ABSTRACT
Severe aortic stenosis is the most frequent valve condition requiring surgery, and its incidence is increasing yearly. Transcatheter aortic valve implantation (TAVI) is the first-line treatment for patients at all levels of surgical risk. Nevertheless, modifications to the procedure often appear to improve clinical outcomes. A major concern after TAVI is the higher rate of permanent pacemaker implantation (PPMI) compared with surgical valve replacement. Optimal implantation depth is crucial to reduce the burden of PPMI without causing serious complications such as valve embolization. The classic implantation technique, where the 3 cusps are aligned in the same plane, has been modified to a cusp overlap projection by isolating the noncoronary cusp and superimposing the left and right cusps. This simple modification provides optimal visualization during deployment and helps to achieve the desired implant depth to reduce conduction disturbances and PPMI. Another limitation after TAVI is coronary reaccess due to the frame of the transcatheter valve obstructing the coronary ostia. Commissural alignment of the prostheses with the native valve may facilitate selective cannulation of the coronary arteries after this procedure. This review will discuss the techniques and supporting evidence for these modifications to the deployment and implant projection methods, and how they can improve TAVI outcomes.
Keywords: Cusp overlap projection. Commissural alignment. Transcatheter aortic valve replacement.
RESUMEN
La estenosis aórtica grave es la valvulopatía más frecuente y su incidencia aumenta cada año. El implante percutáneo de válvula aórtica (TAVI) es la primera línea de tratamiento con cualquier riesgo quirúrgico. Una complicación frecuente del TAVI es una tasa más alta de implante de marcapasos permanente (IMPP) en comparación con la cirugía. La profundidad óptima de implante es fundamental para reducir la tasa de IMPP sin generar otras complicaciones, como la embolización de la válvula. La técnica clásica de implante, en la cual las 3 cúspides están alineadas en el mismo plano, se ha modificado a una proyección de superposición de cúspides, aislando la cúspide no coronaria y superponiendo la izquierda y la derecha. Esta modificación proporciona una visualización óptima durante el despliegue y facilita obtener la profundidad deseada para reducir la tasa de IMPP. Otra limitación del TAVI es el reacceso coronario debido a la obstrucción de la válvula a los ostium coronarios. La alineación comisural de la prótesis con la válvula nativa facilita la canulación selectiva de las coronarias después del procedimiento. En la presente revisión se comentan las técnicas y la evidencia sobre estas modificaciones de la técnica de liberación e implante, y cómo pueden mejorar el TAVI.
Palabras clave: Alineamiento comisural. Proyección de superposición de cúspides. Recambio valvular aórtico percutáneo.
Abbreviations CAD: coronary artery disease. COP: cusp overlap projection. PPMI: permanent pacemaker implantation. TAVI: transcatheter aortic valve implantation. THV: transcatheter heart valve. ID: implantation depth.
INTRODUCTION
Severe symptomatic aortic stenosis (SAS) is the most frequent valve disease in Europe and North America. This disease has been diagnosed in over 7 million patients and accounts for up to 40% of all native valve interventions.1 The absolute number of aortic valve interventions has steadily increased yearly, mainly due to the large number of new diagnoses in the aging population. Some projections estimate that the number of significant valve diseases will double by 2050.2
The treatment of SAS used to require open heart surgery. However, since the first implant in 2002 and its European approval in 2007, transcatheter aortic valve implantation (TAVI) has transformed the landscape, offering a less invasive treatment for SAS.3 TAVI was initially restricted to inoperable patients but since the Partner 34 trial in low-risk patients and SURTAVI5 trial in intermediate-risk patients, it has become the first-line treatment for patients at all levels of surgical risk. The latest European guidelines favor transfemoral TAVI as the treatment choice in patients older than 75 years.4 Moreover, some studies have reported cost-effectiveness analyses favoring TAVI over surgical aortic valve replacement (SAVR),5 while early discharge and outpatient protocols have proven safe, with encouraging results.6 From 2019 to 2021, the number TAVI procedures increased in Spain from 90 to 120 per million people.7 Given this trend, the absolute number of TAVI procedures in both younger and older patients is expected to rise in the coming years.
Considering that the indication for TAVI has been extended, several key aspects may warrant further investigation and might discourage the use of this procedure. First, up to 50% of TAVI patients have significant coronary artery disease (CAD). Since implants are being performed in younger patients with longer life expectancy, it is expected that a large number will develop significant CAD, requiring coronary angiography and treatment.8 Coronary artery catheterization in patients with a transcatheter heart valve is complex since the prosthesis creates a direct obstacle to the arteries to be engaged. Consequently, strategies to facilitate coronary procedures after TAVI are essential.
Second, compared with SAVR, the number of permanent pacemaker implantations (PPMI) is higher, with rates of up to 17.4% for self-expanding valves and 6.5% for balloon-expandable valves.9,10 Recent registries report a PPMI rate of 11.3% for all TAVI procedures. Patients requiring a PPMI after TAVI have worse clinical outcomes, longer hospitalizations, and higher mortality rates during follow-up.11
To mitigate these risks, newer-generation valves are being developed and special considerations during preprocedural planning and transcatheter heart valve (THV) have emerged. These advancements will be discussed in the following review.
