to assess the effectiveness of this approach in reducing recurrent ischemic stroke in patients with atrial fibrillation (AF) who have experienced an AF-related acute ischemic stroke despite being anticoagulated at the time of stroke onset.
METHODS
Study design
We conducted a multicenter randomized controlled trial with patients with a past medical history of ischemic stroke within the past 6 months despite OAC. A total of 6 teaching hospital centers are involved in this study. Eligible patients will be screened and included based on specific criteria (figure 1 illustrates the inclusion and exclusion criteria). In general, patients with stroke despite OAC will include the following data: those with VKA with correct or labile INR and those with DOAC with poor compliance. Poor compliance will be defined as missing a maximum of 1 day dose (1 pill for VKA and 2 for DOAC) over the past week before the index stroke. Poor compliant patients will be included as the risk of non-compliance is an inherent characteristic of OAC. In contrast, patients missing > 1 day dose over the past week before the index procedure will not be included as these patients cannot be considered anticoagulated. A crucial inclusion criterion for randomization purposes will be the absence of an absolute contraindication to OAC, as this population is considered a high-risk cohort for thrombotic events, and post-LAAO OAC discontinuation has been reported to increase thrombotic risk.15 The study flow diagram is shown in figure 2.

Figure 1. Inclusion and exclusion criteria. AF, atrial fibrillation; CT, computed tomography; DOAC, direct oral anticoagulant; Hb, hemoglobin; LAAO, left atrial appendage occlusion; NYHA, New York Heart Association; OAC, oral anticoagulation; PFO, patent foramen ovale; TEE, transesophageal echocardiogram; TIA, transient ischemic attack.
a Cardioembolic ischemic stroke definition: AF-related stroke after ruling out symptomatic ipsilateral great vessel/intracranial vascular disease, and small vessel disease and active endocarditis or neoplasm.
b > 1 dose per antagonist of vitamin K and > 2 doses per DOAC.
c > 50% lumen diameter narrowing on CT, magnetic resonance imaging, or transcranial Doppler with symptoms of ipsilateral transient or visual TIA.
d If general anesthesia is planned for the study procedure.
e If the patient needs CCTA and cannot undergo TEE.
f The active treatment group may confound the results of this trial.

Figure 2. Central illustration. Study protocol. CT, computed tomography; DOAC, direct oral anticoagulant; LAAO, left atrial appendage occlusion; OAC, oral anticoagulation; TEE, transesophageal echocardiogram.
Subject screening, enrollment, and randomization
Patients meeting all inclusion and no exclusion criteria will be approached for the study. Prior to inclusion, transesophageal echocardiography (TEE) or coronary computed computed tomography will rule out the presence intra-cardiac thrombus and assess the LAA anatomy. If suitable for LAAO, the study will be explained, and informed consent from the patients will be obtained. Upon consent, patients will be randomized on a 1:1 ratio to the interventional group—receiving LAAO and long-term OAC—or the control group, receiving the optimal medical therapy as decided by neurologists. The interventional procedure will occur within 2 weeks post-randomization. The optimal medical therapy may involve intensified antithrombotic therapy, switching OAC regimens, reinforcing drug compliance, or a combination of these strategies. Randomization will be managed online, allocating patients in groups of 10 to ensure balanced inclusion across both groups.
OAC strategies
The treating neurologist team will decide on the OAC strategy and dosage for the interventional and control groups. Any DOAC (apixaban, dabigatran, rivaroxaban, or edoxaban) will be accepted. DOAC dosages will be down titrated if the patient’s bleeding risk is high or per product label recommendations. In the interventional group, although DOAC is preferred to minimize bleeding risk,16 VKA will be allowed if necessary, such as for patients with mechanical cardiac valves. The protocol does not restrict other concomitant drugs; each will be assessed for additional hemorrhagic risk by the physician. The OAC duration will be indefinite in the 2 groups unless a new formal contraindication emerges, such as in cases of major bleeding. Furthermore, each situation will be managed individually by investigators at each center.
Interventional group
Patients from the interventional group will undergo LAAO following standard practice. ACO will be discontinued prior to the procedure per product label recommendations for DOAC, and with bridging therapy using low-molecular-weight heparin in patients with an indication for VKA. A prophylactic antibiotic—cephalosporin or vancomycin for beta-lactam allergy—will be administered 2 hour prior. Procedure will be performed under general anesthesia or deep sedation with TEE or intracardiac echocardiography guidance. The femoral vein will be used for vascular acces, followed by a transseptal puncture. A 100 IU/kg bolus of IV heparin will be administered to achieve an activated clotting time ≥ 250 seconds. Fluoroscopy and TEE or intracardiac echocardiography will guide the procedure, allowing for LAA measurement, catheter and device positioning, and early complication detection. Approved LAAO devices include Amulet (Abbott Medical, United States), Watchman FLX (Boston Scientific, United States), and LAmbre (Lifetech Scientific [Shenzhen] Co. Ltd., China). Six to 24 hours after the intervention, transthoracic echocardiography will check for any pericardial effusions or device embolizations that may have occurred. If no complications are found, the patient will be discharged the same or the next day (institution protocol). Anticoagulation therapy (DOAC at the same dose as before LAAO or VKA with the initial dose determined by the thrombosis clinic assessment) will be resumed the day after the procedure. No antiplatelet therapy will be added to anticoagulation therapy in the LAAO group.
Study endpoint and outcome definitions
The primary endpoint of the study is the occurrence of a cardioembolic event—ischemic stroke or peripheral arterial embolism— within the first 12 months after inclusion. Secondary endpoints include evaluating the safety and efficacy profile of the strategies and combining cardioembolic events (efficacy) and major bleeding (safety) within the same period. A stroke is defined as the sudden onset of a focal neurologic deficit consistent with a major cerebral artery territory, categorized as ischemic, hemorrhagic, or unspecified, and confirmed through imaging using computed tomography or magnetic resonance imaging. Systemic cardioembolic events are acute vascular occlusions in an extremity or organ, confirmed by imaging, surgery, or autopsy. Major bleeding will follow the Bleeding Academic Research Consortium (BARC) criteria for types 3 and 5.17
Additional endpoints will assess major and minor bleeding (using the BARC classification), all-cause and cardiovascular death, recurrent stroke severity (using the modified Rankin scale), procedural major adverse events in the interventional group, device-related thrombus, additional hospital admissions, and OAC compliance. All clinical events, including primary and secondary endpoints, will be independently allocated.
The success of the intervention is defined as the implantation of the LAAO device without major complications, such as death, stroke, or those requiring surgical or endovascular treatment.18 Procedural safety will be evaluated by including all clinical events within the first 7 after the intervention. Events will be defined following the Valve Academic Research Consortium (VARC) guidelines, including mortality, myocardial infarction, stroke, systemic embolism, major bleeding, and procedural complications.19 Major bleeding will follow the BARC criteria for types 3 and 5.17 A neurologist will independently grade disabling strokes with an m-RS score of ≥ 3.20 Device-related thrombus will be any thrombus > 1 mm on the LAAO device, and peri-device leaks will be classified by TEE jet width (> 3 mm being significant).21 Complete LAAO is defined as the absence of any leaks > 3 mm on the final TEE.22
Clinical and imaging follow-up
Patients will be followed for 12 months. Clinical visits with neurological assessment and modified Rankin scale evaluation will occur on months 3 and 12. A phone follow-up will be conducted on month 6. The interventional group will undergo post-LAAO additional imaging modalities. Two imaging modalities with TEE or coronary computed tomography angiography will assess device-related thrombus and peri-device leaks 3 (2-4 months) and 12 months (10-12 months) after the intervention. Additionally, standard blood tests, including complete blood count and renal function will be performed on the same day as the imaging modalities to detect hidden hemorrhagic events.
Sample size and statistical analysis
Sample size is determined based on observed event rates in major registries, with an estimated 10% rate of recurrent cardioembolic events in the control group and 2% in the interventional group within the first year. To detect an 8% difference between the 2 groups, with a 5% type I error and 90% power, 183 patients per group are needed for a total of 366. Accounting for potential dropouts (~5%), the study will include 380 patients. The primary analysis will be conducted following the intention-to-treat principle, making sure that all randomized patients are analyzed in the group they have been initially allocated to, regardless of protocol deviations, dropouts, or crossover. This approach will provide an unbiased estimation of the treatment effect under real-world conditions and preserve the benefits of randomization. If a patient from the interventional group experiences a primary endpoint prior to the procedure—as they will be on OAC treatment—they will be allocated to the interventional group. An interim analysis will be performed after including 50% of the population (190 patients), and the study will be stopped if significant differences are detected.
Categorical variables will be expressed as frequencies and compared using the chi-square or Fisher’s exact test. Continuous variables will be expressed as mean ± SD or median (IQR), using the Kolmogorov-Smirnov test for normality. Comparisons will use the Student’s t test or the Mann-Whitney U test. Composite endpoints will be assessed as a time-to-first event. Cumulative incidence will be evaluated using the Kaplan-Meier method and compared using the log-rank test, followed by Cox proportional hazards modeling. Treatment effects will be estimated with hazard ratios and 95% confidence intervals, with 2-sided P-values ≤ .05 considered statistically significant. Analyses will be performed using STATA (Version 14.0 (Stata Corp., United States). The trial has been registered on ClinicalTrials.gov, and the registry No. is pending registration approval.
Current study status
Study recruitment is set to commence. The study is expected to be completed in 23 months. The projected study timeline is shown in figure 3.

Figure 3. Projected study timeline.
DISCUSSION AND CLINICAL IMPLICATIONS
The current study is expected to have a significant impact on the scientific community, particularly in the fields of cardiology and neurology. As previously mentioned, the prevalence of AF and the number of patients experiencing ischemic strokes despite being OAC are on the rise,3-5 which presents a significant challenge due to the absence of clear and uniform treatment recommendations for these patients. Intensification of antithrombotic regimens in this population often leads to an elevated risk of major bleeding, especially among elderly and frail individuals.13 Therefore, assessing a novel therapeutic strategy that combines LAAO with DOAC is essential. LAAO targets the left atrial appendage, which is responsible for more than 90% of thrombus formation in non-valvular AF,16 potentially enhancing the efficacy of OAC while maintaining a lower bleeding risk with DOAC. Recent registries evaluating the LAAO + DOAC strategy have reported promising outcomes, demonstrating a significantly reduced rate of recurrent strokes and major bleeding vs optimal medical therapy alone.23-25 Given these encouraging preliminary data, the timing is ideal for a randomized controlled trial to evaluate this combined approach rigorously.
As far as we know, the proposed study is one of the first randomized clinical trials to compare LAAO + DOAC and optimal medical therapy, as determined by a neurologist, in patients who have experienced ischemic strokes despite being on OAC. The ELAPSE trial has also started recruitment with a similar design (NCT05976685). Should trial results confirm the superiority of the LAAO + DOAC strategy over current medical management protocols, our findings will contribute to a paradigm shift in the treatment guidelines for this patient cohort. Specifically, the LAAO + DOAC combo could offer a more effective and safer therapeutic option, addressing the unmet need for reducing stroke recurrence while minimizing bleeding risks, which could impact future clinical practice and guideline recommendations, ultimately improving patient outcomes in those with AF and a history of ischemic stroke. This trial successful completion and positive outcomes can potentially establish a new standard of care, thus significantly impacting clinical practice and patient quality of life alike in this high-risk population.
