ABSTRACT
Introduction and objectives: The optimal treatment of nonculprit angiographic intermediate lesions (diameter stenosis 40%-69%) in patients with ST-segment elevation myocardial infarction (STEMI) is still unknown. Lesions with fractional flow reserve (FFR) ≤ 0.80 are indicative of ischemia and benefit from revascularization. However, lesions with FFR > 0.80 and optical coherence tomography (OCT) findings of vulnerability have been hypothesized to cause adverse events during follow-up. The study aims to compare the efficacy of a preventive treatment with stent implantation plus optimal medical therapy vs optimal medical therapy alone for nonculprit intermediate lesions with FFR > 0.80 and OCT findings of plaque vulnerability in STEMI patients at 4 years of follow-up.
Methods: This parallel-group, multicenter, controlled, single-blind, and 1:1 randomized trial will enroll a total of 600 STEMI patients with ≥ 1 intermediate nonculprit lesions with FFR > 0.80 and OCT findings of plaque vulnerability. The primary endpoint is target vessel failure, defined as the composite of cardiac death, target vessel myocardial infarction, or target vessel revascularization. The study will include a parallel registry of patients with FFR > 0.80 but without OCT findings of vulnerability. Vulnerable plaques are defined as lipid-rich fibroathermas with plaque burden ≥ 70% and a thin fibrous cap (≤ 80 μm).
Results: The VULNERABLE trial will reveal the role of preventive treatment with stent implantation for nonculprit and functionally nonsignificant vulnerable plaques in STEMI patients.
Conclusions: This is the first randomized trial of OCT-guided treatment of vulnerables plaques. Registered at ClinicalTrials.gov (NCT05599061).
Keywords: Fractional flow reserve. Optical coherence tomography. ST-segment elevation myocardial infarction. Vulnerable plaque.
RESUMEN
Introducción y objetivos: El tratamiento óptimo de las lesiones angiográficas intermedias (diámetro de estenosis 40-69%) no culpables en pacientes con infarto agudo de miocardio con elevación del segmento ST (IAMCEST) está por determinar. La reserva fraccional de flujo (RFF) permite diagnosticar lesiones causantes de isquemia (RFF ≤ 0,80) que se benefician de una revascularización. No obstante, las lesiones con RFF > 0,80 y criterios de vulnerabilidad por tomografía de coherencia óptica (OCT) también se ha hipotetizado que pueden causar eventos adversos en el seguimiento. El objetivo es comparar la eficacia del tratamiento preventivo con implantación de stent más tratamiento médico óptimo de lesiones intermedias no culpables con RFF > 0,80 y características de placa vulnerable frente a solo tratamiento médico óptimo en pacientes con IAMCEST a 4 años de seguimiento.
Métodos: Estudio de grupos paralelos, multicéntrico, controlado, aleatorizado 1:1 y simple ciego. Se incluirán 600 pacientes con IAMCEST y al menos una lesión intermedia no culpable que presenten RFF > 0,80 y características de placa vulnerable por OCT. El objetivo primario se define como fallo del vaso diana, compuesto de muerte cardiaca, infarto del vaso diana y necesidad de revascularización del vaso diana. El estudio incluye un registro paralelo para pacientes con RFF > 0,80 sin características de placa vulnerable. Se define placa vulnerable como fibroateromas lipídicos con carga de placa ≥ 70% y capa fibrosa fina (≤ 80 µm).
Resultados: El estudio VULNERABLE permitirá conocer el papel del tratamiento preventivo con stent de placas vulnerables no culpables funcionalmente no significativas en pacientes con IAMCEST.
Conclusiones: Se trata del primer estudio aleatorizado para el tratamiento de placas vulnerables guiado por OCT. Registrado en ClinicalTrials.gov (NCT05599061).
Palabras clave: Reserva fraccional de flujo. Tomografía de coherencia óptica. Infarto agudo de miocardio con elevación del segmento ST. Placa vulnerable.
Abbreviations
FFR: fractional flow reserve. MLA: minimum lumen area. OCT: optical coherence tomography. OMT: optimal medical therapy. PDE: percent diameter stenosis. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
The presence of multivessel disease, defined as angiographic lesions with a percent diameter stenosis (PDS) ≥ 50% by visual estimation in patients with ST-segment elevation myocardial infarction (STEMI), is estimated to be approximately 50%.1 The COMPLETE trial compared angiography-guided preventive revascularization with stent implantation added to optimal medical therapy (OMT) for nonculprit lesions with a PDS ≥ 70% vs OMT alone.2 The trial found that angiography-guided preventive revascularization significantly reduced adverse cardiovascular events at 3 years of follow-up.2 Although the COMPLETE trial required physiological assessment using fractional flow reserve (FFR) for lesions with a PDS between 50% and 69% to guide the decision on revascularization, in practice, it was performed in only a very small percentage of patients.
