ABSTRACT
All cardiologists should delve into history to understand the current state of the art of their specialty. In the last century, the coronary stent was a pivotal achievement of research and biotechnological engineering. Since then, technology has advanced, and substantial improvements have been incorporated into this device, which has become the gold standard for treating coronary artery disease. This article summarizes the history of the coronary stent from its inception to the present day. The document reviews key historical and scientific milestones that have contributed to making percutaneous angioplasty a safe and highly effective procedure due to coronary stents. The evolution of the stent has been closely linked to the growth and maturation of interventional cardiology to date.
Keywords: Stent. Drug-eluting stent. Percutaneous transluminal coronary angioplasty.
RESUMEN
Todo cardiólogo debe realizar un viaje atrás en la historia para entender el estado actual de su especialidad. El stent coronario es uno de los logros más importantes de la investigación y de la ingeniería biomédica del último siglo. Su tecnología ha ido evolucionando e incorporando mejoras sustanciales que hoy en día hacen de este dispositivo un estándar de gran calidad para el tratamiento de la enfermedad coronaria. En este artículo se resume la historia del stent coronario desde su génesis hasta el presente. Se repasan los hitos históricos y científicos más remarcables que contribuyeron a hacer de la angioplastia percutánea un procedimiento seguro y altamente efectivo gracias al stent coronario. La evolución del stent ha ido de la mano del crecimiento y la maduración de la cardiología intervencionista.
Palabras clave: Stent. Stent liberador de fármaco. Angioplastia coronaria transluminal percutánea.
BEGINNINGS AND DEVELOPMENT OF CORONARY ANGIOPLASTY (1970s AND 1980s)
Spectacular advances have been made in interventional cardiology over the past decades, hand in hand with biotechnological progress. The development of coronary stents has been pivotal by enabling the reliable establishment and expansion of percutaneous angioplasty. Stents were introduced to address the issues posed by plain old balloon angioplasty, which became evident in its early stages. Therefore, it is important to reflect on how it all started (table 1)1.
Table 1. Milestones in the development of interventional cardiology
| Year | Milestone |
|---|---|
| 1929 | Werner Forssmann performs the first transluminal cardiac catheterization |
| 1953 | Sven Seldinger introduces percutaneous access |
| 1958 | Mason Sones performs the first coronary angiography (via surgical brachial access) |
| 1963 | Charles Dotter performs the first peripheral angioplasty |
| 1968 | Eberhard Zeitler expands peripheral angioplasty across Europe |
| 1968 | Melvin Judkins develops the percutaneous coronary angiography technique |
| 1977 | Andreas Grüntzig performs the first percutaneous coronary balloon angioplasty |
| 1979 | Geoffrey Hartzler performs the first coronary angioplasty in acute myocardial infarction |
| 1986 | Jacques Puel implants the first coronary stent (Wallstent) |
| 1991 | Cannon and Roubin report the first stent implantation in acute myocardial infarction |
| 1994 | Regulatory approval of the first scientifically evidence-based stent (Palmaz-Schatz) |
In the early 1970s, the treatment of coronary artery disease was limited to the use of nitroglycerin and propranolol, with few diagnostic tests and very little scientific evidence. However, some important milestones had already been achieved, setting the stage for the significant advancement of percutaneous treatment.1 Coronary angiography was rarely indicated, being restricted to patients with severe symptoms and in anticipation of possible treatment with coronary artery bypass graft surgery, which was the only revascularization modality available at the time. Andreas Roland Grüntzig, a German radiologist and cardiologist who worked in Zurich, Switzerland and later in Atlanta, United States, was a figure of exceptional ability and perseverance who pioneered the balloon angioplasty technique, overcoming the prevailing scepticism and opposition in his field. Having inherited the legacy of peripheral angioplasty from Charles Dotter through Eberhard Zeitler, Grüntzig developed the angioplasty balloon. He initially applied the technique to peripheral artery disease in 1974 and then boldly expanded its use to treat the human coronary tree on September 16th, 1977.2
After the initial clinical success, the limitations of balloon angioplasty began to emerge, especially as it was applied in different clinical and anatomical scenarios. Concerns included acute occlusion due to elastic recoil, dissection, and thrombosis. These issues resulted in perioperative infarctions, the need for cardiac surgery, or repeat angioplasty during follow-up.3 Restenosis was a delayed phenomenon but its high incidence (20%-40%) also posed challenges.4 To prevent elastic recoil and occlusive dissections, the radial force exerted by the angioplasty balloon needed to be maintained with an intraluminal prosthesis.
CREATION AND APPROVAL OF CORONARY STENTS (1980-1994)
The origin of the term stent (recognized by the Royal Spanish Academy)5 is unknown but is widely believed to be named after the British dentist Charles Thomas Stent (1807-1885). In 1856, Stent patented a thermoplastic material for making dental impressions, which he named “Stent’s paste”.6 After the patented paste fell out of use, the term continued to be used for any prosthetic material that could replace biological tissue. Its use expanded to include tubular prostheses used in hepatobiliary and urology surgery.6 Charles Dotter—also a pioneer in this field—reported his experience of inserting metal coils into dog arteries for the first time in 1969 to demonstrate the feasibility of implanting an intraluminal containment device.7 However, it was not until the 1980s, after the limitations of balloon angioplasty became evident, that the term stent gained broader usage. During this time, significant emphasis was placed on developing the technology used today.
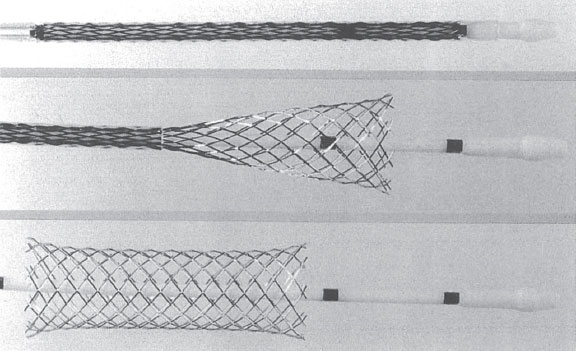
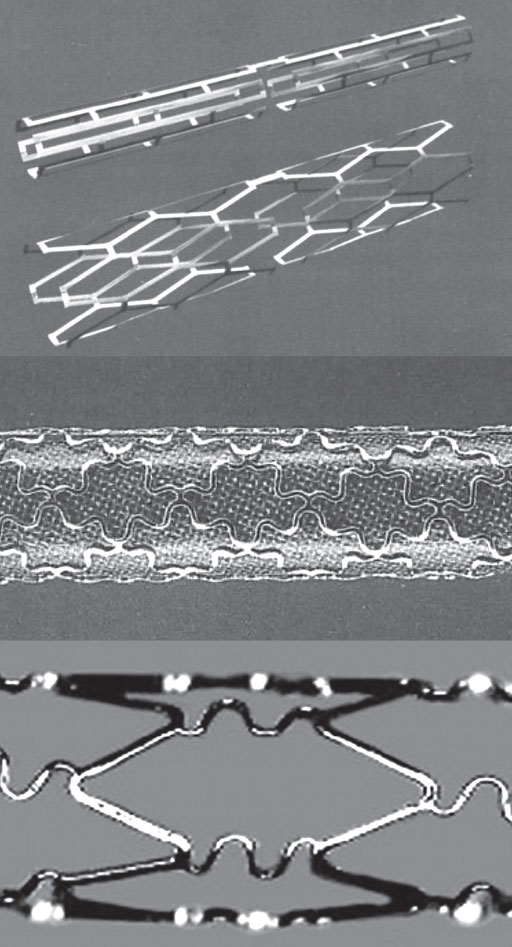
In 1980, a meeting in Switzerland between 2 expatriate Swedes, Åke Senning, a cardiothoracic surgeon who had been a supporter of Andreas Grüntzig, and engineer Hans Wallsten, marked the beginning of a successful project. Along with French engineer Christian Imbert, they eventually developed the first stent for use in coronary arteries: the Wallstent. The term was not an eponym of the engineer but derived from implanting a prosthesis (stent) into the vessel wall.1 The The Wallstent consisted of a self-expandable mesh of stainless steel wire released by a delivery system (figure 1). They founded the company MedInvent (later acquired by Schneider, Switzerland), sought researchers to test the device, and contacted Ulrich Sigwart (Lausanne) and Jacques Puel (Toulouse).1
Figure 1. Self-expanding Wallstent. Stent deployment process demonstrating significant longitudinal shortening (shown from top to bottom).
