ABSTRACT
Introduction and objectives: Distal radial access (DRA) for coronary procedures is currently recognized as an alternative to conventional transradial access, with documented advantages primarily related to access-related complications. However, widespread adoption of DRA as the default approach remains limited. Therefore, this prospective cohort study aimed to present our initial experience with DRA for coronary procedures in any clinical settings.
Methods: From August 2020 to November 2023, we included 1000 DRA procedures (943 patients) conducted at a single center. The study enrolled a diverse patient population. We recommended pre- and postprocedural ultrasound evaluations of the radial artery course, with ultrasound-guided DRA puncture. The primary endpoint was DRA success, while secondary endpoints included coronary procedure success, DRA performance metrics, and the incidence of access-related complications.
Results: The DRA success rate was 97.4% (n = 974), with coronary procedure success at 96.9% (n = 969). The median DRA time was 40 [interquartile range, 30-60] seconds. Diagnostic procedures accounted for 64% (n = 644) of cases, while 36% (n = 356) involved percutaneous coronary intervention (PCI), including primary PCI in 13% (n = 128). Pre-procedure ultrasound evaluation and ultrasound-guided DRA were performed in 83% (n = 830) and 85% (n = 848) of cases, respectively. Access-related complications occurred in 2.9% (n = 29).
Conclusions: This study shows the safety and feasibility of DRA for coronary procedures, particularly when performed under ultrasound guidance in a diverse patient population. High rates of successful access and coronary procedure outcomes were observed, together with a low incidence of access-related complications. The study was registered on ClinicalTrials.gov (NTC06165406).
Keywords: Vascular access. Distal radial artery. Coronary angiography. Percutaneous transluminal coronary angioplasty. Doppler ultrasound. Access-related complications.
RESUMEN
Introducción y objetivos: Actualmente, el acceso radial distal (ARD) para procedimientos coronarios es una alternativa al acceso radial convencional, con algunas ventajas descritas principalmente en términos de complicaciones relacionadas con el acceso. A pesar de la evidencia, pocos centros han establecido el ARD como acceso sistemático para procedimientos coronarios. El objetivo de esta cohorte prospectiva es presentar la experiencia inicial en nuestro centro con el ARD en pacientes con indicación de procedimientos coronarios en cualquier escenario clínico.
Métodos: Se incluyeron 1.000 procedimientos de ARD (943 pacientes) realizados en un único centro de agosto de 2020 a noviembre de 2023. El estudio fue realizado con pacientes en cualquier escenario clínico. Se recomendó la valoración por ultrasonido del trayecto de la arteria radial antes y después del procedimiento, así como la punción ecoguiada. El objetivo principal fue el éxito del ARD. Como objetivos secundarios se consideraron el éxito del procedimiento coronario, el desempeño del ARD y las complicaciones relacionadas con el acceso.
Resultados: El éxito del ARD fue del 97,4% (n = 974) y el éxito del procedimiento coronario fue del 96,9% (n = 969). El tiempo de acceso del ARD fue de 40 segundos [rango intercuartílico, 30-60]. Se realizaron procedimientos diagnósticos en el 64% (n = 644) e intervencionismo coronario percutáneo (ICP) en el 36% (n = 356), incluyendo ICP primario en el 13% (n = 128) de los pacientes. La valoración por ultrasonido antes del procedimiento se llevó a cabo en el 83% (n = 830) y la punción ecoguiada en el 85% (n = 848). La incidencia de complicaciones relacionadas con el acceso fue del 2,9% (n = 29).
Conclusiones: Este estudio muestra la viabilidad y la seguridad del ARD principalmente guiado por ultrasonido para los procedimientos coronarios en cualquier escenario clínico, con un alto porcentaje de éxito del acceso y de éxito del procedimiento, además de una baja incidencia de complicaciones relacionadas con el acceso. El estudio fue registrado en ClinicalTrials.gov (NTC06165406).
Palabras clave: Acceso vascular. Arteria radial distal. Coronariografía. Angioplastia coronaria transluminal percutánea. Ultrasonido Doppler. Complicaciones relacionadas con el acceso.
Abbreviations CAG: coronary angiography. DRA: distal radial access. DRart: distal radial artery. PRart: proximal radial artery. TRA: transradial access.
INTRODUCTION
Currently, distal radial access (DRA) in the anatomical snuffbox for both noncoronary and coronary procedures is gaining popularity. Since its introduction by Babunashvili et al.,1 in 2011, several observational studies have validated the feasibility and safety of DRA,2-4 comparing it with conventional transradial access (TRA). DRA has shown advantages such as a lower incidence of radial artery occlusion (RAO) and shorter hemostasis time, with minimal access-related complications.5,6 The usefulness of ultrasound to guide DRA and evaluate access-related complications has also been described.7,8 Recent randomized trials comparing DRA with TRA have reported conflicting results regarding RAO incidence, crossover rates, and access times.9-11 Nevertheless, meta-analyses consistently support the benefits of DRA, albeit with a higher crossover rate.12-13 One of the limitations of most studies on DRA is the restricted inclusion of patients in emergent situations or complex percutaneous coronary interventions (PCI), such as ST-segment elevation myocardial infarction (STEMI); therefore, the feasibility of the approach in this context is somewhat scarce.2,9-11,14 Despite current evidence, the use of DRA as the default access for coronary procedures is still not widely implemented in most centers. Hence, this prospective single-center cohort aimed to present the experience of our first 1000 DRA in patients undergoing coronary procedures in any clinical settings.