To achieve these goals, the centers participating in this study have been carefully selected based on their annual volume of candidates for LAAO and their expertise in managing these procedures. These centers are recognized as referral centers for both LAAO and stroke code management. Although randomizing 380 patients across 6 hospitals within a reasonable timeframe may appear challenging, we have implemented measures to streamline the process. Dedicated teams for the early identification of eligible patients and standardized follow-up strategies have been established to facilitate recruitment.
We recognize that this is an ambitious undertaking; however, the study design and the cumulative experience of participant centers provide confidence in achieving the goals set within the anticipated timeline. If successful, this trial has the potential to establish a new standard of care, significantly impacting clinical practice and improving the quality of life of this high-risk population.
FUNDING
This work has been funded by a grant from Fundació La Marató de TV3.
ETHICAL CONSIDERATIONS
The study is being conducted following the recommendations outlined in the Declaration of Helsinki on clinical research, has been approved by Hospital Clinic de Barcelona Research Ethics Committee, and endorsed by the remaining ethics committees of all participant centers. Informed consent acceptance and signature are required prior to performing any elective procedures for the study of the non-culprit lesions. Potential sex and gender biases are considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the drafting of this manuscript.
AUTHORS’ CONTRIBUTIONS
X. Freixa and E. Flores-Umanzor drafted this document. The remaining signatories reviewed the document and made changes at their discretion. All the authors revised and approved the final version of the manuscript.
CONFLICTS OF INTEREST
I. Cruz-Gonzalez and X. Freixa are proctors from Abbott Medical, Boston Scientific and Lifetech. R. Estevez-Loureiro and D. Arzamendi are proctors from Abbott Medical, Boston Scientific. L. Nombela- Franco is a proctor from Abbott Medical. The rest of the authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- AF is increasingly prevalent, contributing to a growing number of patients experiencing ischemic strokes despite being on OAC.
- These cases represent a therapeutic dilemma, as current treatment guidelines lack clear recommendations for patients who experience recurrent ischemic events despite adequate anticoagulation.
- Existing anticoagulation strategies alone may not sufficiently prevent strokes while maintaining an acceptable bleeding risk.
- LAAO is a promising intervention that could complement anticoagulation, potentially enhancing stroke prevention while limiting bleeding complications.
- However, the safety and efficacy profile of LAAO + OAC in this high-risk population has not been rigorously evaluated in randomized clinical trials.
WHAT DOES THIS STUDY ADD?
- This study will be one of the first randomized clinical trials to assess whether LAAO + long-term OAC improves outcomes vs optimal medical therapy in patients with AF who experience ischemic stroke despite anticoagulation.
- Comparing LAAO + OAC to standard care will provide critical evidence on the potential for reducing recurrent cardioembolic events within 12 months.
- If the study confirms the benefit of this combination strategy, it could establish a new treatment paradigm for this high-risk population, filling a critical gap of the current clinical practice guidelines.
- The results could guide individualized treatment approaches balancing stroke prevention and bleeding risk.
REFERENCES
1. Hart RG, Pearce LA, Aguilar MI. Meta-analysis:antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
2. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation:a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
4. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
5. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
6. Xian Y, O'Brien EC, Liang L, et al. Association of Preceding Antithrombotic Treatment With Acute Ischemic Stroke Severity and In-Hospital Outcomes Among Patients With Atrial Fibrillation. JAMA. 2017;317:1057-1067.
7. Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann Neurol. 2020;87:677-687.
8. Meinel TR, Branca M, De Marchis GM, et al. Prior Anticoagulation in Patients with Ischemic Stroke and Atrial Fibrillation. Ann Neurol. 2021;89:42-53.
9. Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke:the IAC study. J Neurol Neurosurg Psychiatry. 2021;92:1062-1067.
10. Paciaroni M, Agnelli G, Caso V, et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke. 2019;50:2168-2174.
11. Stretz C, Wu TY, Wilson D, et al. Ischaemic stroke in anticoagulated patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2021;92:1164-1172.
12. Best JG, Cardus B, Klijn CJM, et al. Antithrombotic dilemmas in stroke medicine:new data, unsolved challenges. J Neurol Neurosurg Psychiatry. 2022:jnnp-2020-325249.
13. Yasuda S, Kaikita K, Akao M, et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N Engl J Med. 2019;381:1103-1113.
14. Maarse M, Seiffge D, Fierro N, et al. Left atrial appendage occlusion versus standard of care in patients with atrial fibrillation and a prior thrombo-embolic event despite oral anticoagulant therapy:a propensity score matched comparison. Eur Heart J. 2022;43(Supplement_2).
15. Osmancik P, Herman D, Neuzil P, et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol. 2022;79:1-14.
16. Anduaga I, Affronti A, Cepas-Guillén P, et al. Non-Pharmacological Stroke Prevention in Atrial Fibrillation. J Clin Med. 2023;12:5524.
17. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
18. Tzikas A, Holmes DR, Jr., Gafoor S, et al. Percutaneous left atrial appendage occlusion:the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace. 2017;19:4-15.
19. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438-1454.
20. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale:implications for stroke clinical trials:a literature review and synthesis. Stroke. 2007;38:1091-1096.
21. Price MJ, Ellis CR, Nielsen-Kudsk JE, et al. Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion:An Analysis of the Amulet IDE Trial. JACC Cardiovasc Interv. 2022;15:2127-2138.
22. Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. Europace. 2020;22:184.
23. Masjuan J, Salido L, DeFelipe A, et al. Oral anticoagulation and left atrial appendage closure:a new strategy for recurrent cardioembolic stroke. Eur J Neurol. 2019;26:816-820.
24. Freixa X, Cruz-González I, Regueiro A, et al. Left Atrial Appendage Occlusion as Adjunctive Therapy to Anticoagulation for Stroke Recurrence. J Invasive Cardiol. 2019;31:212-216.
25. Cruz-González I, González-Ferreiro R, Freixa X, et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Rev Esp Cardiol. 2020;73:28-34.
* Corresponding author.
Email address: freixa@clinic.cat (X. Freixa).
ABSTRACT
Introduction and objectives: Several studies have shown that reduced (< 50%) left ventricular ejection fraction (LVEF) is an independent risk factor for cardiovascular events and mortality in patients with severe aortic stenosis (AS) undergoing valve replacement. Although patients with preserved LVEF (> 50%) have a better prognosis, there is a group with supranormal LVEF (≥ 70%) whose prognosis seems to differ due to their characteristics. The aim of this study was to evaluate outcomes after transcatheter aortic valve implantation (TAVI) in patients with severe AS and supranormal LVEF.
Methods: We performed a retrospective cohort study that included 1160 patients undergoing TAVI between 2007 and 2021 at Hospital Clínico San Carlos (Madrid, Spain). The patients were classified according to preoperative LVEF into reduced (< 50%), normal (50% to 69%), and supranormal (≥ 70%). Clinical, echocardiographic variables, and the following outcomes were compared: death from any cause at 30 days and at 1 year, death from cardiovascular causes at 1 year, and rehospitalization due to cardiovascular causes at 1 year.
Results: Of the 1160 patients with severe AS who underwent TAVI during the study period, 276 (23.8%) had reduced LVEF, 702 (60.5%) had normal LVEF, and 182 (15.7%) had supranormal LVEF. Patients with supranormal LVEF were predominantly men (82.9 ± 5.3 years) and had lower ventricular volumes, higher relative wall thickness, and concentric geometry. There were no differences in 30-day or 1-year mortality. However, rehospitalization for cardiovascular causes at 1 year was significantly higher in the supranormal LVEF group (LVEF < 50%: 29.2%; LVEF 50% to 69%: 27.4%; LVEF ≥ 70%: 34.4%; P < .043).
Conclusions: Patients with severe AS and supranormal preprocedural LVEF (≥ 70%) who underwent TAVI had a higher rate of cardiovascular rehospitalization at 1 year, with no differences in mortality.
Keywords: Supranormal ejection fraction. Severe aortic stenosis. TAVI. Rehospitalization.
RESUMEN
Introducción y objetivos: Se ha evidenciado en diversos estudios que la fracción de eyección del ventrículo izquierdo (FEVI) reducida (< 50%) es un factor de riesgo independiente de eventos y mortalidad en pacientes con estenosis aórtica (EA) grave tratados con recambio valvular. A pesar de que aquellos con FEVI conservada (> 50%) muestran mejor pronóstico, existe un grupo con FEVI supranormal (≥ 70%) que parece tener un pronóstico diferente por sus características particulares. El objetivo de este estudio fue evaluar los resultados del implante percutáneo de válvula aórtica (TAVI) en pacientes con EA grave y FEVI supranormal.
Métodos: Estudio de cohorte retrospectiva que incluyó 1.160 pacientes tratados con TAVI en 2007-2021 en el Hospital Clínico San Carlos (Madrid, España). Se clasificaron según su FEVI preoperatoria en reducida (< 50%), normal (50-69%) y supranormal (≥ 70%). Se compararon variables clínicas y ecocardiográficas, y los siguientes desenlaces: mortalidad por cualquier causa a los 30 días y al año, muerte por causa cardiovascular al año y rehospitalización por causa cardiovascular al año.
Resultados: De los 1.160 pacientes con EA grave que recibieron un TAVI durante el periodo del estudio, 276 (23,8%) se registraron con FEVI reducida, 702 (60,5%) con FEVI normal y 182 (15,7%) con FEVI supranormal. Los pacientes con FEVI supranormal eran predominantemente varones (82,9 ± 5,3 años), tenían menores volúmenes ventriculares, mayor grosor parietal relativo y geometría concéntrica. No hubo diferencias en la mortalidad a 30 días ni al año; sin embargo, la rehospitalización por causa cardiovascular al año fue significativamente superior en el grupo de FEVI supranormal (FEVI < 50%, 9,2%; FEVI 50-69%, 27,4%; FEVI ≥ 70%, 34,4%; p < 0,043).
Conclusiones: Los pacientes con EA grave tratados con TAVI que presentaban FEVI supranormal (≥ 70%) preprocedimiento tuvieron una mayor tasa de rehospitalización por causa cardiovascular al año, sin diferencias en la mortalidad.
Palabras clave: Fracción de eyección supranormal. Estenosis aórtica grave. TAVI. Rehospitalización.
Abbreviations
AS: aortic stenosis. LVEF: left ventricular ejection fraction. LVOT: left ventricular outflow tract. RPT: relative parietal thickness. TAVI: transcatheter aortic valve implantation. VTI: velocity time integral.
INTRODUCTION
Aortic stenosis (AS) is the second most common valvular heart disease, affecting 12% of people older than 75 years.1,2 Without treatment, the survival rate for symptomatic severe AS is less than 3 years.3 Transcatheter aortic valve implantation (TAVI) is recommended for symptomatic patients and for asymptomatic patients with a reduced left ventricular ejection fraction (LVEF) of less than 50%.4
Reduced LVEF is recognized as an independent risk factor for events and mortality in patients with severe AS.5 However, the prognosis of severe AS in patients with preserved LVEF (> 50%) remains uncertain, especially in the presence of markers of subclinical myocardial injury, such as hypertrophy and fibrosis.6 Among these patients, those with a supranormal LVEF (≥ 70%) may have a worse prognosis after TAVI due to specific ventricular geometry and functional characteristics.7
The aim of this study was to evaluate the prognosis of patients with supranormal LVEF (≥ 70%) undergoing TAVI and study their echocardiographic and clinical characteristics.