OPTIMAL IMPLANTATION DEPTH
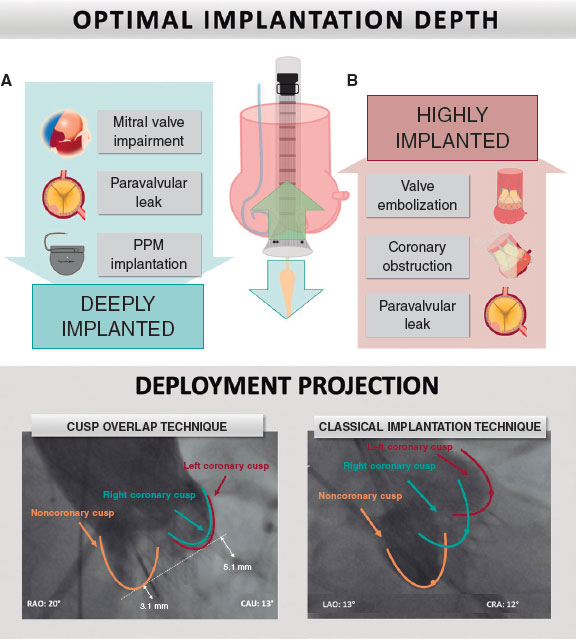
There has been much discussion regarding the optimal implantation depth (ID), particularly its effect on valve performance and ability to modify other clinical endpoints. High THV implantation may lead to dreaded complications, such as valve embolization, coronary obstruction, and paravalvular leak (PVL). Conversely, deep vale implantation increases the risk of PPMI, PVL, and impaired mitral valve function. Therefore, ensuring optimal ID is essential to obtain better results (figure 1).12
Figure 1. A: achieving optimal implantation depth is fundamental to improving outcomes. A deeply implanted valve may impair mitral valve function, produce paravalvular leaks, and interact with the conduction system, increasing permanent pacemaker rates. In contrast, high valve implantation may produce coronary obstruction, valve embolization, and paravalvular leak. B: cusp overlap projection where the left and right cusp overlap on the right side of the screen, isolating the noncoronary cusp, has been shown to optimize implantation depth. CAU, caudal; CRA, cranial; PPM, permanent pacemaker; RAO, right anterior oblique.
One of the main reasons ID produces conduction disturbances is its interaction with the membranous septum, a fibrous structure of the interventricular septum located at the base of the triangle of Koch. The conduction system travels within the membranous septum and continues as the left bundle branch superficially as it reaches the muscular septum. This is why left bundle branch block (LBBB) is the most common conduction disturbance after TAVI, depending on the length of the membranous septum and THV depth. An optimal ID has been proven to minimize membranous septum interaction, conduction disturbances, and PPMI rates.13
DOUBLE S CURVE
The classic implantation technique, which aligns the 3 cusps in the same plane, usually results in the delivery system being foreshortened and eliminates parallax, which deviates the prosthesis from the annular plane. A double S-shaped curve consisting of the intersection point where the annulus and the delivery system are in the optimal position may facilitate a more controlled deployment of the THV.
In a study by Ben-Shoshan et al.,14 100 patients underwent TAVI, which was deployed using the double S curve model with the Medtronic self-expanding valve. More than 80% of the patients had a double S curve in the right anterior oblique and caudal quadrant. The authors reported procedural success in 98% of the patients, and the rates of PPMI and other complications were similar to those described in previous studies. They also specified that they did not intend a higher ID. Therefore, PPMI rates were similar to those in previous studies in patients at the same risk. This technique has not been widely adopted because the S curve requires intraprocedural image analysis, which is not available in all centers.14
CUSP OVERLAP
The cusp overlap projection (COP) technique was proposed by Tang et al.15 to optimize the implantation of self-expanding THV using the classic implantation technique (CIT) by overlapping the left coronary cusp and the right coronary cusp, thereby isolating the noncoronary cusp. The angulation required during implantation is predicted by multislice computed tomography (MSCT). This view offers several benefits: it elongates the outflow tract and overlaps the right coronary cusp and left coronary cusp (LCC) along the basal plane of the annulus, isolates the noncoronary cusp (NCC), and centers the right noncommissure in the center of the fluoroscopic view. This allows more controlled deployment, achieving a higher ID.15 Compared with the double S curve, COP was highly concordant in over 80% of the patients, reducing the need for intraprocedural imaging (figure 1).14
A simplified summary of the COP technique is as follows: a) a preprocedural MSCT isolates the NCC and overlaps the right and left cusps. In most patients, this results in a right anterior oblique/caudal view; b) a high-support wire, such as a Safari (Boston Scientific, USA) or a double-curved Lunderquist (Cook Medical, USA), maintains the position during deployment; c) a pigtail catheter is placed in the NCC, and deployment begins by positioning the ring marker in the mid-portion of the pigtail (in the case of the latest Evolut FX [Medtronic Inc, USA] valve, in the lowest portion of the pigtail) to achieve an ID of approximately 3 mm; d) when the valve reaches 80% deployment, parallax is eliminated in a left anterior oblique view for depth assessment. The valve should be recaptured and repositioned if the ID is < 1 mm or > 5 mm; and e) if the inflow portion of the valve is infra-annular, the valve is slowly released from the delivery catheter (figure 2).16
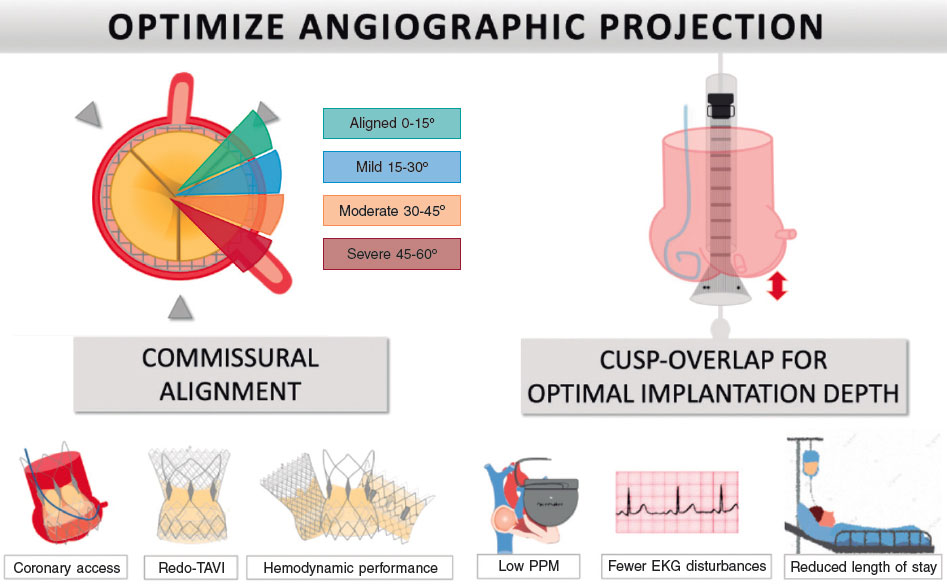
Figure 2. Central illustration. Minor modifications during deployment can achieve commissural alignment (left side of the panel), facilitate coronary access in future procedures, reduce the burden of coronary occlusion in redo-TAVI, and improve valve hemodynamics. A cusp overlap projection (right side of the panel) can improve the implanter’s view to help optimize implantation depth, reduce conduction disturbances and permanent pacemaker rates, and subsequently improve outcomes and length of stay. EKG, electrocardiogram; PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
SELF-EXPANDING VALVES
Evolut R, Evolut PRO, Evolut PRO+ and Evolut FX from Medtronic Inc, United States