METHODS
Population and study design
The Distal Radial Access for Diagnostic and Interventional Coronary Procedures in an all-comer population (DISTAL) registry is a prospective observational investigation aiming to assess the performance of DRA and compare clinical and procedural characteristics in a diverse population undergoing coronary procedures. This interim analysis presents our initial experience with DRA conducted at a single center. All DRA procedures performed by 4 experienced operators, previously proficient in TRA, were included in the study from August 2020 to November 2023.
This study was approved by the Ethics Committee of our institution (CEIC-2804) and was conducted following the principles of the Declaration of Helsinki. All patients gave their informed written consent before the procedure.
Inclusion and exclusion criteria
The study included patients aged 18 years and older undergoing diagnostic or therapeutic coronary procedures using DRA in any clinical setting. Patients with an unsuitable distal radial artery (DRart) assessed by ultrasound (non-permeable or diameter <1 .8 mm) were excluded, as were patients with no palpable pulse of DRart with such unsuitability characteristics. Additional exclusion criteria encompassed participation in other clinical trials, known allergy to iodinated contrast, inability to provide informed consent, and women of childbearing age without a negative pregnancy test. While the Barbeau test was recommended, it was not mandatory for inclusion.15
Endpoints
The primary endpoint was the success of DRA and the main secondary endpoint was the success of the coronary procedure. Other secondary endpoints included DRA procedure time, total procedure duration, the incidence of radial artery spasm, exposure to ionizing radiation, patient comfort levels, hemostasis time, access-related complications, and the impact of ultrasound guidance on DRA performance. Detailed definitions of these endpoints are provided in the supplementary data.
Distal radial access technique
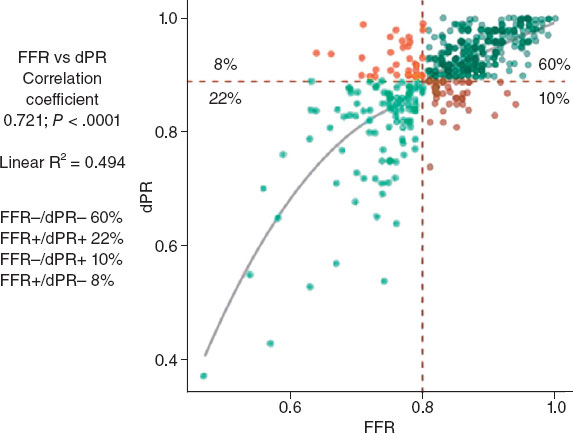
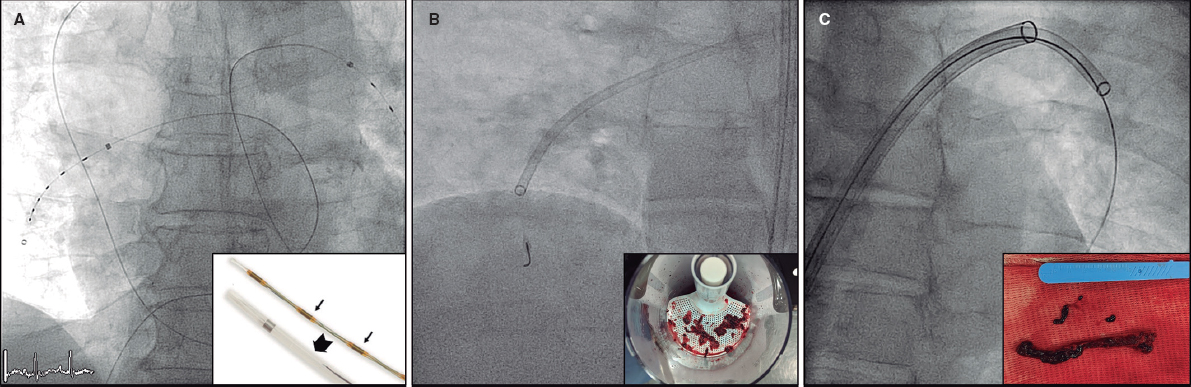
The DRA technique has been previously described,2,4,16-18 and is explained in detail in the supplementary data. Key aspects of interest included patient selection, the decision to use ultrasound-guided puncture19 (figure 1) vs blind with palpation puncture at the discretion of the operator, patient positioning for right (r) or left (l) DRA, the puncture technique itself, and the hemostasis procedure (figure 2).
