ABSTRACT
Introduction and objectives: Radiofrequency (RF) renal denervation (RDN) has been shown to be a safe and effective treatment option for patients with uncontrolled hypertension. This analysis sought to explore the cost-effectiveness of this therapy in Spain.
Methods: A decision-analytic Markov model projected clinical events, quality-adjusted life years (QALY) and costs over the patients’ lifetime. Treatment effectiveness in the base case analysis was informed by the change in office systolic blood pressure observed in the full cohort of the SPYRAL HTN-ON MED trial (–4.9 mmHg vs sham control). Alternate scenarios were calculated for effect sizes reported in the HTN-ON MED subcohort of patients on 3 antihypertensive medications treated outside the United States, the HTN-OFF MED trial, and the Global SYMPLICITY Registry high-risk and very high-risk cohorts. The analysis was conducted from the Spanish National Health System perspective and a willingness-to-pay a threshold of €25 000 per QALY gained was considered.
Results: RF RDN therapy resulted in clinical event reductions (10-year relative risk 0.80 for stroke, 0.88 for myocardial infarction, and 0.72 for heart failure) and a lifetime gain of 0.35 (13.99 vs 13.63) QALYs. Incremental lifetime costs were €5335 (€26 381 vs €21 045), resulting in an incremental cost-effectiveness ratio of €15 057 per QALY gained. Cost-effectiveness was further improved among all the other clinical evidence scenarios.
Conclusions: The results of this study suggest that RF RDN can provide a cost-effective alternative in the treatment of uncontrolled hypertension in Spain.
Keywords: Denervation. Hypertension. Cost-effectiveness analysis. Spain.
RESUMEN
Introducción y objetivos: La denervación renal (DNR) por radiofrecuencia (RF) es una alternativa terapéutica eficaz y segura en pacientes con hipertensión no controlada. Este estudio evalúa el coste-efectividad de esta terapia en España.
Métodos: Se empleó un modelo de Markov para estimar los eventos clínicos, los años de vida ajustados por calidad (AVAC) y los costes durante toda la vida de los pacientes. La eficacia del tratamiento en el caso base se obtuvo del cambio en la presión arterial sistólica en consulta observado en la cohorte completa del estudio SPYRAL HTN-ON MED (–4,9 mmHg frente a control simulado). Se exploraron escenarios alternativos empleando el tamaño del efecto observado en el subgrupo de pacientes del estudio HTN-ON MED en 3 fármacos antihipertensivos tratados fuera de Estados Unidos, el estudio HTN-OFF MED, y las cohortes de alto y muy alto riesgo del registro Global SYMPLICITY. Se consideró la perspectiva del Sistema Nacional de Salud y con un umbral de disposición a pagar de 25.000 €/AVAC.
Resultados: La DNR por RF se asoció a una reducción de los eventos clínicos (riesgo relativo a 10 años de 0,80 en ictus, 0,88 en infarto de miocardio y 0,72 en insuficiencia cardiaca). Durante un horizonte temporal de toda la vida se observaron una ganancia de 0,35 AVAC (13,99 vs 13,63) y un coste incremental de 5.335 € (26.381 frente a 21.045 €), obteniendo una ratio coste-efectividad incremental de 15.057 €/AVAC. En los demás escenarios analizados se obtuvieron mejores resultados.
Conclusiones: Los resultados de este estudio sugieren que la DNR por RF puede representar una alternativa coste-efectiva en el tratamiento de la hipertensión no controlada en España.
Palabras clave: Denervación. Hipertensión. Análisis coste-efectividad. España.
Abbreviations
HT: hypertension. ICER: incremental cost-effectiveness ratio. SBP: systolic blood pressure. RDN: renal denervation. R-HT: resistant hypertension. QALY: quality-adjusted life year.