METHODS
We conducted a retrospective cohort study of patients with severe AS who underwent TAVI at Hospital Clínico San Carlos in Madrid, Spain, between June 2007 and December 2021. Severe AS was defined according to current guideline criteria: mean gradient > 40 mmHg, peak velocity > 4 m/s, aortic valve area < 1 cm², or indexed aortic valve area < 0.6 cm²/m². The decision to perform TAVI was made by a multidisciplinary medical-surgical team. Patients were categorized into 3 groups based on their preprocedural LVEF, as assessed by echocardiography: reduced (< 50%), normal (50%-69%), and supranormal (≥ 70%). Clinical data were collected from medical records. Patients were excluded if they did not survive the procedure, had previous cardiac valve surgery, had cardiomyopathy unrelated to valvular disease, had a life expectancy of less than 1 year, or had missing data in their preprocedural echocardiographic study or clinical follow-up.
The clinical endpoints used to evaluate the prognosis of patients with supranormal LVEF (≥ 70%) undergoing TAVI were all-cause mortality at 30 days and 1 year, cardiovascular mortality at 1 year, and cardiovascular-related rehospitalization at 1 year. We also assessed their correlation with echocardiographic and clinical characteristics.
The study was conducted in accordance with the principles of the Declaration of Helsinki by the World Medical Association and received approval from the ethics committee of Hospital Clínico San Carlos in Madrid, Spain. Since the study was retrospective and posed no risk to patients, informed consent was not required. All data were handled with the utmost confidentiality by the researchers.
Echocardiography
Two-dimensional Doppler echocardiography was performed using the available equipment and following clinical practice guidelines.8 Measurements included septal thickness, posterior wall thickness, end-diastolic diameter, and left ventricular outflow tract (LVOT) diameter in the parasternal long-axis view. Peak and mean valvular gradients were assessed using continuous Doppler in multiple windows to obtain the highest velocity. The velocity-time integral (VTI) was measured with pulsed Doppler by placing the sample volume just before the aortic valve annulus. The aortic valve area was then calculated using the continuity equation:
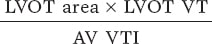
Ventricular volumes and LVEF were calculated using the biplane Simpson method. The left ventricular (LV) mass was calculated using the Devereux formula and indexed to body surface area. Relative parietal thickness (RPT) was calculated using the following formula:
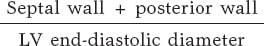
The indexed stroke volume was obtained using the following formula:

Statistical analysis
The statistical analysis was conducted using available commercial software (IBM SPSS 28.0). Normally distributed continuous variables are expressed as the mean and standard deviation, with a 95% confidence interval (95%CI). Categorical variables are expressed as absolute numbers and percentages. The Student t-test was used to compare variables with a normal distribution. Analysis of variance and the Tukey post hoc test were used to compare means, while the chi-square test was used to compare prevalences among the 3 groups. A univariable logistic regression analysis was applied to evaluate predictors of hospitalization and mortality. P values < .05 were considered statistically significant.
RESULTS
Of the 1228 patients who underwent TAVI during the study period, 1160 were included in the analysis. Among these, 276 patients (23.7%) had a reduced LVEF (< 50%), 702 patients (60.5%) had a normal LVEF (50%-69%), and 182 patients (15.6%) had a supranormal LVEF (≥ 70%). Sixty-eight patients were excluded based on the following criteria: 23 due to death during the procedure, 15 with previous cardiac valve surgery, 6 with cardiomyopathy unrelated to valvular disease, 18 with a life expectancy of less than 1 year, and 6 with missing data in the preprocedural echocardiographic study or clinical follow-up (figure 1).
Figure 1. Flow chart illustrating the patients included and excluded from the study, the final sample analyzed, and its distribution among the 3 study groups. LVEF, left ventricular ejection fraction.
The baseline characteristics of the study population are shown in table 1. The mean age was 82.2 ± 5.8 years and was slightly lower in the reduced LVEF group than in the other 2 groups. Male sex was more common in the group with LVEF ≥ 70% (P < .005). Patients with LVEF < 50% had a higher prevalence of prior myocardial infarction, coronary artery disease, and revascularization, along with a higher EUROSCORE II (22.5 [14.7-32.0]; P < .001). This group also more frequently required the intervention as an emergency procedure (P < .001).
Table 1. Patients’ baseline characteristics
| Characteristics | LVEF < 50% (n = 276) | LVEF 50%-69% (n = 702) | LVEF ≥ 70% (n = 182) | P value |
|---|---|---|---|---|
| Age (years) | 81.6 ± 6.3 | 82.2 ± 5.9 | 82.9 ± 5.3 | < .050 |
| Male sex | 38.1% | 58.2% | 68.3% | < .001 |
| Hypertension | 80.7% | 82.9% | 86.0% | .363 |
| Diabetes mellitus | 41.7% | 35.6% | 33.9% | .182 |
| Body mass index | 27.1 ± 4.4 | 28.4 ± 5.2 | 27.7 ± 5.1 | < .002 |
| Hyperlipidemia | 56.9% | 59.8% | 56.0% | .254 |
| Previous PTA | 30.6% | 19.4% | 16.8% | < .001 |
| Previous CABG | 9.6% | 4.5% | 3.3% | < .002 |
| Previous infarction | 20.6% | 9.1% | 7.6% | < .001 |
| Coronary artery disease | 45.6% | 32.7% | 34.7% | < .002 |
| Left main coronary artery disease | 5.6% | 3.4% | 1.8% | .222 |
| Incomplete revascularization | 20.7% | 30.4% | 35.3% | .174 |
| COPD | 16.7% | 15.1% | 14.5% | .714 |
| Smoking | 37.2% | 41.7% | 14.4% | .034 |
| Atrial fibrillation | 38.6% | 37.8% | 42.1% | .570 |
| Glomerular filtration | 61.2 (46.0-77.9) | 63.1 (46.8-79.4) | 60.9 (45.5-75.2) | .311 |
| Cancer | 16.0% | 15.5% | 18.7% | .725 |
| EuroSCORE II | 22.5 (14.7-32.0) | 14.3 (7.4-18.0) | 11.8 (8.9-18.9) | < .001 |
| Dyspnea | 87.5% | 87.5% | 91.7% | .289 |
| Emergency procedure | 33.9% | 17.5% | 14.1% | < .001 |
| Valve-in-valve | 3.6% | 3.3% | 2.7% | .881 |
| Post-TAVI outcome | ||||
| Peak gradient (mmHg) | 18.3 ± 7.3 | 19.3 ± 8.9 | 19.4 ± 8.7 | .223 |
| Mean gradient (mmHg) | 9.3 ± 3.8 | 9.9 ± 4.8 | 10.0 ± 5.7 | .229 |
|
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; PTA, percutaneous transluminal angioplasty; TAVI, transcatheter aortic valve implantation. |
||||
Echocardiographic data
Patients with LVEF ≥ 70% had smaller LV end-diastolic and end-systolic volumes, and greater septal wall thickness and RPT than the other 2 groups. In this group, left ventricular mass index (LVMI) was 126.3 ± 32.8 g/m², reflecting a predominant phenotype of concentric hypertrophy and remodelling. A similar pattern was observed in patients with normal LVEF (50%-69%), although this group had a larger end-diastolic LV volume (table 2). In contrast, patients with LVEF < 50% had a greater LV mass, with an LVMI of 147.6 ± 40.2 g/m² (P < .001), a low RWT (< 0.42), and an elevated end-diastolic volume, indicating a predominant phenotype of eccentric hypertrophy. In addition, this group had a lower indexed stroke volume (32.5 ± 11.8; P < .001).
Table 2. Patients’ baseline characteristics
| Characteristics | LVEF < 50% | LVEF 50%-69% | LVEF ≥ 70% | P value |
|---|---|---|---|---|
| RPT | 0.48 (0.41-0.58) | 0.57 (0.50-0.65) | 0.60 (0.52-0.69) | < .001 |
| Indexed LVESV (mL/m²) | 31 (25-39) | 38 (31-45) | 39 (31-49) | < .001 |
| Indexed LVEDV (mL/m²) | 63 (48-80) | 48 (38-59) | 45 (35-56) | < .001 |
| LVMI (g/m²) | 147.6 ± 40.2 | 128.8 ± 34.2 | 126.3 ± 32.8 | < .001 |
| IVS (mm) | 12.1 ± 2.6 | 13.6 ± 2.4 | 14.1 ± 2.7 | < .001 |
| Indexed stroke volume (mL/m²) | 32.5 ± 11.8 | 38 ± 11.5 | 40 ± 11.6 | < .001 |
|
IVS, interventricular septum; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVMI, left ventricular mass index; RPT, relative parietal thickness. |
||||
Perioperative clinical endpoints
There were no significant differences among the 3 groups regarding intra- and postoperative mortality.
Clinical endpoints at follow-up
During the 1-year follow-up, 164 patients (14.13%) died, with no significant differences among the 3 groups (LVEF < 50%, 14.6%; LVEF 50%-69%, 12.6%; LVEF ≥ 70%, 12.7%; P < .736). However, significant differences were found in the rate of cardiovascular rehospitalization at 1 year, with higher rates in the supranormal LVEF group (LVEF ≥ 70%, 34.4%; LVEF < 50%, 29.2%; LVEF 50%- 69%, 27.4%; P < .043). Clinical endpoints are shown in table 3.
| Variables | LVEF < 50% (n = 276) | LVEF 50%-69% (n = 702) | LVEF ≥ 70% (n = 182) | P value |
|---|---|---|---|---|
| Perioperative | ||||
| Intraoperative mortality | 0.4% | 1.4% | 0.6% | .345 |
| Postoperative mortality | 2.8% | 3.7% | 4.3% | .676 |
| Follow-up | ||||
| All-cause mortality at 30 days | 2.4% | 3.9% | 5.0% | .359 |
| Cardiovascular mortality at 1 year | 12.8% | 9.6% | 15.2% | .370 |
| All-cause mortality at 1 year | 14.6% | 12.6% | 12.7% | .736 |
| Cardiovascular rehospitalization at 1 year | 29.2% | 27.4% | 34.4% | < .043 |
|
LVEF, left ventricular ejection fraction. |
||||
Univariable regression analysis
In patients with supranormal LVEF, coronary artery disease and increased interventricular septal thickness were predictors of cardiovascular hospitalization at 1 year (table 4). In this group, indexed LV end-diastolic volume and a history of coronary artery disease were predictors of all-cause mortality at 1 year (table 5). In the general population, no predictors of 1 year mortality were identified, except for age (table 6).
