ABSTRACT
Introduction and objectives: The bidirectional Glenn shunt (BDG) is an essential step in the repair of a physiologically single-ventricle heart. BDG increases pulmonary blood flow, allows growth of the pulmonary arteries, and improves SaO2. The procedure also allows unloading of ventricular volume, thereby improving survival. Our aim was to register all patients who developed collaterals following BDG, document the management methods used, and assess their impact.
Methods: We included 56 patients who underwent BDG procedures at a median age of 2.08 (1-3) years. After BDG, peripheral pulmonary stenting was used in 2 patients. Symptomatic hyperviscosity was present in 10 patients (17.86%), who underwent venesection. BDG was unsuccessful in 2 patients. Venovenous collaterals were observed in 41 patients (73.2%), and aortopulmonary collaterals in 37 (66.1%).
Results: Hematocrit levels were significantly higher in patients with venovenous collaterals (50.00 ± 8.76) than in those without (P = .031). Mean pulmonary artery pressure was also significantly higher in patients with venovenous collaterals (15 [12-18] mmHg; P = .025). One patient had undergone successful closure of venovenous collaterals to epicardial veins and abdominal veins 3 years previously. Seven patients underwent transcatheter closure (TCC) of collaterals. Of these, 4 patients underwent TCC of venovenous collaterals to left and right pulmonary veins; 1 patient underwent closure of an aortopulmonary collateral; 1 patient underwent a failed attempt at venovenous collateral closure that was complicated by an ischemic stroke; and 1 patient had localized extravasation upon separation of the cable. A highly statistically significant increase in SaO2 was observed after TCC of venovenous collaterals (69.83 ± 10.91 vs 82.83 ± 9.87; P = .008).
Conclusions: TCC of collaterals is a technically demanding but effective management strategy following BDG to improve patients’ SaO2 and quality of life. Awareness of possible complications and their effective management is crucial.
Keywords: Venovenous. Aortopulmonary collaterals. Pulmonary vein. Coil embolization. Device embolization. Transcatheter closure.
RESUMEN
Introducción y objetivos: La derivación bidireccional de Glenn (DBG) es un paso esencial en la reparación cardiaca fisiológica del ventrículo único. La DBG aumenta el flujo sanguíneo pulmonar, permite el crecimiento de las arterias pulmonares y mejora la saturación arterial de oxígeno. También permite la descarga del volumen ventricular, mejorando así la supervivencia. El objetivo del estudio fue registrar a todos los pacientes tras DBG que desarrollaron canales colaterales, los métodos de abordaje y su impacto.
Métodos: Se incluyeron 56 pacientes que habían sido tratados con DBG, con una mediana de edad de 2,08 (1-3) años. Se colocó un stent pulmonar periférico tras la DBG a 2 pacientes. De todos ellos, 10 (17,86%) presentaban hiperviscosidad sintomática y se les realizó una flebotomía. La DBG falló en 2 pacientes. Cuarenta y un pacientes (73,2%) tenían colaterales venovenosas y 37 (66,1%) colaterales aortopulmonares.
Resultados: Los pacientes con colaterales venovenosas presentaban valores de hematocrito significativamente mayores (50,00 ± 8,76), desde el punto de vista estadístico, en comparación con los pacientes sin colaterales venosas (p = 0,031). Los pacientes con colaterales venovenosas presentaban una presión arterial pulmonar media significativamente mayor (15 [12-18] mmHg), desde el punto de vista estadístico (p = 0,025). Se llevó a cabo el cierre percutáneo (CP) de las colaterales en 7 pacientes. Uno de ellos tuvo un cierre satisfactorio de las colaterales venovenosas a las venas epicárdicas y abdominales 3 años antes. Cuatro pacientes se sometieron a CP de colaterales venovenosas a venas pulmonares izquierdas y derechas. Se realizó un cierre de una colateral aortopulmonar a 1 paciente. En 1 paciente se falló en un intento de cierre de colaterales venosas que se complicó con un accidente vascular cerebral. Un paciente presentó extravasación localizada al separar el cable. Se produjo un aumento estadísticamente muy significativo de la saturación de oxígeno tras el CP de las colaterales venovenosas (69,83 ± 10,91 frente a 82,83 ± 9,87; p = 0,008).
Conclusiones: El CP de las colaterales es técnicamente exigente, pero es un tratamiento eficaz tras la DBG para mejorar la saturación y la calidad de vida del paciente. Es crucial conocer las posibles complicaciones y su tratamiento eficaz.
Palabras clave: Venovenoso. Colaterales aortopulmonares. Vena pulmonar. Embolización con coils. Embolización del dispositivo. Cierre percutáneo.
Abbreviations
APC: aortopulmonary collateral. BDG: bidirectional Glenn shunt. MBT: modified Blalock-Taussig. TCC: transcatheter closure.