Most of the available literature on the COP technique has focused on the Medtronic self-expanding valve. In a single-center experience, Pascual et al.17 evaluated COP with the Evolut R and PRO valves. This center modified all implants from the CIT to COP and compared 226 patients, with 113 in each arm. The results showed that, in patients in the COP group, implant depth was 1 mm lower (4.8 mm ± 2.2 vs 5.7 mm ± 3.1; P =.011) and the PPMI rate decreased from 23% to 12.4% (odds ratio: 0.45; 95% confidence interval [95%CI], 0.21-0.97; P =.043).17 Although the sample size in this single-center study was relatively small, similar results were obtained in a second analysis involving 2 high-volume centers with a propensity score-matched analysis of 444 patients (175 in the COP group). The analysis demonstrated a mean depth reduction of 1 mm (4.2 mm vs 5.3 mm; P < .001) and lower PPMI rates in the first 30 days (11.8% vs 21.7%; P = .03; relative risk: 0.54; 95%CI, 0.32-0.91) with a similar incidence of other complications.18 This latter study included patients with the newer Evolut PRO+ generation.
In a 3-center experience, Mendiz et al.19 analyzed new LBBB and PPMI rates in 257 patients (101 in the COP group). The rates were lower for the COP group, with 12.9% vs 5.8% (P = .05) for new LBBB and 17.8% vs 6.4% (P = .004) for PPMI. Similarly, Maier et al.20 recruited 759 patients in a single-center from 2016 to 2021 and used a propensity score analysis. The results mirrored those previously mentioned, with a PPMI rate of 8.0% for the COP group vs 16.8% for the CIT (P = .028) and fewer conduction disturbances. Even more interesting is that the reduced PPMI rates led to shorter hospital stay in the COP group (8.4 ± 4.0 vs 10.3 ± 6.7 days; P = .007). A study by Ochiai et al.21 included 258 patients from 2017 to 2022. Using the COP technique, these authors aimed for a higher ID. New-onset LBBB was numerically lower (4.2% vs 11.3%), and PPMI rates were significantly lower in patients undergoing COP (0.0% vs 10.8%; P = .02).
The newest valve generation from Medtronic (Evolut FX) was designed to improve deliverability, trackability, and deployment accuracy. Merdler et al.22 included 200 consecutive patients in their study; the first 100 received the Evolut PRO+ while the remaining 100 received the Evolut FX. No significant differences were found in PPMI rates (12% vs 9%; P = .21) and clinical outcomes were similar. Another series showed a reduction in PPMI rates from 11.2% to 7% in the first 43 patients, although this difference was not statistically significant (P = .25). Given these results and the modifications made to the valve, it is expected that the benefits of the COP technique will be maintained with the latest generation of valves. Therefore, best practice supports the use of the COP technique for this generation as well.23
In a meta-analysis including 11 studies with 1464 patients in the COP group and 1743 in the CIT group, the odds ratio for PPMI was 0.48 (95%CI, 0.33-0.70), achieving a higher ID with a mean difference of almost 1 mm (0.83; 95%CI, 1.2 to −0.45; P < .001). No statistically differences were found in new rates of LBBB, and similar complication rates were observed for moderate/severe PVL, valve dislocation, need for a second THV, 30-day mortality, stroke, conversion to surgery, coronary obstruction, and post-TAVI mean gradients (mmHg).24 However, this meta-analysis did not include the most extensive analysis to date by Wieneman et al.25 These authors recruited 2209 patients from 2016 to 2022, with 1151 patients undergoing the COP technique. The rates of PPMI (17.0% vs 12.3%; P = .002) and PVL (4.6% vs 2.4%; P = .006) were significantly lower in the COP cohort.
The only prospective analysis currently underway is the Optimize PRO study (NCT04091048), a nonrandomized analysis comparing the safety and efficacy of COP using the Evolut PRO and Evolut PRO+ valves. Preliminary data have been reported by Grubb et al.26 Among 400 attempted implants, the PPMI rate was 9.8% and decreased to 5.8% if 4 critical steps from the COP protocol were met.26 The 30-day complication rates were also low, with an all-cause mortality of 0.8%, disabling stroke of 0.7%, hospital readmission of 10.1%, cardiovascular rehospitalization of 6.1%, and no instances of moderate or severe aortic regurgitation at discharge. These promising results should to be confirmed when the final results are published.
Accurate Neo2
Kim et al.27 compared 901 TAVI procedures using the self-expanding Accurate Neo 2 (Boston Scientific Corporation, United States) valve: 631 using the CIT and 270 with the COP technique. There were no significant differences in the primary combined outcome of PPMI, new-onset LBBB, technical failure, and ≥ moderate PVL (23.1% vs 21.5%; P = .586). When PPMI rates were analyzed separately, they were similar among groups (CIT7.3% vs COP 6.3%; P = .592) with no differences in ID. The authors point out that initial anchoring of the upper crown limits repositioning of the valve, and ID is not affected by COP. Nevertheless, the projection proved safe and feasible for this valve, and the complication rates were similar for the 2 techniques. To document commissural alignment during the procedure, Meduri et al.28 used the COP view to confirm that the THV was positioned correctly. Therefore, it is arguably a better projection for this valve since it is equivalent in most aspects but can favor commissural alignment.