The experimental protocol for the Wallstent initially involved use in animals, followed by application in human peripheral arteries, and finally in the coronary arteries of patients. The Toulouse center encountered fewer difficulties in initiating animal experimentation and reached human trials sooner. Thus, in December 1985, Hervé Rousseau and Francis Joffre, both radiologists from Jacques Puel’s department in Toulouse, France, implanted the first peripheral stent-graft. In March 1986, Jacques Puel implanted the first coronary stent-graft in a patient who developed restenosis after balloon angioplasty in the left anterior descending coronary artery.1 Meanwhile, in June 1986, Ulrich Sigwart implanted the first coronary stent-graft to treat an acute occlusive dissection in a proximal left anterior descending coronary artery following balloon angioplasty. This was the first time a patient avoided emergency surgery for this complication.1,8
Later, Sigwart became a spokesperson in the public arena and in publications, perhaps aided by his better command of the English language.1 In March 1987, the first report of the joint experience was published in The New England Journal of Medicine.9 The article reported the implantation of 24 coronary stents in 19 patients to treat restenosis (n = 17), acute occlusion following balloon angioplasty (n = 4), or deterioration of coronary artery bypass grafts (n = 3). Years later, Sigwart recounted that the journal requested he avoid the verb stenting and instead use the noun stent to refer to the new device.10 The initial multicenter experiences with the Wallstent were led by centers in Toulouse, Lausanne, and Rotterdam. In 1991, Serruys et al.11 described the follow-up of the first 105 treated patients: the mortality rate was 7.6%; the incidence of occlusion was 24% (mostly within the first 2 weeks), and the rate of restenosis was between 14% and 32% (depending on the definition).
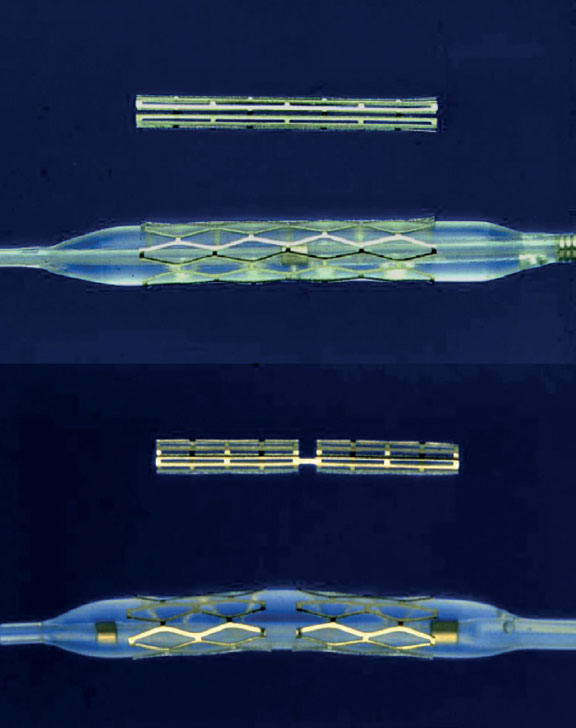
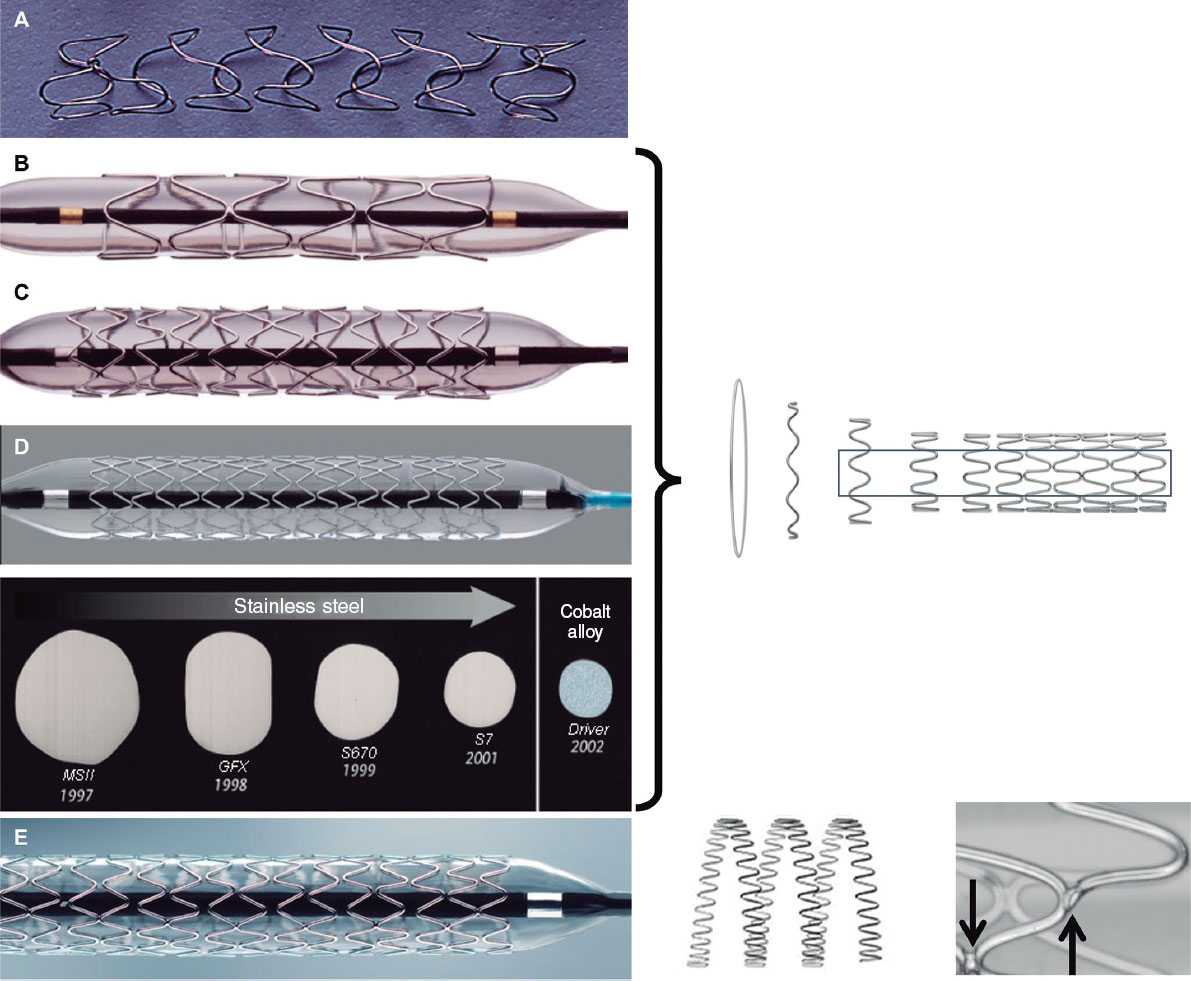
At the same time, across the ocean, Julio Palmaz, an Argentine interventional radiologist based in the United States, attended Grüntzig’s live sessions in 1977. Witnessing the complications of angioplasty, he spotted the opportunity to develop a device for their prevention. He designed his first prototype in his kitchen using copper wire and a soldering iron. He later used stainless steel and invented the first balloon-expandable stent, which he implanted in dog aortas.1,12 Palmaz subsequently relocated to San Antonio (Texas, United States), where he refined the device using cutting machines on steel tubes.13 In the United States, he met Richard Schatz, a military cardiologist who assisted him in adapting the model for use in coronary arteries by connecting 2 small stents with a bridge, thereby enhancing the flexibility and navigability of the entire system (figure 2). After securing the necessary investment, they founded Expandable Grafts Partnership (later acquired by Johnson & Johnson, United States) to manufacture the prototypes and fund further research.12
Figure 2. Balloon-expandable Palmaz-Schatz stent (top) and PS 153 series (bottom), consisting of 3 shorter stents connected by a bridge to enhance flexibility and navigation.