Table 4. Supranormal left ventricular ejection fraction and predictors of cardiovascular hospitalization at 1 year
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.077 | 0.991-1.169 | .080 |  |
| Hypertension | 1.687 | 0.546-5.213 | .364 | |
| Diabetes mellitus | 1.846 | 0.767-4.440 | .171 | |
| Body mass index | 1.012 | 0.933-1.099 | .770 | |
| Coronary artery disease | 0.327 | 0.137-0.780 | .012 | |
| Smoking | 1.796 | 0.650-4.965 | .259 | |
| EuroSCORE II | 1.046 | 0.998-1.096 | .060 | |
| RPT | 1.004 | 0.041-24.392 | .998 | |
| Indexed LVEDV | 0.979 | 0.949-1.010 | .188 | |
| IVS | 0.965 | 0.933-0.998 | .036 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
Table 5. Supranormal left ventricular ejection fraction and predictors of 1-year mortality
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.180 | 0.976-1.426 | .087 |  |
| Hypertension | 2.181 | 0.167-28.575 | .552 | |
| Diabetes mellitus | 0.875 | 0.154-4.968 | .154 | |
| Body mass index | 1.004 | 0.796-1.265 | .976 | |
| Coronary artery disease | 3.372 | 0.612-18.575 | .012 | |
| Smoking | 7.453 | 0.691-61.024 | .259 | |
| EuroSCORE II | 0.921 | 0.831-1.022 | .120 | |
| RPT | 0.011 | 0.000-154.979 | .998 | |
| Indexed LVEDV | 1.094 | 1.018-1.177 | .015 | |
| IVS | 1.004 | 0.943-1.068 | .912 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
Table 6. Predictors of 1-year mortality in the general population
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.070 | 1.002-1.143 | .043 |  |
| Hypertension | 1.268 | 0.545-2.947 | .582 | |
| Diabetes mellitus | 1.458 | 0.764-2.784 | .253 | |
| Body mass index | 0.949 | 0.882-1.020 | .152 | |
| Coronary artery disease | 1.593 | 0.867-2.929 | .134 | |
| Smoking | 1.794 | 0.899-3.581 | .097 | |
| EuroSCORE II | 1.046 | 0.973-1.033 | .868 | |
| RPT | 0.252 | 0.022-2.836 | .264 | |
| Indexed LVEDV | 0.986 | 0.967-1.006 | .188 | |
| IVS | 1.000 | 0.974-1.027 | .036 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
DISCUSSION
This study demonstrates that LVEF is an important prognostic factor in patients with severe AS treated with TAVI. While no differences in mortality were observed at 1 month or 1 year, patients with supranormal LVEF (≥ 70%) had a higher rate of rehospitalization at 1 year than those with reduced (< 50%) or normal (50%-69%) LVEF.
LVEF has been widely recognized in the literature as a prognostic factor in various clinical contexts. A study by Wehner et al.9 reported that an LVEF of 60% to 65% is associated with the best prognosis, while patients with LVEF ≥ 70% have a 5-year mortality rate similar to those with reduced LVEF. A study by Gu et al.,10 found higher mortality and hospitalization rates at 5 years in patients hospitalized for heart failure with LVEF > 65% than in those with normal LVEF.
In patients with AS undergoing TAVI, the OCEAN-TAVI registry found that LVEF > 65% was an independent predictor of death and rehospitalization at the 3-year follow-up (hazard ratio [HR], 1.16; 95%CI, 1.02-1.31; P = .023).¹¹ There were no significant differences in mortality among the study groups, except for the rehospitalization rate. It remains to be elucidated whether longer-term follow-up could also detect differences in mortality.
In patients with AS undergoing surgical interventions, LVEF is a recognized prognostic marker. In a study by Dahl et al.,¹² reduced LVEF (< 50%) was a clear predictor of 5-year risk. The study revealed that patients with supranormal LVEF experienced longer hospital stays, increased need for mechanical ventilation, a higher incidence of hemodialysis, and a greater rate of rehospitalization. This latter finding is consistent with the findings of the present study. There is no clear explanation for these results, but they may be related to the persistence of myocardial hypertrophy or diastolic dysfunction following the intervention.13
According to previous studies, increased (> 80 mL/m²) and reduced (< 55 mL/m²) ventricular volumes are risk factors to consider in patients with severe AS.14,15 In this analysis, in the subgroup of patients with supranormal LVEF, indexed LV end-diastolic volume was a predictor of 1-year mortality (HR, 1.094; 95%CI, 1.018-1.177; P < .015). A low indexed stroke volume has also been associated with worse prognosis in patients with AS, both with reduced and preserved LVEF.16 Patients with preserved LVEF may have a low stroke volume when the ventricular cavity is small and they have restrictive physiology that limits the stroke volume, even with a supranormal ejection fraction.17 In most studies, these patients have a worse prognosis, with a higher mortality risk and less event-free time.18,19
Supranormal LVEF represents a new phenotype in patients with preserved LVEF (> 50%), with distinctive clinical and hemodynamic characteristics. There is no universal agreement on the exact LVEF value to define supranormal. According to the American College of Cardiology, a LVEF ≥ 70% is considered supranormal,20 while other groups set this threshold at ≥ 65%. For this study, LVEF ≥ 70% was used as the reference to better highlight clinical and echocardiographic differences among the study groups, which likely influenced the prevalence observed in the population.
In a study by Wehner et al.,9 which reviewed 403 977 echocardiograms from 203 135 patients without prespecified diagnoses, an LVEF ≥ 70% was found in 3% (13 553) of participants. In the present study of patients with severe AS, 15% had LVEFs ≥ 70%. Other studies, such as the OCEAN-TAVI registry,¹¹ reported a higher percentage of patients with supranormal LVEF and AS (47%), likely because they used a lower cutoff for supranormal LVEF (≥ 65%). These findings suggest that severe AS is associated with a higher-than-normal LVEF, likely due to left ventricular (LV) remodelling and concentric hypertrophy resulting from elevated afterload.21-24 In our study, the LVMI was elevated in most patients, regardless of LVEF. Patients with normal and supranormal LVEF predominantly exhibited concentric geometry, characterized by a reduced LV cavity and increased septal thickness. In contrast, patients with reduced LVEF showed predominantly eccentric geometry with a dilated LV.
Finally, our results suggest that while widely used risk scales like EuroSCORE II remain valid, echocardiographic factors should also be considered when determining the timing and type of intervention.25
Limitations
This retrospective, observational study was conducted at a single center. All patients underwent TAVI, and there was no comparison with those treated with surgical valve replacement. The medical and pharmacological treatment were not specified, which is an important omission given recent advancements in heart failure management. In addition, a 1-year follow-up may be too short to detect differences in mortality between the groups and a longer-term follow-up might reveal differences.
CONCLUSIONS
LVEF remains an important prognostic factor in decision-making for patients with severe AS. In this study, patients with reduced (< 50%), normal (50%-69%), or supranormal (≥ 70%) preprocedural LVEF who underwent TAVI showed no differences in 1-year mortality. However, those with supranormal LVEF (≥ 70%) had a higher rate of cardiovascular-related rehospitalization at 1 year, suggesting that this subgroup may have unfavorable factors, such as significant diastolic dysfunction. Further research is needed to investigate and confirm these findings.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study adhered to the Declaration of Helsinki and received approval from the ethics committee of Hospital Clínico San Carlos in Madrid, Spain. As the study was retrospective and posed no risk to patients, informed consent was not required. All information was handled with strict confidentiality by the researchers. Consecutive patients were recruited during the study period without sampling or randomization, so sex or gender biases were not considered in the analysis
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used during the performance of the study.
AUTHORS’ CONTRIBUTIONS
E. Martínez Gómez, X. Solar, D. Faria, L. Nombela Franco, and J.A. de Agustín contributed to the conception and design, data acquisition, analysis, and interpretation of the study. E. Martínez Gómez, X. Solar, D. Faria, L. Nombela Franco, P. Jiménez Quevedo, G. Tirado, E. Pozo Osinalde, C. Olmos Blanco, P. Mahía Casado, P. Marcos Alberca, M. Luaces, J.J. Gómez de Diego, L. Collado Yurrita, A. Fernández-Ortiz, J. Pérez-Villacastín, and J.A. de Agustín contributed to the drafting of the article or its critical revision. All authors approved the final version of the article.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- LVEF is a highly significant prognostic marker in cardiology. Paradoxically, studies have shown that patients with a supranormal LVEF have a worse prognosis in some scenarios, such as AS.
WHAT DOES THIS STUDY ADD?
- This study shows that patients undergoing TAVI with supranormal LVEF (≥ 70%) have higher rehospitalization rates at 1 year than those with reduced (< 50%) or normal (50%-69%) LVEF.
REFERENCES
1. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly:disease prevalence and number of candidates for transcatheter aortic valve replacement:a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002-1012.
2. D'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people:the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515-3522.
3. Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61-67.
4. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
5. Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8:e002917.
6. Bing R, Cavalcante JL, Everett RJ, et al. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:283-296.
7. Shah S, Segar MW, Kondamudi N, et al. Supranormal Left Ventricular Ejection Fraction, Stroke Volume, and Cardiovascular Risk:Findings From Population-Based Cohort Studies. JACC Heart Fail. 2022;10:583-594.
8. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
9. Wehner GJ, Jing L, Haggerty CM, et al. Routinely reported ejection fraction and mortality in clinical practice:where does the nadir of risk lie?Eur Heart J. 2020;41:1249-1257.
10. Gu J, Ke JH, Wang Y, Wang CQ, Zhang JF. Characteristics, prognosis, and treatment response in HFpEF patients with high vs. normal ejection fraction. Front Cardiovasc Med. 2022;9:944441.
11. Imamura T, Hida Y, Ueno H, et al. Clinical Implication of Supra-Normal Left Ventricular Ejection Fraction in Patients Undergoing Transcatheter Aortic Valve replacement. J Clin Med. 2023;12:7429.
12. Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8:e002917.
13. Mariage JL, Bulpa P, Michaux I, et al. Impact of myocardial hypertrophy and preoperative left ventricular ejection fraction on post operative complications after aortic valve replacement for aortic stenosis. Chest. 2005;128:268S.
14. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling concepts and clinical implications:a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569-582.
15. Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure overloaded human heart:structural deterioration and compensatory mechanisms. Circulation. 2003;107:984-991.
16. Kwak S, Everett RJ, Treibel TA, et al. Markers of Myocardial Damage Predict Mortality in Patients With Aortic Stenosis. J Am Coll Cardiol. 2021;78:545-558.
17. Severino P, Maestrini V, Mariani MV, et al. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail Rev. 2020;25:9-17.
18. Ito S, Nkomo VT, Orsinelli DA, et al. Impact of Stroke Volume Index and Left Ventricular Ejection Fraction on Mortality After Aortic Valve Replacement. Mayo Clin Proc. 2020;95:69-76.
19. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction:implications for diagnosis and treatment. Eur Heart J. 2010;31:281-289.
20. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
21. Forrest L, Rocheleau G, Bafna S, et al. Genetic and phenotypic profiling of supranormal ejection fraction reveals decreased survival and underdiagnosed heart failure. Eur J Heart Fail. 2022;24:2118-2127.
22. Dumesnil JG, Shoucri RM. Effect of the geometry of the left ventricle on the calculation of ejection fraction. Circulation. 1982;65:91-98.
23. González Gómez A, Fernández Golfín C, Monteagudo JM, et al. Severe aortic stenosis patients with preserved ejection fraction according to flow and gradient classification:Prevalence and outcomes. Int J Cardiol. 2017;248:211-215.
24. Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864.
25. Strachinaru M, Van Mieghem NM. Low-gradient severe aortic stenosis with preserved ejection fraction:how fast should we act?Int J Cardiovasc Imaging. 2021;37:3177-3180.
* Corresponding author.
E-mail address: eimartin1980@gmail.com (E. Martínez Gómez).