Portico and Navitor valves
The Portico valve (Abbott Cardiovascular, United States) with the second-generation FlexNav delivery system was tested in 3 tertiary centers. A total of 85 patients undergoing transfemoral TAVI were recruited, 42 with the COP view. The target depth was 3 to 5 mm from the NCC to the inflow of the heart valve frame. The primary endpoints were ID and a combination of new-onset LBBB and PPMI. COP was associated with a higher ID (4.9mm vs 7.4mm; P = .005) and a lower rate of the combined outcome (31.0% vs 58.1%; P = .012). However, when the endpoints were analyzed separately, there was only a tendency toward fewer PPMI (14.3% vs 30.2%; P = .078).29 Despite the similarities between the Portico and the Evolut valves, they seems to be a different impact on conduction disturbances while achieving a higher ID. These differences may be explained by the opening force and distribution of the radial force, with lower overall PPMI rates for the Portico system (13.5% vs 19%).30
A larger trial by Wang et al.31 included the Portico valve and its newest generation, the Navitor valve. These authors compared 366 patients and compared deployment using COP vs the standard 3-cusp coplanar projection. They analyzed 183 pairs in a propensity score-matched analysis. The PPMI rate was 12.6% in the COP group vs 18% in the CIT group, but this difference was not statistically significant (P = .15). However, like other self-expanding valves, commissural alignment was obtained in the COP projection, and the complication rate was similar in the 2 groups. It is worth noting that after matching, the Portico valve was used in 183 patients in the CIT group, whereas the newest generation Navitor valve was used in 183 of the COP group.
BALLOON-EXPANDABLE VALVES
While cusp overlap was initially developed for self-expanding valves due to the asymmetrical nature of their deployment, Sammour et al.32 applied the same principles to the Sapien 3 valve (Edwards Lifesciences, United States) using the double S curve and COP technique. In most patients, a right anterior oblique/caudal projection will isolate the NCC and overlap the LCC and right coronary cusp. Following this concept, they developed a high-deployment technique (HDT): the valve is deployed in a right anterior oblique/caudal view, and the parallax of the crimped valve is eliminated. Then, the valve is positioned by aligning the radiolucent line of the crimped valve at the base of the NCC. Finally, a flush catheter is located at the base of the NCC as a marker for the deployment aortogram to confirm stent coverage. The authors recruited 622 patients (60.5%) for conventional deployment, while HDT was used in 406 patients (39.5%). ID was significantly shallower with HDT (1.5 vs 3.2 mm; P < .001). The rates of PPMI (5.5% vs 13.1%; P < .001), complete heart block (3.5% vs 11.2%; P < .001), and LBBB (5.3% vs 12.2%; P < .001) were lower with HDT. Multivariable logistic regression showed that HDT was an independent predictor for 30-day PPMI (OR, 0.439; 95%CI, 0.246–0.781; P = .005). Complication rates were similar, with 1 case of valve embolization and no cases of coronary obstruction.
The aforementioned study by Ochiai et al.21 included 258 patients with Sapien 3 THV, 108 with HDT, and 150 with conventional deployment. The results were similar to those of Sammour et al., with fewer conduction disturbances. However, PPMI rates were low in both groups, occurring in only around 2% of the patients. The position of the coronary ostia relative to the THV was assessed using post-TAVI MSCT. There were no differences in the interference of the THV skirt with the coronary ostia. Conversely, the incidence of interference of the stent frame with access to the coronary ostia was significantly higher in the HDT group (97.2% vs 89.3%; P = .02).
The most recent analysis by Stephan et al.33 recruited 280 patients undergoing transfemoral TAVI with the Sapien 3 valve. The authors used the COP technique in 143 patients, resulting in significantly higher IDs. However, there were no significant differences in new-onset LBBB. Although PPMI rates were numerically lower (7.3% vs 4.9%), the difference was not statistically significant (P = .464).
The evidence on a higher ID for balloon-expandable valves is contradictory. Some studies suggest a reduction in PPMI and conduction disturbances, while more recent analyses show equipoise between HDT and the CIT. However, in most cases, there is at least a tendency toward fewer PPMI, low complication rates, and high success rates with HDT. More extensive prospective studies are warranted to accurately determine outcomes with HDT in this type of prosthesis.
DRAWBACKS OF CUSP OVERLAP AND HIGHER IMPLANTATION DEPTH
The COP is a safe and feasible technique that requires minimal modifications to the standard procedure for most commercially available THVs. This projection facilitates commissural alignment and provides better visual orientation to obtain an optimal ID, reducing conduction disturbances and PPMI. Although most studies have not reported significant differences in complication rates, several considerations must be taken into account. Valve embolization is a potentially severe complication with a risk of < 1%. Operators must be skillful and resourceful in managing this complication by positioning the valve safely in the aorta while preparing a second THV for deployment. In patients with a lower calcium burden and without prior conduction disturbances, which may be the case for younger patients, the benefit of a higher ID must be weighed against the risk of valve embolization. Another risk of a higher ID is that it could hamper proper cannulation of the coronary arteries during subsequent interventions.
Second, a higher ID may complicate coronary access and has been identified as a predictor of unsuccessful cannulation. Although this risk may be mitigated by commissural alignment, there is a potential risk that high valve implantation will cause obstruction of the coronary ostia, where a pericardial skirt covers the inflow of the frame. This poses a risk of occluding native arteries. Furthermore, in younger patients, who may require a valve-in-valve TAVI procedure in the future, high deployment may preclude a second procedure because the leaflets of the first valve could create a neoskirt that potentially obstructs the coronary ostia.35
In addition, patients with previous aortic valve replacement have a lower risk of PPMI after redo-TAVI but a significantly higher risk of coronary obstruction, especially those with narrow sinuses of Valsalva and lower coronary ostia. In these patients, aiming for a higher ID may not enhance outcomes.