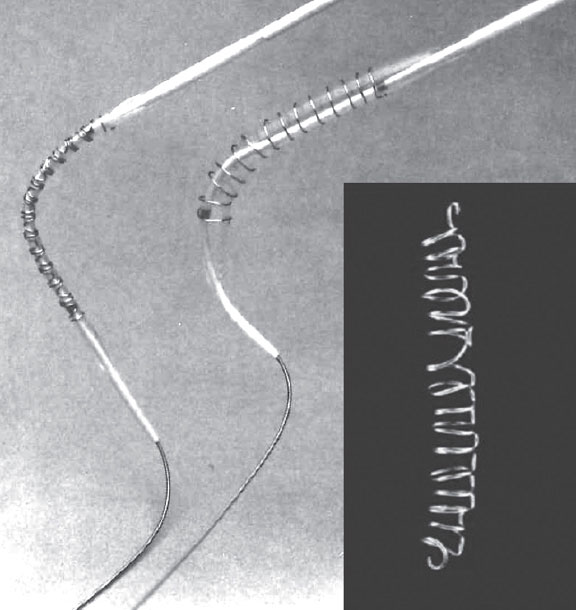
Due to research restrictions in the United States, the first human trials were conducted abroad.1,12 In October 1987, Julio Palmaz and Goetz Richter implanted the first Palmaz-Schatz stent in peripheral arteries in Freiburg, Germany. Later that year, Palmaz, Schatz, and. Eduardo Sousa implanted the first Palmaz-Schatz stent in coronary arteries in Sao Paulo, Brazil (21 months after the first coronary Wallstent). Unfortunately for Julio Palmaz, the milestone of the first balloon-expandable stent implantation had been achieved 3 months earlier by Gary Roubin and Spencer King III at Emory University, Atlanta, Georgia, United States. Their device was a metal wire structure coiled around a balloon (figure 3) invented by the Italian radiologist Cesare Gianturco, who had previously worked with Grüntzig.
Figure 3. Balloon-expandable Gianturco-Roubin stent, featuring a helical coil formation.
The US Food and Drug Administration approved the Gianturco- Roubin stent (Cook Medical Inc., United States) in 1993, but not the Palmaz-Schatz, which required2 randomized clinical trials. These trials were completed and published in 1994, leading to Food and Drug Administration approval.1,12,14,15 Here we review these 2 landmark trials that scientifically validated the use of the stent in cardiology.
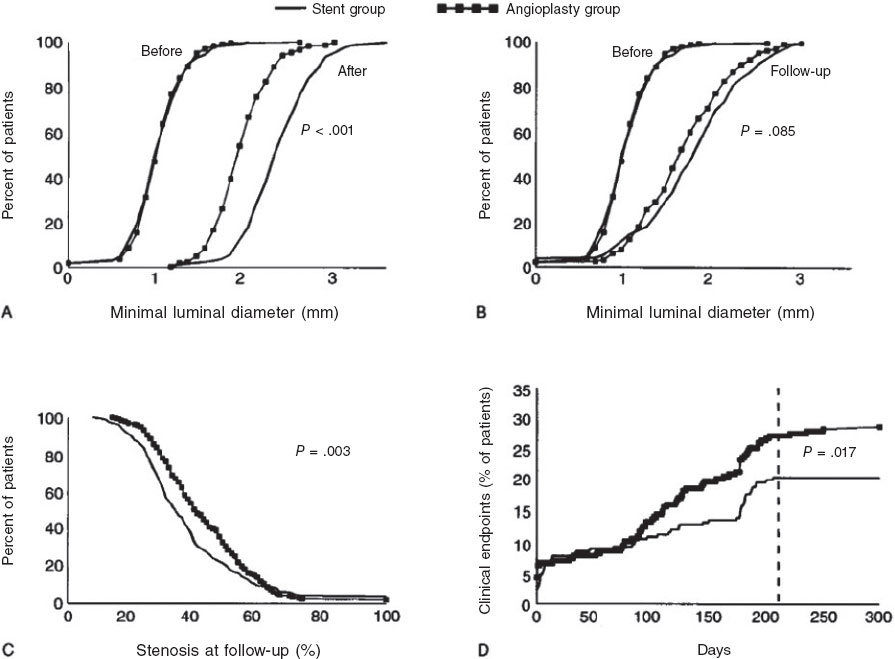
The Belgian Netherlands Stent (BENESTENT) trial, presented by Serruys et al.15 in 1994, randomized 520 patients with stable angina and single-vessel coronary artery disease to undergo balloon angioplasty or Palmaz-Schatz stent implantation. The trial included 28 centers, mostly in Europe. All patients received aspirin for 6 months, and those who underwent stent implantation also received warfarin for 3 months. At 7 months, stent treatment decreased the composite rate for adverse events by 32%, primarily due to a lower need for repeat revascularization. The rate of binary restenosis (≥ 50%) was 22% in the stent group vs 32% in the balloon group (figure 4). Stent thrombosis occurred in 3.5% of the patients. Stent-treated patients experienced more vascular and hemorrhagic complications and longer hospital stay. At 1-year follow-up, the relative reduction in combined events remained at 26% in favor of the stent, with an incidence of repeat angioplasty of 10% vs 21% in the balloon group16.
Figure 4. Graphs from the BENESTENT study illustrating acute luminal gain (A) and follow-up (B). Frequency distribution of restenosis (C) and cumulative clinical events (D). (Reproduced with permission from Serruys et al.15).
The Stent Restensosis Study (STRESS), reported by Fischman et al.14 the same year, randomized 410 patients from 20 centers, mostly in North America. The antithrombotic regimen included indefinite aspirin for all patients and a 1-month regimen of warfarin for those receiving the Palmaz-Schatz stent. At 6 months, the incidence of combined adverse events was similar (19.5% in the stent group vs 23.8% in the balloon group; P = .16), but there was a trend toward a lower need for repeat revascularization in the stent group (10.2% vs 15.4%; P = .06). The rate of binary restenosis was also lower in stent-treated patients (32% vs 42%; P < .05). Stent thrombosis occurred in 3.4% (7/205) of the patients treated per protocol and in 21.4% (3/14) of those who underwent stent implantation as a bailout therapy for angioplasty (crossover). Again, vascular and hemorrhagic complications, and the length of stay were more significant in stent-treated patients. At nearly 1 year of follow-up, numerical differences favored the stent, although they were not statistically significant (unplanned revascularization: 12% vs 17%; P = .09).17
Finally, despite the obstacles and delays, the Palmaz-Schatz stent became the gold standard for a time due to the supporting evidence, its greater safety profile, and its ease of use. Other stents, despite their significant initial expansion, lacked study support and concerns remained about the incidence of thrombosis and restenosis. In terms of these complications, the Gianturco-Roubin stent proved inferior to the Palmaz-Schatz stent,18 while the Wallstent showed issues of longitudinal shortening, implantation imprecision, and side branch compromise due to its small cell size. Because of these factors, these stents were gradually phased out and eventually disappeared from the market.
Starting in 1994, the use of stents expanded due to the BENESTENT and STRESS trials. However, doubts remained about whether the costs associated with this new intervention would translate into significant benefits. Several subsequent studies convinced the medical community of the superiority of stents over simple balloon angioplasty in various scenarios. Two landmark studies showed clear benefits in reducing restenosis rates: one in chronic occlusions (32% vs 74%; P > .001) in 199619 and another in isolated disease of the proximal left anterior descending coronary artery (19% vs 40% at 12 months; P = .02) in 1997.20 In addition, the strategy of stent angioplasty vs balloon angioplasty with the possibility of bailout stenting favored the first-line use of stents for their clinical benefits and cost-effectiveness.21 In acute myocardial infarction, the Stent-PAMI trial established the indication for the use of stents over balloon angioplasty.22
PROGRESS AND PLATFORM MODERNIZATION (1990S)
During the 1990s, several important advancements enhanced the safety and efficacy of stents (table 2).1 These included the use of intravascular ultrasound to optimize implantation, advances in hemostasis, and the expansion of radial access. In addition, the shift from anticoagulation to dual antiplatelet therapy reduced the hemorrhagic complications observed in the BENESTENT and STRESS trials.14,15 Last but not least, technological improvements in stent platforms were key to making this treatment more widespread.
Table 2. Advances in angioplasty in the 1990s
| Years | Advances | Resultados |
|---|---|---|
| 1989-1993 | Radial access for coronary angiography and coronary angioplasty | Beginning of a new era in minimally invasive arterial access |
| 1993-1994 | Reduction in access gauge down to 6-Fr Femoral hemostatic closures | Fewer hospitalizations and hemorrhagic complications |
| 1994 | Publication of the BENESTENT15 and STRESS14 trials | The stent demonstrates its effectiveness in angioplasty |
| The Palmaz-Schatz stent is established as the gold standard | ||
| Use of intravascular ultrasound to optimize stent implantation | Adequate expansion due to high implant pressures led to minimal thrombosis and reduced restenosis | |
| 1995-1998 | Studies on dual antiplatelet therapy | Minimization of thrombosis |
| Discontinuation of oral anticoagulation | ||
| Less bleeding | ||
| 1994-2000 | Enhancements to the Palmaz-Schatz (Cordis, United States) and emergence of new modern platforms: Micro Stent (Arterial Vascular Engineering, United States), Multi-Link (Advanced Cardiovascular Systems, United States), etc. | Expansion of the indication for stent angioplasty |
| Tubular/modular stents outperform self-expanding and helical stents |
The initial stents had clear technical shortcomings that needed to be addressed to expand their use to various anatomical scenarios, such as tortuosities, bifurcations, and calcified lesions. At the initiative of interventional cardiologists themselves, the possibility of cutting the Palmaz-Schatz stent at the articulated bridge was proposed to provide a short stent (“disarticulated Palmaz” or “hemi-Palmaz”) for short lesions with more challenging anatomical access.23 However, it was the incorporation of laser cutting technology that revolutionized stent design. Cordis, a Johnson & Johnson company based in the United States, improved the Palmaz-Schatz platform by introducing the Spiral and later the Crown24 (figure 5). This evolution eventually led to the Bx Velocity, a laser-cut tubular stent with zigzag rings and wavy connectors that provided greater flexibility while maintaining the closed-cell design (connectors at all the bending angles of the rings), which limited its navigation in curves (figure 5). Building on the Bx Velocity platform, Cordis launched the first drug-eluting stent in history: the Cypher.12
Figure 5. Cordis stents: evolution of the Palmaz-Schatz platform, from the articulated PS 153 series (top), through the Crown (center), to the Bx Velocity platform (bottom).
In 1990, Medtronic Inc. (United States) introduced the Wiktor—a balloon-expandable helical coil stent similar to the Gianturco-Roubin stent but made of tantalum, which is more radiopaque (figure 6). However, due to its fragility and weak radial force, the Wiktor was surpassed by newer platforms and was eventually withdrawn from the market.24 Around 1994, Arterial Vascular Engineering (AVE, United States) launched the Micro Stent, featuring a modular design: round cobalt alloy wire with smooth curved angles forming rings that were then joined with welds at alternate vertices, creating open cells. This design improved flexibility and navigation without losing radial strength.24 AVE was acquired by Medtronic in 1998. In subsequent iterations of the Micro Stent (GFX, GFX2, S670, S7), the strut thickness (the wire that composes the stent mesh) was gradually reduced, and stainless steel was replaced by a cobalt-nickel alloy in the Driver stent in 2002 (figure 6). Well into the 21st century, Medtronic used the Driver platform to create the Endeavor drug-eluting stent. In 2010, the transition to the Integrity platform involved using a continuous sinusoidal-shaped wire, laser welded at multiple points to protect its integrity.
Figure 6. Medtronic and AVE stents: helical Wiktor stent (A) and modular AVE microstent (B), with their GFX2 (C) and Driver (D) iterations. Diagram showing strut developments throughout successive platforms. Image of the Integrity platform (E) with laser welded continuous wire technology.