The FLOWER-MI and FRAME-AMI trials3,4 investigated preventive stenting of FFR-guided nonculprit lesions—obtained through intracoronary pressure wire—compared with angiography-guided complete revascularization (visual estimation). Both trials mainly included intermediate lesions and demonstrated that pressure wire-guided preventive revascularization significantly reduces the need for revascularization, with similar or superior efficacy to angiography-guided complete revascularization.3,4 Despite these findings, clinical practice guidelines based on the COMPLETE trial recommend preventive stenting of nonculprit lesions guided by angiography alone.5,6
It is important to note that FFR is considered the gold standard for detecting myocardial ischemia (FFR ≤ 0.80). However, deferring treatment of nonculprit lesions that do not cause ischemia (FFR > 0.80) through OMT raises concerns in selected cases in which the anatomical features of the lesion suggest signs of vulnerability. In the FLOWER-MI trial, the group of patients randomized to undergo pressure-wire-guided revascularization with an FFR > 0.80 (referred for OMT) had more adverse events than those in the same group with FFR values ≤ 0.80 (referred for percutaneous revascularization).7 Several studies using intravascular imaging modalities have also demonstrated an association between the presence of fibro-lipid plaques with high lipid content and thin fibrous caps—known as vulnerable plaques—and the development of future adverse events due to plaque rupture.8,11
The VULNERABLE trial aims to evaluate the efficacy of a combined strategy using intracoronary physiological techniques and intravascular imaging to guide the treatment of intermediate nonculprit lesions in STEMI patients. The study hypothesis is that preventive stenting—in addition to OMT—in intermediate nonculprit lesions with FFR values > 0.80 and characteristics of vulnerable plaque will be superior to OMT alone. The present article includes the rationale and design of the study.
METHODS
Design
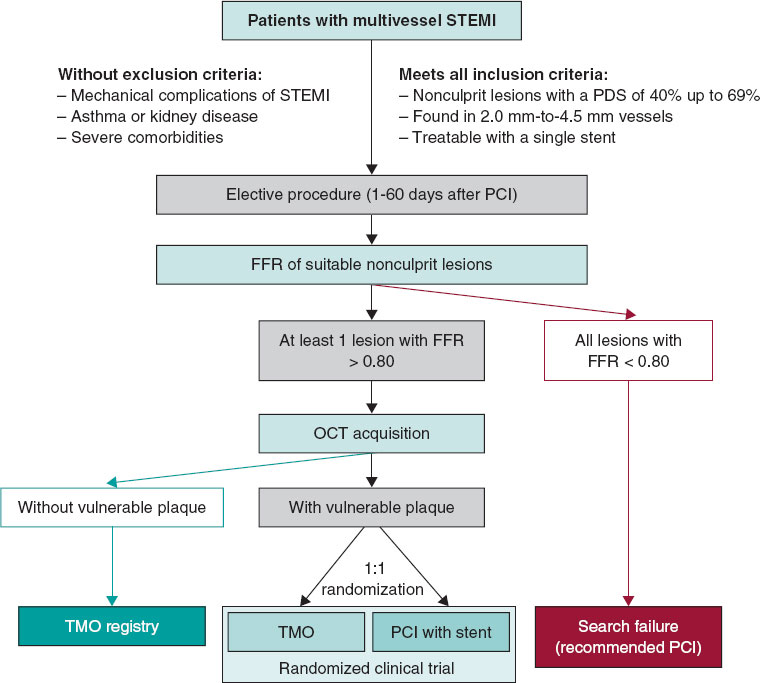
The VULNERABLE trial (NCT05599061) includes 3 groups based on the results obtained during the combined functional and anatomical assessment using pressure wires and optical coherence tomography (OCT). Figure 1 shows the study flowchart, which illustrates the 3 groups: patients with FFR ≤ 0.80 treated with stent (search failures), patients with FFR > 0.80 without vulnerable plaque characteristics (included in the registry group), and patients with FFR > 0.80 and vulnerable plaque characteristics (included in the randomized clinical trial).
This is a multicenter, controlled, prospective, randomized, parallel-group, single-blind study with patients included in the clinical trial group. The study will be conducted in accordance with the recommendations outlined in the Declaration of Helsinki on clinical research and has been approved by the lead ethics committee (Hospital Universitari de Bellvitge) and endorsed by the remaining ethics committees of participating centers. The participating centers and principal investigators are shown in table 1 of the supplementary data.