COMMISSURAL ALIGNMENT
One of the main concerns in expanding the indication of TAVI to younger and low-risk patients is the feasibility of coronary access post-TAVI, mainly due to the potential need for percutaneous coronary intervention. This underscores the practical importance of achieving commissural alignment (CA). Additionally, considerations of durability and the potential for redo-TAVI are crucial when contemplating the expansion of indications to younger patients.35
The concept of CA has gained prominence in recent years, leading to improvements in the design of the newest valve generations to facilitate its achievement.35 MSCT data from studies without intentional CA technique show that approximately 80% of patients undergoing TAVI experience commissural misalignment.36 In low-risk patients who underwent balloon-expandable TAVI, approximately 13% had a commissural post obstructing the coronary ostium. Commissural misalignment is as high as 16% with self-expanding TAVI.37 In the RE-ACCESS study, Barbanti et al.38 showed that only 7.7% of patients underwent unsuccessful coronary cannulation after TAVI.
The ALIGN-TAVI consortium defined CA based on the angle between the native and new valve commissures. The definition of CA was established among different categories: aligned (angle deviation < 15°), mild commissural misalignment (CMA) (15°-30°), moderate CMA (30°-45°), and severe CMA (> 45°) (figure 3).39
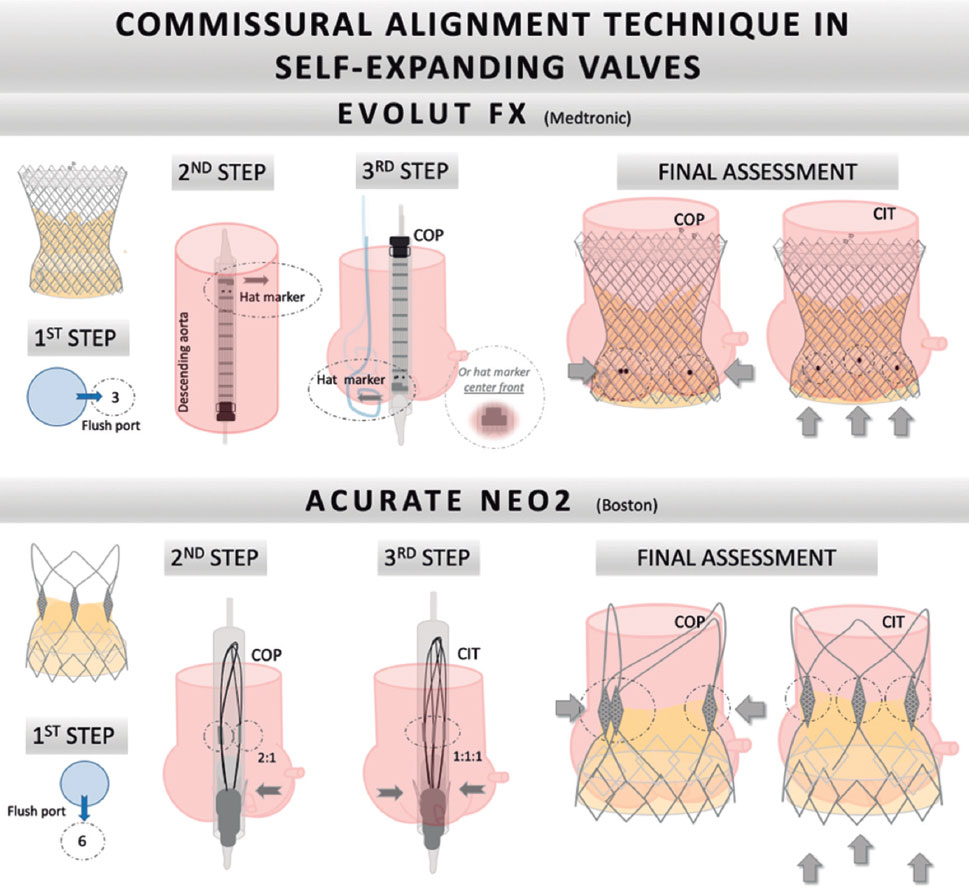
Figure 3. Step-by-step tutorial for commissural alignment in self-expanding valves. A: Evolut FX valve. First, the flush port is positioned at 3 o’clock. The hat marker band must be placed toward the outer curvature when advancing the valve in the descending aorta. Second, during deployment, the hat marker faces the NCC. Finally, 2 of the radiopaque markers of the Evolut FX valve should be viewed on the left side of the screen and the other marker on the right. B: Accurate Neo2 valve. First, insert the valve with the flush port positioned to 6 o’clock. Second, torque the delivery catheter counterclockwise. Finally, during valve deployment, 2 radiopaque posts should be viewed in the major curve of the aorta and 1 on the other side. Using the classic implantation technique, 1 post should be viewed in the middle of the aortic annulus and the other 2 on each side. CIT, classic implantation technique; COP, cusp overlapping projection.
Commissural alignment in self-expanding valves
Evolut R, Evolut PRO, Evolut PRO+, Evolut FX Medtronic valves
The optimal technique for CA starts with a preprocedural MSCT analysis to select a patient-specific fluoroscopic projection. The most commonly used technique for the Medtronic self-expanding valve begins with the flush port positioned at 3 o’clock. The hat marker band must be placed toward the outer curvature when the valve is advanced in the descending aorta. During deployment, the gantry must be placed in the COP, with the left and right commissures of the THV appearing on the right side of the screen and the hat marker facing the NCC (in some cases, it may face center front). In the newest generation Evolut FX valve, there are 3 markers in the inflow portion of the skirt of the valve, corresponding to each commissure. These marks enhance the fluoroscopic view and are associated with fewer cases of CMA (figure 3).39
ACURATE neo2
The technique for CA in the ACURATE neo2 platform varies. The insertion must be made with the flush port positioned at 6 o’clock. The THV has 3 radiopaque posts that mark each commissure. Correct CA can be ensured with fluoroscopy by torquing the delivery catheter counterclockwise. In the COP, 2 posts should overlap in the major curvature of the aorta and the last post on the lesser curvature. Using the CIT, one post should be viewed in the middle of the aortic annulus and the other 2 on each side (figure 3).40,41
Commissural alignment in balloon-expandable valves
There is very little reliable evidence on the topic, but extended methods exist to obtain CA with balloon-expandable THV. A small study by Santos-Martínez et al.42 evaluated the feasibility of CA with the Myval THV (Meril Life Sciences Pvt. Ltd, India). A preprocedural MSCT-simulated TAVI in a silico model predicted the optimal rotation of the valve for achieving CA using a self-developed script. The Myval devices were then crimped in the rotation predicted by the silico model to avoid CMA. This strategy was tested in 10 patients, with only 4 showing minor CMA and none showing moderate-severe CMA. The mean CMA angle was 16.7°. Although the results are promising, the need for a silico model before the procedure limits the usage of this technique.