INTRODUCTION
Uncontrolled hypertension (HT) poses a significant global clinical and economic burden. The prevalence of uncontrolled HT varies greatly, based on the population evaluated and the definition adopted.1 In Spain it is estimated that 32.9% of the adult population aged 30 to 79 have HT, with 57.1% of those treated achieving well-controlled levels.2 Uncontrolled HT is most common among aging, obese, or chronic kidney disease patient populations, although various risk factors and secondary causes (including poor medication adherence) can also contribute to its development.1 As is well established, patients with uncontrolled HT have an increased risk of cardiovascular events, including stroke, myocardial infarction (MI), and heart failure (HF), as well as their sequelae.1,3
Radiofrequency (RF) renal denervation (RDN) is a device-based interventional treatment option intended to permanently disrupt sympathetic nervous signaling to the kidneys, achieving lasting reductions in blood pressure.4
Over more than a decade, a large body of trials and real-world evidence has supported the viability, safety, and effectiveness of RF RDN, with the most recent SPYRAL HTN-ON MED5 and HTN-OFF MED trials6 contributing data from second-generation RF RDN devices. The SPYRAL HTN-ON MED5 and HTN-OFF MED trials6 were sham-controlled studies that evaluated the therapy in the presence and absence of antihypertensive medications, respectively. Other trial data and findings from the international, multicenter open-label Global SYMPLICITY Registry (GSR),7 which has enrolled more than 3000 participants to date, provide evidence on the safety, effectiveness, and longer-term outcomes of RF RDN treatment.7
Most recently, the latest guidelines from the European Society of Hypertension, and the joint expert statement from the Spanish Society of Hypertension-Spanish League for the Fight Against Hypertension and the Interventional Cardiology Association of the Spanish Society of Cardiology, recommend RDN as an adjunctive treatment option for uncontrolled HT, including resistant hypertension (R-HT).8,9 This consensus statement specifically recognizes the value of RDN for patients at high cardiovascular risk with hypertension-mediated organ damage or cardiovascular disease. Furthermore, RF RDN has recently received approval from the United States Food and Drug Administration as an adjunct therapy in hypertensive patients without adequate blood pressure control.10
While its clinical viability is widely established, less is currently known about the potential cost-effectiveness of RF RDN based on the latest clinical evidence. The present study aimed to address this gap by assessing the cost-effectiveness of RF RDN treatment within the Spanish health system.
METHODS
A decision-analytic, state-transition Markov model was used to project outcomes, including costs and health benefits associated with RF RDN, over a lifetime. This analysis model, adopting the perspective of the Spanish National Health System, was built on the foundation of an earlier model.11 Key parameter inputs can be found in table 1.
Table 1. Model inputs
| Parameter | Value | Distribution | SE | Source |
|---|---|---|---|---|
| Age, y | 55.0 | Normal | 0.53 | Kandzari et al.5 |
| Gender (% female) | 19.9% | Beta | 0.02 | Kandzari et al.5 |
| Baseline systolic BP | 163 mmHg | Normal | 0.40 | Kandzari et al.5 |
| Treatment effect | 4.9 mmHg | Normal | 0.54 | Kandzari et al.5 |
| Discount rate (costs) | 3.00% p.a. | - | - | López-Bastida et al.12 |
| Discount rate (health outcomes) | 3.00% p.a. | - | - | López-Bastida et al.12 |
| Costs | ||||
| HT (year 1+) | €251 | Gamma | €25 | Soto et al.13 |
| Stroke (acute) | €4787a | Gamma | €479 | Ribera et al.14; Navarrete-Navarro et al.15 |
| Stroke (remainder of year 1) | €6647a | Gamma | €665 | |
| Stroke (year 2+) | €4135a | Gamma | €414 | |
| MI (acute) | €7674 | Gamma | €96 | Darbà et al.16 |
| MI (year 1+) | €950 | Gamma | €135 | Escobar et al.17 |
| Stable AP (year 1+) | €615 | Gamma | €74 | Schwander et al.18 |
| Unstable AP (acute) | €2910 | Gamma | €51 | Schwander et al.18 |
| Unstable AP (year 1+) | €615 | Gamma | €74 | Schwander et al.18 |
| HF (year 1+) | €5808 | Gamma | €300 | Delgado et al.19 |
| ESRD (year 1+) | €25 574b | Gamma | €2557 | Villa et al.20 |
| RF RDN therapy | €7484 | Gamma | €748 | Estimated by Medtronic |
| Utilities | ||||
| HT | 0.96 | Beta | 0.10 | Sullivan et al.21 |
| Stroke | 0.63 | Beta | 0.03 | Grosso et al.22; Darlington et al23 |
| MI (months 1-6) | 0.76 | Beta | 0.09 | Aasa et al.24; Glasziou et al.25 |
| MI (months 6+) | 0.88 | Beta | 0.02 | Grosso et al.22; Pignone et al.26 |
| Stable AP | 0.84 | Beta | 0.02 | Sullivan et al.21 |
| Unstable AP | 0.74 | Beta | 0.02 | Glasziou et al.25 |
| HF | 0.71 | Beta | 0.07 | Chen et al.27; Fryback et al.28 |
| ESRD | 0.63 | Beta | 0.06 | Lee et al.29 |
|
AP, angina pectoris; BP, blood pressure; ESRD, end-stage renal disease; HF, heart failure; HT, hypertension; MI, myocardial infarction; p.a., per annum; RF RDN, radiofrequency renal denervation; SE, standard error. a Stroke costs were determined assuming 85% ischemic stroke costs from Ribera A et al.14 and 15% hemorrhagic stroke costs from Navarrete-Navarro et al.15 b ESRD costs were determined based on epidemiological data and the cost associated with the different treatment modalities.20,30 |
||||
Model structure
The Markov model consisted of 7 primary health states: HT alone, stroke, MI, other symptomatic coronary heart disease (CHD) or angina pectoris (AP), HF, end-stage renal disease (ESRD), and death (figure 1 and supplementary material in Sharp et al.11). Transitions could occur monthly, and half-cycle correction was implemented. The model was encoded in Microsoft Excel (Microsoft, United States), with supporting statistical analyses conducted in JMP Pro 16 (SAS Institute, United States).