INTRODUCTION
The occurrence of congenital heart disease is between 6 and 13 per 1000 live births.1 In developed countries, prenatal diagnosis is currently used to detect congenital heart disease (CHD) before birth. In developing countries, only a minority of children with CHD are detected and few benefit from surgical treatment, causing a pattern of late presentation accompanied by a high complication rate.2
The term single ventricle is generally used to describe any CHD with 1 functioning ventricle, including double inlet left ventricle, single ventricle, common ventricle, and univentricular atrioventricular (AV) connection. Other lesions, such as hypoplastic left heart syndrome (HLHS), tricuspid atresia, unbalanced AV septal defect, mitral atresia with normal aortic root, and heterotaxy syndromes with 1 functioning ventricle, can also be added to this group.3
The bidirectional Glenn shunt (BDG) and hemi-Fontan are surgical techniques used to create a superior cavopulmonary anastomosis (CPA) in patients with an anatomic or functional single ventricle. Whether it is right or left, the single ventricle must provide blood supply to the higher resistance systemic circulation and the lower resistance pulmonary circulation until surgical correction is undertaken. The BDG procedure or hemi-Fontan helps to eliminate the volume load on the single ventricle and facilitate subsequent Fontan surgery.4
Systemic venous collateral channels in patients with univentricular hearts after CPA or Fontan operations may cause significant systemic desaturation. After CPA, the pressure difference between the superior vena cava (SVC) and inferior vena cava leads to the development of venous connections between the 2 systems to decompress elevated pressure in the SVC system. Venovenous collaterals can emerge at any time following the CPA.5
Evaluation for venous collaterals should be performed routinely in all patients undergoing pre-Glenn and pre-Fontan catheterization. Venovenous collaterals draining below the heart will be separated from the systemic circulation after Fontan completion. These collaterals need not be embolized unless the patient has an interrupted inferior vena cava with the exclusion of the hepatic veins from the Fontan circulation, as in the Kawashima operation. In contrast, venovenous collaterals that drain into pulmonary veins or the atrium will continue to cause cyanosis due to right-to-left shunts and should be embolized.6
Spicer et al.7 reported an 84% incidence of aortopulmonary collaterals (APC) in children undergoing pre-Fontan cardiac catheterizations. APCs developed in the univentricular heart often have extensive communication and commonly involve networks of smaller lacy vessels between larger collaterals. Total closure of the APCs is not feasible in such situations. In addition, extensive embolization of all APCs adds to the total catheterization procedure time and potential complications without further clinical benefit. Bradley et al.8 recommend selective embolization of moderate to large APCs in patients undergoing univentricular heart repair. Furthermore, the pulmonary blood flow supplied by the APCs may be important in cyanotic patients. Closure of APCs in such patients may decrease the systemic saturation to dangerously low levels.
We aimed to register all patients referred to our hospital following BDG from March 2022 to February 2023. We conducted a full assessment of their hemodynamics and collateral channels, including venovenous collaterals to systemic veins or pulmonary veins and APCs. We also explored different management methods and evaluated the impact of this management on patients with single-ventricle physiology.
METHODS
The study included 56 patients who underwent BDG. We excluded critically ill patients. All patients underwent a comprehensive medical history, which included demographic data (current age, sex, weight, height, body surface area), perinatal history, developmental history, history of venesection, history of hospitalization, and surgical data. The surgical data included age at the time of the intervention, date of intervention, and any other surgical procedures performed prior to BDG, such as previous pulmonary artery banding or a modified Blalock-Taussig (MBT) shunt. The history also included information on previous invasive hemodynamic studies, previous transcatheter interventions (such as pulmonary artery stenting or closure of venovenous collaterals), and current medical treatment. The clinical examination consisted of assessing arterial blood pressure, pulse, respiratory rate, and baseline SaO2. We also conducted a local cardiac examination, observation of subcutaneous superficial collaterals on the chest, and examination of thoracic scars. Heart sounds and murmurs, as well as both lungs, were auscultated. Twelve-lead surface electrocardiography was performed to assess heart rate and rhythm, axis, the presence of any conduction disturbances, and arrhythmias. A chest X-ray was performed to assess the cardiac shadow, pulmonary vasculature, and the presence of previous stents, embolization devices, and sutures from previous sternotomy.
A full transthoracic echocardiographic examination was performed to determine cardiac and visceral situs, the location of the cardiac apex, AV and ventriculoarterial connections, the relationship and abnormalities of the great vessels, description of the AV connection as being double inlet, atresia of 1 of the inlets, or a common AV valve, description of the ventriculoarterial connection, and determination of the morphology and systolic function of the dominant ventricle (right, left, or indeterminate).
Available multislice computed tomography data were included to confirm the anatomy, determine systemic and pulmonary venous drainage, evaluate the peripheral pulmonary tree, assess the BDG size and patency, and determine the presence of venovenous collaterals and APC. Routine laboratory investigations were conducted before catheterization, including a complete blood count, international normalized ratio, kidney function tests, and virology.
Invasive cardiac catheterization involved a complete hemodynamic study of patients before Fontan completion and desaturated patients. The procedure was performed under 100% oxygen supplementation. The usual access points were the right femoral artery and right or left subclavian veins. Injection of the BDG was carried out in the posteroanterior (PA) view to assess BDG patency and the size of the pulmonary tree. Innominate vein injection in the PA view was used to assess the presence of venovenous collaterals. Descending aorta injection in the PA view was conducted to determine the presence of APC. Pressures and saturations were recorded from various cardiac chambers.
Percutaneous interventions were performed when indicated, including peripheral pulmonary stenting or closure of venovenous collaterals or APC. Venovenous collaterals were only closed in desaturated patients who were not candidates for Fontan completion (due to impaired ventricular function, severe AV valve regurgitation, or mean pulmonary artery pressure [PAP] > 14 mmHg), after excluding patients with pulmonary hypertension or SVC syndrome. Major APCs were closed in patients with evidence of ventricular volume overload (eg, elevated ventricular end-diastolic pressure), causing back pressure on pulmonary veins and arteries.