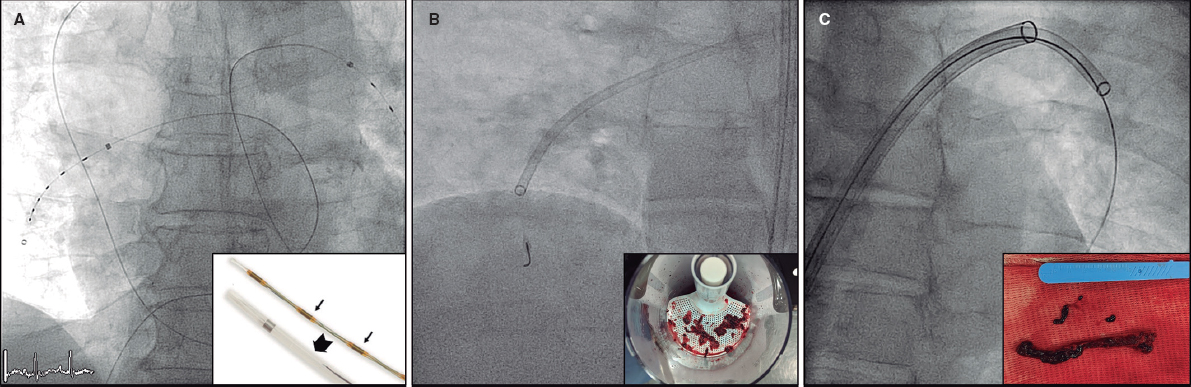
ABSTRACT
Introduction and objectives: Most patients with acute pulmonary embolism (PE) receive anticoagulation only. Reperfusion is required in high-risk and a minority of intermediate-risk PE patients. Systemic thrombolysis (ST) is the first-line reperfusion therapy, but due to contraindications and major bleeding concerns, the use of catheter-directed therapies (CDT) is increasing as a suitable alternative. The objective of the present study was to detect predictors of the use of CDT compared with other therapies in patients with acute PE.
Methods: This registry included consecutive intermediate- and high-risk PE patients in 2 tertiary centers with a 24/7 PE response team from 2014 to 2022. The patients were grouped according to the primary treatment: anticoagulation only, CDT, or ST. We evaluated predictors of treatment assignment and safety endpoints.
Results: A total of 274 patients were included. Of them, 112 received anticoagulation only, 96 received ST as the primary treatment, and 66 underwent CDT first. Comorbidities were higher in the CDT group than in the other 2 groups. Patients undergoing ST/CDT had higher PE severity parameters at hospital admission. On multivariable analysis, independent predictors for the use of CDT were Charlson Comorbidity Index (OR, 1.29; 95%CI, 1.05-1.59), recent surgery (OR, 11.07; 95%CI, 3.07-39.87), and bilateral central PE (OR, 2.42; 95%CI, 1.10-5.32). Analysis of early safety outcomes showed that intracranial bleeding occurred only in the ST group (1.8% of patients).
Conclusions: This contemporary registry used CDT as the primary treatment in 24% of intermediate- and high-risk patients, mainly in comorbid and postsurgical patients. CDT was a safe and effective alternative to medical therapy in selected patients.
Keywords: Catheter-directed therapies. Pulmonary embolism. Systemic thrombolysis. Anticoagulation. Local thrombolysis.
RESUMEN
Introducción y objetivos: La mayoría de los pacientes con embolia pulmonar (EP) aguda reciben únicamente anticoagulación. La reperfusión es necesaria en los pacientes con EP de alto riesgo y en una minoría de pacientes con EP de riesgo intermedio-alto. La trombólisis sistémica (TS) es el tratamiento de reperfusión de primera línea, pero debido a las contraindicaciones y a la preocupación por las hemorragias graves, las terapias dirigidas por catéter (TDC) están surgiendo como una alternativa adecuada. El objetivo del presente estudio fue detectar predictores del uso de TDC con respecto a otras terapias en pacientes con EP aguda.
Métodos: Este registro incluyó pacientes consecutivos con EP de riesgo intermedio y alto en dos centros terciarios, con un equipo de respuesta a la EP, desde 2014 hasta 2022. Los pacientes se agruparon según la terapia inicial: solo anticoagulación, TDC o TS; y se evaluaron los predictores de selección de terapia y variables de seguridad.
Resultados: Se incluyó a un total de 274 pacientes. De ellos, 112 recibieron solo anticoagulación, 96 recibieron TS como tratamiento primario y 66 fueron sometidos a TDC en un primer momento. Las comorbilidades fueron mayores en el grupo TDC que en los otros dos. Los pacientes sometidos a TS o TDC presentaban mayores parámetros de gravedad de la EP al ingreso hospitalario. Tras el análisis multivariable, el índice de comorbilidad de Charlson (OR = 1,29; IC95%, 1,05-1,59), la cirugía reciente (OR = 11,07; IC95%, 3,07-39,87) y la EP central bilateral (OR = 2,42; IC95%, 1,10-5,32) siguieron siendo predictores independientes del uso de TDC. En cuanto a los resultados precoces de seguridad, sólo se produjeron hemorragias intracraneales en el grupo TS (1,8% de los pacientes).
Conclusiones: Este registro contemporáneo utilizó TDC como terapia inicial en el 24% de los pacientes de riesgo intermedio y alto, principalmente en pacientes comórbidos y posquirúrgicos. La TDC fue una alternativa segura y eficaz al tratamiento médico en pacientes seleccionados.
Palabras clave: Terapia dirigida por catéter. Intervencionismo dirigido por catéter. Embolia pulmonar. Trombólisis sistémica. Anticoagulación. Trombólisis local.
Abbreviation
AC: anticoagulation alone. CDT: catheter-directed therapies. HR: high risk. IHR: intermediate-high risk. PE: pulmonary embolism. ST: systemic thrombolysis.
INTRODUCTION
Pulmonary embolism (PE) is the third leading cause of cardiovascular death and the first avoidable cause of death in hospitalized patients.1 According to the European Society of Cardiology (ESC) guidelines, the treatment of PE is based on patient risk assessment.2 Reperfusion therapy with systemic thrombolysis (ST) is indicated as the first-line therapy in patients with high-risk (HR) PE and in those with intermediate-high risk (IHR) PE who deteriorate on anticoagulant drugs.2 However, ST is underused because of contraindications in roughly 30% of patients and even in those with HR-PE and no formal contraindications.3-5 Moreover, this therapy carries a significant risk of major bleeding (≈10%-15%), especially in patients with advanced age, recent surgery, or active cancer.3
Catheter-directed therapies (CDT) have emerged as an alternative to ST for reperfusion in patients with acute PE.6-10 These techniques may improve surrogate right parameters of ventricular failure and clinical outcomes with lower bleeding rates. In a meta-analysis of observational studies comparing catheter-directed thrombolysis vs ST, the risk of in-hospital death and intracranial hemorrhage was reduced in patients undergoing percutaneous intervention. 11 The current ESC guidelines state that CDT should be considered in patients with HR-PE an unsuccessful attempt at thrombolysis or a contraindication to this treatment, and as a rescue treatment for IHR-PE patients with clinical deterioration.2 However, the penetration of interventional therapies is increasing, showing a discrepancy between guideline recommendations and clinical practice.
There is currently scarce evidence in the literature on the contemporary choice of reperfusion therapy, the parameters leading physicians to select one reperfusion therapy over the others, and the target population who may derive the greatest benefit from CDT. Therefore, the main objective of the present study was to identify the clinical factors associated with the choice of CDT as PE therapy in a contemporary cohort of patients with acute PE.
METHODS
Study design
This study was based on an ambispective multicenter registry that included consecutive patients with intermediate-risk (IR) and HR-PE, evaluated by local Pulmonary Embolism Response Teams (PERT), classified according to ESC guidelines,2 and treated with CDT.12 Two tertiary care centers also recruited patients evaluated by the PERT and treated medically, as previously reported in a single-center experience.13 This study analyzed all consecutive patients evaluated by the local PERT in these 2 hospitals from 2014 to 2022.
The inclusion criteria were patients aged more than 18 years with a confirmed diagnosis of acute IR- or HR-PE (by computed tomography or transthoracic echocardiogram plus clinical suspicion in unstable patients unable to undergo computed tomography). We excluded patients with an uncertain diagnosis of PE, those with > 7 days from symptom onset to diagnosis, and those with low-risk PE according to ESC guidelines.2 The registry was observational, with no recommendation on PE management. Thus, treatment was established according to the criteria of the treating physicians, and the use of CDT was chosen according to availability and the decision of the PERT. The reporting of this study adheres to the Strengthening The Reporting of Observational studies in Epidemiology (STROBE) guideline for cohort studies.14
Data collection and variable definitions
A secure web-based database stored anonymized data in both centers. Data were self-reported by local investigators from digital clinical records and included vital signs and laboratory values. Initial admission to the cardiac intensive care unit included more granular data with recording of hourly clinical vital signs, shock parameters at admission, and worsening during cardiac intensive care unit admission and subsequently after reperfusion (if the patient underwent reperfusion). After hospital discharge, structured follow-up was conducted with visits at 1-month, 3- to 6-months, and 12-months. However, 30-day follow-up results are included in this study. The right ventricle/left ventricle ratio was mainly derived from computed tomography except in patients with no baseline computed tomography due to instability. Bilateral central PE was diagnosed when a thrombus was detected in both main pulmonary arteries by computed tomography or angiography. PE risk was stratified according to ESC guidelines.2 In all patients, we calculated the shock index, defined by the heart rate to systolic blood pressure ratio, Pulmonary Embolism Severity Index score,15 Bova score,16 and Charlson Comorbidity Index.17 For most patients who underwent CDT, hemodynamic parameters (such as systolic and mean pulmonary artery pressure) were measured invasively, with a catheter placed in the pulmonary artery.
Pulmonary embolism therapies
Parenteral anticoagulation was started immediately after PE diagnosis. ST was given through a peripheral vein following the doses recommended in the ESC guidelines.2 CDT included: a) catheter-directed thrombolysis using a multiperforated catheter(s) inserted into the pulmonary artery and left for 6 to 24 hours to infuse low-dose thrombolytics (usually alteplase 0.25 mg/kg or the tenecteplase equivalent); b) mechanical thrombus fragmentation; c) thrombus aspiration using either nondedicated catheters (usually 8-Fr coronary guiding catheters) or dedicated catheters (Indigo 8-Fr [Penumbra, United States], Nautilus 10-Fr [iVascular, Spain], or FlowTriever 24-Fr [Inari medical, United States]); or d) a combination of them. The dose of fibrinolytic therapy (both for ST and catheter-directed thrombolysis) was decided by the treating physician. See figure 1 for an illustration of different CDT options.
Figure 1. Catheter-directed therapies used in the study. Representative images of catheter-directed therapies. A: ultrasound-assisted thrombolysis, EKOS system (Boston Scientific, United States). B: percutaneous thrombectomy with Indigo system (Penumbra, United States). C: large-bore thrombus aspiration, FlowTriever catheter (Inari, United States).
Objectives
The main endpoint of the present study was to detect predictors of the use of CDT in patients with IR- or HR-PE requiring reperfusion therapy. Another endpoint was to compare the characteristics of the patients who received the different therapies for acute PE: anticoagulation alone (AC), ST, or CDT. If more than 1 reperfusion therapy was used, the patients were grouped according to the first administered therapy. The analysis focused on identifying variables associated with the choice of different therapies by the treating physician. Thirty-day all-cause mortality was reported as a safety outcome. We also analyzed in-hospital adverse events, such as bleeding events according to the International Society of Thrombosis and Hemostasis classification18 and acute kidney injury. In patients undergoing CDT, we also recorded procedural results (eg, hemodynamic improvement).