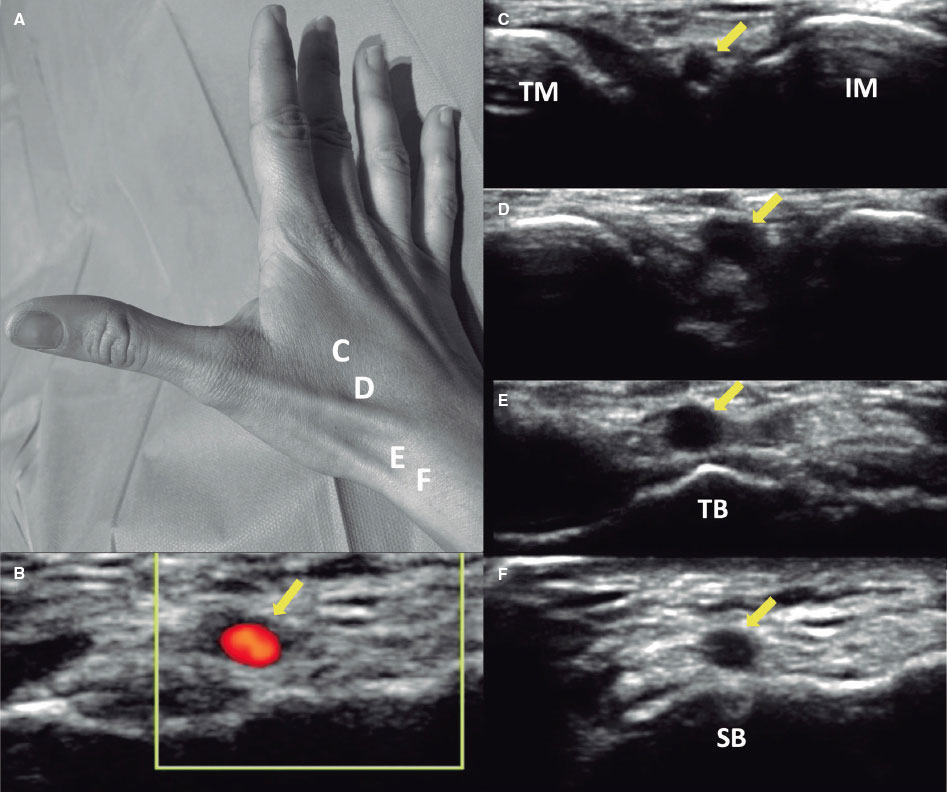
Figure 1. A: markers for ultrasound positioning in the anatomical snuffbox. B: patency of the distal radial artery (DRart) confirmed by color Doppler ultrasound. C-D: course of DRart between the metacarpal bones. E-F: recommended puncture sites of the DRart on a surface bone. IM, index metacarpal; SB, scaphoid bone; TB, trapezium bone; TM, thumb metacarpal.
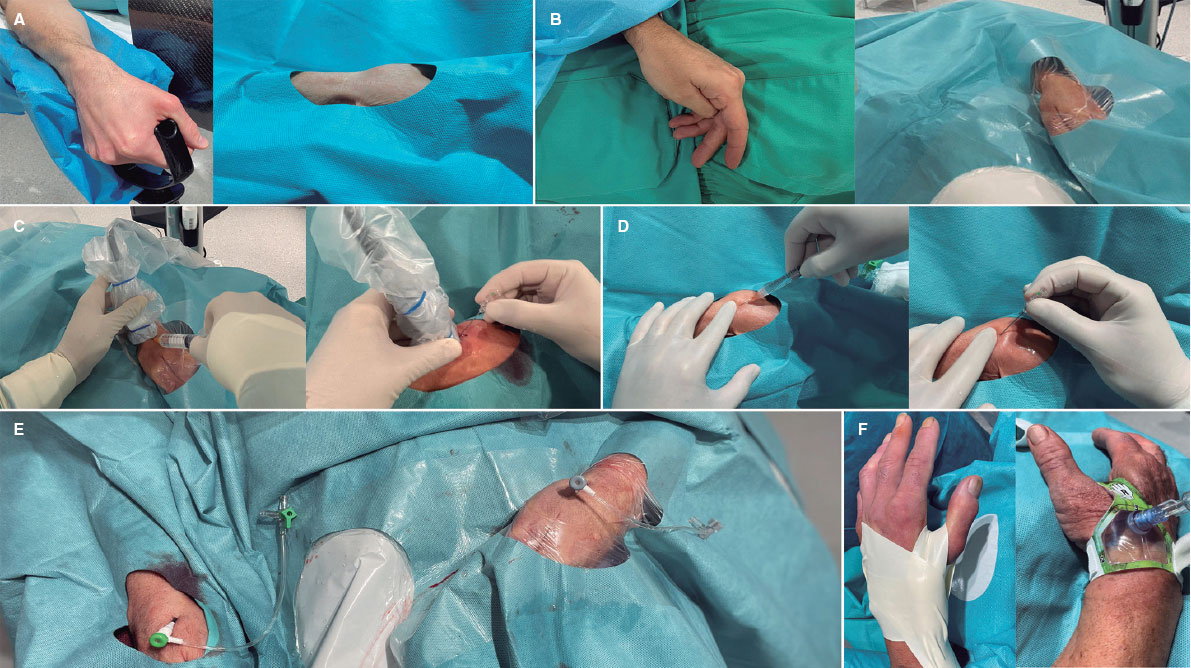
Figure 2. Distal radial access (DRA) technique. Position of the hand for A) right DRA and B) left DRA. C: ultrasound-guided DRA technique. D: blind with palpation DRA puncture. E: final position of the introducer sheaths on the right and left DRA. F: hemostasis devices in DRA.
Statistical analysis
Sample size and statistical power calculations were performed using the GRANMO calculator.20 A sample size of 1000 procedures was determined to provide a statistical power greater than 99% to detect a difference of 3% or more in the proportion of DRA success (primary endpoint) at our center, assuming an alpha risk of 1%. This calculation was based on a reference proportion from previous medical literature estimated around 95%.11,18,21
Categorical variables are presented as counts (percentages), while continuous variables were assessed for normal distribution using the Kolmogorov-Smirnov test. Normally distributed variables are expressed as mean (standard deviation), and nonnormally distributed variables as median [interquartile range].
To evaluate the impact of the learning curve, comparisons were made among quartiles of the study period for variables including access failure, DRA time, total procedure time, and access-related complications. Analysis of variance or the Kruskal-Wallis test was used depending on the normality of the variable. Logistic regression analysis (logit command) was used with the first quartile as the reference to compare percentages among quartiles.
Statistical analyses were conducted using SPSS Statistics 20.0 software (IBM, United States) and STATA 12 (StataCorp, College Station, United States). A p-value < 0.05 was considered statistically significant for all tests.