Table 1. Objectives of the VULNERABLE trial
| Primary endpoint |
|---|
| Compare the percentage of TVF between the 2 groups of patients assigned to the randomized clinical trial (FFR > 0.80 with characteristics of vulnerable plaque by OCT): preventive revascularization with stent + OMT vs OMT alone |
| Key secondary endpoints |
| Compare the percentage of TVF between patients allocated to the registry group (FFR > 0.80 without characteristics of vulnerable plaque by OCT and treated with the OMT) and patients allocated to the randomized OMT group (FFR > 0.80 with characteristics of vulnerable plaque) |
| Other secondary endpoints |
| Compare the rate of all-cause mortality reported between the 2 subgroups of randomized patients |
| Compare the percentage of cardiac deaths reported between the 2 subgroups of randomized patients |
| Compare the percentage of all myocardial infarctions reported between the 2 subgroups of randomized patients |
| Compare the percentage of target vessel myocardial infarctions reported between the 2 subgroups of randomized patients |
| Compare the percentage of target vessel revascularization needs between the 2 subgroups of randomized patients |
| Evaluate the percentage of restenosis and stent thrombosis in the preventive revascularization group with stent + OMT of the randomized clinical trial |
| * Although all objectives are marked with a complete 4-year follow-up, an interim study will be conducted at 2 years. ** All objectives will be calculated on an intention-to-treat basis according to the statistical plan. An exploratory per-protocol analysis will also be conducted based on the assessment by the study’s core imaging laboratory. |
|
FFR: fractional flow reserve; OCT: optical coherence tomograph; OMT: optimal medical treatment; TVF: target vessel failure. |
The study has been entirely designed and initiated by researchers and is sponsored by the Spanish Society of Cardiology Working Group on Intracoronary Diagnostic Techniques, which includes a steering committee, a data and safety monitoring board, and an independent event adjudication committee. The members of these committees are listed in table 2 of the supplementary data. The steering committee and all study investigators are committed to accurate data collection and adherence to the study protocol. The funding entity (Abbott Vascular, United States) plays no role in the study design, data collection, analysis, or the writing of the study results. The study sponsor (Foundation for Education in Interventional Cardiology Procedures [EPIC]), along with the principal investigators, is responsible for data management and confidentiality.
Table 2. Inclusion and exclusion criteria of the VULNERABLE trial
| Inclusion criteria |
|---|
| Patients older than 18 years |
| With STEMI (ST-segment elevation > 1 mm in, at least, 2 contiguous leads or true posterior ST-segment elevation with > 2 mm depression in anterior leads or new onset left bundle branch block) treated with successful revascularization of the culprit lesion within 72 hours from symptom onset |
| Presenting with multivessel disease with, at least, 1 angiographically intermediate lesion (PDS of 40% up to 69% by visual estimation) in a native vessel different from the culprit vessel |
| Planned FFR-guided percutaneous revascularization with a single 2.0 mm-to- 4.5 mm stent |
| Between 1 and 60 days after the index procedure (revascularization of the STEMI culprit vessel) |
| Exclusion criteria |
| Life expectancy < 4 years |
| Women of childbearing age who wish to become pregnant |
| Known intolerance to acetylsalicylic acid, heparin, everolimus, or iodinated contrast |
| Unresolved mechanical complications or infarct-related cardiogenic shock |
| Lesions suitable for the study located in the left main coronary artery, vessels with previous revascularization, in coronary bifurcations with > 2.5 mm side branches, severe angulations, or segments with severe calcification |
| History of severe asthma |
| Chronic kidney disease with glomerular filtration rate < 45 mL/min |
|
FFR: fractional flow reserve; PDS: percent diameter stenosis; STEMI: ST-segment elevation myocardial infarction. |
Endpoints
The primary objective of the VULNERABLE study (NCT05599061) is to compare the efficacy of preventive stenting combined with OMT vs OMT alone for intermediate lesions in noninfarct-related arteries with an FFR > 0.80 and vulnerable plaque characteristics as identified by OCT over a 4-year follow-up period. The primary endpoint of the study is the rate of target vessel failure (TVF), which is defined as a composite of cardiac death, target vessel myocardial infarction, or the need for target vessel revascularization.