Ethics and funding
The registry protocol was approved by the clinical research ethics committee at Hospital Clínico San Carlos as the central committee for the registry, following local research regulations (code 18/010-E). All prospectively included patients signed an informed consent form. An informed consent waiver was granted from the ethics research committee for patients recruited retrospectively. The investigation was an academic, unfunded, investigator-initiated study.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables as mean ± standard deviation (SD) or median [interquartile range (IQR)], as appropriate. Group comparisons (AC, CDT, and ST) for continuous variables were performed using the ANOVA and chi-square tests for categorical variables. Comparisons between groups were performed with the Student t-test or Wilcoxon test, as appropriate, for continuous variables and the chi-square test for categorical variables. The predictors for using the different reperfusion techniques (ie, CDT or ST) were determined using a logistic regression analysis. The univariate analysis included baseline and clinical variables at PE diagnosis. Variables with P values < .10 in the univariable analysis were included in the multivariable model. Paired t-tests were used to analyze the change in hemodynamic parameters after transcatheter procedures. Missing values for covariates, if any, were not imputed. Statistical analyses were performed using Stata 16 (StataCorp, College Station, United States).
RESULTS
Baseline characteristics and risk stratification
From 2014 to 2022, a total of 274 patients were included in the registry (9.5% with intermediate-low risk, 74.7% with IHR, and 15.8% with HR-PE) (figure 2). Of them, 112 patients (40.9%) received AC only: 57% low molecular weight heparin and 43% unfractionated heparin. The remaining 162 patients (59.1%) underwent reperfusion therapy: 35% received ST as the primary treatment and 24% underwent CDT first. Of the ST group, all the patients received alteplase as fibrinolytic treatment and 5 patients underwent rescue CDT after unsuccessful ST. Notably, 58% of IHR-PE patients in our cohort received reperfusion therapies.
Figure 2. Study patients and selected therapy.
Patients’ baseline characteristics according to the treatment strategy are shown in table 1. The study was well balanced regarding gender (52% men); however, there were more men in the AC group than in the ST group (58.0% vs 42.7%, P = .027). Patients in the AC and CDT groups were significantly older than those in the ST group (65.9 ± 16.2 and 62.3 ± 14.7 vs 57.4 ± 16.6 years, respectively, P < .001). Regarding comorbidities, previous cancer was more common among patients in the CDT group than in those in the ST group. The Charlson Comorbidity Index was higher in the CDT group than in the other 2 groups. Among precipitating factors for PE, a history of recent surgery was more frequent in patients in the CDT group than in the other 2 groups, while a recent hospital admission was more frequent in the AC and CDT groups than in the ST group.
Table 1. Baseline characteristics
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Male sex | 142 (51.8%) | 65 (58.0%) | 41 (42.7%) | 36 (54.5%) | .077 | .027 | .650 | .138 |
| Age, years | 62.1 (16.4) | 65.9 (16.2) | 57.4 (16.6) | 62.3 (14.7) | < .001 | < .001 | .136 | .056 |
| Body mass index (kg/m2) | 29.4 (6.7) | 29.4 (6.0) | 29.7 (8.8) | 29.2 (5.0) | .921 | .765 | .890 | .724 |
| Obesity | 133 (48.5%) | 52 (46.4%) | 54 (56.3%) | 27 (40.9%) | .136 | .167 | .533 | .078 |
| Prior venous thromboembolism | 53 (19.4%) | 20 (17.9%) | 22 (23.2%) | 11 (16.7%) | .511 | .345 | .840 | .316 |
| Previous cancer | 42 (15.3%) | 15 (13.4%) | 11 (11.5%) | 16 (24.2%) | .065 | .674 | .065 | .032 |
| Hypertension | 135 (49.5%) | 55 (49.1%) | 46 (47.9%) | 34 (52.3%) | .857 | .864 | .681 | .585 |
| Diabetes mellitus | 51 (18.7%) | 18 (16.1%) | 19 (19.8%) | 14 (21.5%) | .628 | .484 | .362 | .788 |
| Heart failure | 14 (5.1%) | 8 (7.1%) | 3 (3.1%) | 3 (4.5%) | .411 | .197 | .487 | .638 |
| Chronic kidney disease | 20 (7.3%) | 10 (8.9%) | 4 (4.2%) | 6 (9.1%) | .342 | .172 | .971 | .201 |
| Charlson Comorbidity Index | 1.0 (1.6) | 0.8 (1.4) | 0.9 (1.5) | 1.5 (1.8) | .026 | .676 | .010 | .043 |
| Recent surgery | 35 (12.8%) | 12 (10.8%) | 4 (4.2%) | 19 (28.8%) | < .001 | .074 | .002 | <.001 |
| Recent immobilization | 48 (17.5%) | 14 (12.5%) | 17 (17.7%) | 17 (25.8%) | .080 | .293 | .024 | .216 |
| Recent hospital admission | 28 (10.3%) | 14 (12.6%) | 4 (4.2%) | 10 (15.2%) | .044 | .032 | .633 | .014 |
|
AC, anticoagulation; CDT, catheter-directed therapies; ST, systemic thrombolysis. Data are shown as mean (SD) for continuous variables and No. (%) for categorical variables. P values denote the significance of the differences between the groups for continuous variables analyzed by the ANOVA test and Student t-test, as appropriate. The chi-square test was used to assess the significance of between-group differences for categorical variables. Obesity was defined as body mass index ≥ 30 kg/m2. Statistically significant values are highlighted in bold letters. |
||||||||
Clinical and risk stratification parameters at hospital admission are shown in table 2. Patients who received reperfusion therapies, either with CDT or ST, had higher severity parameters than those in the AC group (eg, shock index, right ventricular involvement, or lactate levels). The Pulmonary Embolism Severity Index score, which incorporates comorbidities and PE severity parameters, was higher in CDT patients than in the other 2 groups (P < .001).
Table 2. Risk stratification parameters at hospital admission
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Systolic blood pressure, mmHga | 118.7 (25.3) | 126.8 (23.1) | 114.5 (25.9) | 110.8 (24.6) | < .001 | < .001 | < .001 | .359 |
| Heart rate, bpm | 106.9 (18.8) | 99.5 (19.7) | 112.9 (16.3) | 110.9 (16.2) | < .001 | < .001 | < .001 | .459 |
| Shock Index | 0.96 (0.36) | 0.82 (0.28) | 1.06 (0.39) | 1.07 (0.35) | < .001 | < .001 | < .001 | .953 |
| Respiratory failure | 71 (28.9%) | 28 (26.4%) | 29 (34.9%) | 14 (24.6%) | .314 | .205 | .796 | .191 |
| Syncope | 57 (20.8%) | 23 (20.5%) | 18 (18.8%) | 16 (24.2%) | .696 | .747 | .564 | .399 |
| Deep vein thrombosis | 74 (27.6%) | 34 (30.6%) | 23 (24.5%) | 17 (27.0%) | .612 | .326 | .612 | .723 |
| Right ventricular involvement | 249 (94.0%) | 93 (87.7%) | 94 (98.9%) | 62 (96.9%) | .002 | .002 | .042 | .346 |
| Bilateral pulmonary embolism | 175 (63.9%) | 70 (62.5%) | 57 (59.4%) | 48 (72.7%) | .204 | .645 | .163 | .080 |
| Lactate, mmol/L | 2.9 (2.9) | 2.2 (2.0) | 3.7 (3.8) | 3.0 (2.6) | .006 | .002 | .039 | .315 |
| Elevated troponin levels | 209 (86.0%) | 85 (83.3%) | 73 (89.0%) | 51 (86.4%) | .539 | .271 | .600 | .642 |
| Elevated NT-proBNP levels | 167 (78.4%) | 74 (77.9%) | 57 (78.1%) | 36 (80.0%) | .958 | .977 | .777 | .804 |
| High-risk PEb | 43 (15.8%) | 8 (7.1%) | 18 (18.8%) | 17 (26.2%) | .002 | .012 | < .001 | .264 |
| PESI score | 105.1 (35.1) | 97.6 (29.3) | 104.9 (36.1) | 118.2 (39.4) | < .001 | .109 | < .001 | .028 |
| Bova score | 4.7 (1.5) | 4.2 (1.5) | 5.1 (1.4) | 5.0 (1.5) | < .001 | < .001 | .002 | .526 |
|
AC, anticoagulation; CDT, catheter-directed therapies; PE, pulmonary embolism; PESI, pulmonary embolism severity index; ST, systemic thrombolysis. Statistically significant values are highlighted in bold. Data are shown as mean ± standard deviation for continuous variables and No. (%) for categorical variables. P values denote the significance of the differences between the groups for continuous variables analyzed by the ANOVA test and Student t-test, as appropriate. The chi-square test tested the significance of between-group differences for categorical variables. aThis variable reflects systolic blood pressure at hospital admission, but some of these patients were under vasopressors, and others were stable on admission and later deteriorated hemodynamically. bAs defined by the European Society of Cardiology guidelines. |
||||||||
Reperfusion therapies
Figure 3 shows the trend in the choice between the 2 primary reperfusion therapies over time. There was a progressive increase in the use of CDT and a consequent decrease in the use of ST. The variables that might have led the treating physicians to choose between the 2 reperfusion therapies are shown in table 3. In the univariate analysis, the variables associated with the choice of CDT instead of ST were those reflecting comorbidities, such as older age, previous cancer, and the Charlson Comorbidity Index. Recent surgery and hospital admission were also associated with the choice of CDT. After multivariable analysis in this cohort of patients with acute PE, the only independent predictors of the choice of CDT over ST were the Charlson Comorbidity Index and recent surgery. In addition, this analysis showed that the presence of bilateral central PE was associated with the treating physician’s choice of CDT instead of ST.
Figure 3. Choice of reperfusion therapy over the years.