Statistical analysis
Qualitative data are expressed as frequencies and percentages and quantitative data as mean ± standard deviation. A standard t-test, 1-way analysis of variance, and independent samples t-test were used. Linear regression and Pearson correlation analysis were used to determine the correlation of variables of interest. The data were analyzed using commercially available software (SPSS version 19.0), and P < .05 was considered statistically significant. All data and materials from the study are available upon request.
RESULTS
Our registry included 56 patients who underwent BDG and were referred to our hospital between March 2022 and February 2023. The median age of the patients was 9.67 (7.42-12.17) years (min-max, 2.25-34.67 years), with 31 male patients (55.4%) and 25 female patients (44.6%).
Thirty patients (53.6%) had only BDG, while 26 patients (46.4%) had undergone other procedures before BDG: pulmonary artery banding in 15 patients (26.8%), MBT shunt in 10 patients (17.9%), and both in 1 patient (1.7%). Of the 10 patients who had MBT shunt operations, 7 (70%) had a right MBT shunt, and 3 (30%) had a left MBT shunt. Three patients (5.4%) underwent surgical septectomy with BDG. One patient (1.8%) underwent permanent pacemaker insertion via redo sternotomy.
Five patients (8.9%) underwent percutaneous interventions: Rashkind balloon atrial septostomy after birth and before in 2 patients (3.6%); peripheral pulmonary stenting after BDG in 2 patients (3.6%), and venovenous collateral closure in 1 patient (1.8%).
The median age at BDG was 2.08 (1-3) years (min-max, 0.42-17 years). A total of 47 patients (83.9%) had a right-sided BDG, 8 patients (14.3%) had bilateral BDG, and only 1 patient (1.8%) had a left-sided BDG.
All patients had intact peripheral pulsations, and 54 patients (96.4%) had clubbing. Only 1 patient (1.8%) had SVC syndrome (figure 1 of the supplementary data), and another patient (1.8%) exhibited surface venous collaterals on the chest and abdomen associated with deep cyanosis shortly after BDG (figure 2 of the supplementary data). Both patients underwent surgical intervention to reverse the BDG procedure. Mean baseline SaO2 was 78.27 ± 8.47%, ranging from 55% to 99%. The hemoglobin indices of the studied patients are presented in Table 1 of the supplementary data.
Table 1. Basic anatomy by transthoracic echocardiography
| Basic anatomy | N | Percentage |
|---|---|---|
| D-TGA | 10 | 17.9 |
| DORV | 16 | 28.6 |
| Tricuspid atresia | 9 | 16.1 |
| Tetralogy of Fallot | 4 | 7.1 |
| Unbalanced CAVC | 3 | 5.4 |
| Pulmonary and tricuspid atresia | 3 | 5.4 |
| DILV | 6 | 9.8 |
| Anatomical single ventricle | 1 | 1.8 |
| DIRV, DORV, DOLV, D-malposed great vessels | 2 | 3.6 |
| CAVC balanced type (Rastelli type A) | 1 | 1.8 |
|
CAVC, common atrioventricular canal; DILV, double inlet left ventricle; DIRV, double inlet right ventricle; D-malposed, double-malposed; DOLV, double outlet left ventricle; DORV, double outlet right ventricle; D-TGA, dextro-transposition of the great arteries. |
||
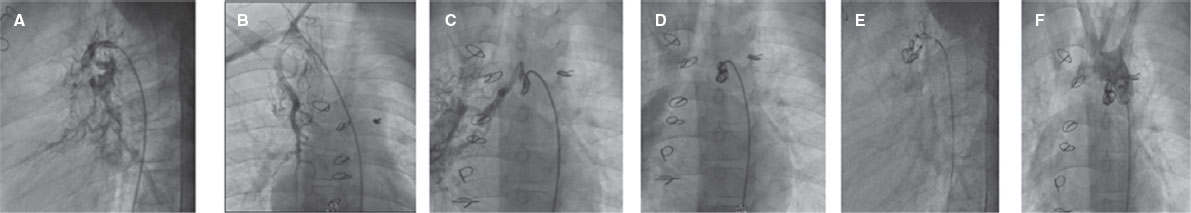
Figure 1. Aortography in lateral and right anterior oblique cranial views showing 2 major aortopulmonary collateral arteries, one from the right internal mammary artery and the other from the posterior part of aortic arch filling both pulmonary arteries. A, B, successful transcatheter closure of aortopulmonary collaterals. C, D, E, closure of aortopulmonary collaterals by 3 coils. F, final injection after aortopulmonary collateral coil closure.
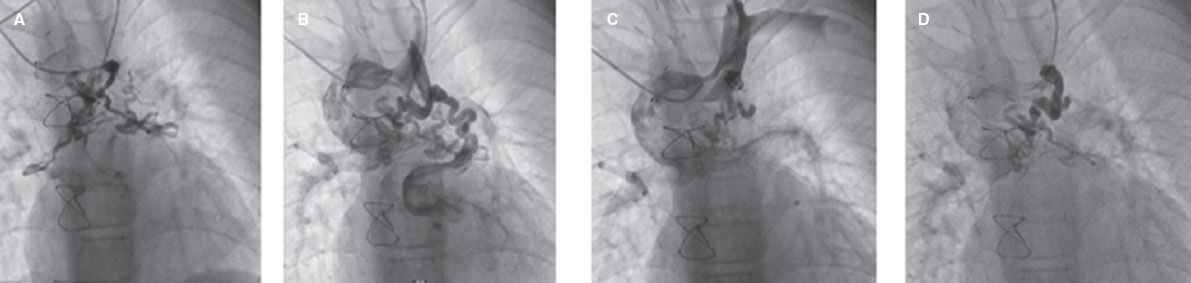
Figure 2. Successful transcatheter closure of venovenous collaterals. A, venovenous collaterals draining into right and left upper pulmonary veins. B, coil closure of the proximal part of the collaterals. C, D, result after transcatheter closure of venovenous collateral by the coil shows residual sluggish flow to collaterals.