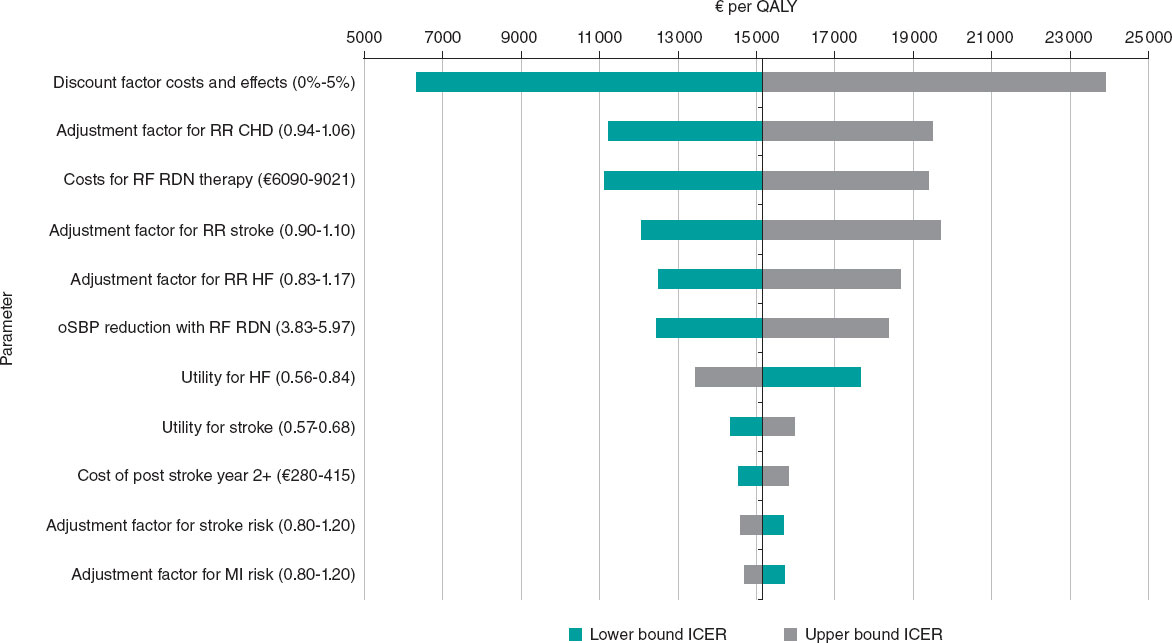
Figure 1. Tornado diagram illustrating the deterministic sensitivity analysis results. CHD, coronary heart disease; HF, heart failure; ICER, incremental cost-effectiveness ratio; MI, myocardial infarction; oSBP, office systolic blood pressure; RF RDN, radiofrequency renal denervation; RR, relative risk.
Transition probabilities and relative risk reductions
Transition probabilities to subsequent health states were informed by multivariate risk equations derived from large cohort studies.31-34 Baseline risks for the control cohort were calculated by applying cohort characteristics and office systolic blood pressure (SBP) level to these equations. Corresponding transition probabilities for the RF RDN arm were determined by multiplying these baseline risks by office SBP reduction-specific relative risks (RR), derived from a meta-regression of 47 randomized controlled trials (RCT) of intentional HT treatment.35 Mortality rates were informed by Spanish general population lifetable data and postevent survival data specific to Spain where available (table 1 of the supplementary data, and Sharp et al.11).
Clinical data
Cohort characteristics and treatment efficacy for the base case analysis were obtained from the SPYRAL HTN-ON MED full cohort trial.5 Study participants were, on average, aged 55 years, with a baseline office SBP of 163 mmHg, and were prescribed 1 to 3 medications (mean, 1.9).5 The RF RDN arm received denervation treatment with the Symplicity Spyral multielectrode renal denervation system (Medtronic, United States) plus maintained antihypertensive medications, while the sham control group received antihypertensive therapy only. The trial-reported office SBP reduction observed at 6 months for the RF RDN arm was –9.9 mmHg vs –5.0 mmHg for the sham arm, resulting in an effect size of –4.9 mmHg.5 Additional scenario analyses were conducted using evidence from several other subcohorts and studies. These included the SPYRAL HTN-ON MED subcohort of patients on 3 medications treated outside the United States36 to represent an R-HT cohort more comparable to the European setting (office SBP effect size vs sham –6.9 mmHg), the SPYRAL HTN-OFF MED trial6 in which patients received the therapy in the absence of antihypertensives (effect size –6.6 mmHg), the high-risk and very high-risk cohorts of the GSR37 (effect sizes –21.5 mmHg and –31.6 mmHg vs baseline, respectively, calculated as the average of the reductions reported at 6, 12, 24, and 36 months), and, for completeness, a scenario with the SPYRAL HTN-ON MED5 effect size of –4.9 mmHg calculated based on reported cohort characteristics for a Spanish R-HT sample38. Scenarios based on SPYRAL HTN-OFF MED6 and GSR7 were calculated using the cohort characteristics of these respective study cohorts and sub-cohorts where applicable.