The study also aims to evaluate several secondary endpoints, which are summarized in table 1. Among these secondary objectives, a key focus is the comparison of the TVF rate (the primary endpoint) between the registry group (patients with FFR > 0.80 without vulnerable plaque characteristics treated with OMT) and the randomized OMT arm of the clinical trial (patients with FFR > 0.80 and vulnerable plaque characteristics). The study endpoints are defined in table 3 of the supplementary data.12,13
Patient inclusion and exclusion criteria
The inclusion and exclusion criteria for the study are detailed in table 2. In brief, all patients with STEMI who have undergone successful revascularization of the culprit lesion and have at least 1 intermediate lesion (visually defined as having a DS of 40%-69%) in a noninfarct-related artery will be eligible for the study if percutaneous revascularization with a single stent guided by FFR is being considered. The study procedure must be conducted between 1 and 60 days after the revascularization of the culprit lesion. Patients must provide informed consent prior to the elective procedure for evaluating the nonculprit lesion.
Study protocol for nonculprit lesions and randomization
Eligible lesions will first be assessed with a pressure wire following the standard procedures in each center. Lesions with an FFR ≤ 0.80 will be considered search failures, and revascularization will be recommended based on clinical indications.5,6
Lesions with an FFR > 0.80 will be further evaluated with OCT according to the standard acquisition methods to detect vulnerable plaques in each center. The decision on whether a lesion meets the criteria for vulnerable plaque will be made by an accredited local investigator during the study procedure.
Patients with at least 1 lesion with an FFR > 0.80 without vulnerable plaque characteristics on OCT will be included in the registry group of the study. The protocol recommends OMT for all lesions with an FFR > 0.80 without vulnerable plaque characteristics. These patients will receive the same clinical follow-up as those in the randomized clinical trial group.
Patients with at least 1 lesion with an FFR > 0.80 that meets the criteria for a vulnerable plaque on OCT will be included in the clinical trial group. These patients will be randomized 1:1 to either preventive stenting combined with OMT or OMT alone (figure 1). Randomization will be conducted without stratification by center or clinical condition, using telematic algorithms. This process will be carried out online via the data collection platform provided by pInvestiga (Pontevedra, Spain).
Figure 1. Study diagram. FFR, fractional flow reserve; OCT, optical coherence tomography; OMT, optimal medical treatment; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The supplementary data provide additional details on the FFR assessment method, including special situations where the lesion under study could not be fully evaluated, instances of unstable nonculprit plaques, complications related to diagnostic techniques, or patients with more than 1 nonculprit lesion.
Study device and implantation procedure
Patients with an FFR > 0.80 and vulnerable plaque characteristics identified by OCT assigned to the percutaneous coronary intervention group will be treated with an everolimus-eluting stent (Xience, Abbott, United States). According to the protocol, stent implantation must be guided by OCT. The criteria for OCT-guided stent implantation are detailed in table 4 of the supplementary data.
Optimal medical therapy
All patients included in both the randomized clinical trial and the registry must receive treatment in accordance with the European Society of Cardiology guidelines for managing acute coronary syndromes.5 The study protocol emphasizes managing modifiable risk factors—such as diet, smoking, obesity, exercise, and psychological status—as well as nonmodifiable risk factors, with set targets for blood pressure (systolic < 130 mmHg and diastolic < 80 mmHg), low-density lipoprotein cholesterol (< 55 mg/dL), and glycated hemoglobin A1c (< 7%). Pharmacological therapy should include beta-blockers and renin-angiotensin system inhibitors. Dual antiplatelet therapy is also recommended, but only during the first year after the index procedure, at the discretion of each center. As per the protocol, patient treatment details will be reported annually, and 2 lipid profile tests will be conducted throughout the study.
Vulnerable plaque criteria on optical coherence tomography and investigator training
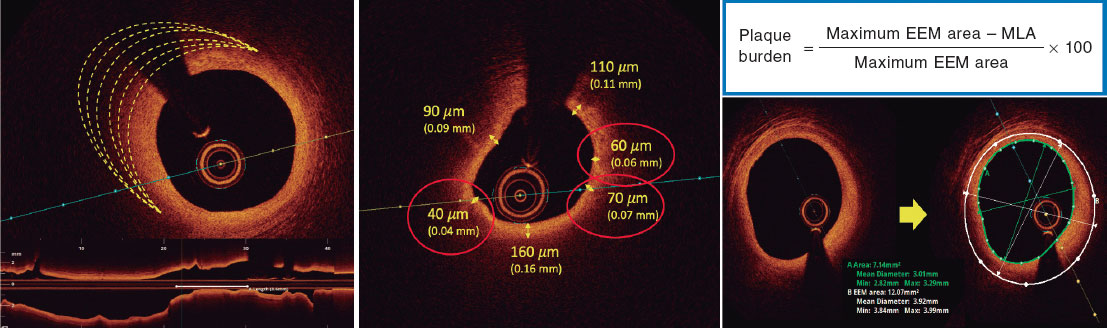
Based on histopathological data, a plaque is defined as vulnerable when it is caused by a fibroatheroma with a large necrotic core composed of cellular debris and a high number of inflammatory cells, covered by a thin fibrous cap (≤ 65 µm).14 The criteria for identifying a vulnerable plaque in the study are adapted from the classic histopathological definition but modified for OCT assessment. These criteria are shown in figure 2.