Two of the patients (3.6%) were anemic with hemoglobin levels below the normal range for age and sex (1 girl aged 2 years and 3 months with a hemoglobin level of 8 g/dL, and a 13-year-old boy with a hemoglobin level of 11.8 g/dL). Thirty-eight patients (67.9%) had polycythemia with hemoglobin levels ranging from 14.5 to 21.1 g/dL and a mean of 16.92 ± 1.75 g/dL. Additionally, 31 patients (55.4%) had elevated hemoglobin values ranging from 44.8 to 71, with a mean of 54.37 ± 6.43. Ten patients (17.9%) required venesection before cardiac catheterization. Among them, 4 (7.14%) had venesection for the first time, while the other 6 patients (10.7%) had multiple previous venesections due to symptoms of hyperviscosity (eg, fatigue, headache, dyspnea, and visual disturbances). Echocardiographic data are included in Table 1 and Table 2.
Table 2. Echocardiographic data
| Variables | N |
|---|---|
| Morphology of dominant ventricle | |
| Left ventricle | 26 (46.4%) |
| Right ventricle | 27 (48.2%) |
| Undetermined single ventricle | 1 (1.8%) |
| Ventricular systolic function | |
| Preserved | 48 (85.7%) |
| Impaired | 8 (14.3%) |
| Glenn location | |
| Right | 48 (85.7%) |
| Left | 1 (1.8%) |
| Bilateral | 7 (12.5%) |
A total of 30 patients underwent multislice computed tomography. Of these patients, 2 (6.7%) were found to have aneurysmally dilated BDG (figure 3 of the supplementary data).
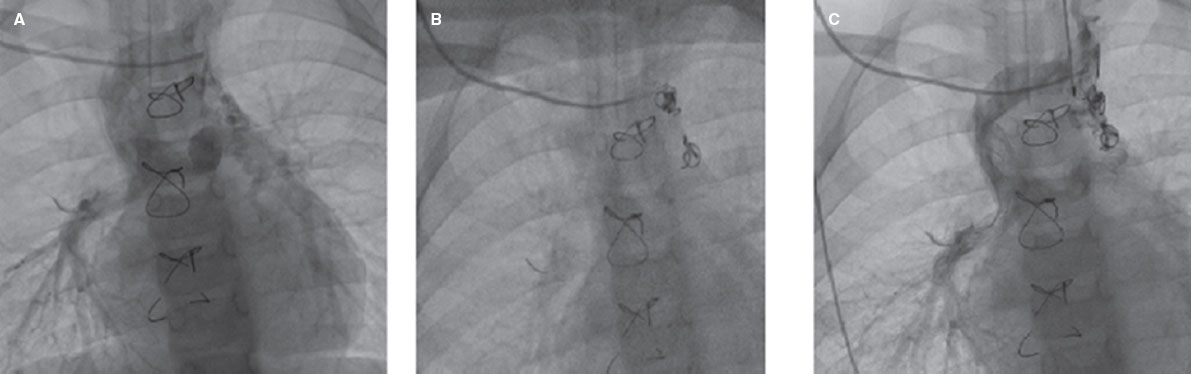
Figure 3. Successful transcatheter closure of a venovenous collateral. A, Glenn shunt and venovenous collateral to the left upper pulmonary vein. B, transcatheter closure of the venovenous collateral by 2 coils. C, final injection after closure of the venovenous collateral with significantly diminished flow to the left upper pulmonary vein.
Three patients experienced significant left pulmonary artery stenosis, which was later successfully treated with transcatheter stenting.
All patients underwent cardiac catheterization, which included invasive assessment of pressures and SaO2 from various cardiac chambers. The results of this assessment can be found in Table 2 of the supplementary data. Venovenous collaterals and APC were comprehensively assessed, and all the data collected are presented in Table 3.