Costs and health-related quality of life
Clinical event costs were sourced from published literature.13-20 Given the perspective of the analysis; only direct medical costs were considered. All costs were expressed in 2022 euros, with relevant consumer price index data used to adjust historical costs, where necessary.39 The cost of RF RDN therapy was assessed using a micro-costing approach that considered preprocedure and procedure costs including personnel, device and catheterization laboratory overhead costs, as well as postoperative hospitalization. Health-state specific utilities, expressed as a numerical value ranging from 0 (death) to 1 (perfect health), were derived from published literature and were age-adjusted in the analysis.21-29 In conjunction with life years (LY) gained, these values inform the resulting quality-adjusted life years (QALY), a measure of the quantity and quality of life, in the model. Where multiple Spanish publications could be sourced, we prioritized contemporary publications with a greater sample size, after consideration by the clinical authors. Where Spanish publications could not be sourced, we reverted to non-Spanish values.
Model validations
Comprehensive model validations were conducted. The approach and validation results are shown in supplementary data and table 2, 3, 4, and 5 of the supplementary data.
Table 2. Base case results: clinical events over 10 years and a lifetime, and cost-effectiveness result over a lifetime
| 10-year time horizon | Lifetime horizon | |||||||
|---|---|---|---|---|---|---|---|---|
| Base case | SoC | RF RDN | Diff | RR | SoC | RF RDN | Diff | RR |
| Stroke | 9.0% | 7.2% | 1.8% | 0.80 | 34.4% | 28.8% | 5.6% | 0.84 |
| MI | 7.5% | 6.6% | 0.9% | 0.88 | 35.4% | 34.7% | 0.7% | 0.98 |
| AP/other CHD | 14.5% | 13.0% | 1.6% | 0.89 | 28.2% | 26.4% | 1.9% | 0.93 |
| HF | 5.0% | 3.6% | 1.4% | 0.72 | 19.5% | 15.2% | 4.2% | 0.78 |
| ESRD | 0.40% | 0.40% | 0.0% | 0.96 | 1.04% | 1.08% | 0.04% | 1.04 |
| CVD | 5.3% | 4.5% | 0.8% | 0.85 | ||||
| ACD | 11.2% | 10.5% | 0.7% | 0.94 | ||||
| Costs | €21 045 | €26 381 | €5335 | |||||
| LYs | 15.8 | 16.08 | 0.28 | |||||
| QALYs | 13.63 | 13.99 | 0.35 | |||||
| ICER | €15 057 per QALY | |||||||
|
ACD, all-cause death; AP, angina pectoris; CHD, coronary heart disease; CVD, cardiovascular death; Diff., difference; ESRD, end-stage renal disease; HF, heart failure; ICER, incremental cost-effectiveness ratio; LY, life years (discounted); MI, myocardial infarction; QALYs, quality-adjusted life years (discounted); RF RDN, radiofrequency renal denervation; RR, relative risk; SoC, standard of care. |
||||||||
Analysis outcomes and interpretation
The primary analysis outcome was the incremental cost-effectiveness ratio (ICER), calculated by dividing the incremental costs gained between the RF RDN cohort and the comparator by the incremental QALYs gained, and measured in euros per QALY observed. Additional and supporting outcomes included strategy- specific costs, LY, and QALY gain over a lifetime, and clinical events over 10 years and lifetime with associated risk reductions from RF RDN. Costs and QALYs were discounted at 3% per annum and cost-effectiveness was evaluated against a willingness-to-pay (WTP) threshold of €25 000 per QALY gained, which is commonly referenced for Spain.12,40
Sensitivity analysis
Comprehensive deterministic and probabilistic sensitivity analyses (DSA and PSA) were conducted to evaluate the robustness of results under varied assumptions, including differences in the cohort characteristics and effect sizes modeled, and higher or lower baseline event risks, achieved by applying adjustment factors of 2.0 and 0.5 to the underlying risk equations. The PSAs involved 10 000 repeated calculation runs each, with random sampling from the distribution of input parameters in each analysis cycle (table 6 of the supplementary data).