Figure 2. Vulnerable plaque criteria by optical coherence tomography. EEM, external elastic membrane; minimal lumen area.
According to the protocol, 3 simultaneous criteria are required to define a vulnerable plaque by OCT:
The presence of a fibro-lipid plaque with a necrotic core covering more than 90º of the perimeter of the vessel over a length of more than 5 mm. A necrotic core is defined as a hypointense image with poorly defined borders that attenuates the OCT light beam, preventing visualization of the artery behind the core.
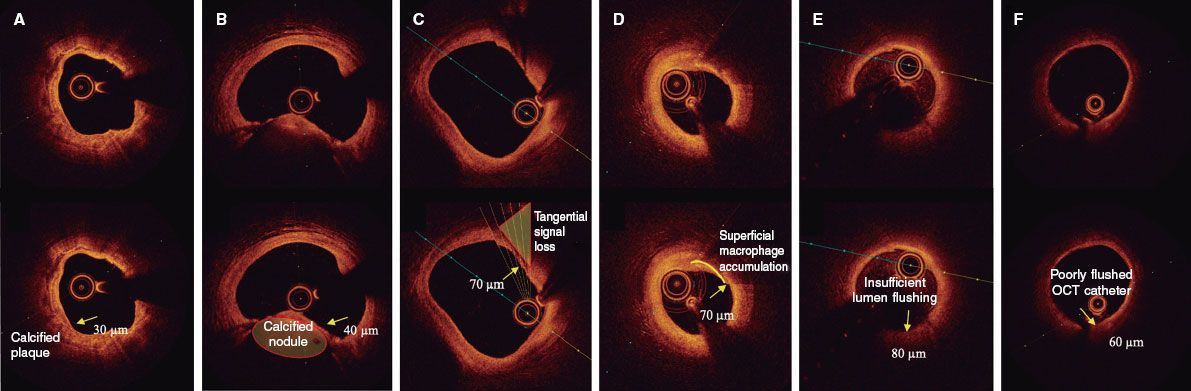
The presence of a thin fibrous cap, defined as ≤ 80 µm (65 + 15 µm axial resolution) in ≥ 3 consecutive images. The fibrous cap is defined as the tissue separating the necrotic core from the vessel lumen. Investigators will be trained to differentiate other findings that could be mistaken for a thin cap on OCT. Figure 3 shows examples of analogous OCT images that may mimic a thin fibrous cap but do not correspond to vulnerable plaques.
Figure 3. Distinction between vulnerable plaques and other findings by optical coherence tomography (OCT). A: plaque with superficial calcium (hypointense core with well-defined margins that do not attenuate the passage of light; arrow) and a thin fibrous cap. B: calcified nodule (arrow) protruding into the lumen and attenuating the signal, despite being composed of calcium. C: tangential signal loss (arrow) due to insufficient light beams caused by the peripheral, noncentral position of the OCT probe. D: superficial accumulation of macrophages (arrow) with a hyperintense appearance relative to the adjacent intima, with signal attenuation behind. E: presence of blood in the lumen due to inadequate flushing (arrow) during image acquisition, which distorts the arterial wall image, creating the appearance of hypointense regions. F: presence of blood between the probe and the OCT catheter (arrow) due to inadequate flushing, which distorts the arterial wall image and mimics hypointense regions.
