Medicine and technology have long advanced hand-in-hand. Clinicians push boundaries, expertly yielding the tools at their disposal. Scientists and engineers, in turn, respond to the demands and needs of clinicians by developing the next generation of tools, thereby expanding the space in which clinicians can operate and explore. This boundary-pushing partnership is particularly prevalent in the field of interventional cardiology, which has enthusiastically and effectively embraced advancements in percutaneous and imaging technologies to revolutionize cardiovascular medicine. Continuing this tradition, several developments in computational processing and modeling promise to enhance the utility and efficacy of arterial imaging, with some tools having already entered the clinical setting. For example, a tool that uses computational models built from computed tomography angiography to assess the fractional flow reserve has improved the clinical decision-making process and lowered the rates of unnecessary invasive procedures.1 Additional tools will further unify the physical and virtual realms of medicine through the bridge provided by imaging, offering both simple tools to label and quantify individual images and advanced tools to simulate and profile entire lesions. The future of the cooperative alliance between medicine and technology must be continuously nurtured and will continue to thrive with the enthusiastic and critical contributions of well-informed interventional cardiologists.
WHAT IS COMPUTATIONAL PROCESSING AND MODELING?
Computational processing is the application of algorithms and software to perform specified and encoded procedures. Computational processing can be applied to intravascular images to enhance, characterize or detect and quantify features depicted in the images. One application of such processing is to extract physiological features used to generate computational models of the imaged vessel region. Computational modeling is the creation and use of virtual representations of physical systems. Such representations can be programmed with sets of rules that prescribe how they should behave and respond under different conditions, and in that way the models can be used to simulate the behavior of the physical system under various hypothetical scenarios.
Unmet NEEDs IN INTERVENTIONAL CARDIOLOGY
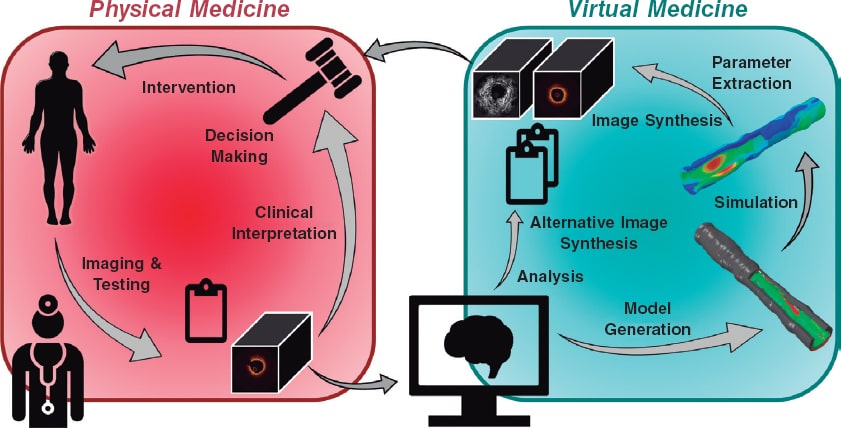
In moving towards a more personalized and precise provision of medical care, the convergence of intravascular imaging with computational processing and modeling will be a pivotal step to empower interventional cardiologists. The role and need for this convergence (figure 1), part of a wider vision of computational cardiology,2 is highlighted by several key challenges faced by the current interventional cardiology practice.
Figure 1. Our vision for the future of cardiovascular medicine is one in which physical and virtual medicine forms a continuum. Clinicians collect data including images and other test results from a patient. Anatomical and morphological information will be automatically extracted by algorithmic processing routines, distilled into reported quantitative metrics, and used to generate patient-specific computational models. Various simulated tests and procedures will be performed on the virtual patient. Results of the analyses and simulations will be transformed back into clinical data to enable a seamless integration and assessment by the heart team; the outcomes could inform the decision-making process and guide the patient's procedure.