Table 3. Angiographic assessment of collaterals
| Catheterization | N = 56 | |
|---|---|---|
| Aortopulmonary collaterals | ||
| Presence | Yes | 37/56 (66.1%) |
| Number | One | 9/37 (24.3%) |
| Multiple | 28/37 (75.7%) | |
| Size | Small | 28/37 (75.7%) |
| Moderate/large | 9/37 (24.3%) | |
| Origin | Descending aorta | 23/37 (62.2%) |
| Aorta | 11/37 (29.7%) | |
| Left subclavian artery | 0/37 (0.0%) | |
| RIMA and aortic arch | 1/37 (2.7%) | |
| LIMA | 1/37 (2.7%) | |
| Aorta and left subclavian artery | 1/37 (2.7%) | |
| Drainage | Left | 19/37 (51.4%) |
| Right | 7/37 (18.9%) | |
| Both | 11/37 (29.7%) | |
| Venovenous collaterals | ||
| Presence | Yes | 41/56 (73.2%) |
| Number | One | 7/41 (17%) |
| Few | (2-3) 3/41 (7.3%) | |
| Multiple | 31/41 (75.6%) | |
| Size* | Small | 11/41 (26.8%) |
| Moderate/large | 31/41 (75.6%) | |
| Origin** | Left innominate vein | 35/41 (85.4%) |
| Right innominate vein | 1/41 (2.4%) | |
| Subclavian vein | 1/41 (2.4%) | |
| Azygous and hemi-azygous | 1/41 (2.4%) | |
| SVC | 1/41 (2.4%) | |
| Undefined | 2/41 (4.8%) | |
| Drainage*** | Pericardium | 3/41 (7.3%) |
| Epicardial | 14/41 (34%) | |
| IVC | 9/41 (22%) | |
| Coronary sinus | 7/41 (17%) | |
| Abdominal vein | 2/41 (4.8%) | |
| Azygous | 3/41 (7.3%) | |
| Left pulmonary | 5/41 (12.2%) | |
|
IVC, inferior vena cava; LIMA, left internal mammary artery; RIMA, right internal mammary artery; SVC, superior vena cava. * One patient had both small and large venovenous collaterals. ** Two patients had undefined origin of venovenus collaterals. *** Two patients had different draining sites of venovenous collaterals. |
||
Among the 56 patients who underwent catheterization, 52 (92.9%) had collaterals, including venovenous and/or APC. Only 4 patients (7.1%) had no collaterals. Of the total, 19 patients (36.5%) were managed with medical treatment and regular follow-up. Another 20 patients (38.5%) were referred for surgical assessment for Fontan completion. Seven patients (13.5%) underwent TCC of collaterals (Table 4 and figure 1, figure 2, and figure 3). Two patients (3.8%) were scheduled for TCC of collaterals in a subsequent session due to financial obstacles. Additionally, 2 patients underwent unsuccessful attempts to close venovenous collaterals (3.8%), 2 patients (3.8%) were referred for revision of the Glenn shunt due to a failed procedure, and 2 patients (3.8%) were referred for revision of the Glenn shunt and biventricular repair.
Table 4. Details of transcatheter closure of collaterals in 6 patients
| Effect on SO2 | Complications | Access and course | Coil/device | Collateral course | Type of collaterals |
|---|---|---|---|---|---|
| SaO2 increased from 55% before the procedure to 75% after | Localized extravasation | Right subclavian vein- innominate vein- VV collaterals | AGA ADO II 4x6 device | Medium-sized (4 mm) from innominate vein to one of the left pulmonary veins | VV collateral |
| SaO2 increased from 83% before the procedure to 93% after | First attempt failed due to high tortuosity Second attempt was successful | Left internal jugular vein- innominate vein- VV collaterals Right subclavian vein- innominate vein- VV collaterals | 2 AGA ADO I devices (8/6 and 6/4) | Two large collaterals arising from the innominate vein to the left upper pulmonary vein | VV collaterals |
| SaO2 increased from 80% before the procedure to 92% after | No complications | Right subclavian vein- innominate vein- VV collaterals Left internal jugular vein- innominate vein- VV collaterals | Two Cook detachable coils (5/3 and 5/5) | Large tortuous collaterals from innominate vein to left upper pulmonary vein | VV collaterals |
| SaO2 increased from 83% before the procedure to 80% after | No complications | Right femoral artery - aorta- APC | Three Cook detachable coils (6.5/5; 5/5, and 5/3) | Two MAPCAs one from the RIMA and a large one from the posterior part of aortic arch filling both pulmonary arteries | MAPCAs collaterals |
| SaO2 increased from 69% before the procedure to 90% after | No complications | Right subclavian vein- innominate vein -venovenous collaterals Left internal jugular vein- innominate vein- venovenous collaterals to left and right upper pulmonary veins | Cook detachable coil (6.5/5) | From left innominate vein to right upper pulmonary vein | VV collaterals |
| SaO2 increased from 60% before the procedure to 76% after | No complications | Right subclavian vein- Innominate vein VV collaterals Left internal jugular vein- innominate vein VV collaterals | Cook detachable Coil (5x5) | Large VV collateral from innominate vein to paravertebral systemic veins | VV collaterals |
|
ADO, Amplatzer Duct Occlude; APC, aortopulmonary collateral; MAPCAS, major aortopulmonary collaterals; RIMA, right internal mammary artery; SaO2, oxygen saturation; VV, venovenous. |
|||||
Three patients developed complications during TCC of collaterals. The first patient experienced complications due to coil embolization in the innominate vein during the attempt to close venovenous collaterals; however, the coil was successfully snared.
The second patient was an 18-year-old woman who underwent elective TCC of a venovenous collateral using an Amplatzer Duct Occluder II (Abbott, United States). After the device was delivered to the collateral, occluding its proximal portion, difficulties arose in separating the device cable. However, after some manipulations and selective injection at the origin of the collateral, localized extravasation was observed at the proximal site. The catheter was retracted to the innominate vein and reinjected after several minutes, revealing decreased extravasation and successful sealing of the perforation. A follow-up chest X-ray showed no further complications (figure 4).