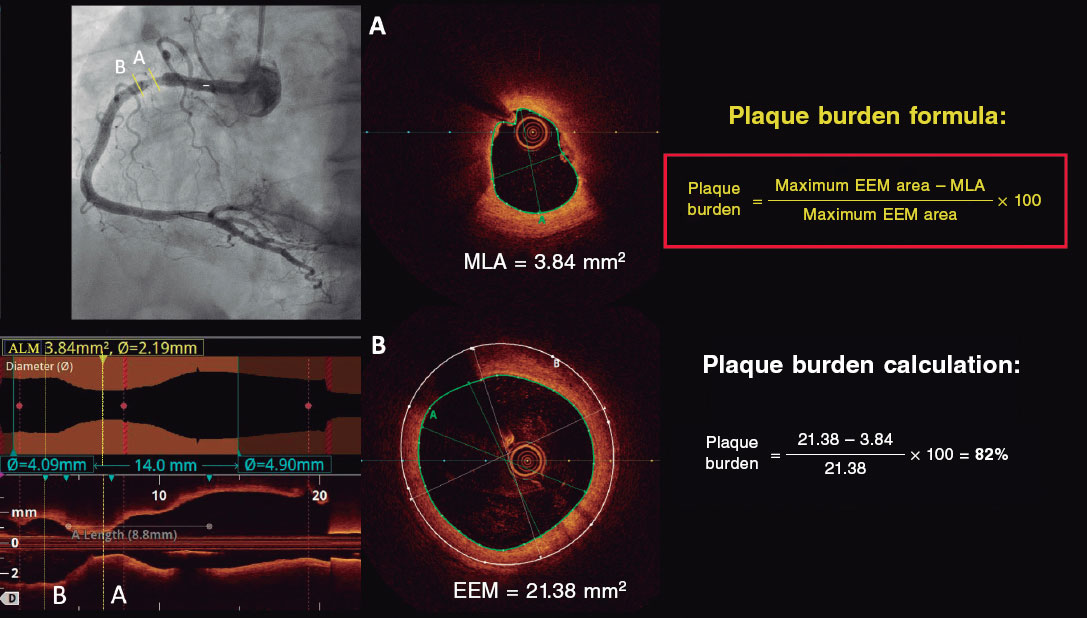
Investigators will be required to measure a plaque burden of ≥ 70% in the cross-sectional area corresponding to the minimal luminal area (MLA) within the lesion. To perform this assessment, it is necessary to measure the vessel perimeter by delineating the external elastic membrane (EEM). Due to the difficulty of assessing the vessel perimeter in fibro-lipid plaques, especially at the MLA site, investigators will be trained to choose a section as close as possible to the MLA, where at least 60% of the vessel perimeter can be visualized if it is not possible at the same point. This allows for calculation using the following formula (figure 4):
Figure 4. Plaque burden assessment by optical coherence tomography. A: cross-section of the minimal lumen area. B: cross-section where the external elastic membrane (EEM) was measured. Since the EEM cannot usually be assessed in the cross-section corresponding to the MLA, an approximate estimation is made by measuring the EEM within 10 mm proximal or distal to the MLA (preferably distal) in the absence of side branches. The EEM will be assessed in the first cross-section where 60% of the EEM perimeter can be evaluated.
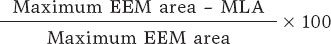
As per protocol, at least 1 local investigator from each participating center must have completed an online training course for the detection and assessment of vulnerable plaques using OCT, following the study criteria. Upon completing this course and passing a specific questionnaire, the investigator will be certified and approved to participate in the study.
Angiographic and optimal coherence tomography quantification analyses
The study includes an independent imaging laboratory for angiographic quantification and OCT analysis (Barcelona Cardiac Imaging Core Laboratory [BARCICORE-Lab]) to monitor adherence to the study criteria for diagnosing vulnerable plaques. A blinded analysis of the study results will be conducted, and patients will be assigned according to the protocol for exploratory analysis. A detailed explanation of the angiographic and OCT analysis conducted by the study laboratory is shown in the supplementary data.
Clinical follow-up and blinding
Patients in both the registry group and the randomized clinical trial group will undergo clinical follow-up for 4 years. Follow-up will include telephone consultations at 1 and 3 years, and in-person visits at 2 and 4 years. Each follow-up will involve an electrocardiogram and blood tests with cholesterol determination.
Patients in the randomized clinical trial group will be blinded to their assigned treatment group (single-blind). The details of blinding and monitoring are specified in the supplementary data.
Sample size calculation
The sample size has been calculated for the randomized clinical trial group. The number of patients included in the registry and search failures will depend on the total number needed to achieve the estimated sample size for the randomized trial.
According to previous studies on patients with acute coronary syndrome, theTVF rate for nonculprit lesions meeting vulnerable plaque criteria treated with OMT is estimated to be around 8% to 10% at 4 years. In similar lesions treated with stenting, the rate is approximately 4%.2,7,9 The studies used for the sample size calculation are summarized in table 5 of the supplementary data. Based on the study hypothesis, preventive stenting in nonculprit lesions with an FFR > 0.80 and vulnerable plaque characteristics is expected to reduce the primary endpoint by 60%. The estimated rate of TVF in the OMT group at 4 years is 10%. Assuming an annual loss to follow-up rate of 1.5% (total 6%), randomizing 600 participants 1:1 to preventive stenting plus OMT vs OMT alone will provide 80% power to demonstrate the superiority of preventive stenting with a 2-sided alpha error of .05.