Standard assessments with less interventional workload
Among the most critical and urgent roles of computational processing is integrating and augmenting—not displacing—the role played by interventional cardiologists. Computational methods can remove the inter- and intra-observer variability, time consumption, and monotony associated with extensive manual measurements of intravascular images. Such analysis can be performed in the background and continuously without the constraints of busy clinical schedules. Support from processing technologies can also assist physicians with limited training, experience or expertise who may otherwise be unable to identify important features in intravascular images. Additionally, modeling offers relevant quantitative metrics of the vascular state that simply cannot be directly measured, such as estimates of stress along and within the vessel walls.
Patient profiling and stratification
The core premise of precision medicine is that patient populations can be segmented into narrow classes that respond differently to interventions. The cardiology community has proposed broad classifications to divide atherosclerotic plaques, typically driven by the prevailing tissue type and presumed degree of progression.3 Despite or in deference to its simplicity, there have been few significant updates to this classification in recent decades, even as intravascular imaging offers more and richer information on lesion geometry and morphology and as treatment of the disease has evolved. Computational processing and modeling may offer improved profiling of individual patients on the basis of clinical presentation, disease state, detailed lesion phenotype, and even mechanical condition. By computing series of quantitative measures to describe patient and lesion, the possibility of building a more robust patient profile becomes real. Such granular profiling could enable stratification to better assess who benefits the most from which interventions, thus guiding the therapeutic decisions.4
Prediction and risk assessments for clinical decision support
In addition to improved patient profiling and stratification, modeling offers the ability to engage in truly personalized risk assessment and prediction of disease progression under various treatment regimens. Because much of the interpretation of intravascular images is currently qualitative, decision-making during patient care can be an exquisite art and formulaic science alike and depend on each cardiologist’s personal experiences (and biases), training, and institutional practices. Advancing beyond personal clinician experience and intuition, computer processing and modeling offer repeatable, standardized quantification to inform the decision-making process. For example, simulations of detailed patient- and lesion-specific models, or “Digital Twins”,5 could facilitate various virtual interventions or intervention parameters to be tested before selecting an optimal strategy to minimize risk. Alternatively, disease progression and plaque growth models may help to predict which vessels and mild lesions may progress dangerously—suggesting the need for prophylactic action—and which are likely to remain benign and inconsequential over time.
TECHNOLOGICAL Tools in DEVELOPMENT
To fulfill the needs of computational processing and modeling in interventional cardiology, various new technologies are being developed that leverage the rich data available from intravascular imaging. The detection and measurement of geometric features is already available in limited cases, and it is likely to expand. The automated delineation of the lumen and external elastic lamina is incorporated in some intravascular ultrasound systems, and developments in computational processing have recently yielded promising results to identify these, as well as the internal elastic lamina, in optical coherence tomography images. Facilitated by automated detection of inner and outer vessel borders, automatic measurements from pullbacks such as lumen area, plaque burden, eccentricity, and remodeling index will reduce the need for manual identification and annotation of the most critical frames and will enable better visualization of diseased vessels. This information may also be used, for example, in the proper sizing of balloons and stents.
Advances in image processing also offer improved availability of information on lesion morphology. While experts are generally adept at determining the composition and distribution of plaque from cross-sectional images, doing so is a slow process that requires extensive expertise. Increased availability of automated virtual histology will improve the characterization, profiling, and stratification of lesion phenotypes. New image-based methods to characterize the stiffness of diseased tissue also promise greater insight into the mechanical profile of a lesion. Altogether, this information on plaque distribution and properties will help cardiologists to plan and guide interventions (eg, by informing the need for lesion preparation or modification prior to ballooning or stenting).
Computational modeling is a central focus of ongoing technological research and development. The ability to simulate disease progression and interventions is an enticing challenge that has been drawing the attention of multidisciplinary teams. Among these efforts, major collaborative, international European projects have sought to develop and refine advanced predictive models of atherosclerotic plaque processes and angioplasty by integrating patient risk factors, blood panel results, and imaging data.6 Robust longitudinal validation of such models remains an obstacle.