Table 3. Univariate and multivariable predictors of the choice of CDT over ST or AC as a first-line therapy in acute pulmonary embolism
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | OR (95%CI) | P | OR (95%CI) | P |
| Male sex | 1.61 (0.86-3.03) | .139 | ||
| Age (per year) | 1.02 (1.00-1.04) | .058* | ||
| Body mass index (per kg/m2) | 0.99 (0.94-1.04) | .722 | ||
| Prior venous thromboembolism | 0.66 (0.30-1.48) | .317 | ||
| Previous cancer | 2.47 (1.06-5.75) | .035* | ||
| Hypertension | 1.19 (0.63-2.24) | .585 | ||
| Diabetes mellitus | 1.11 (0.51-2.42) | .788 | ||
| Heart failure | 1.48 (0.29-7.55) | .640 | ||
| Chronic kidney disease | 2.30 (0.62-8.49) | .211 | ||
| Recent surgery | 9.30 (2.99-28.90) | < .001 | 11.07 (3.07-39.87) | < .001 |
| Recent immobilization | 1.61 (0.75-3.45) | .219 | ||
| Recent hospital admission | 4.11 (1.23-13.72) | .022 | 1.25 (0.29-5.43) | .767 |
| Systolic blood pressure (per mmHg) | 0.99 (0.98-1.01) | .357 | ||
| Heart rate (per bpm) | 0.99 (0.97-1.01) | .457 | ||
| Respiratory failure | 0.61 (0.29-1.29) | .193 | ||
| Syncope | 1.39 (0.65-2.97) | .400 | ||
| Deep vein thrombosis | 1.14 (0.55-2.36) | .723 | ||
| Right ventricular involvement | 0.33 (0.03-3.72) | .369 | ||
| Bilateral central pulmonary embolism | 1.82 (0.93-3.59) | .082 | 2.42 (1.10-5.32) | .028 |
| Lactate (per mmol/L) | 0.94 (0.83-1.06) | .317 | ||
| Elevated troponin levels | 1.00 (1.00-1.00) | .312 | ||
| Elevated NT-proBNP levels | 1.12 (0.45-2.81) | .804 | ||
| Charlson Comorbidity Index | 1.21 (1.00-1.47) | .048 | 1.29 (1.05-1.59) | .018 |
|
OR, ods ratio; 95%CI, 95% confidence interval. *Age and previous cancer were not included in the multivariable model despite being significant in the univariate analysis to avoid problems of collinearity because they are included in the Charlson Comorbidity Index. |
||||
Procedural characteristics in the CDT group are displayed in table 4. The median treatment delay from diagnosis of acute PE to percutaneous treatment was 6.0 [interquartile range [IQR], 3.5-19.0] hours and the mean procedure length was 89.0 ± 44.4 minutes. Catheter-directed thrombolysis was used in 35 patients (53.0%), and the most frequently used thrombolytic drug was alteplase (71.4%), with a mean dose of 16.7 ± 7.2 mg. The median bolus dose in patients treated with alteplase was 4 [IQR, 2.9-6.3] mg and the median perfusion time of the remaining dose was 16.0 [IQR, 12.0-20.0] hours. In all patients treated with tenecteplase, the drug was administered as a bolus. Thrombus aspiration was performed in 42 patients (63.6%). The most commonly used aspiration devices were coronary catheters (42.9%), followed by FlowTriever catheter (Inari Medical, United States) (38.1%). A combined thrombolysis plus aspiration technique was performed in 11 patients. Systolic pulmonary artery pressure decreased from 57.9 ± 15.4 to 47.6 ± 12.6 mmHg (mean: −10.3 ± 11.3 mmHg, P < .001) after the percutaneous procedure, while the mean pulmonary artery pressure decreased from 35.0 ± 9.1 to 28.6 ± 8.8 mmHg (mean: −6.4 ± 6.8 mmHg, P < .001). Systolic blood pressure significantly increased after the procedure from 127.8 ± 23.4 to 138.8 ± 22.0 mmHg (mean: +11.0 ± 24.5 mmHg, P = .028).
Table 4. Procedural characteristics in the catheter-directed therapies group
| Patients with percutaneous intervention (N = 66) | |
|---|---|
| Therapy delay, hours* | 6.0 [3.3-19.0] |
| Procedure length, minutes | 89.0 (44.4) |
| Vascular access | |
| Femoral | 64 (97.0%) |
| Brachial | 2 (3.0%) |
| Maximum sheath diameter, French | 8.0 [6.0-20.0] |
| Catheter-directed thrombolysis | 35 (53.0%) |
| Thrombolytic drug | |
| Alteplase | 25 (71.4%) |
| Tenecteplase | 10 (28.6%) |
| Drug dose | |
| Alteplase, mg | 16.7 (7.2) |
| Tenecteplase, units | 3737.5 (1947.8) |
| Ultrasound-assisted | 2 (5.7%) |
| Thrombus aspiration | 42 (63.6%) |
| Catheter | |
| Coronary catheters | 18 (42.9%) |
| FlowTriever | 16 (38.1%) |
| Indigo | 6 (14.3%) |
| Nautilus | 2 (4.8%) |
| sPAP change, mmHg | −10.3 (11.3) |
| mPAP change, mmHg | −6.4 (6.8) |
| sBP change, mmHg | +11.0 (24.5) |
| mBP change, mmHg | +5.3 (17.6) |
|
mBP, mean blood pressure; mPAP, mean pulmonary artery pressure; rTPA, alteplase; sBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; TNK, tenecteplase. Data are shown as mean ± standard deviation or median [interquartile range] for continuous variables, as appropriate, and No. (%) for categorical variables. * Therapy delay was defined as the time that elapsed between diagnosis of pulmonary embolism and the procedure. |
|
Safety outcomes
Early clinical outcomes and in-hospital events according to the treatment strategy are shown in table 5. The median length of hospitalization was 8 [IQR, 6.0-13.0] days. In-hospital major bleeding, as defined by the International Society of Thrombosis and Hemostasis, occurred in 7 patients (7.3%) in the ST group and in 9 patients (13.6%) in the CDT group. Intracranial bleeding occurred in 5 patients, all of them in the ST group, during hospital admission. Vascular access complications, including minor and major events, were found in 6 (10.6%) of the patients who underwent CDT. Of note, 5 of these patients received catheter-directed thrombolysis (4 with alteplase and 1 with tenecteplase) and the tenecteplase-treated patient underwent aspiration with a nonspecific catheter. One of the vascular complications was a hematoma related to extracorporeal membrane oxygenation implantation and which, therefore, bore no direct relationship with the CDT procedure. The remaining events were 1 incident of femoral access bleeding leading to hypovolemic shock and eventual death (a local thrombolysis CDT case), 2 hematomas requiring transfusion, and another 2 hematomas not requiring transfusion. The incidence of 30-day all-cause mortality was 4.6%, 10.4% and 15.9% for the AC, ST and CDT groups, respectively (P = .045). Twenty-two patients died due to hemodynamic or respiratory deterioration related to PE, 2 patients died from anoxic encephalopathy (both in the CDT group), and 1 patient died from severe intracranial bleeding (ST group).
Table 5. Early safety outcomes in patients with acute pulmonary embolism
| Total | AC | ST | CDT | P | ||||
|---|---|---|---|---|---|---|---|---|
| N = 274 | N = 112 | N = 96 | N = 66 | Global | AC vs ST | AC vs CDT | ST vs CDT | |
| Admission length, days | 8.0 (6.0-13.0) | 7.0 (6.0-11.0) | 9.0 (6.0-12.5) | 10.0 (6.0-23.0) | .132 | 0.394 | .052 | .178 |
| In-hospital events | ||||||||
| Major bleeding* | 18 (6.6%) | 2 (1.8%) | 7 (7.3%) | 9 (13.6%) | .008 | 0.052 | .002 | .184 |
| Intracranial bleeding | 5 (1.8%) | 0 (0.0%) | 5 (5.2%) | 0 (0.0%) | .009 | 0.014 | - | .060 |
| Acute kidney injury | 22 (8.0%) | 11 (9.8%) | 9 (9.4%) | 2 (3.0%) | .228 | 0.913 | .093 | .115 |
| Vascular access complication | - | - | - | 6 (10.6%) | - | - | - | - |
| 30-day all-cause death | 25 (9.3%) | 5 (4.6%) | 10 (10.4%) | 10 (15.9%) | .045 | 0.110 | .011 | .310 |
|
AC, anticoagulation; CDT, catheter-directed therapies; ST, systemic thrombolysis. Data are shown as median [interquartile range] for continuous variables and No. (%) for categorical variables. *As defined by the International Society of Thrombosis and Hemostasis. |
||||||||
DISCUSSION
The present study explores the clinical characteristics, risk profile and outcomes of patients with IR and HR-PE in 2 tertiary care referral centers with a 24/7 PERT team. The main findings were as follows: a) in this contemporary PE cohort, the factors associated with the choice of CDT over ST in the multivariable analysis were a higher Charlson Comorbidity Index, a history of recent surgery, and a proximal, bilateral PE; b) the choice of CDT as reperfusion therapy has increased; and c) CDT significantly improves hemodynamic parameters, suggesting that the effectiveness of the treatment is preserved in this comorbid population; nonetheless, the risk of complications is not negligible and should be considered in decision-making.
To our knowledge, this is the first study that focuses on the parameters associated with treating physicians’ choice between the available treatment strategies in patients with acute IR and HR-PE. As expected, patients undergoing reperfusion had worse hemodynamic status and more frequently had right ventricular impairment or higher lactate levels. ST was more frequently used in patients with fewer comorbidities (eg, younger age, recent surgery, or hospital admission), which is in agreement with previous studies.3,5 In contrast, CDT was chosen for patients with a greater number of comorbidities and probably with a higher bleeding risk (recent surgery). However, there were no differences in age, sex or previous comorbidities between the group of patients treated with AC and those who underwent CDT, with only PE severity as a driver for CDT reperfusion.
Catheter-directed therapies as an increasingly chosen option
In the last 10 years, CDT has emerged as a promising alternative to ST, but randomized studies vs standard medical therapy are lacking. The PE landscape currently has 2 scenarios on the opposite side of the innovation curve. On the one side, the early adopters (United States scenario) are using CDT with a very low threshold as an elective therapy for submassive PE (including the entire IR spectrum) despite the lack of randomized evidence or strong guideline recommendations. Conversely, awareness of CDT and its availability might be relatively low in late-adopter countries and nonacademic nontertiary centers, leading to inequalities in patients’ access to advanced therapies for PE.
The rise in CDT treatments is due to the growing market and the promising results of early studies showing nearly immediate improvement in right ventricular function and hemodynamic status compared with conservative treatment,7,10,19,20 with very low bleeding risk.21,22 The variety of techniques (figure 1) might add some heterogeneity but discussion of the various CDTs is beyond the scope of this manuscript.
The significant number of patients treated with reperfusion in our cohort (59% of IHR-PE patients and 81% of HR-PE patients) may reflect that PERTs are currently activated only for a higher-risk segment of patients, but also reflects the optimal accessibility to reperfusion when ST and CDT are available together.
Systemic thrombolysis vs catheter-directed therapies
ST is the treatment of choice for patients with hemodynamic instability and PE-related cardiopulmonary arrest, although the mortality benefit is mainly based on a small clinical trial (n = 8) that was prematurely terminated.23 Risk factors for PE are age, multiple comorbidities and especially past or active cancer,24 which also confer an exceedingly high bleeding risk,25 especially when treated with ST. Previous studies have shown that major bleeding occurs in ≈10% to 15% of acute PE patients treated with ST, while intracranial bleeding events occur in around 1.5% to 2% of this patient population.3,4,26,27 It is probably for this reason that this treatment is not frequently applied in older patients with previous comorbidities, as shown in the present study and other previous publications.3-5 Thus, managing older, comorbid and oncologic patients with ongoing acute PE remains a real challenge for clinicians, and in this particular scenario, CDT may be a safe and effective option for PE treatment. In fact, the multivariable analysis performed in our study showed that increasing comorbidities was an independent factor for the use of CDT over ST as the preferred reperfusion therapy. These results suggest a new choice for this group of highly vulnerable patients who would not otherwise be treated with reperfusion and therefore would have a higher mortality risk due to the conservative approach.3 However, these results should be interpreted with caution because of the low percentage of patients treated with ST in the present study (35.0%) and the low percentage of HR-PE patients included (15.8%). Furthermore, given the large time period covered by the study, a significant percentage of IHR-PE patients undergoing ST were included. Following the publication of the PEITHO trial28 and the emergence of specific catheters for PE treatment, the administration of ST in IHR-PE patients became less frequent, even in those with worse progress within this subgroup. Therefore, it is likely that our study population does not accurately represent patients in current clinical practice.
Postsurgical patients are especially complex because surgery is a risk factor for PE and is a formal contraindication for ST. Percutaneous thrombectomy has shown a low incidence of major bleeding in single-arm studies and seems a good alternative in these patients.8,9,29 However, to use these devices, the thrombus must be in the proximal segment of the main pulmonary arteries. Indeed, bilateral central PE was an independent variable that prompted the choice of CDT in our study.