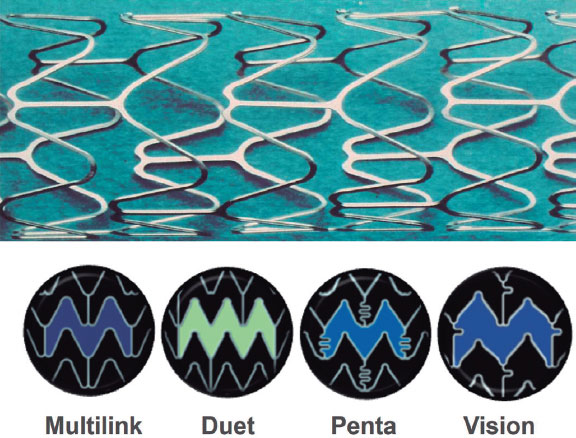
On the other hand, Advanced Cardiovascular Systems (United States), a company previously acquired by Eli Lilly’s Medical Device and Diagnostics Division (United States) and later by Guidant, created the Multi-Link stent, which was approved for use in Europe in 1995 and in the United States in 1997. This tubular stainless steel stent had an open-cell design, with flat struts and rounded angles at the rings.24 Its modern design made it highly competitive and it dominated the market alongside the AVE stent.12 Guidant continued to enhance the Multi-Link platform by thinning the struts, incorporating curved connectors, and switching to a chromium-cobalt alloy in the Vision model (figure 7). The Multi-Link Rx (50-μm strut) demonstrated superiority over the Bx Velocity stent (140 μm strut) in terms of 12-month restenosis in the ISAR-STEREO-2 trial (18% vs 31%; P < .001),25 which demonstrated the importance of strut thickness in reducing vessel wall damage and the occurrence of restenosis. The chromium-cobalt Multi-Link platform later served as the foundation for the drug-eluting stents Xience V (Abbott Vascular, United States) and Promus (Boston Scientific, United States).
Figure 7. ACS-Guidant stents. Multi-link platform in its successive iterations with modifications in cell structure and connectors.
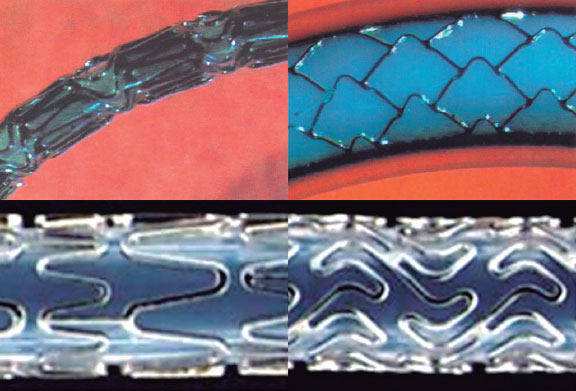
The NIR stent by Medinol (Israel), distributed by Boston Scientific, was a closed-cell stainless steel stent designed for flexible navigation. Once expanded, its cell geometry provided substantial rigidity and, therefore, radial strength24 (figure 8). This platform was used for the first paclitaxel drug-eluting stent, the Taxus, launched by Boston Scientific in 2003. In the late 1990s, Boston Scientific, which had distributed the Wallstent and the NIR, developed and marketed its own stents, the Express and Veriflex/Liberté, which would later serve as the basis for subsequent versions of the Taxus (figure 8).
Figure 8. Platforms used by Boston to develop the Taxus. NIR stent from Medinol (top); Express platform (bottom left); Veriflex platform used for the Taxus Liberté (bottom right).
Many stents launched during the 1990s were compared based on their technical characteristics and direct comparison studies generally yielded equivalent data.26 After this technological revolution at the end of the century, it became evident that balloon-expandable tubular stents (Palmaz-Schatz and Multi-Link) and modular design stents (Micro Stent) had outperformed self-expanding and helical stents. Advances in angioplasty during these years (table 2) firmly established stents as the standard for percutaneous treatment of coronary artery disease. However, restenosis remained a significant issue for both stents and angioplasty in general. The incidence of restenosis had decreased from 30% to 40% with balloon angioplasty to 20% to 30% in the early studies of the Palmaz-Schatz stent.14,15 After successive improvements in stent platforms and implantation techniques, restenosis rates were reduced, but still hovered around 20% 1 year after implantation.27 Furthermore, the expanded use of stents in more complex scenarios (saphenous vein grafts, small vessels, long lesions, etc.) suggested an even higher incidence of restenosis. Addressing this issue became a priority, leading to the next revolution in interventional cardiology as the 21st century began.
TACKLING RESTENOSIS IN THE 21ST CENTURY: THE ERA OF DRUG-ELUTING STENTS
Once the use of stents became widespread, restenosis and thrombosis emerged as complications that needed to be understood and addressed. Initially, heparin coatings were devised for stents to prevent these 2 processes. While they seemed to have a protective effect against thrombosis, their effect on restenosis was uncertain. Despite the clear advancement that coronary stents represented for angioplasty, they triggered a vascular response leading to sustained inflammatory processes, tissue growth, and late lumen loss.28 It became evident that restenosis primarily resulted from the proliferative activity of vascular smooth muscle cells.29 Consequently, efforts focused on halting this cellular response. Brachytherapy, involving the transcatheter delivery of ionizing radiation to the lesion, emerged as a method to mitigate this proliferative response. However, the difficulty of applying this therapy, coupled with the occurrence of very late thrombosis, likely related to inhibition of endothelialization and restenosis at the irradiated segment edges, limited its success.30 Subsequently, attention shifted to the development of antiproliferative drugs.
Sirolimus (rapamycin) is an antifungal agent first isolated in 1965 from a bacterium found on Easter Island (Chile).31 This agent is an inhibitor of the mTOR (mammalian target of rapamycin) protein with antiproliferative and immunosuppressive effects that had already been used in cancer treatment and as a therapy after organ transplantation. This molecule was selected by the research team at Cordis to create the first drug-eluting stent, the Cypher. Sirolimus was incorporated into a polymer carrier that coated the metal surface of the stent, allowing its controlled release to the endothelium. In contrast, paclitaxel (taxol) is an antimitotic agent extracted from the bark of the Pacific yew tree that was first isolated in 1967.32 Paclitaxel exerts a cytotoxic effect by blocking microtubule disassembly, thereby disrupting the cell cycle and mitosis. Boston Scientific chose paclitaxel to develop the first generation of the Taxus stent, embedding the drug in a polymer carrier as well. Concurrently, paclitaxel was also being used in the development of drug-eluting balloons by Bruno Scheller and Ulrich Speck’s team in Germany, aiming to address the issue of restenosis.33
The first implantation of a drug-eluting stent was a Cypher and took place in December 1999 in Sao Paulo, Brazil. The team included Eduardo Sousa and Patrick Serruys. The experience with the initial 30 patients and their 1-year follow-up without any cases of restenosis marked the beginning of a new era.34 This was followed by the RAVEL clinical trial with 238 patients randomized to receive either a Bx Velocity or a Cypher. At 6 months, late lumen loss was 0.80 ± 0.53 mm with the Bx Velocity and −0.01 ± 0.33 mm with the Cypher (P < .001). Binary restenosis was 26.6% and 0%, respectively (P < .001).35 In addition, the TAXUS II trial randomized 536 patients to receive either an NIR or a Taxus stent with 2 different forms of paclitaxel release (slow or moderate). At 6 months, late lumen loss on intravascular ultrasound was > 20% with NIR and < 8% with Taxus. The restenosis rate decreased from 19% to 2.3% with the slow-release Taxus stent and to 4.7% with the moderate-release stent (P < .001). After 1 year, events were halved, similar to the findings of the RAVEL trial.36
A few years after the widespread adoption of drug-eluting stents, certain data emerged that tempered the initial enthusiasm. Late thrombosis events (beyond the first month) began to be reported, raising concerns.37 Antiproliferative drugs led to delayed endothelialization, and there were reports of local inflammatory reactions, presumably related to the polymer.38 It was hypothesized that these reactions could explain the observed cases of late thrombosis. Subsequent pathology studies demonstrated more frequent and earlier development of neoatherosclerosis in drug-eluting stents compared with conventional stents.39 Meta-analyses confirmed a very slight increase in the risk of thrombosis overall, with no differences in mortality, while also confirming the surprising effectiveness of the new stents.40
After the initial success of Cypher and Taxus, new and improved stents began emerging with advancements in drug formulation, polymer coatings, and metal platforms 41 (table 3). These innovations allowed the treatment of more complex lesions due to improved delivery systems. Stainless steel platforms gave way to chrome-cobalt and chrome-platinum alloys, enabling thinner struts that reduced vascular damage while maintaining radial strength. Open-cell designs with fewer connectors became standard among brands. Companies developed sirolimus analogs and used new, biocompatible polymers with thinner coatings on the strut surface.