Computational processing offers another little-explored function to synthesize and enhance images. As intravascular imaging is the cornerstone of interventional cardiology, these generative abilities could be used with great effect to improve diagnostic image quality and efficacy, convey information generated from computational models, and facilitate education and training for reading and interpreting images.
Several technologies in development may require changes to future clinical practice. For example, some methods require multiple image pullbacks or simultaneous measurement of pressure and matched image acquisition. Changes will be limited by the corresponding progress in hardware development and adoption, demonstrated cost-benefit tradeoffs for patients, and receptiveness of the interventional cardiology field.
THE Indispensible ROLE OF INTERVENTIONAL CARDIOLOGISTS
Interventional cardiologists will not only have a pivotal role in the future adoption of computational processing and modeling technologies but also an important present role in defining and achieving that future. Those experienced in managing and treating patients are needed to direct, develop, and shepherd new technologies—their deep knowledge of the demands and practical limitations of clinical care are indispensable to scientists and engineers. There is also ongoing profound need for data with which to train and validate new methods and models. Here, too, the involvement, expertise, and contributions of collaborative cardiologists are essential.
The increasing integration of more complex technologies also introduces a growing imperative for cardiologists to further cultivate their technical literacy. While medical and health sciences must remain a priority in the training of interventional cardiologists, broader training is an important prerequisite to critically assess new claims and make informed decisions on the applicability and reliability of new techniques as they enter the medical arena. Clinicians will need to understand the assumptions, uncertainties, and conditions under which these tools should be beneficially applied to treat their patients. Interventional cardiologists and other medical professionals are already well-equipped for many of these tasks. Looking beyond the novelty and flair of the methods, familiar and fundamental concepts considered for other diagnostic tests, such as sensitivity and specificity, should be equally applied to scrutinize advanced new software tools.
As computational processing and modeling converge with intravascular imaging in the coming years, interventional cardiologists will be empowered to deliver more personalized and precise medical care. Those in the field should expect to play an active role in the development, assessment, and adoption of new technologies, and equip themselves with the evolving knowledge and skills necessary to make the most of these tools in the management of their patients.
FUNDING
This work was supported by the U.S. National Institutes of Health (Bethesda, MD, United States; grant number 5R01GM049039-24) and the Massachusetts Institute of Technology (Cambridge, MA, United States; MathWorks Engineering Fellowship).
CONFLICTS OF INTEREST
M. Olender and E.R. Edelman have the patent “Arterial Wall Characterization in Optical Coherence Tomography Imaging” (16/415,430) pending, and the patent “Systems and Methods for Utilizing Synthetic Medical Images Generated Using a Neural Network” (62/962,641) pending. In addition M. Olender reports grants from MathWorks while conducting the study; and E.R. Edelman reports grants from the U.S. National Institutes of Health while conducting the study; grants from Abiomed, grants from Edwards LifeSciences, grants from Boston Scientific, grants from Medtronic, grants from Autus Medical, other from Biodevek, other from Panther Therapeutics, personal fees from Abbvie, outside the submitted work.
REFERENCES
1. Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease:The prospective longitudinal trial of FFRCT:Outcome and resource impacts stud. Eur Heart J. 2015;36:3359-3367.
2. Athanasiou LS, Nezami FR, Edelman ER. Computational Cardiology. IEEE J Biomed Health Inform. 2019;23:4-11.
3. Stary HC, Chandler AB, Dinsmore RE, et al. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation. 1995;92:1355-1374.
4. Gray RA, Pathmanathan P. Patient-Specific Cardiovascular Computational Modeling:Diversity of Personalization and Challenges. J Cardiovasc Transl Res. 2018;11:80-88.
5. Corral-Acero J, Margara F, Marciniak M, et al. The 'Digital Twin'to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556-4564.
6. Sakellarios AI, Pelosi G, Fotiadis DI, et al. Predictive Models of Coronary Artery Disease Based on Computational Modeling:The SMARTool System. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:7002-7005.
* Corresponding author: 77 Massachusetts Avenue, Cambridge, MA 02139, United States.
E-mail address: molender@mit.edu (M. Olender).