Anticoagulation vs catheter-directed therapies
Anticoagulation only is recommended for low-risk and stable IR-PE patients.2 ST in IR-PE decreased the risk of hemodynamic decompensation but at a high cost of bleeding,28 and consequently reperfusion therapies are intended for patients with hemodynamic deterioration.2 Nonetheless, the irruption of transcatheter therapies, especially large-bore aspiration devices, could provide the advantages of pulmonary reperfusion observed in the PEITHO trial28 without the worrisome adverse effects (mainly bleeding events). Our study shows that the use of CDTs has clearly increased in recent years but they were still being reasonably reserved for the higher-risk PE spectrum. Ongoing large clinical trials, such as PEERLESS (NCT: 05111613), HI-PEITHO (NCT: 04790370), and PE-TRACT (NCT: 05591118), will definitely clarify the indication for CDT in patients with acute IHR-PE.
Early safety outcomes in patients with acute pulmonary embolism
Our study showed an incidence of 30-day all-cause mortality of 9.3%, which is lower than that in other observational studies.21,30 However, the cited studies included only patients undergoing reperfusion therapies (either CDT or ST) and the present study also included patients undergoing conservative management, who can be expected to have lower severity and therefore better prognosis. In contrast to the findings of other published literature,19,21,31 the incidence of in-hospital major bleeding and early all-cause death was relatively high in the CDT group in our cohort. These results can be explained by 2 main reasons: first, patients in the CDT group in our cohort were older and had more comorbidities, with 30% having a formal contraindication for ST; and second, the CDT group included almost 50% of patients receiving thrombolytic drugs, which are associated with a higher risk of bleeding than thrombus aspiration alone. Furthermore, among the group of patients who underwent catheter-guided thrombolysis, tenecteplase was used in 28.6%, with this drug demonstrating a high incidence of major bleeding in the PEITHO trial.28 Finally, the vascular access used in the vast majority of patients in the present study was femoral (97.0%), with an incidence of vascular complications of 10.6% (all of them occurring in patients undergoing catheter-directed thrombolysis or aspiration with a nonspecific catheter). Previous studies have shown a low incidence of major bleeding when catheter-directed thrombolysis is performed through brachial access.32 However, specific devices, especially large-bore aspiration devices, can currently only be used via femoral access due to their large caliber. In addition, there were no intracranial bleeding events in patients undergoing CDT in our cohort.
On the other hand, our study showed a significant hemodynamic improvement in patients who underwent CDT, in accordance with previous studies.7-10,33 This benefit is important, but the futility of interventional treatments must be considered in very old and comorbid patients, balancing cost-effectiveness and clinical judgment.34 More data are needed to establish the risk-benefit balance of CDT compared with anticoagulation and ST in older patients or patients with a high comorbidity burden.
Limitations
Several limitations should be considered when interpreting the results of this study. Due to its observational nature, the presence of unmeasured confounders could have influenced the conclusions of the study. The total number of patients admitted with PE in the study period in the 2 centers is unknown, and consequently a survival bias should be acknowledged. The percentage of intermediate-low risk patients included was relatively low, suggesting that PERT activation was selected for the most severe patients. Thus, a selection bias may have occurred in this study. Specific devices for the percutaneous treatment of PE were not initially available at the beginning of this study, and were incorporated as they became available (first specific devices in 2018). This was a registry with self-reported data without external monitoring, and consequently local investigators are responsible for the integrity of the data.
CONCLUSIONS
The results of this study show that the factors associated with the choice of CDT on multivariable analysis were a higher Charlson comorbidity index, a history of recent surgery, and proximal, bilateral PE. The choice of CDT over ST as reperfusion therapy increased during the study period. CDT was an effective option for older, comorbid patients with PE, but the management of acute PE patients is challenging and should be individualized.
FUNDING
This research did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONS
The registry protocol was approved by the clinical research ethics committee at Hospital Clínico San Carlos as the central committee for the registry, following local research regulations (code 18/010-E). All prospectively included patients signed an informed consent form. An informed consent waiver was granted from the ethics research committee for patients recruited retrospectively. Sex but not gender data were included in the database design in 2018.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
C. Real designed the study outline, performed the statistical analysis, and drafted the article. C. Ferrera designed the study outline and drafted the article. M.E. Vázquez-Álvarez participated in data collection and data interpretation, M. Huanca, F.J. Noriega, E. Gutiérrez-Ibañes, A.M. Mañas-Hernández, N. Ramos-López, M. Juárez, P. Jiménez-Quevedo, J. Elízaga, and A. Viana-Tejedor participated in data collection and critically revised the manuscript. P. Salinas designed the protocol, database and study outline, coordinated the data analysis and interpretation, and critically revised the manuscript. All authors gave final approval of the version to be published.
CONFLICTS OF INTEREST
The authors report no conflicts of interest with respect to the content of this manuscript.
WHAT IS KNOWN ABOUT THE TOPIC?
- Catheter-directed therapies (CDT) have emerged as a safe and effective reperfusion therapy in patients with acute pulmonary embolism (PE). According to ESC guidelines, these therapies should be considered in patients with HR-PE and failed thrombolysis or a contraindication to this therapy and as a rescue treatment for IHR-PE patients with clinical deterioration. However, several studies aiming to establish the indication for these therapies in a broader spectrum of patients have been published in recent years. Furthermore, reperfusion therapy with systemic thrombolysis (ST) is known to be underused due to concerns about bleeding, and consequently CDT may be a feasible option in this profile of patients who would otherwise go untreated.
WHAT DOES THIS STUDY ADD?
- In clinical practice in two tertiary centers, the factors associated with the choice of CDT over ST were comorbidities, a history of recent surgery, and proximal, bilateral PE. However, the risk profile of patients treated with the 2 therapies was similar in each risk stratum. Therefore, we conclude that CDT could be a safe and effective alternative in patients requiring reperfusion therapy.
ACKNOWLEDGMENTS
The authors thank María Beneito-Durá for providing statistical advice.
REFERENCES
1. Götzinger F, Lauder L, Sharp ASP, et al. Interventional therapies for pulmonary embolism. Nat Rev Cardiol. 2023 Oct;20:670-684.
2. Konstantinides S V, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
3. Keller K, Hobohm L, Ebner M, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. 2020;41:522-529.
4. Jiménez D, Bikdeli B, Barrios D, et al. Epidemiology, patterns of care and mortality for patients with hemodynamically unstable acute symptomatic pulmonary embolism. Int J Cardiol. 2018;269:327-333.
5. Stein PD, Matta F. Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism:Saves Lives but Underused. Am J Med. 2012;125:465-470.
6. Pruszczyk P, Klok FK, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism:a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022;18:e623-e638.
7. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation. 2014;129:479-486.
8. Tu T, Toma C, Tapson VF, et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism. JACC Cardiovasc Interv. 2019;12:859-869.
9. Sista AK, Horowitz JM, Tapson VF, et al. Indigo Aspiration System for Treatment of Pulmonary Embolism. JACC Cardiovasc Interv. 2021;14:319-329.
10. Kroupa J, Buk M, Weichet J, et al. A pilot randomised trial of catheter-directed thrombolysis or standard anticoagulation for patients with intermediate-high risk acute pulmonary embolism. EuroIntervention. 2022;18:e639-e646.
11. Pasha AK, Siddiqui MU, Siddiqui MD, et al. Catheter directed compared to systemically delivered thrombolysis for pulmonary embolism:a systematic review and meta-analysis. J Thromb Thrombolysis. 2022;53:454-466.
12. Salinas P, Vázquez-Álvarez M-E, Salvatella N, et al. Catheter-directed therapy for acute pulmonary embolism:results of a multicenter national registry. Rev Esp Cardiol. 2024;77:138-147.
13. Ramos-López N, Ferrera C, Luque T, et al. Impact of a pulmonary embolism response team initiative on hospital mortality of patients with bilateral pulmonary embolism. Med Clin. 2023;160:469-475.
14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement:guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
15. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and Validation of a Prognostic Model for Pulmonary Embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
16. Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014;44:694-703.
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:Development and validation. J Chronic Dis. 1987;40:373-383.
18. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non?surgical patients. J Thromb Haemost. 2005;3:692-694.
19. Ismayl M, Machanahalli Balakrishna A, et al. Meta-Analysis Comparing Catheter-Directed Thrombolysis Versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. Am J Cardiol. 2022;178:154-162.
20. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT). Chest. 2015;148:667-673.
21. Beyer SE, Shanafelt C, Pinto DS, et al. Utilization and Outcomes of Thrombolytic Therapy for Acute Pulmonary Embolism. Chest. 2020;157:645-653.
22. Pei DT, Liu J, Yaqoob M, et al. Meta-Analysis of Catheter Directed Ultrasound-Assisted Thrombolysis in Pulmonary Embolism. Am J Cardiol. 2019;124:1470-1477.
23. Jerjes-Sanchez C, Ramírez-Rivera A, de Lourdes García M, et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism:A randomized controlled trial. J Thromb Thrombolysis. 1995;2:227-229.
24. Blom JW. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA. 2005;293:715.
25. de Winter MA, Dorresteijn JAN, Ageno W, et al. Estimating Bleeding Risk in Patients with Cancer-Associated Thrombosis:Evaluation of Existing Risk Scores and Development of a New Risk Score. Thromb Haemost. 2022;122:818-829.
26. Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism:a systematic review and meta-analysis. Eur Heart J. 2015;36:605-614.
27. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage. JAMA. 2014;311:2414.
28. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N Engl J Med. 2014;370:1402-1411.
29. Toma C, Jaber WA, Weinberg MD, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18:1201-1212.
30. Hobohm L, Schmidt FP, Gori T, et al. In-hospital outcomes of catheter-directed thrombolysis in patients with pulmonary embolism. Eur Hear J Acute Cardiovasc Care. 2021;10:258-264.
31. Stein PD, Matta F, Hughes MJ. Catheter-Directed Thrombolysis in Submassive Pulmonary Embolism and Acute Cor Pulmonale. Am J Cardiol. 2020;131:109-114.
32. Portero-Portaz JJ, Córdoba-Soriano JG, Gallardo-López A, Gutiérrez-Díez A, Melehi El-Assali D, Jiménez-Mazuecos JM. Resultados de la terapia dirigida por catéter en la tromboembolia pulmonar aguda. Rev Esp Cardiol. 2020;73:953-954.
33. Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose TFibrinolysis for Acute Massive and Submassive Pulmonary Embolism. JACC Cardiovasc Interv. 2015;8:1382-1392.
34. Lindman BR, Alexander KP, O'Gara PT, Afilalo J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2014;7:707-716.
* Corresponding author.
E-mail address: salinas.pablo@gmail.com (P. Salinas).
- Use of a pediatric risk score for cardiac catheterization in a Spanish population with congenital heart disease
- Left atrial appendage occlusion vs oral anticoagulants in AF and coronary stenting. The DESAFIO registry
- Transcatheter aortic valve implantation via percutaneous alternative access routes: outcomes
- The MANTA vascular closure device in transfemoral TAVI: a real-world cohort
Editorials
All for one or one for all!
Original articles
Editorials
Fast-track TAVI: establishing a new standard of care
Departamento de Cardiología, Hospital de la Santa Creu i Sant Pau, Institut de Recerca Sant Pau (IR Sant Pau), Barcelona, Spain
Original articles
Debate
Debate: TAVI prosthesis selection for severe calcification
The balloon-expandable technology approach
Servicio de Cardiología, Hospital Regional Universitario de Málaga, Málaga, Spain
The self-expandable technology approach
Servicio de Cardiología, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain