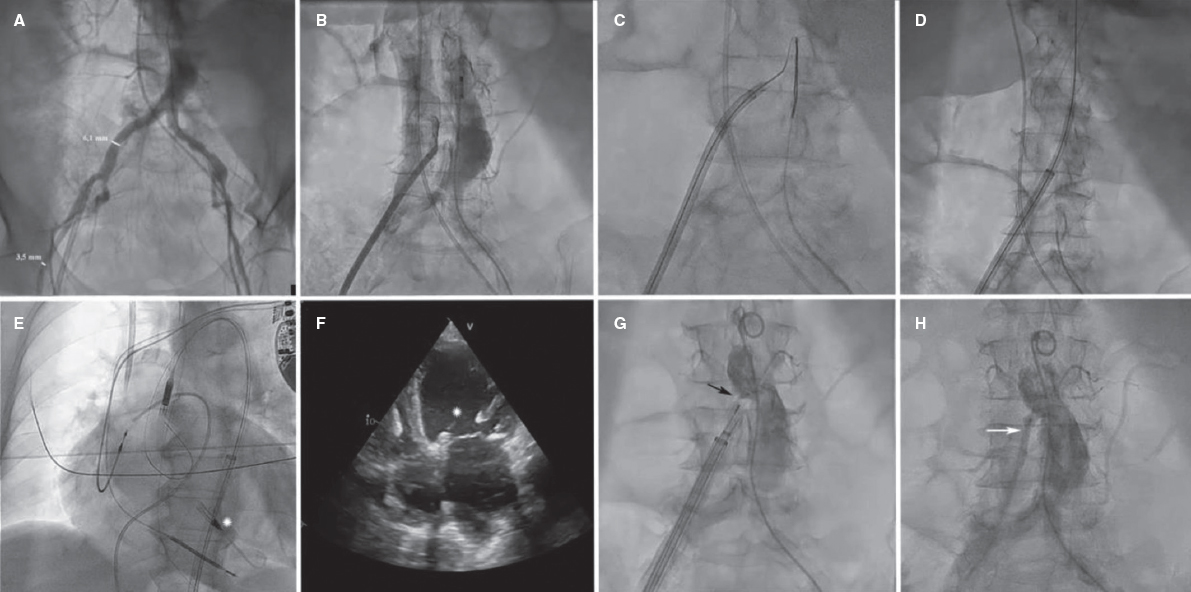
We present 2 cases which required transcaval access for mechanical circulatory support device implantation.
Case #1 is a 65-year-old woman with ischemic dilated cardiomyopathy and severe ventricular dysfunction who was admitted due to cardiogenic shock (SCAI D, SOFA 15, vasoactive-inotropic score 55, and lactate levels of 5.7 mg/dL). An Impella-CP device (Abiomed, United States) was implanted via transcaval access due to the lack of femoral (figure 1A) and subclavian access. To identify the appropriate projection angle for incision, 2 pigtail catheters were overlapped in the aorta and vena cava. The incision was performed through electrification of a heavyweight guidewire that eventually crossed the aorta (figure 1B,C, and video 1 of the supplementary data). The guidewire was exchanged for a 0.35 mm extra-stiff guidewire, and a 16-Fr × 65 cm GORE DrySeal introducer sheath was advanced (Gore, United States) (figure 1D and video 1 of the supplementary data), on which the Impella device was implanted (figure 1E,F; asterisk: Impella inlet). The Impella device was removed 7 days later, and the transcaval incision was closed (figure 1G, arrow, and video 2 of the supplementary data) leaving an untreated minimal residual leak behind (figure 1H, arrow), which was not visualized on follow-up angiography performed 3 weeks later.
Figure 1.
Case #2 is a 59-year-old man with peripheral vascular disease who was admitted due to Killip IV myocardial infarction with left main coronary artery occlusion. Despite revascularization and use of an intra-aortic balloon pump, the patient remained in shock (SCAI E, SOFA 18, vasoactive-inotropic score 170, and lactate levels of 15.7 mg/dL). Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) was implanted using the technique described above (video 3 of the supplementary data) with a 17-Fr × 55 mm arterial cannula that was inserted directly after dilatation. VA-ECMO was removed 5 days later with good patient progress (video 4 of the supplementary data). There were no complications associated with either one of the 2 procedures.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study was approved by Hospital Universitari Vall d’Hebron research ethics committee. The corresponding informed consent forms were obtained from both patients for publication.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
All the authors participated in the design, drafting, and review of this manuscript.
CONFLICTS OF INTEREST
None declared.
SUPPLEMENTARY DATA
Vídeo 1. Uribarri A. DOI: 10.24875/RECICE.M23000431
Vídeo 2. Uribarri A. DOI: 10.24875/RECICE.M23000431
Vídeo 3. Uribarri A. DOI: 10.24875/RECICE.M23000431
Vídeo 4. Uribarri A. DOI: 10.24875/RECICE.M23000431