RESULTS
From August 2020 to November 2023, a total of 1000 DRA procedures (943 patients) were performed. Table 1 shows the patients’ baseline clinical characteristics. The mean age was 68 years, and 29% of the patients were women. A total of 47% of the procedures were performed on an outpatient basis. In 35% of cases, the indication was acute coronary syndrome (13% STEMI).
Table 1. Baseline clinical characteristics
| n = 1000 | |
|---|---|
| Age, (years), mean (SD) | 68.1 (11.7) |
| Female, n (%) | 289 (28.9) |
| Weight, (kg), mean (SD) | 78.0 (14.8) |
| Height, (cm), mean (SD) | 167.9 (8.1) |
| Body mass index, (kg/m2), mean (SD) | 28.0 (4.5) |
| Hypertension, n (%) | 735 (73.5) |
| Dyslipidemia, n (%) | 578 (57.8) |
| Diabetes mellitus, n (%) | 353 (35.3) |
| Current smoker, n (%) | 246 (24.6) |
| Family history of premature coronary heart disease, n (%) | 54 (5.4) |
| Previous peripheral artery disease, n (%) | 50 (0.5) |
| Previous stroke, n (%) | 41 (4.1) |
| Previous heart failure, n (%) | 252 (25.2) |
| GFR (mL/minute/1.73m2), mean (SD) | 72.4 (20.0) |
| Dialysis, n (%) | 27 (2.7) |
| Left ventricular ejection fraction, mean (SD) | 52.6 (16.2) |
| Atrial fibrillation, n (%) | 170 (17.0) |
| OAC | |
| Acenocoumarin, n (%) | 170 (17.0) |
| Direct OAC, n (%) | 81 (8.1) |
| Previous CAG, n (%) | 251 (25.1) |
| Previous CABG, n (%) | 43 (4.3) |
| Previous PCI, n (%) | 218 (21.8) |
| Previous ischemic heart disease | |
| Previous STEMI, n (%) | 133 (13.3) |
| Previous NSTEMI, n (%) | 69 (6.9) |
| Previous CCS, n (%) | 53 (5.3) |
| CAG indication | |
| Chronic coronary syndrome, n (%) | 207 (20.7) |
| STEMI, n (%) | 128 (12.8) |
| NSTEMI, n (%) | 224 (22.4) |
| Staged PCI, n (%) | 60 (6.0) |
| Diagnostic, n (%) | 381 (38.1) |
| Preoperative CAG in patients with VHD, n (%) | 183 (18.3) |
| Dilated cardiomyopathy, n (%) | 158 (15.8) |
| Ventricular tachycardia, n (%) | 24 (2.4) |
| Others, n (%) | 16 (1.6) |
| Outpatient coronary arteriography, n (%) | 470 (47) |
CABG, coronary artery bypass grafting; CAG, coronary angiography; CCS, chronic coronary syndrome; GFR, glomerular filtration rate; NSTEMI, non−ST-segment elevation myocardial infarction; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; VHD, valvular heart disease. Data are expressed as No. (%) or mean ± standard deviation. | |
Table 2 presents the characteristics of the radial artery and the DRA procedure. High rates of preprocedure ultrasound evaluation and ultrasound-guided technique for DRA were noted (83% and 85%, respectively). Notably, the percentage of coronary procedures showing insufficient catheter length due to DRA was low (3.7%).
Table 2. Characteristics of the DRA procedure
| n = 1000 | |
|---|---|
| Preprocedure characteristics | |
| Arterial pulse strength scale | |
| Absent, n (%) | 12 (1.2) |
| Weak, n (%) | 167 (16.7) |
| Normal, n (%) | 652 (65.2) |
| Strong, n (%) | 169 (16.9) |
| Radial artery preprocedure ultrasound evaluation, n (%) | 830 (83.0) |
| Arterial tortuosity | |
| Radial, n (%) | 23 (2.3) |
| Subclavian, n (%) | 62 (6.2) |
| Calcified radial artery, n (%) | 26 (2.6) |
| Distal radial artery size, mm (SD) | 2.3 (0.3) |
| Proximal radial artery size, mm (SD) | 2.5 (0.4) |
| Depth of the distal radial artery, mm (SD) | 3.8 (1.0) |
| DRA technique | |
| CAG by the same DRA, n (%) | 57 (5.7) |
| Ultrasound-guided access, n (%) | 848 (84.8) |
| DRA side | |
| Right DRA, n (%) | 627 (62.7) |
| Left DRA, n (%) | 373 (37.3) |
| Introducer size | |
| 5 French, n (%) | 256 (25.6) |
| 6 French, n (%) | 744 (74.4) |
| Introducer sheath type | |
| Prelude Ideal (Merit Medical) Introducer Kit, n (%) | 950 (95.0) |
| Radifocus Introducer II Kit A (Terumo Corporation), n (%) | 50 (5.0) |
| Short length of the radial catheter | 37 (3.7) |
| Postprocedure arterial patency evaluation, n (%) | 907 (90.7) |
| Postprocedure puncture site bleeding, n (%) | 55 (5.5) |
CAG, coronary angiography; DRA, distal radial access. Data are expressed as No. (%) or mean ± standard deviation. | |
Table 3 summarizes the characteristics of coronary procedures, including the extent of coronary artery disease, types of procedures, and features of patients who underwent PCI. In general, 64% of the procedures were only diagnostic, while 36% included PCI.
Table 3. Characteristics of the coronary procedure
| Procedure characteristics | n = 1000 |
|---|---|
| Coronary disease extent | |
| One vessel, n (%) | 285 (28.5) |
| Two vessels, n (%) | 174 (17.4) |
| Three vessels, n (%) | 176 (17.6) |
| LMCAD, n (%) | 55 (5.5) |
| Coronary bypass graft, n (%) | 27 (2.7) |
| Characteristics of the coronary procedure | |
| Type of coronary procedures | |
| Diagnostic, n (%) | 644 (64.4) |
| PCI, n (%) | 356 (35.6) |
| Ambulatory PCI, n (%) | 90 (9.0) |
| PCI culprit lesion | |
| LMCAD, n (%) | 9 (0.9) |
| Left anterior descending artery, n (%) | 164 (16.4) |
| Circumflex coronary artery, n (%) | 95 (9.5) |
| Right coronary artery, n (%) | 100 (10.0) |
| Coronary bypass graft | 2 (0.2) |
| Specific techniques | |
| Wire-based intracoronary physiological assessment, n (%) | 57 (5.7) |
| Optical coherence tomography, n (%) | 21 (2.1) |
| Intravascular ultrasound, n (%) | 30 (3.0) |
| Guide catheter extension system, n (%) | 15 (1.5) |
| Rotational atherectomy, n (%) | 16 (1.6) |
| Cutting balloon, n (%) | 34 (3.4) |
| Intracoronary lithotripsy, n (%) | 8 (8.0) |
| Thrombus aspiration, n (%) | 81 (8.1) |
| Intracoronary perfusion catheter, n (%) | 7 (0.7) |
| Special PCI procedures | |
| Complex bifurcation, n (%) | 60 (6.0) |
| Chronic total occlusion, n (%) | 16 (1.6) |
| Volume of contrast, (mL), mean (SD) | 85.0 (53.1) |
| Heparin dose, (IU), median [IQR] | 5000 (3000-8500) |
LMCAD, left main coronary artery disease; PCI, percutaneous coronary intervention. | |
Table 4 depicts the clinical endpoints. The DRA success rate was 97.4% and the coronary procedure success rate was 96.9%. The median access time was 40 (interquartile range [IQR], 30-60) seconds, and 4% of patients experienced radial artery spasm. The overall rate of access-related complications was low (2.9%).
Table 4. Clinical endpoints
| n = 1000 | |
|---|---|
| Primary endpoint | |
| DRA success, n (%) | 974 (97.4) |
| Coronary procedure success by DRA, n (%) | 969 (96.9) |
| Secondary endpoints | |
| Access time, (sec), median [IQR] | 40 (30-60) |
| Procedure time, (min), median [IQR] | 29.0 [17.3-45.0] |
| Radial artery spasm, n (%) | 44 (4.4) |
| DAP, (Gy.m2), median [IQR] | 32.7 [19.2-63.0] |
| Fluoroscopy time (min), median [IQR] | 4.6 [2.5-10.0] |
| VAS patient comfort for access, mean (SD) | 2.2 (0.6) |
| VAS patient comfort for hemostasis, mean (SD) | 2.1 (0.4) |
| Hemostasis time, (hour), mean, (SD) | 2.9 (1.1) |
| Access-related complications (all), n (%) | 29 (2.9) |
| Radial artery occlusion, n (%) | 10 (1.0) |
| Hematoma, n (%) | |
| Type I-a, n (%) | 11 (1.1) |
| Type I-b, n (%) | 1 (0.1) |
| Type II, n (%) | 1 (0.1) |
| Type III, n (%) | 1 (0.1) |
| Type IV, n (%) | 0 (0) |
| Radial pseudoaneurysm, n (%) | 0 (0) |
| Radial dissection, n (%) | 5 (0.5) |
| Arteriovenous fistula, n (%) | 0 (0) |
DAP, dose-area product; DRA, distal radial access; VAS, visual analog scale. Data are expressed as No. (%), mean ± standard deviation, or median [interquartile range]. | |
Combined preprocedure ultrasound evaluation and ultrasound-guided puncture were performed in 82.8% of cases, with successful DRA achieved in 97.7% compared with 95.9% in those who did not undergo ultrasound guidance (P = .183). Based on the strength of the arterial pulse—absent, weak, normal, and strong—ultrasound-guided puncture was performed in 100%, 91%, 89.7%, and 45.5% of cases, respectively. Access time was longer with ultrasound-guided puncture than with nonultrasound-guided puncture (40 s [30-70] vs 35 s [30-45]; P < .001). The success of DRA in relation to the use of ultrasound-guided technique among all strengths of arterial pulse is detailed in table 1 of the supplementary data.
Arterial patency after removal of the hemostatic device was assessed in 907 patients (90.7%), revealing RAO in only 1% (n = 10).
In the quartile analysis, a shift in the selection of DRA side was observed, with lDRA initially more commonly used, shifting to rDRA as the preferred access in later quartiles (figure 3A). DRA failure rates were low in all quartiles but decreased significantly from the third quartile onwards (figure 3B). Access time decreased significantly from the second quartile onwards and remained stable thereafter (figure 3C). However, no significant differences were found in total procedure duration between quartiles (figure 3D).
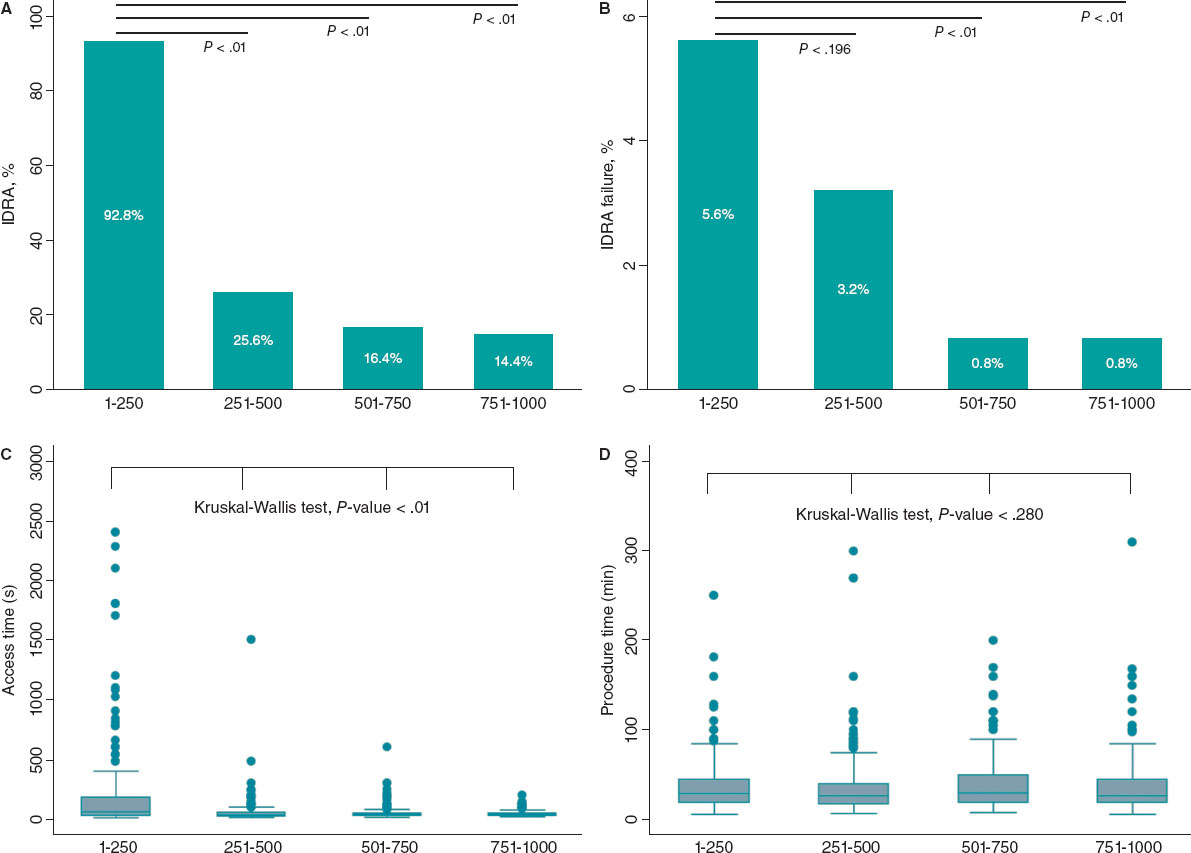
Figure 3. Stratified analysis by quartiles of patients over the study period. A: use of left vs right distal radial access (DRA). B: DRA access failure rate by quartile. C: DRA access time in seconds. D: total procedural time in minutes.
DISCUSSION
Using data from a large prospective registry of patients who underwent DRA for coronary procedures, with high use of ultrasound-guided techniques, our study showed that DRA achieves high rates of access and procedural success, coupled with a low incidence of access-related complications in an all-comer population.
The usefulness of ultrasound in the distal radial access technique
Understanding the anatomy of the anatomical snuffbox is crucial for successful DRA, and ultrasound serves as a valuable tool in achieving this, offering demonstrated advantages.5,16,17,22 In our study, preprocedure ultrasound evaluation and ultrasound-guided DRA techniques were used in most patients. In addition to assessing arterial diameters and evaluating calcification and tortuosity, ultrasound enabled us to exclude patients with unsuitable distal radial arteries. Overall, we found no significant differences between ultrasound-guided and nonultrasound-guided DRA, although the former was associated with longer access times. However, the role of ultrasound is particularly noteworthy in cases of weak or absent arterial pulses, which are often underrepresented in prior studies. The presence of a suboptimal arterial pulse can stem from various factors, including small DRart, hypotension, collateral blood supply, or depth of DRart.11 In our study, most patients with weak pulses underwent ultrasound-guided puncture, with a favorable trend toward successful access in those who did. However, in patients with normal to strong pulses, no differences in DRA success were found, and even prolongation of access time was observed with its use. Therefore, in this type of pulse, an ultrasound-guided puncture is probably not necessary.
Feasibility, safety, and technical issues in distal radial access
This study corroborates the previously reported advantages of DRA,3,9,10,12,13,18 such as a low rate of radial artery spasm, acceptable access time, short hemostasis time, and adequate patient comfort.
Furthermore, the absence of an increased risk of hand dysfunction after DRA has been demonstrated,23 even compared with TRA at 12 months of follow-up, documented by Al-Azizi et al.24 Here, we focus on controversial issues that may have hampered wider adoption of this technique, and our results may provide additional support for DRA.