RESULTS
Base case analysis
Over 10 years, the base case results indicate that RF RDN treatment results in the following risk reductions vs sham control: RR, 0.80 for stroke; 0.88 for MI; 0.72 for HF; 0.89 for AP/other symptomatic CHD; 0.96 for ESRD; 0.85 for cardiovascular death, and 0.94 for all-cause death. Lifetime risk reductions were somewhat less pronounced. Over the lifetime, survival with RF RDN was improved by 0.57 years (23.21 vs 22.64 years). Lifetime costs were €26 381 for RF RDN vs €21 045 for standard of care (an increment of €5335) and total QALYs were 13.99 and 13.63 (an increment of 0.35 QALYs), resulting in a cost-effective lifetime ICER of €15 057 per QALY gained. Cost savings with RF RDN resulted primarily from acute and follow-on costs for stroke, followed by HF and AP (table 2 and figure 1 of the supplementary data).
Sensitivity and scenario analyses
RF RDN remained cost-effective among all conducted sensitivity and scenario analyses, which included a broad range of cohort characteristics, effect sizes, cost and utility assumptions, and general population mortality rates (table 3).
Table 3. Results of scenario analyses (different cohorts and effect sizes)
| Base case | Costs (€) | QALYs | Δ Costs (€) | Δ QALYs | ICER (€ per QALY) | ||
|---|---|---|---|---|---|---|---|
| RF RDN | SoC | RF RDN | SoC | ||||
| HTN-ON MED (office SBP effect size –4.9 mmHg vs sham) | 26 381 | 21 045 | 13.99 | 13.63 | 5335 | 0.35 | 15 057 |
| HTN-ON MED (office SBP effect size –9.9 mmHg vs BL) | 25 418 | 21 045 | 14.13 | 13.63 | 4372 | 0.49 | 8884 |
| HTN-ON MED subcohort on 3 AH medications treated OUS (office SBP effect size –6.9 mmHg vs sham) | 25 989 | 21 045 | 14.04 | 13.63 | 4944 | 0.41 | 12 043 |
| HTN-OFF MED (office SBP effect size –6.6 mmHg vs sham) | 26 286 | 21 320 | 15.22 | 14.82 | 4967 | 0.39 | 12 701 |
| GSR high-risk cohort (office SBP effect size –21.5 mmHg vs BL) | 25 174 | 22 967 | 12.21 | 11.35 | 2207 | 0.86 | 2569 |
| GSR very high-risk cohort (office SBP effect size –31.6 mmHg vs BL) | 23 941 | 23 292 | 12.00 | 10.89 | 649 | 1.12 | 580 |
| Spanish resistant hypertension cohort (office SBP effect size –4.9 mmHg vs sham) | 21 277 | 15 437 | 9.58 | 9.31 | 5840 | 0.27 | 21 675 |
| Risk function adjustment factor of 2.0 for MI/CHD/stroke (office SBP effect size –4.9 mmHg vs sham) | 30 782 | 25 691 | 12.71 | 12.31 | 5091 | 0.41 | 12 555 |
| Risk function adjustment factor of 0.5 for MI/CHD/stroke (office SBP effect size –4.9 mmHg vs sham) | 23 191 | 17 558 | 14.91 | 14.63 | 5633 | 0.27 | 20 702 |
|
AH, antihypertensive; BL, baseline; CHD, coronary heart disease; GSR, Global SYMPLICITY Registry; ICER, incremental cost-effectiveness ratio; MI, myocardial infarction; SBP, systolic blood pressure; OUS, outside the United States; QALYs, quality-adjusted life years; RF RDN, radiofrequency renal denervation; SBP, systolic blood pressure; SoC, standard of care. |
|||||||
In the DSA, the most influential parameters were the discount rate applied to costs and effects, the adjustment factor for CHD risk, and the cost of RF RDN therapy, followed by variations in adjustment factors of the underlying risk functions and treatment effect size. For the tested ranges, the WTP of €25 000 per QALY was not exceeded (figure 1).
In the PSA, the probability that simulations were below the cost-effectiveness threshold of €25 000 per QALY ranged from 97.4% to 100% (figure 2).
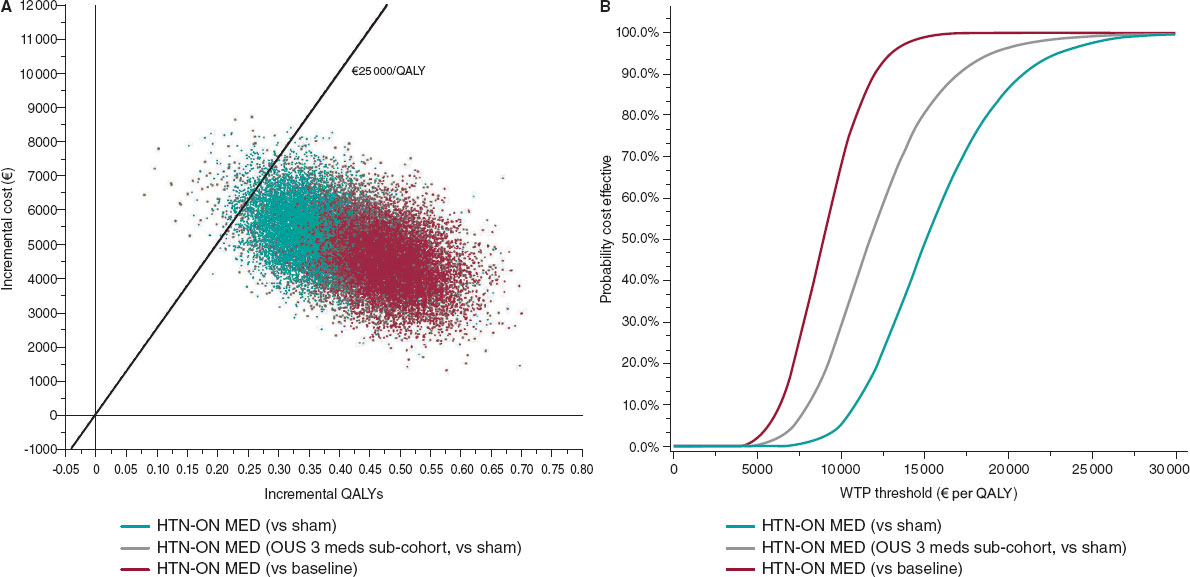
Figure 2. Central illustration. Probabilistic sensitivity analysis scatterplot (A) and cost-effectiveness acceptability curves (B) for the HTN-ON MED full cohort vs sham, and vs baseline, and for the HTN-ON MED subcohort of patients on 3 antihypertensives, treated outside the United States. The figure on the left shows the simulation results of probabilistic sensitivity analyses conducted for the HTN-ON MED base case (vs sham), for the subcohort on 3 medications treated outside the United States (vs sham), and for assumed effect size vs baseline blood pressure. The line in the graph represents the Spanish WTP threshold of €25 000 per QALY. Combinations of QALY gain and costs to the right of this line are considered cost-effective. The figure on the right provides the probability of the therapy being cost-effective at different WTP thresholds and demonstrates a high likelihood that RF RDN is a cost-effective intervention. OUS, outside the United States; QALY, quality-adjusted life year; WTP, willingness-to-pay.
DISCUSSION
This study explored the health-economic value of RF RDN treatment within the Spanish National Health System, using contemporary clinical evidence and cost data. The results of the analysis suggest that RF RDN treatment is associated with clinically meaningful reductions in cardiovascular events, resulting in improved health outcomes and cost savings that partly, but not fully, amortize the upfront cost of RF RDN treatment. The results of the model demonstrate that, compared with current standard practice and with an ICER below Spain’s WTP threshold, RF RDN is a cost-effective treatment option for patients with uncontrolled HT—including resistant HT—and hypertensive patients with high and very high cardiovascular risk. The results were found to be robust among a variety of tested cohort characteristics, effect sizes, and adjustments of the projected baseline event risks, and applied to patients not treated with antihypertensive medications, as demonstrated by the analysis using SPYRAL HTN-OFF MED6 data.
These findings are in line with those recently published for the United Kingdom (UK) health system, where RF RDN resulted in comparable QALY gains and an ICER well below the UK NICE cost-effectiveness threshold, suggesting RF RDN is a cost-effective treatment option in that health care system.11
Among the strengths of the current analysis is its reliance on a granular modeling framework able to model cohort-specific baseline risks and effect size-specific risk reductions derived from a large-scale meta-regression of HT RCTs. At the same time, the analysis has several limitations. First, any model representation is only an approximation of clinical reality and may not reflect all possible disease progression pathways experienced by the analyzed cohort. Nevertheless, the clinical events modeled encompass the events and disease states most relevant to HT and its treatment and are in line with prior assessments of HT treatments.41-43 Second, the analysis relies on the currently available 6-month data of the SPYRAL HTN-ON MED5 trial and assumes this effect size is maintained over a lifetime. This assumption, however, seems well supported by the large body of RF RDN evidence available to date, which suggests that treatment effects are maintained, might even increase over time rather than decrease, and do not require retreatment to be maintained.7,44-46 Third, the use of the SPYRAL HTN-ON MED5 observed effect size of –4.9 mmHg change in office SBP vs sham control in the base case is among the lowest effects in the more recent body of RF RDN evidence. Nevertheless, the SPYRAL HTN-ON MED trial5 is the largest sham-controlled RCT of latest-generation RF RDN devices. Finally, quality of life data for Spain are still limited. For this reason, international data were used to inform utility estimates.
CONCLUSIONS
The results of the present analysis, based on contemporary clinical evidence, suggest that RF RDN can be a cost-effective treatment option and might meaningfully reduce clinical events in patients with uncontrolled HT in Spain.
FUNDING
This study was supported by funding from Medtronic plc.