Table 3. First and second generation drug-eluting stents (polymer and thin struts)
| Name | Company | Platform | Metal | Strut thickness | Drug | Polymer thickness |
|---|---|---|---|---|---|---|
| Cypher | Cordis (J&J) | Bx Velocity | Stainless steel | 140 µm | Sirolimus | 12.6 µm |
| Taxus Express | Boston Scientific | Express | Stainless steel | 132 µm | Paclitaxel | 16 µm |
| Taxus Liberté | Boston Scientific | Veriflex | Stainless steel | 97 µm | Paclitaxel | 16 µm |
| Endeavor | Medtronic | Driver | Chromium-cobalt | 91 µm | Zotarolimus | 4.1 µm |
| Resolute Onyx | Medtronic | Integrity | Nickel-chrome + platinum-iridium | 81-91 µm | Zotarolimus | 4.1 µm |
| Xience V/Promus | Abbott/Boston Scientific | Multi-link | Chromium-cobalt | 81 µm | Everolimus | 7.6 µm |
| Promus Element | Boston Scientific | Omega | Chromium-platinum | 81 µm | Everolimus | 6.0 µm |
Numerous head-to-head randomized clinical trials directly compared second-generation drug-eluting stents with first-generation and traditional bare-metal stents.42 While the superiority of drug-eluting stents over bare-metal stents was generally accepted in most scenarios, demonstrating the advantages of the new generations was more challenging. From 2008 onward, several studies used optical coherence tomography to assess vascular responses to different stents. These findings were corroborated by pathological studies, which showed increased inflammatory responses and fibrin accumulation with first-generation stents43 This generational shift led to the phased withdrawal of Cypher and Taxus, with Xience (Abbott Vascular, USA) emerging as the preferred stent due to its superior outcomes, establishing it as the best-in-class for subsequent comparisons.
LATEST ADVANCES AND BIORESORBABLE STENTS
Drug-eluting stents (1999) represented one of the major revolutions in interventional cardiology following angioplasty balloons (1977) and conventional stents (1986). Starting from the second generation, drug-eluting stents have become the gold standard due to their safety and effectiveness. Subsequent generations of stents have incorporated biodegradable polymers, eliminated polymers, or included special coatings to promote endothelialization and biocompatibility (table 4). Moreover, further advancements achieved even thinner struts. However, advances in stent coating types have not consistently demonstrated a benefit.44 Nonetheless, some data suggest that the evolution toward ultra-thin struts may indeed offer an advantage in terms of reducing the incidence of repeat revascularizations at the target lesion in the long term.44,45 Nowadays, drug-eluting stents available in the market navigate very satisfactorily and are highly effective. The differences between various models are subtle, and the purported advantages are challenging to demonstrate. The prevalence of direct comparison studies with noninferiority designs reflects this sort of stagnation in progress.46 Despite this, technological development continues in pursuit of improvements.41
Table 4. Drug-eluting stents with biodegradable polymer or polymer-free
| Name | Company | Metal | Strut thickness | Polymer | Drug |
|---|---|---|---|---|---|
| Biomatrix Flex | Biosensors | Stainless steel | 112 µm | Yes | Biolimus A9 |
| Biomatrix Alfa | Biosensors | Chromium-cobalt | 84-88 µm | Yes | Biolimus A9 |
| Nobori | Terumo | Stainless steel | 112 µm | Yes | Biolimus A9 |
| Ultimaster | Terumo | Chromium-cobalt | 80 µm | Yes | Sirolimus |
| Synergy | Boston Scientific | Chromium-platinum | 74-81 µm | Yes | Everolimus |
| Orsiro | Biotronik | Chromium-cobalt | 60-80 µm | Yes | Sirolimus |
| Biomime | Meril | Chromium-cobalt | 65 µm | Yes | Sirolimus |
| supraflex Cruz | SMT | Chromium-cobalt | 60 µm | Yes | Sirolimus |
| Coroflex ISAR Neo | Braun | Chromium-cobalt | 55-65 µm | No | Sirolimus + probucol |
| Biofreedom | Biosensors | Stainless steel | 112 µm | No | Biolimus A9 |
| Biofreedom Ultra | Biosensors | Chromium-cobalt | 84-88 µm | No | Biolimus A9 |
| Cre8 | Alvimedica | Chromium-cobalt | 70-80 µm | No | Sirolimus + fatty acid |
Special mention goes to the concept of the bioresorbable stent, which aimed to avoid the drawbacks of leaving a permanent metal scaffold in the coronary artery. In the 1990s, Japanese engineer Keiji Igaki and interventional cardiologist Hideo Tamai developed a platform made from polylactic acid polymer with a 170 μm strut and no drug. It required heating to expand during implantation (using contrast heated to 80º C). Theoretically, the polymer was supposed to begin degrading after 6 months to 2 years, gradually losing its radial strength. Hideo Tamai implanted the first bioresorbable stent in history (the Igaki-Tamai stent, Kyoto Medical) in Japan in 1998. The initial publication reported 15 patients with a 6-month follow-up showing a 10.5% restenosis incidence per treated lesion.47 However, a 10-year follow-up of 50 patients revealed a 28% incidence of vessel revascularization and 2.4% thrombosis.48
In 2006, John Ormiston implanted the first drug-eluting bioresorbable stent, the Absorb Bioresorbable Vascular Scaffold (Abbott Vascular, USA), with everolimus embedded in a polylactic acid polymer matrix and 150 μm struts.1 Following promising data from pilot studies, the ABSORB II study was initiated, which randomized 501 patients to Absorb BVS vs Xience, aiming for superiority in vasomotor response of the treated segment (theoretical advantage of a resorbable platform) and noninferiority in terms of late lumen loss. Unfortunately, the 3-year analysis presented in 2016 showed failure to achieve either objective, with an added increase in subacute thrombosis (2.8% vs 0%; P = .03) and target vessel myocardial infarction (7.1% vs 1.2%; P = .006).49 This was followed by unfavorable long-term results from other randomized clinical trials and meta-analyses,50 eventually leading Abbott to withdraw its device from the market.