Statistical analysis plan
The primary and secondary endpoints will be analyzed using the intention-to-treat principle at the 4-year follow-up. Comparisons will estimate event proportions between groups using logistic regression and will be reported as odds ratios with 95% confidence intervals. Only 1 event per patient will be counted for the primary endpoint. P values < .05 will be considered statistically significant for the primary endpoint. Kaplan-Meier curves will be used to visualize the time to the first event between groups.
For primary endpoint composites with missing data, a specific monitoring plan will determine if the missing data are random. In cases where data are adjudicated as missing at random, imputation methods will be used. For nonrandom missing data, sensitivity analyses using worst-case and last observation carried forward methods will be conducted.
Subgroup analyses will be performed for the primary and secondary endpoints, which involves comparing TVF rates between registry patients and those randomized to OMT in the clinical trial. Prespecified subgroups include: age > 75 years, sex, diabetes mellitus, left ventricular ejection fraction ≤ 35% at the time of the procedure, lesions in the proximal or mid-left anterior descending artery, and lesions in vessels with a reference diameter ≤ 2.75 mm.
Additionally, a hypothesis-generating parallel analysis will be conducted according to the study protocol. Patients will be included in the analysis only if the imaging laboratory confirms that their assigned treatment group, as determined by the local investigator, is consistent with the presence of vulnerable plaque identified by OCT. Patients will be excluded if there is a discrepancy between the investigator’s assignment and the imaging laboratory’s findings.
Interim analysis
After 2 years of follow-up, an interim analysis of the data is planned to monitor the primary endpoint in the randomized clinical trial group. Clinical follow-up will be extended if the events observed in the OMT arm of the randomized clinical trial are less than 4%.
DISCUSSION
The VULNERABLE trial aims to investigate the combined use of intracoronary physiology and images to guide the treatment of intermediate nonculprit lesions in STEMI patients.
Several lipid-lowering and anti-inflammatory drugs have been shown to reduce thrombotic events in patients with STEMI, likely by stabilizing functionally nonsignificant vulnerable plaques.15,17 In the PACMAN-AMI trial, treatment with alirocumab in addition to statins significantly reduced atheroma, decreased lipid content, and led to thickening of the fibrous cap compared with placebo in coronary regions with angiographically nonobstructive atherosclerosis (DS, 20%-50%).18 However, it is noteworthy that only 31% of patients in that study exhibited all 3 markers of reduced atherosclerosis, and data on more significant plaques (eg, 40%-69% stenosis with vulnerability criteria) were not specified.19
The use of stents in patients with vulnerable plaques is intended to enhance neointimal healing of the struts, which thickens the fibrous cap and stabilizes the plaque. The randomized PREVENT trial assessed the effectiveness of preventive stenting for functionally nonsignificant vulnerable lesions in patients with chronic coronary syndrome compared with OMT. Vulnerable plaques were identified using various intravascular imaging techniques, with most being guided solely by intravascular ultrasound. The study found that preventive stenting resulted in a statistically significant reduction in the rate of TVF at 2 years of follow-up (0.4% vs 3.4%; P = .0003).11
Finally, several observational trials have demonstrated that OCT is an effective method for detecting vulnerable plaques and monitoring the response to intensive treatments aimed at stabilizing these plaques through fibrous cap thickening.18,20 The PECTUS-obs trial included 438 acute coronary syndrome patients with nonculprit lesions with FFR > 0.80 treated with the OMT alone.10 All lesions were examined using OCT, with criteria similar to those used in the VULNERABLE trial to define vulnerable plaques. In that study, 34% of patients had at least 1 vulnerable lesion, which was associated with a higher risk of adverse events (15.4% vs 8.2% for the composite endpoint of death, myocardial infarction, or revascularization in the groups with and without vulnerable plaques, respectively). The VULNERABLE trial is the first to use OCT to guide the treatment of vulnerable plaques in functionally nonsignificant lesions.
CONCLUSIONS
The VULNERABLE trial aims to evaluate the effectiveness of preventive stenting plus OMT vs OMT alone for vulnerable plaques, as defined by OCT, in functionally nonsignificant intermediate lesions in nonculprit vessels of patients with STEMI. In addition, the study will provide information on the clinical relevance of the presence of vulnerable plaques in nonculprit lesions.