ETHICAL CONSIDERATIONS
Ethics committee approval was not applicable to this work due to the nature of the study, which is an economic evaluation of a health technology. As this study does not involve the participation of individuals, informed consent was not required. Additionally, possible sex and gender biases have been considered and addressed in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tool was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
Methodology was developed by J.B. Pietzsch and K.N. Cao, and was reviewed by O. Rodríguez-Leor, F. Jaén-Águila, T. García- Camarero, and J.A. García-Donaire. Model inputs research was conducted by J.B. Pietzsch, K.N. Cao, A.M. Ryschon, C. Mansilla-Morales, M. Álvarez-Orozco and M. Kolovetsios. The analysis was carried out by K.N. Cao, A.M. Ryschon, and J.B Pietzsch. K.N. Cao, J.B. Pietzsch, and A.M. Ryschon prepared the original draft, while O. Rodríguez-Leor, F. Jaén-Águila, T. García-Camarero, J.A. García-Donaire, C. Mansilla-Morales, M. Álvarez-Orozco and M. Kolovetsios contributed to the review and editing process. Supervision was provided by J.B. Pietzsch, O. Rodríguez-Leor, and J.A. García-Donaire.
CONFLICTS OF INTEREST
O. Rodríguez-Leor, J.A. García-Donaire, F. Jaén-Águila, and T. García-Camarero acknowledge receiving grants from Medtronic to conduct this project. O. Rodríguez-Leor has received grants from Shockwave outside the submitted work. T. García-Camarero has received honoraria from Boston Scientific and Palex outside the submitted work. A.M. Ryschon, K.N. Cao, and J.B. Pietzsch are employed by Wing Tech Inc., a health-economic consulting firm providing consulting services to Medtronic, including for the development of the health-economic analysis framework underlying the current study, and to develop this work. C. Mansilla-Morales, M. Álvarez-Orozco, and M. Kolovetsios are employed full-time by Medtronic.
The authors hereby declare that this economic support has not interfered with the conduct of this project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
WHAT IS KNOWN ABOUT THE TOPIC?
- It is well established that reductions of elevated blood pressure benefit patients by lowering their cardiovascular event risks.
- Such event reductions not only improve patient survival and quality of life, but concurrently also reduce health care utilization and costs.
- RF RDN is an adjunctive treatment option for patients with uncontrolled HT, including R-HT.
WHAT DOES THIS STUDY ADD?
- In the current analysis, blood pressure reductions observed in recent RF RDN studies were used to calculate the expected lifetime benefit and cost implications for the therapy in the Spanish health care system.
- The analysis found that RF RDN, based on an assumed long-term treatment effect, can contribute to a meaningful patient benefit at acceptable incremental costs to the Spanish health care system, rendering the therapy a cost-effective intervention relative to Spain’s WTP threshold of €25 000 per QALY gained.
REFERENCES
1. Mancia G, Cappuccio F, Burnier M, et al. Perspectives on improving blood pressure control to reduce the clinical and economic burden of hypertension. J Intern Med. 2023;294:251-268.
2. Banegas JR, Sánchez-Martínez M, Gijón-Conde T, et al. Numerical values and impact of hypertension in Spain. Rev Esp Cardiol. 2024;77:767–778.
3. Sarafidis PA. Epidemiology of resistant hypertension. J Clin Hypertens (Greenwich). 2011;13:523-528.
4. Whitbourn R, Harding SA, Walton A. Symplicity multi-electrode radiofrequency renal denervation system feasibility study. Eurointervention. 2015;11:104-109.
5. Kandzari DE, Townsend RR, Kario K, et al. Safety and Efficacy of Renal Denervation in Patients Taking Antihypertensive Medications. J Am Coll Cardiol. 2023;82:1809-1823.
6. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal):a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444-1451.
7. Mahfoud F, Boehm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes:3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474-3482.
8. Rodríguez-Leor O, Jaen-Aguila F, Segura J, et al. Renal denervation for the management of hypertension. Joint position statement from the SEH-LELHA and the ACI-SEC. REC:Interv Cardiol. 2022;4:39-46.
9. Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension. Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hyperten. 2023;41:1874-2071.
10. United States Food and Drug Administration. Premarket Approval Application (PMA) for Medtronic, Inc.'s Symplicity Spyral Radiofrequency Renal Denervation System. 2023. Available at:https://www.fda.gov/media/171411/download. Accessed 3 Apr 2024.
11. Sharp ASP, Cao KN, Esler MD, et al. Cost-effectiveness of catheter-based radiofrequency renal denervation for the treatment of uncontrolled hypertension:an analysis for the UK based on recent clinical evidence. Eur Heart J Qual Care Clin Outcomes. 2024;0:1–11.
12. López-Bastida J, Oliva J, Antonanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11:513-520.
13. Soto M, Sampietro-Colom L, Sagarra J, Brugada-Terradellas J. InnovaSEC in action:cost-effectiveness of Barostim in the treatment of refractory hypertension in Spain. Rev Esp Cardiol. 2016;69:563-571.