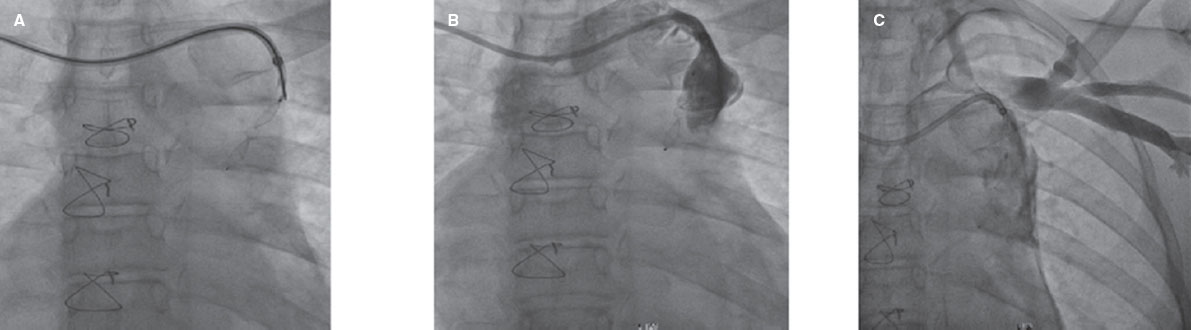
Figure 4. Venovenous collateral angiography in posteroanterior view. A, well-seated Amplatzer Duct Occluder II in venovenous collateral. B, extravasation at the proximal origin of collateral after cable separation. C, injection after several minutes showing sealing of extravasation.
The third patient was a 13-year-old boy who underwent injection of the azygos vein, revealing 2 large right and left venovenous collaterals to the pulmonary veins. Closure of these collaterals was attempted but was unsuccessful. Due to the prolonged procedure, the patient experienced acute left-sided weakness after catheterization. Cerebral magnetic resonance imaging and angiography showed an acute hemorrhagic infarction in the right basal ganglionic and periventricular areas, along with occlusion of the right middle cerebral artery starting from the distal M1 segment, consistent with an embolic event. The patient subsequently underwent mechanical thrombectomy and experienced minimal residual weakness on the left side and dysarthria.
SaO2 significantly increased after TCC of venovenous collaterals (69.83 ± 10.91 vs 82.83 ± 9.87), with a P value of .008 (Table 5).
Table 5. Comparison between SaO2 at baseline and after management among patients who underwent transcatheter closure of venovenous collaterals
| SaO2 (%) | Baseline | After management | Difference | Test value | P | Sig |
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| Mean ± SD | 69.83 ± 10.91 | 82.83 ± 9.87 | 13.00 ± 3.09 | 4.210* | .008 | HS |
| Range | 55-83 | 71-93 | ||||
|
SD, standard deviation; SaO2, oxygen saturation. P value > .05: nonsignificant; P value < .05: significant; P value < .01: highly significant. * Paired t-test. |
||||||
Basal SO2 was lower in patients with venovenous collaterals, although this difference was not statistically significant. Hematocrit levels and mean PAP were significantly higher in patients with venovenous collaterals than in those without venovenous collaterals (P = .031 and P = .025, respectively; Table 3 of the supplementary data).
End-diastolic pressure (EDP) was positively correlated with mean PAP with a P value of .001 (figure 4 of the supplementary data).
Age at BDG procedure was positively correlated with aemoglobin (HCT) and aemoglobin levels (P = .001 and P = .002, respectively), with patients who underwent BDG at older ages showing higher hemoglobin and HCT levels.
Length of time since the BDG procedure was negatively correlated with baseline SO2 (P = .023), with the longer the time since Glenn shunting, the lower the patient’s baseline SO2. In addition, there was a highly significant positive correlation between the length of time since the Glenn procedure and HCT level (P = .002), indicating that the longer the time since Glenn shunting, the higher the patient’s HCT level.
DISCUSSION
Our patients underwent BDG surgery at a median age of 2.08 [IQR, 1-3] years. We found 3 other recent registries conducted in developing countries similar to ours: Azhar et al.9 in Saudi Arabia, Meyer et al.10 in South Africa, and Tariq et al.11 in Pakistan. The median ages of patients in these registries were 10 months, 2.5 years, and 1.9 years, respectively. In older registries, such as those reported by Talwar et al.12 in India, Al-Dairy et al.13 in Iran, and Sen et al.14 in India, the median ages of patients were 3 years, 5 years, and 7.5 years, respectively, indicating a younger age at BDG in recent registries.9-14 In contrast to registries in developing countries, the Western literature reports a median age at BDG of less than 1 year, as reported by Kogon et al.15 in Atlanta (United States), LaPar et al.16 in Virginia (United States), Reddy et al.17 in California (United States), and Shuler et al.18 in Cincinnati (United States).
Ideally, children should undergo BDG between the ages of 6 and 12 months followed by Fontan completion 1 year later.19 The relatively older age of children undergoing the BDG in developing countries may be due to late presentation, delayed diagnosis, lack of primary health care facilities—especially in rural areas—, lack of timely referral, and families’ reluctance to allow their children to undergo the staged surgical correction for financial reasons.20
Regarding post-BDG interventions, 2 patients (3.6%) underwent peripheral pulmonary artery stenting before presenting to our hospital, and we performed left pulmonary artery stenting in 3 patients (5.4%). In contrast to our study, only 1 patient in the study by Yamada et al. 21 underwent right pulmonary artery stenting. The discrepancy in intervention rates between the 2 studies can be explained by the older age of our patients and efforts to reduce their comorbidities to enhance functional capacity and improve their quality of life, especially since most were not suitable for Fontan completion.
The most common basic anatomy included transposition of the great arteries, double outlet right ventricle, and tricuspid atresia. This finding is consistent with those of Naik et al.22 and Sen et al.1 In contrast, Atz et al.23 studied 382 patients in the United States and found that the most common basic anatomy was HLHS (25.6%), followed by tricuspid atresia (18%) and double inlet left ventricle (13%). The absence of HLHS in registries conducted in developing countries may be attributed to its poor prognosis and low survival rate. Among the 56 patients in our study, 41 (73.2%) had venovenous collaterals. McElhinney et al.24 studied 54 patients, with only 18 (33.3%) having venovenous collaterals. The higher percentage in our study may be due to the longer interval from surgery to catheterization, as the median interval from surgery to catheterization was 1.3 years in McElhinney et al.24 and 7.5 years in our study.