High success rates of DRA in coronary procedures have been reported in numerous studies.2-4,17,18,25 In addition, recent clinical trials and meta-analyzes describe a higher crossover rate compared with TRA.9-13
In contrast to our results, trials comparing DRA with TRA have reported lower access success and longer puncture times.9-11 Conversely, our study demonstrates remarkably high success rates for DRA and coronary procedures, as well as shorter access time, consistent with registries in which DRA is the default approach among experienced operators, as shown by the largest registries published to date, the DISTRACTION and KODRA studies.2-4,18,21
The KODRA trial included 4977 DRA procedures from a Korean registry.21 The authors reported a DRA success rate of 94.4%, with a crossover rate of 6.7%. In contrast to our work, the use of ultrasound-guided puncture in KODRA was low (6.4%). Additionally, the authors found predictors of DRA failure, such as the presence of a weak pulse and limited operator experience (less than 100 cases).
The equivalence of rDRA and lDRA has previously been demonstrated, and contemporary studies use mainly rDRA.9-11,17 As in the first registries, which suggested a potential advantage of lDRA, we started our experience with lDRA but, based on operator comfort and preference, the use of the rDRA increased over time.
Although the feasibility and benefits of DRA over TRA in STEMI have been observed, the literature on the topic remains scarce.2,9-11 In our registry, all attempted DRA procedures in patients with STEMI were successful. However, the first DRA in STEMI was performed after the operators had surpassed the learning curve for the technique (up to case 320). Similarly, the use of DRA for complex PCI has been previously described.22,26,27 In our cohort, all complex PCI procedures were performed without crossover.
The puncture site in DRA, situated 5 cm distal to TRA, may lead to an adequate catheter length in specific contexts (such as tall patients, dilated aorta, subclavian artery tortuosity, and the need for retrograde access to PCI for chronic total occlusions).28 We found a low incidence of short catheter length during DRA procedures, with only 1 crossover due to severe tortuosity of the subclavian artery.
DRA-related complications have been consistently reported to be low.2,9-11,18 Similarly, we found a very low rate of complications, the most common being type I-a hematoma. In our study, the incidence of in-hospital RAO was 1%.
The number of DRA procedures to overcome the learning curve and maintain a success rate above 94% is around 150 to 200.2,8 However, in our early experience, we achieved this percentage after the first 20 cases per operator.17 In this study, operators navigated the learning curve in the first quartile; however, success significantly improved to more than 99% in the last 2 quartiles, probably because DRA became the default access for coronary procedures among operators.
Limitations
First, this study was an interim analysis of the leading participating site and coordinator of the DISTAL registry (NTC06165406), conducted because substantial enrollment from other sites was lacking. Although the data cannot be fully extrapolated to other centers, recalculation of the sample size was considered sufficient to evaluate the results.
Second, patient enrollment was not consecutive because the decision to use DRA was at the operators’ discretion. Only one-third of coronary procedures during the study period used this approach. However, we included all patients in whom operators intended to use DRA in any clinical setting were included, with only 21 patients excluded due to DRart ≤1.8mm. Third, this was a descriptive cohort of DRA, without a comparison control group. Fourth, the scale used to assess the arterial pulse is subjective. However, this scale is widely used in routine clinical practice and has been used in multiple DRA studies. Finally, radial artery patency was not evaluated in 9.7% of the patients before discharge, and no evaluation was conducted at 1 month; therefore, the in-hospital rate of radial artery occlusion may be underestimated and no mid-term data are available on the patency of the DRart.
CONCLUSIONS
This study shows the safety and feasibility of DRA primarily guided by ultrasound for coronary procedures in an all-comer population, with high rates of both access and procedural success, in addition to a very low rate of access-related complications.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by the Ethics Committee of our institution (CEIC-2804) and was conducted following the principles of the Declaration of Helsinki. All patients gave their informed written consent before the procedure.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Not used.
AUTHORS’ CONTRIBUTIONS
K. Rivera and D. Fernández-Rodríguez conceived and designed the study. K. Rivera, D. Fernández-Rodríguez, M. García-Guimarães, J. Casanova-Sandoval, and J. L. Ferreiro analyzed data, and drafted the manuscript. All authors contributed to the treatment of patients, data acquisition and mining, and review and approval of the final version of the manuscript.
CONFLICTS OF INTEREST
J. L. Ferreiro reports a) honoraria for lectures from Eli Lilly Co, Daiichi Sankyio, Inc, AstraZeneca, Pfizer, Abbott, Boehringer Ingelheim, Bistol-Myers Squibb, Rovi, Terumo and Ferrer; b) consulting fees from AstraZeneca, Eli Lilly Co, Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Inc, Bristol-Myers Squibb and Biotronik; c) research grants from AstraZeneca. The remaining authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- Previous studies have demonstrated the safety and feasibility and safety DRA. Compared with TRA, DRA has several advantages, despite the high prevalence of crossover and controversial incidence of radial artery occlusion.
WHAT DOES THIS STUDY ADD?
- The results of this cohort show the safety and feasibility of DRA in an all-comer population throughout the spectrum of DRart pulses. Our study demonstrates that preprocedure ultrasound evaluation and the ultrasound-guided DRA technique help to achieve a low crossover rate, which is especially useful in patients with an unfavorable arterial pulse. According to our observations, DRA in urgent/emergent procedures and complex PCI is feasible and safe once the learning curve has been overcome and the operator is familiar with the technique.
REFERENCES
1. Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Catheter Cardiovasc Interv. 2011;77:530-536.
2. Lee JW, Park SW, Son JW, Ahn SG, Lee SH. Real-world experience of the left distal transradial approach for coronary angiography and percutaneous coronary intervention:A prospective observational study (LeDRA). EuroIntervention. 2018;14:e995-e1003.
3. Oliveira MDP, Navarro EC, Kiemeneij F. Distal transradial access as default approach for coronary angiography and interventions. Cardiovasc Diagn Ther. 2019;9:513-519.
4. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851-857.
5. Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv. 2018;11:2113-2119.
6. Lu H, Wu D, Chen X. Comparison of Distal Transradial Access in Anatomic Snuffbox Versus Transradial Access for Coronary Angiography. Heart Surg Forum. 2020;23:E407-E410.
7. Ghose T, Kachru R, Dey J, Khan WU, et al. Safety and Feasibility of Ultrasound-Guided Access for Coronary Interventions through Distal Left Radial Route. J Interv Cardiol. 2022;2022:2141524.
8. Roh JW, Kim Y, Lee OH, et al. The learning curve of the distal radial access for coronary intervention. Sci Rep. 2021;11:13217.
9. Tsigkas G, Papageorgiou A, Moulias A, et al. Distal or Traditional Transradial Access Site for Coronary Procedures:A Single-Center, Randomized Study. JACC Cardiovasc Interv. 2022;15:22-32.
10. Aminian A, Sgueglia GA, Wiemer M, et al. Distal Versus Conventional Radial Access for Coronary Angiography and Intervention:The DISCO RADIAL Trial. JACC Cardiovasc Interv. 2022;15:1191-1201.
11. Kozin´ski Ł, Orzałkiewicz Z, Da˛browska-Kugacka A. Feasibility and Safety of the Routine Distal Transradial Approach in the Anatomical Snuffbox for Coronary Procedures:The ANTARES Randomized Trial. J Clin Med. 2023;12:7608.
12. Ferrante G, Condello F, Rao SV, et al. Distal vs Conventional Radial Access for Coronary Angiography and/or Intervention:A Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2022;15:2297-2311.
13. Barbarawi M, Barbarawi O, Jailani M, Al-Abdouh A, Mhanna M, Robinson P. Traditional versus distal radial access for coronary angiography:A meta-Analysis of randomized controlled trials. Coron Artery Dis. 2023;34:274-280.
14. Erdem K, Kurtogˇlu E, Küçük MA, Ilgenli TF, Kizmaz M. Distal transradial versus conventional transradial access in acute coronary syndrome. Turk Kardiyoloji Dernegi Arsivi. 2021;49:257-265.
15. Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R. Transradial coronary catheterization and intervention across the whole spectrum of allen test results. J Am Coll Cardiol. 2014;63:1833-1841.
16. Sgueglia GA, Lee BK, Cho BR, et al. Distal Radial Access:Consensus Report of the First Korea-Europe Transradial Intervention Meeting. JACC Cardiovasc Interv. 2021;14:892-906.
17. Rivera K, Fernández-Rodríguez D, Casanova-Sandoval J, et al. Comparison between the Right and Left Distal Radial Access for Patients Undergoing Coronary Procedures:A Propensity Score Matching Analysis. J Interv Cardiol. 2022;2022:7932114.
18. Oliveira MD, Navarro EC, Caixeta A. Distal transradial access for coronary procedures:A prospective cohort of 3,683 all-comers patients from the DISTRACTION registry. Cardiovasc Diagn Ther. 2022;12:208-219.
19. Hadjivassiliou A, Kiemeneij F, Nathan S, Klass D. Ultrasound-guided access to the distal radial artery at the anatomical snuffbox for catheter-based vascular interventions:A technical guide. EuroIntervention. 2021;16:1342-1348.
20. Calculadora de tamaño muestral GRANMO. Available at:https://www.imim.cat/media/upload/arxius/granmo/granmo_v704.html. Accessed 25 Mar 2024.
21. Lee JW, Kim Y, Lee BK, et al. Distal Radial Access for Coronary Procedures in a Large Prospective Multicenter Registry:The KODRA Trial. JACC Cardiovasc Interv. 2024;17:329-340.
22. Zong B, Liu Y, Han B, Feng CG. Safety and feasibility of a 7F thin-walled sheath via distal transradial artery access for complex coronary intervention. Front Cardiovasc Med. 2022;9:959197.
23. Sgueglia GA, Hassan A, Harb S, et al. International Hand Function Study Following Distal Radial Access:The RATATOUILLE Study. JACC Cardiovasc Interv. 2022;15:1205-1215.
24. Al-Azizi K, Moubarak G, Dib C, et al. Distal Versus Proximal Radial Artery Access for Cardiac Catheterization:1-Year Outcomes. Am J Cardiol. 2024;220:102-110.
25. Rivera K, Fernández-Rodríguez D, Bullones J, et al. Impact of sex differences on the feasibility and safety of distal radial access for coronary procedures:a multicenter prospective observational study. Coron Artery Dis. 2024. https://doi.org/10.1097/mca.0000000000001348.
26. Rivera K, Fernández-Rodríguez D, García-Guimarães M, Ramírez Martínez T, Casanova-Sandoval J. Intravascular ultrasound-guided percutaneous exclusion of a complicated coronary artery aneurysm presenting as ST-segment elevation myocardial infarction. Coron Artery Dis. 2023;34:527-528.
27. Nikolakopoulos I, Patel T, Jefferson BK, et al. Distal Radial Access in Chronic Total Occlusion Percutaneous Coronary Intervention:Insights From the PROGRESS-CTO Registry. J Invasive Cardiol. 2021;33:E717-E722.
28. Davies RE, Gilchrist IC. Back hand approach to radial access:The snuff box approach. Cardiovasc Revasc Med. 2018;19:324-326.
* Corresponding authors.
E-mail addresses: psrivera.lleida.ics@gencat.cat (K. Rivera); dfernandez.lleida.ics@gencat.cat (D. Fernández-Rodríguez).