CORONARY REACCESS
Recent studies on coronary reaccess after TAVI in patients without CA have shown that the rate of unsuccessful selective coronary reengagement is approximately 7.7%.38 Tarantini et al.43 compared Sapien valves with aligned and nonaligned supra-annular self-expanding valves (Evolut R/PRO and ACURATE Neo). These authors found that only 5% of patients receiving Sapien 3 valves had nonselective coronary access, and no patients had unfeasible coronary access. However, with self-expanding THVs, the group undergoing the nonaligned commissural technique showed a 43% rate of nonselective access, and 11% had unfeasible access. Conversely, in the group with CA, only 3% had unfeasible access, while 26% had nonselective access.43
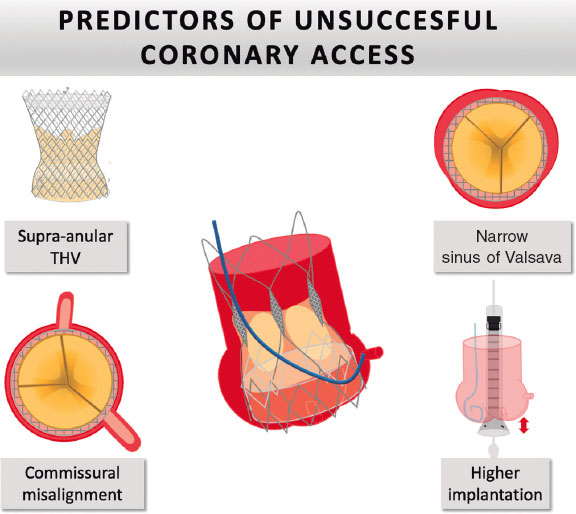
The most frequent predictors of unsuccessful coronary access are patient anatomy (narrow sinus of Valsalva), THV type (self-expanding valves), and TAVI technique (higher ID). Regarding patient anatomy, cusp symmetry and coronary ostial eccentricity are fundamental in predicting the feasibility of CA and potential coronary reaccess.38 Despite the achievement of commissural alignment, some patients show coronary eccentricity or cusp asymmetry, in which commissural alignment does not prevent obstruction of the coronary ostium by the THV post. This is most frequently observed in patients with bicuspid valves. Consequently, the concept of coronary alignment has emerged (figure 4).
Figure 4. Major predictors of impaired coronary reaccess. THV, transcatheter heart valve.
In a study evaluating 1851 computed tomography scans of patients undergoing TAVI evaluation, virtual valves were placed, simulating CA and coronary alignment in the aortic root to evaluate moderate and severe coronary overlap from the THV post. The findings revealed that severe CMA is rare when CA is used and that coronary alignment only improved the right ostium overlap (coronary 0.52% left, 0.52% right; commissural 0.30% left, 3.27% right). The incidence of no overlap with the left coronary ostium was lower in the CA group than in the coronary alignment group. This was due to the higher prevalence of eccentricity of the right coronary ostium; intentional alignment with the right coronary ostium may increase the risk of overlap with the left coronary ostium. The prevalence of coronary asymmetry and eccentricity was low.44
VALVE HEMODYNAMICS: PERFORMANCE AND DURABILITY
Better hemodynamic results are important, as the indications for TAVI are broadened to include low-risk and younger patients. Fuch et al.45 compared surgical aortic valves with TAVI and conducted a computed tomography (CT) study after TAVI. These authors divided the participants into groups based on CA and observed no differences in transvalvular gradients, coronary filling, or PVL. However, they showed a significant increase in central aortic regurgitation.
A retrospective study included 324 patients who underwent random implantation of a balloon-expandable THV. Post-TAVI MSCT was performed to define CMA as deviations of more than 30°. Among these patients, CMA was present in 52.8%. At the 30-day analysis, there were no differences among patients with and without CMA regarding aortic regurgitation rates, transvalvular gradients, or significant residual gradients. Similarly, the incidence of PPMI and long-term clinical outcomes—including death and stroke—did not vary between the 2 groups.46
CMA has been associated with changes in flow patterns and increased leaflet stress, leading to an increased risk of leaflet thrombosis. Consequently, detection of hypo-attenuated leaflet thickening (HALT) in CT studies has gained attention in recent studies, as it is a marker of subclinical leaflet thrombosis and may predict valve durability.39 A case-control study comparing CA in patients with and without HALT (85 patients per group) showed that severe CMA was present in 32% of the patients with HALT and in only 17.2% of those without HALT.47
REDO-TAVI
The indications for TAVI have expanded, particularly in low-risk and younger patients. However, data on TAVI-in-TAVI procedures are scarce. According to the landmark analysis of the EXPLANTORREDO-TAVR registry, 30-day and 1-year mortality were lower in redo-TAVI patients, with no differences in mortality at 4 years. Arguably, SAVR will be reserved for specific situations, such as PVL or unfavorable anatomy for redo-TAVI, whereas TAVI-in-TAVI will grow exponentially in the coming years.48
The main problem with redo-TAVI is the risk of coronary occlusion and the potential difficulty of coronary reaccess after the procedure. Predictive models based on CT studies suggest a higher risk of coronary occlusion in patients without CA. Buzzati et al.49 reported that 10% to 20% of redo-TAVI procedures carry an increased risk of coronary occlusion, and more than 50% have impaired coronary access. Another study using CT data post-TAVI with Evolut and Sapien valves predicted that 45.5% of Evolut patients and 2% of Sapien patients were at risk of coronary obstruction due to sinus sequestration. The risk was predicted based on the distance between the valves and the sinotubular junction.21
Experience with valve-in-valve procedures is derived from THVs implanted within previously placed surgical valves with CA. Conversely, most degenerated THVs were implanted without accounting for CA. Aggressive techniques like BASILICA, which enable leaflet modification to reduce the risk of coronary obstruction, are less effective than those performed in surgical valves.22
CONCLUSIONS
In summary, the role of implant projection in optimizing TAVI can help reduce the most common drawbacks of this procedure. There is abundant evidence supporting the potential benefits of the COP technique in reducing conduction disturbances and PPMI by making a small modification during deployment without increasing the risks compared with the CIT.