In our study, 37 patients (66.1%) had APC. This percentage is similar to that reported by Triedman et al.,25 who diagnosed APC in 65% of patients.
In our study, 6 patients underwent TCC of venovenous collaterals: 5 patients during this registry and 1 patient in 2019. There was a statistically significant increase in SaO2 (%) after the procedure (69.83 ± 10.91 vs 82.83 ± 9.87; P = .008). McElhinney et al.24 also reported successful coil embolization of 10 collateral channels in 6 patients, resulting in an increase in SaO2 of between 9% and 20% (median increase in SaO2 of 16%).
Lu et al.26 reported a cohort study of 9 patients aged 5 to 15 years (median 9 years) with progressive cyanosis after BDG. Successful TCC of the azygos/hemiazygos veins was achieved using coils in 4 patients, patent ductus arteriosus occluders in 3 patients, atrial septal defect occluders in 2 patients, and a patent ductus arteriosus occluder together with coils in 1 patient. Femoral artery SaO2 increased from 81% to 88%.
In our registry, out of the 6 patients who underwent TCC of venovenous collaterals, only 2 had venovenous collaterals to systemic veins (1 had collaterals to epicardial and abdominal veins, and the other to paravertebral veins), whereas the other 4 patients had venovenous collaterals to pulmonary veins.
We also found that 7 out of 8 patients with a pre-existing bilateral SVC connection developed venovenous collaterals. Additionally, 10 out of 11 patients with inadequate PA distribution/PA distortion and 6 out of 8 patients with impaired ventricular systolic function developed venovenous collaterals. However, these findings were not statistically significant. According to Magee et al.,27 venovenous collateral development was associated with an abnormal superior vena cava connection, pulmonary artery distortion, increased mean SVC pressure, increased mean PA pressure, lower right atrium mean pressure, and an increased mean gradient between the SVC and right atrium. Only the last factor was independently associated with collateral development.
McElhinney et al.24 reported that, in patients who developed venous collateral channels, the mean transpulmonary pressure gradient was higher early the BDG procedure (P = .005). This correlation was no longer significant at follow-up, mostly due to decompression of the SVC system through the venous collateral channels. In our patients, no correlation between transpulmonary pressure gradient (SVC-left atrium) and the presence of venovenous collaterals at follow-up.
In our center, we avoid closure of venovenous collaterals in patients with marked elevation in PAP (pulmonary hypertension) as it is a contraindication; venovenous collaterals decompress the congested venous system. However, in patients with normal PAP, or mean PAP ranging from 14 to 20 mmHg (no pulmonary hypertension, who are nevertheless not good candidates for Fontan completion) plus desaturation, we proceed with venovenous collateral closure.
In the present study, 37 of the 56 patients (66.1%) were found to have APCs. One patient underwent successful TCC of aortopulmonary collaterals. Patients with these collaterals were younger at the time of the Glenn procedure and time since the operation was longer. This finding is consistent with that of Grosse-Wortmann et al.,28 who measured APC blood flow noninvasively in BDG and Fontan patients using MRI. These authors found that a higher Qp/Qs ratio was associated with younger age at the time of CPA and concluded that patients who proceeded to CPA at a younger age were more likely to develop APCs.
We found a highly significant positive correlation between EDP and mean PAP (P value of .001). Consistent with our findings, Schwartz et al.29 reported that, at pre-Fontan catheterization, high mean PAP was associated with high single-ventricle EDP. These authors suggested that this association underscores the significance of EDP in individuals with single-ventricle heart disease. They also noted that EDP plays a major role in determining pulmonary artery and central venous pressures, elevations of which are linked to increased morbidity.
CONCLUSIONS
TCC of collaterals is a technically demanding but effective post-BDG management strategy to improve SaO2 and quality of life. Awareness of potential complications and their effective management is essential.
FUNDING
None.
ETHICAL CONSIDERATIONS
This study was approved by the research ethics committee of the faculty of medicine of Ain Shams University (FMASU MS 507/2022). Verbal and written informed consent (for study participation, tests, and publication—including photographs—) was obtained from participants aged 18 years and older or from the participant’s guardian in patients aged less than 18 years after the aim of the study was explained to them. Patient images have been adapted as much as possible to preserve privacy while retaining sufficient information to illustrate research. Our research was carried out in accordance with internationally accepted recommendations for clinical investigation (Declaration of Helsinki of the World Medical Association). Possible sex/gender biases have been considered in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
Y.A. Ali collected data, revised the statistical analysis, and drafted the manuscript. N. El-Sayed Nour El Deen collected data and performed the statistical analysis. G.S. Elshahed supervised data collection, revised the results, and edited the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
WHAT IS KNOWN ABOUT THE TOPIC?
- Closure of venous collaterals to pulmonary veins improves SaO2.
WHAT DOES THIS STUDY ADD?
- TCC of collaterals post-BDG is technically demanding.
- Awareness of possible complications and their effective management is essential.
REFERENCES
1. Liu S, Joseph KS, Lisonkova S, et al. Association between maternal chronic conditions and congenital heart defects:a population-based cohort study. Circulation. 2013;128:583-589.