The first bioresorbable drug-eluting stent failed in comparisons with the gold standard Xience, which demonstrated its high reliability. Nonetheless, important lessons were learned for future progress.51 The Absorb was a device with thick struts (150 μm vs 81 μm of the Xience), which limited its navigability, compromised the side branches even more, had worse endothelialization, and increased thrombogenicity. These issues required improvement for this stent to be more competitive. In additional, Absorb had lower radial strength than bare-metal stents, making optimal implantation technique crucial. This included proper plaque preparation, precise vessel measurement using intracoronary imaging guidance, and high-pressure postdilation.52 These shortcomings became evident in pragmatic postmarketing studies. This project ended partly due to the early (perhaps premature) widespread use of a first-generation device in scenarios of greater anatomical complexity, such as long lesions, small vessels, bifurcations, and even chronic occlusions,53 which undoubtedly highlighted its disadvantages compared with the standard stent at the time.
Other companies developed bioresorbable polymer platforms,51 but, unable to overcome the limitations of the Absorb stent, clinical experimentation in this area slowed until the development of new technological advancements. Additionally, clinical practice guidelines advised against the use of the Absorb stent outside research protocols.54 In contrast, the sirolimus-eluting magnesium bioresorbable stent DREAMS (Biotronik AG, Switzerland) appears to offer a brighter outlook. The new generation features thinner radial struts and increased radial strength achieved by modifying the composition. Data from the first-in-man study—the BIOMAG-I trial—are also promising.55 However, more safety data will be needed before off-protocol use of this stent is allowed. The combination of technological development and application of the lessons learned from the Absorb stent will undoubtedly provide new opportunities to use this technology in cath labs.51
CONCLUSIONS AND FUTURE DIRECTIONS
The invention of the stent has been one of the greatest advances in the history of cardiology and medicine in general. This article recounts the success of the collaboration between innovative minds and the biomedical industry, which invested the necessary resources to develop a much-needed therapy (figure 9). This feat also provided important lessons for research in interventional cardiology. The need to evaluate the safety and effectiveness of successive advancements quickly matured research methodologies and led to the creation of large collaborative networks. The coordination of protocols, data collection and auditing, and subsequent analysis were made possible by the hard work of dedicated researchers and significant funding from companies and academic institutions. As a result, interventional cardiology today benefits from a valuable systematic approach and infrastructure for continued innovation.
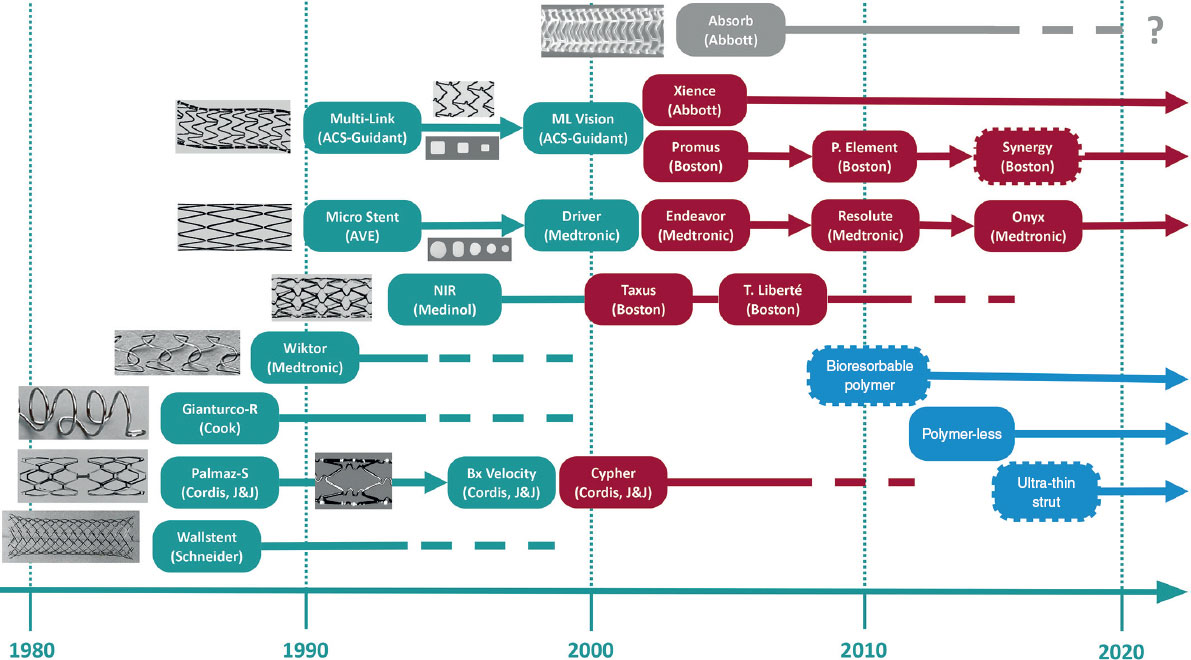
Figure 9. Timeline of milestones in the development of coronary stents. Conventional metal stents are shown in gray; drug-eluting stents in green; bioresorbable polymeric stents in blue; and new drug-eluting stents in orange. The dashed border denotes a resorbable polymer. ACS, Advanced Cardiovascular Systems; AVE, Arterial Vascular Engineering; J&J, Johnson & Johnson.
The practice of angioplasty has become highly safe and effective, largely due to the modern stent, which incorporates numerous improvements developed over its history. Today, the incidence of stent thrombosis is less than 1% in the acute, late, and very late phases.56 Due to the safety of these devices and improvements in technique, the use of potent antithrombotic treatment is being minimized.1 The annual incidence of stent restenosis requiring revascularization is currently 1% to 2% after implantation.57 Although these figures are very low—considering that millions of stents are implanted annually worldwide—it remains a significant health concern from an epidemiological perspective. There are still issues requiring research attention: patients with a propensity to recurrent restenosis, calcified lesions that prevent optimal outcomes, and the deleterious effect of antiproliferative drugs on endothelial function with the consequent development of neoatherosclerosis.41 All these challenges present opportunities for innovation in the stent industry. Moreover, the prospect of performing effective angioplasties without leaving a permanent device remains open with the development of bioresorbable stents, alongside the potential widespread use of drug-coated balloons in various clinical and anatomical scenarios where a permanent stent could pose disadvantages.58
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONS
F. Macaya-Ten: conception and design and writing of the first draft. N. Gonzalo: critical review of the manuscript. J. Escaned: critical review of the manuscript. C. Macaya: conception and design, and critical review of the manuscript.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors appreciate the visual materials and bibliographic documentation provided by various companies mentioned in the present article.
REFERENCES
1. Gaspard P, Whitin H. The History of Coronary Angioplasty. Toulouse:Europa Digital &Publishing/Europa Group;2017.
2. Gruentzig A, Myler R, Hanna E, Turina M. Coronary transluminal angioplasty (abstract). Circulation.. 1977;56:84.
3. Gruentzig A. Results from coronary angioplasty and implications for the future. Am Heart J.. 1982;103:779-783.
4. Gruentzig AR, King SB, Schlumpf M, Siegenthaler W. Long-term follow-up after percutaneous transluminal coronary angioplasty. The early Zurich experience. N Engl J Med. 1987;316:1127-1132.
5. Navarro FA. Stent. Rev Esp Cardiol.. 2018;71:694.
6. Roguin A. Stent:The Man and Word Behind the Coronary Metal Prosthesis. Circ Cardiovasc Interv. 2011;4:206-209.
7. Dotter CT. Transluminally-placed coilspring endarterial tube grafts. Long-term patency in canine popliteal artery. Invest Radiol.. 1969;4:329-332.