The risk of conduction disturbances and PPMI is a significant obstacle after TAVI. Careful MSCT evaluation and preprocedural planning are required to select the correct strategy for each patient. Ultimately, the risk-benefit of a higher ID using the COP technique should be tailored to patient-specific characteristics. The technique should be favored in patients at high risk for PPMI and discouraged in those at high risk of coronary obstruction and a higher burden of coronary disease. However, in most patients, especially when self-expanding valves are used, it should be classified as the standard deployment projection.
FUNDING
None.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this review.
AUTHORS’ CONTRIBUTIONS
Writing the original draft: R. Álvarez Velasco and M. Almendárez. Image acquisition and edition: R. del Valle and A. Alperi. Critical revision: P. Antuña and I. Pascual. Final approval: I. Pascual.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
1. Ferrer-Sistach E, Teis A, Bayés-Genís A, Delgado V. Multimodality imaging in aortic stenosis:new diagnostic and therapeutic frontiers. Rev Esp Cardiol. 2023;76:40-46.
2. D'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people:the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515-3522.
3. Nguyen V, Willner N, Eltchaninoff H, et al. Trends in aortic valve replacement for aortic stenosis:a French nationwide study. Eur Heart J. 2022;43:666-679.
4. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
5. Pinar E, García de Lara J, Hurtado J, et al. Cost-effectiveness analysis of the SAPIEN 3 transcatheter aortic valve implant in patients with symptomatic severe aortic stenosis. Rev Esp Cardiol. 2022;75:325-333.
6. García-Carreño J, Zatarain E, Tamargo M, Elízaga J, Bermejo J, Fernández-Avilés F. Feasibility and safety of early discharge after transcatheter aortic valve implantation. Rev Esp Cardiol. 2023;76:660-663.
7. Freixa X, Jurado-Román A, Cid B, Cruz-González I. Spanish cardiac catheterization and coronary intervention registry. 31st official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2021). Rev Esp Cardiol. 2022;75:1040-1049.
8. Aurigemma C, Giannico MB, Burzotta F, et al. Clinical impact of the extent of jeopardized myocardium in patients undergoing transcatheter aortic valve intervention. Rev Esp Cardiol. 2023;76:157-164.
9. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. New England Journal of Medicine. 2023;389(21):1949-1960.
10. Van Mieghem NM, Deeb GM, Sondergaard L, et al. Self-expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients:5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022;7:1000-1008.
11. Rodés-Cabau J, Ellenbogen KA, Krahn AD, et al. Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement:JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:1086-1106.
12. Breitbart P, Minners J, Hein M, Schröfel H, Neumann FJ, Ruile P. Implantation depth and its influence on complications after TAVI with self-expanding valves. Int J Cardiovasc Imaging. 2021;37:3081-3092.
13. Jilaihawi H, Zhao Z, Du R, et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:1796-1807.
14. Ben-Shoshan J, Alosaimi H, Lauzier PT, et al. Double S-Curve Versus Cusp-Overlap Technique:Defining the Optimal Fluoroscopic Projection for TAVR With a Self-Expanding Device. JACC Cardiovasc Interv. 2021;14:185-194.
15. Tang GHL, Zaid S, Michev I, et al. “Cusp-Overlap”View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:1663-1665.
16. Gada H, Vora A, Siddique S, et al. TCT CONNECT-457 Reduction of Rates of Permanent Pacemaker Implantation With 34-MM Evolut R Using Cusp Overlap Technique. J Am Coll Cardiol. 2020;76(Suppl. S):B196.
17. Pascual I, Almendárez M, Avanzas P, et al. Cusp-overlapping TAVI technique with a self-expanding device optimizes implantation depth and reduces permanent pacemaker requirement. Rev Esp Cardiol. 2022;75:412-420.
18. Pascual I, Hernández-Vaquero D, Alperi A, et al. Permanent Pacemaker Reduction Using Cusp-Overlapping Projection in TAVR:A Propensity Score Analysis. JACC Cardiovasc Interv. 2022;15:150-161.
19. Mendiz OA, NocˇM, Fava CM, et al. Impact of Cusp-Overlap View for TAVR with Self-Expandable Valves on 30-Day Conduction Disturbances. J Interv Cardiol. 2021;9991528.
20. Meier D, Tzimas G, Akodad M, et al. TAVR in TAVR:Where Are We in 2023 for Management of Failed TAVR Valves?Curr Cardiol Rep. 2023;25:1425-1431.
21. Ochiai T, Yamanaka F, Shishido K, et al. Impact of High Implantation of Transcatheter Aortic Valve on Subsequent Conduction Disturbances and Coronary Access. JACC Cardiovasc Interv. 2023;16:1192-1204.
22. Merdler I, Case B, Bhogal S, et al. Early experience with the Evolut FX self-expanding valve vs. Evolut PRO+for patients with aortic stenosis undergoing TAVR. Cardiovasc Revasc Med. 2023;56:1-6.
23. Khera S, Krishnamoorthy P, Sharma SK, et al. Improved Commissural Alignment in TAVR With the Newest Evolut FX Self-Expanding Supra-Annular Valve:First-in-Human Experience. JACC Cardiovasc Interv. 2023;16:498-500.