FUNDING
This study has been funded by Abbott Vascular.
ETHICAL CONSIDERATIONS
The study is being conducted following the recommendations outlined in the Declaration of Helsinki on clinical research, has been approved by Hospital Universitari de Bellvitge research ethics committee, and endorsed by the remaining ethics committees of participating centers. Informed consent acceptance and signature are required prior to performing any elective procedures to study the nonculprit lesion. Potential sex and gender biases are considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the drafting of this manuscript.
AUTHORS’ CONTRIBUTIONS
J. Gómez-Lara and E. Gutiérrez-Ibañes drafted this document. The remaining signatories reviewed the document, made changes at their discretion, and approved the final text.
CONFLICTS OF INTEREST
J. Gómez-Lara and E. Gutiérrez-Ibañes received a grant from Abbott Vascular for this study. A. Jurado-Román has received fees from Abbott, Boston, and Shockwave. E. Fernández received fees from Abbott and Hexacath. C. Cortés received a Río Hortega Contract from Instituto de Salud Carlos III. S. Brugaletta received fees from Abbott, Microport, and General Electric. T. García-Camarero received fees from Medtronic and Boston. J.A. Linares Vicente received fees from Abbott Vascular, Braun, AstraZeneca, Bayer, and IZASA. O. Rodríguez-Leor received fees from Shockwave, WorlsMedica, and Medtronic. S. Ojeda received fees from Abbott, Boston, WorldMedica, and Biosensors. A. Pérez de Prado received grants and fees from Abbot, Boston, iVascular, and Terumo. H.M. García-García received fees from ACIST, Boston Scientific, Medis, Biotronik, InfraRedx/Nipro, Chiesi, and Cordis. S. Ojeda and A. Pérez de Prado are associate editors of REC: Interventional Cardiology; the journal’s editorial procedure to ensure impartial processing of the manuscript has been followed. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Thin-cap fibroatheromas, also known as vulnerable plaques, are responsible for most acute coronary syndromes. Approximately 50% of patients with STEMI have additional angiographic lesions beyond the culprit lesion, which are associated with a significant number of adverse ischemic events. Preventive stenting for severe nonculprit lesions (DS ≥ 70%) has been shown to reduce the number of adverse events. However, the effectiveness of preventive stenting for angiographically intermediate nonculprit lesions (SD, 40%-69%) that have characteristics of vulnerable plaques remains to be determined.
WHAT DOES THIS STUDY ADD?
- VULNERABLE is the first randomized trial to evaluate the preventive treatment of angiographically intermediate, nonculprit lesions that exhibit features of vulnerability identified by OCT in patients with STEMI.
REFERENCES
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 2014;312:2019-2027.
2. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381:1411-1421.
3. Lee JM, Kim HK, Park KH, et al. Fractional flow reserve versus angiography-guided strategy in acute myocardial infarction with multivessel disease:a randomized trial. Eur Heart J. 2023;44:473-484.
4. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med. 2021;385:297-308.
5. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
6. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization:Executive Summary:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:4-17.
7. Denormandie P, Simon T, Cayla G, et al. Compared Outcomes of ST-Elevation Myocardial Infarction Patients with Multivessel Disease Treated with Primary Percutaneous Coronary Intervention and Preserved Fractional Flow Reserve of Non-Culprit Lesions Treated Conservatively and of Those with Low Fractional Flow Reserve Managed Invasively:Insights from the FLOWER MI trial. Circ Cardiovasc Interv. 2021;14:011314.
8. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226-235.
9. Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II):a prospective natural history study. Lancet. 2021;397:985-995.
10. Mol JQ, Volleberg R, Belkacemi A, et al. Fractional Flow Reserve-Negative High-Risk Plaques and Clinical Outcomes After Myocardial Infarction. JAMA Cardiol. 2023;8:1013-1021.
11. Park SJ, Ahn JM, Kang DY, et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT):a multicentre, open-label, randomised controlled trial. Lancet. 2024;403:1753-1765.
12. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344-2351.
13. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
14. Virmani R. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13-8.
15. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
16. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-2107.
17. Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505.
18. Raber L, Ueki Y, Otsuka T, et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction:The PACMAN-AMI Randomized Clinical Trial. JAMA. 2022;327:1771-1781.
19. Biccire FG, Haner J, Losdat S, et al. Concomitant Coronary Atheroma Regression and Stabilization in Response to Lipid-Lowering Therapy. J Am Coll Cardiol. 2023;82:1737-1747.
20. Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve:the COMBINE OCT-FFR trial. Eur Heart J. 2021;42:4671-4679.