2. Mocumbi AO, Lameira E, Yaksh A, et al. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol. 2011;148:285-288.
3. Rao PS. Single ventricle A comprehensive review. Children. 2021;8:441.
4. Salik I, Mehta B, Ambati S. Bidirectional Glenn procedure or hemi-Fontan [Internet], Treasure Island (FL):StatPearls Publishing;2022. Available at:https://www.ncbi.nlm.nih.gov/books/NBK563299/. Accessed 17 Jun 2024.
5. Dilawar M, Gottliebson WM, Bradley SM, et al. Rapid development of a large systemic-to-pulmonary vein fistula after bidirectional Glenn shunt and successful closure with an Amplatzer duct occluder. Circulation. 2001;104:E41-E42.
6. Tomita H, Ishikawa Y, Hasegawa S, et al. Use of a 0.052“Gianturco Coil to embolize a persistent right superior vena cava following extracardiac total cavopulmonary connection. Catheter Cardiovasc Interv. 2001;52:481-483.
7. Spicer RL, Uzark KC, Moore JW, et al. Aortopulmonary collateral vessels and prolonged pleural effusions after modified Fontan procedures. Am Heart J. 1996;131:1164-1168.
8. Bradley SC, McCall MM, Sistino JJ, et al. Aortopulmonary collateral flow in the Fontan patient:Does it matter?Ann Thorac Surg. 2001;72:408-815.
9. Azhar A, Eid R, Elakaby A. al. Outcomes of bidirectional Glenn surgery done without prior cardiac catheterization. Egypt Heart J. 2022;74:57.
10. Meyer HM, Marange-Chikuni D, Zühlke L, et al. Outcomes After Bidirectional Glenn Shunt in a Tertiary-Care Pediatric Hospital in South Africa. J Cardiothorac Vasc Anesth. 2022;36:1573-1581.
11. Tariq M, Zahid I, Hashmi S, et al. The Glenn procedure:Clinical outcomes in patients with congenital heart disease in Pakistan. Ann Card Anaesth. 2021;24:30-35.
12. Talwar S, Sandup T, Gupta S, et al. Factors determining early outcomes after the bidirectional superior cavopulmonary anastomosis. Indian J Thorac Cardiovasc Surg. 2018;34:457-467.
13. Al-Dairy A, Dehaki MG, Omrani G, et al. The Outcomes of Superior Cavopulmonary Connection Operation:a Single Center Experience. Braz J Cardiovasc Surg. 2017;32:503-507.
14. Sen S, Bandyopadhyay B, Eriksson P, et al. Functional capacity following univentricular repair--midterm outcome. Congenit Heart Dis. 2012;7:423-432.
15. Kogon BE, Plattner C, Leong T, et al. The bidirectional Glenn operation:a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237-1242.
16. LaPar DJ, Mery CM, Peeler BB, et al. Short and long-term outcomes for bidirectional Glenn procedure performed with and without cardiopulmonary bypass. Ann Thorac Surg. 2012;94:164-171.
17. Reddy VM, McElhinney DB, Moore P, et al. Outcomes after bidirectional cavopulmonary shunt in infants less than 6 months old. J Am Coll Cardiol. 1997;29:1365-1370.
18. Shuler JM, Statile C, Heydarian H, et al. Surgical Timing and Outcomes of Unilateral Versus Bilateral Superior Cavopulmonary Anastomosis:An Analysis of Pediatric Heart Network Public Databases. Pediatr Cardiol. 2021;42:662-667.
19. Silvilairat S, Cabalka AK, Cetta F, et al. Protein-losing enteropathy after the Fontan operation:Associations and predictors of clinical outcome. Congenit Heart Dis. 2008;3:262-268.
20. Tewfik M, El-Sayed M, Roushdy A, et al. The Glenn Shunt Revisited, A Single Center Registry in Ain Shams University Cardiology Department. Congenit Heart Dis. 2022;17:71-85.
21. Yamada K, Roques X, Elia N, et al. The short- and mid-term results of bidirectional cavopulmonary shunt with additional source of pulmonary blood flow as definitive palliation for the functional single ventricular heart. Eur J Cardiothorac Surg. 2000;18:683-689.
22. Naik RB, Srivastava CP, Arsiwala S, et al. Early outcomes after the on pump bidirectional Glenn procedure:A single center experience. J Card Surg. 2021;36:3207-14.
23. Atz AM, Travison TG, McCrindle BW, et al. Cardiac performance and quality of life in patients who have undergone the Fontan procedure with and without prior superior cavopulmonary connection. Cardiol Young. 2013;23:335-343.
24. McElhinney DB, Reddy VM, Hanley FL, et al. Systemic venous collateral channels causing desaturation after bidirectional cavopulmonary anastomosis:Evaluation and management. J Am Coll Cardiol. 1997;30:817-824.
25. Triedman JK, Bridges ND, Mayer JE, et al. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207-15.
26. Lu M, Wu W, Zhang G, et al. Transcatheter occlusion of azygos/hemiazygos vein in patients with systemic venous collateral development after the bidirectional Glenn procedure. Cardiology. 2014;128:293-300.
27. Magee AG, McCrindle BW, Mawson J, et al. Systemic venous collateral development after the bidirectional cavopulmonary anastomosis:prevalence and predictors. J Am Coll Cardiol. 1998;32:502 508.
28. Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion:quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219-25.
29. Schwartz MC, Brock MA, Nykanen D. al. Risk factors for an elevated ventricular end-diastolic pressure prior to the Fontan operation. Pediatr Cardiol. 2018;39:315-323.