24. Michel Pompeu S, Van den Eynde J, Jacquemyn X, et al. Cusp-overlap versus coplanar view in transcatheter aortic valve implantation with self-expandable valves:A meta-analysis of comparative studies. Catheter Cardiovasc Interv. 2023;101:639-650.
25. Wienemann H, Maier O, Beyer M, et al. Cusp overlap versus standard three-cusp technique for self-expanding Evolut transcatheter aortic valves. EuroIntervention. 2023;19:E176-E187.
26. Grubb KJ, Gada H, Mittal S, et al. Clinical Impact of Standardized TAVR Technique and Care Pathway:Insights From the Optimize PRO Study. JACC Cardiovasc Interv. 2023;16:558-570.
27. Kim WK, Toggweiler S, Renker M, et al. Comparison of 3-Cusp Coplanar and 2-Cusp Overlap Views for the Implantation of a Self-Expanding Transcatheter Heart Valve. JACC Cardiovasc Interv. 2023;16:1422-1424.
28. Meduri CU, Rück A, Linder R, et al. Commissural Alignment With ACURATE neo2 Valve in an Unselected Population. JACC Cardiovasc Interv. 2023;16:670-677.
29. Asmarats L, Gutiérrez-Alonso L, Nombela-Franco L, et al. Cusp-overlap technique during TAVI using the self-expanding Portico FlexNav system. Rev Esp Cardiol. 2023;76:767-773.
30. Kim WK. The cusp overlap technique for the Portico valve:it works!Rev Esp Cardiol. 2023;76:755-756.
31. Wang X, Wong I, Bajoras V, et al. Impact of implantation technique on conduction disturbances for TAVR with the self-expanding portico/navitor valve. Catheter Cardiovasc Interv. 2023;101:431-441.
32. Sammour Y, Banerjee K, Kumar A, et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circ Cardiovasc Interv. 2021;14:E009407.
33. Stephan T, Krohn-Grimberghe M, von Lindeiner Genannt von Wildau A, et al. Cusp-overlap view reduces conduction disturbances and permanent pacemaker implantation after transcatheter aortic valve replacement even with balloon-expandable and mechanically-expandable heart valves. Front Cardiovasc Med. 2023;10:1269833.
34. Siddique S, Khanal R, Vora AN, Gada H. Transcatheter Aortic Valve Replacement Optimization Strategies:Cusp Overlap, Commissural Alignment, Sizing, and Positioning. US Cardiology Review. 2022;16:10.
35. Barbanti M, Valvo R, Costa G. Predicting neocommissural orientation during TAVI workup. Rev Esp Cardiol. 2022;75:194-195.
36. Tang GHL, Zaid S, Fuchs A, et al. Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR):Impact on Final Valve Orientation and Coronary Artery Overlap. JACC Cardiovasc Interv. 2020;13:1030-1042.
37. Lim Y, Tan KA, Kuntjoro I, Hon JKF, Yip J, Tay E. Coronary Artery Disease in Patients Undergoing Transvalvular Aortic Valve Implantation. Interventional Cardiology:Reviews, Research, Resources. Interv Cardiol. 2022;17:13.
38. Barbanti M, Costa G, Picci A, et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement:The RE-ACCESS Study. JACC Cardiovasc Interv. 2020;13:2542-2555.
39. Tang GHL, Amat-Santos IJ, De Backer O, et al. Rationale, Definitions, Techniques, and Outcomes of Commissural Alignment in TAVR:From the ALIGN-TAVR Consortium. JACC Cardiovasc Interv. 2022;15:1497-1518.
40. Redondo A, Santos-Martínez S, Delgado-Arana R, Baladrón Zorita C, San Román JA, Amat-Santos IJ. Fluoroscopic-based algorithm for commissural alignment assessment after transcatheter aortic valve implantation. Rev Esp Cardiol. 2022;75:185-188.
41. Redondo A, Valencia-Serrano F, Santos-Martínez S, et al. Accurate commissural alignment during ACURATE neo TAVI procedure. Proof of concept. Rev Esp Cardiol. 2022;75:203-212.
42. Santos-Martínez S, Redondo A, González-Bartol E, et al. Feasibility of precise commissural and coronary alignment with balloon-expandable TAVI. Rev Esp Cardiol. 2023;76:19-24.
43. Tarantini G, Nai Fovino L, Scotti A, et al. Coronary Access After Transcatheter Aortic Valve Replacement With Commissural Alignment:The ALIGN-ACCESS Study. Circ Cardiovasc Interv. 2022;15:E011045.
44. Vinayak M, Tang GHL, Li K, et al. Commissural vs Coronary Alignment to Avoid Coronary Overlap With THV-Commissure in TAVR:A CT-Simulation Study. JACC Cardiovasc Interv. 2024;17:715-726.
45. Fuchs A, Kofoed KF, Yoon SH, et al. Commissural Alignment of Bioprosthetic Aortic Valve and Native Aortic Valve Following Surgical and Transcatheter Aortic Valve Replacement and its Impact on Valvular Function and Coronary Filling. JACC Cardiovasc Interv. 2018;11:1733-1743.
46. Raschpichler M, Flint N, Yoon SH, et al. Commissural Alignment After Balloon-Expandable Transcatheter Aortic Valve Replacement Is Associated With Improved Hemodynamic Outcomes. JACC Cardiovasc Interv. 2022;15:1126-1136.
47. Jung S, Ammon F, Smolka S, Moshage M, Marwan M, Achenbach S. Commissural misalignment independently predicts leaflet thrombosis after transcatheter aortic valve implantation. Clin Res Cardiol. 2024;113:29-37.
48. Tang GHL, Zaid S, Kleiman NS, et al. Explant vs Redo-TAVR After Transcatheter Valve Failure:Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc Interv. 2023;16:927-941.
49. Buzzatti N, Montorfano M, Romano V, et al. A computed tomography study of coronary access and coronary obstruction after redo transcatheter aortic valve implantation. EuroIntervention. 2020;16:E1005-E1013.