8. Sigwart U, Urban P, Golf S, et al. Emergency stenting for acute occlusion after coronary balloon angioplasty. Circulation.. 1988;78:1121-1127.
9. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med.. 1987;316:701-706.
10. Sigwart U. What is a stent and where can you get one? Am J Cardiol. 1997;80:1122.
11. Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13-17.
12. Tan C, Schatz RA. The History of Coronary Stenting. Interv Cardiol Clin.. 2016;5:271-280.
13. Palmaz JC, Sibbitt RR, Reuter SR, Tio FO, Rice WJ. Expandable intraluminal graft:a preliminary study. Work in progress. Radiology.. 1985;156:73-77.
14. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med.. 1994;331:496-501.
15. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med.. 1994;331:489-495.
16. Macaya C, Serruys PW, Ruygrok P, et al. Continued benefit of coronary stenting versus balloon angioplasty:one-year clinical follow-up of Benestent trial. Benestent Study Group. J Am Coll Cardiol.. 1996;27:255-261.
17. George CJ, Baim DS, Brinker JA, et al. One-year follow-up of the Stent Restenosis (STRESS I) Study. Am J Cardiol.. 1998;81:860-865.
18. Lansky AJ, Roubin GS, O'Shaughnessy CD, et al. Randomized comparison of GR-II stent and Palmaz-Schatz stent for elective treatment of coronary stenoses. Circulation.. 2000;102:1364-1368.
19. Sirnes PA, Golf S, Myreng Y, et al. Stenting in Chronic Coronary Occlusion (SICCO):a randomized, controlled trial of adding stent implantation after successful angioplasty. J Am Coll Cardiol.. 1996;28:1444-1451.
20. Versaci F, Gaspardone A, Tomai F, Crea F, Chiariello L, GioffrèPA. A comparison of coronary-artery stenting with angioplasty for isolated stenosis of the proximal left anterior descending coronary artery. N Engl J Med. 1997;336:817-822.
21. Serruys PW, de Bruyne B, Carlier S, et al. Randomized comparison of primary stenting and provisional balloon angioplasty guided by flow velocity measurement. Doppler Endpoints Balloon Angioplasty Trial Europe (DEBATE) II Study Group. Circulation.. 2000;102:2930-2937.
22. Grines CL, Cox DA, Stone GW, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med.. 1999;341:1949-1956.
23. Colombo A, Hall P, Thomas J, Almagor Y, Finci L. Initial experience with the disarticulated (one-half) Palmaz-Schatz stent:a technical report. Cathet Cardiovasc Diagn.. 1992;25:304-308.
24. Serruys PW, Rensing BJ. Handbook of Coronary Stents, Fourth Edition. Kentucky, USA:Taylor &Francis;2002.
25. Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results:strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol.. 2003;41:1283-1288.
26. Colombo A, Stankovic G, Moses JW. Selection of coronary stents. J Am Coll Cardiol.. 2002;40:1021-1033.
27. Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117-1124.
28. Gaspardone A, Versaci F. Coronary stenting and inflammation. Am J Cardiol.. 2005;96:65L-70L.
29. Kearney M, Pieczek A, Haley L, et al. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation.. 1997;95:1998-2002.
30. SabatéM, Pimentel G, Prieto C, et al. Intracoronary brachytherapy after stenting de novo lesions in diabetic patients:results of a randomized intravascular ultrasound study. J Am Coll Cardiol.. 2004;44:520-527.
31. Hobby G, Clark R, Woywodt A. A treasure from a barren island:the discovery of rapamycin. Clin Kidney J.. 2022;15:1971-1972.
32. Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell.. 2014;25:2677-2681.
33. Scheller B, Speck U, Abramjuk C, Bernhardt U, Böhm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation.. 2004;11:810-814.
34. Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents:one-year angiographic and intravascular ultrasound follow-up. Circulation.. 2001;104:2007-2011.
35. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med.. 2002;346:1773-1780.
36. Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation.. 2003;108:788-794.
37. Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents:an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol.. 2006;48:2584-2591.
38. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans:delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202.
39. Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis:overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J.. 2015;36:2147-2159.
40. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med.. 2007;356:998-1008.
41. Torii S, Jinnouchi H, Sakamoto A, et al. Drug-eluting coronary stents:insights from preclinical and pathology studies. Nat Rev Cardiol.. 2020;17:37-51.
42. Garg S, Serruys PW. Coronary Stents:Current Status. J Am Coll Cardiol.. 2010;56:S1-S42.
43. Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211-223.
44. Taglieri N, Bruno AG, Ghetti G, et al. Target Lesion Failure With Current Drug-Eluting Stents:Evidence From a Comprehensive Network Meta-Analysis. JACC Cardiovasc Interv.. 2020;13:2868-2878.
45. Madhavan MV, Howard JP, Naqvi A, et al. Long-term follow-up after ultrathin vs. 2nd-generation drug-eluting stents:a systematic review and meta-analysis of randomized controlled trials. Eur Heart J.. 2021;42:2643-2654.
46. Macaya F, Ryan N, Salinas P, Pocock SJ. Challenges in the Design and Interpretation of Noninferiority Trials. J Am Coll Cardiol.. 2017;70:894-903.
47. Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation.. 2000;102:399-404.
48. Nishio S, Kosuga K, Igaki K, et al. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents:Igaki-Tamai stents. Circulation.. 2012;125:2343-2353.
49. Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II):a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet.. 2016;388:2479-2491.
50. Ali ZA, Serruys PW, Kimura T, et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease:a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet.. 2017;390:760-772.
51. Katagiri Y, Serruys PW, Asano T, et al. How does the failure of Absorb apply to the other bioresorbable scaffolds?An expert review of first-in-man and pivotal trials. EuroIntervention J.. 2019;15:116-123.
52. Stone GW, Abizaid A, Onuma Y, et al. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation:Analysis From the ABSORB Trials. J Am Coll Cardiol.. 2017;70:2863-2874.
53. Smits PC, Chang CC, Chevalier B, et al. Bioresorbable vascular scaffold versus metallic drug-eluting stent in patients at high risk of restenosis:the COMPARE-ABSORB randomised clinical trial. EuroIntervention.. 2020;16:645-653.
54. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J.. 2019;40:87-165.
55. Haude M, Wlodarczak A, van der Schaaf RJ, et al. Safety and performance of the third-generation drug-eluting resorbable coronary magnesium scaffold system in the treatment of subjects with de novo coronary artery lesions:6-month results of the prospective, multicenter BIOMAG-I first-in-human study. EClinicalMedicine.. 2023;59:101940.
56. Gori T, Polimeni A, Indolfi C, Räber L, Adriaenssens T, Münzel T. Predictors of stent thrombosis and their implications for clinical practice. Nat Rev Cardiol.. 2019;16:243-256.
57. Giustino G, Colombo A, Camaj A, et al. Coronary In-Stent Restenosis:JACC State-of-the-Art Review. J Am Coll Cardiol.. 2022;80:348-372.
58. Jeger RV, Eccleshall S, Wan AWA, et al. Drug-Coated Balloons for Coronary Artery Disease. JACC Cardiovasc Interv.. 2020;13:1391-1402.