14. Ribera A, Vela E, García-Altés A, Clèries M, Abilleira S. Trends in healthcare resource use and expenditure before and after ischaemic stroke. A population-based study. Neurología (Engl Ed). 2022;37:21-30.
15. Navarrete-Navarro P, Hart W, Lopez-Bastida J, Christensen M. The societal costs of intracerebral hemorrhage in Spain. Eur J Neurol. 2007;14:556-562.
16. DarbàJ, MarsàA. Burden of ischemic heart disease in Spain:incidence, hospital mortality and costs of hospital care. Expert Rev Pharmacoecon Outcomes Res. 2022;22:1147-1152.
17. Escobar C, Morales C, Capel M, Simón S, Pérez-Alcántara F, Pomares E. Cost-effectiveness analysis of dapagliflozin for the treatment of type 2 diabetes mellitus in Spain:results of the DECLARE-TIMI 58 study. BMC Health Serv Res. 2022;22:1-9.
18. Schwander B, Gradl B, Zöllner Y, et al. Cost-Utility Analysis of Eprosartan Compared to Enalapril in Primary Prevention and Nitrendipine in Secondary Prevention in Europe—The HEALTH Model. Value Health. 2009;12:857-871.
19. Delgado JF, Oliva J, Llano M, et al. Health care and nonhealth care costs in the treatment of patients with symptomatic chronic heart failure in Spain. Rev Esp Cardiol. 2014;67:643-650.
20. Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant. 2011;26:3709-3714.
21. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410-420.
22. Grosso AM, Bodalia PN, MacAllister RJ, Hingorani AD, Moon JC, Scott MA. Comparative clinical-and cost-effectiveness of candesartan and losartan in the management of hypertension and heart failure:a systematic review, meta-and cost-utility analysis. Int J Clin Pract. 2011;65:253-263.
23. Darlington AS, Dippel DW, Ribbers GM, van Balen R, Passchier J, Busschbach JJ. Coping strategies as determinants of quality of life in stroke patients:a longitudinal study. Cerebrovasc Dis. 2007;23:401-407.
24. Aasa M, Henriksson M, Dellborg M, et al. Cost and health outcome of primary percutaneous coronary intervention versus thrombolysis in acute ST-segment elevation myocardial infarction—Results of the Swedish Early Decision reperfusion Study (SWEDES) trial. Am Heart J. 2010;160:322-328.
25. Glasziou P, Alexander J, Beller E, Clarke P, Group AC. Which health-related quality of life score?A comparison of alternative utility measures in patients with Type 2 diabetes in the ADVANCE trial. Health Qual Life Outcomes. 2007;5:1-11.
26. Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women:a cost-utility analysis. Arch Intern Med. 2007;167:290-295.
27. Chen L, Hay JW. Cost-effectiveness of primary implanted cardioverter defibrillator for sudden death prevention in congestive heart failure. Cardiovasc Drugs Ther. 2004;18:161-170.
28. Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med care. 2007;45:1162.
29. Lee CP, Chertow GM, Zenios SA. An empiric estimate of the value of life:updating the renal dialysis cost-effectiveness standard. Value Health. 2009;12:80-87.
30. Organización Nacional de Trasplantes. Registro Español de Enfermos Renales (REER):Informe 2020. Available at:https://www.senefro.org/modules.php?name=webstructure&idwebstructure=29 Accessed 22 Sept, 2022.
31. D'Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal:new results from the Framingham study. Am Heart J. 2000;139:272-281.
32. D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile:adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40-43.
33. Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057-2062.
34. Voss R, Cullen P, Schulte H, Assmann G. Prediction of risk of coronary events in middle-aged men in the Prospective Cardiovascular Münster Study (PROCAM) using neural networks. Int J Epidemiol. 2002;31:1253-1262.
35. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285-2295.
36. Townsend RR, Ferdinand KC, Kandzari DE, et al. Impact of Antihypertensive Medication Changes after Renal Denervation among Different Patient Groups:SPYRAL HTN-ON MED. Hypertension. 2024;81:1095-1105.
37. Rodríguez-Leor O, Mahfoud F, Schmieder R, et al. Reducción de la presión arterial en pacientes de riesgo alto y muy alto tras la denervación renal:resultados a 36 meses del Registro Global Symplicity. Rev Esp Cardiol. 2022;75(Supl 1):552.
38. Gijón-Conde T, Graciani A, Banegas JR. Resistant hypertension:demography and clinical characteristics in 6292 patients in a primary health care setting. Rev Esp Cardiol. 2014;67:270-276.
39. Instituto Nacional de Estadistica. Update a personal income or spending with the overall CPI (CPI system base 2021) for complete annual periods. Available at:https://www.ine.es/calcula/index.do?L=1. Accessed 1 Sept 2022.
40. Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27:746-761.
41. Bress AP, Bellows BK, King JB, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377:745-755.
42. Marra C, Johnston K, Santschi V, Tsuyuki RT. Cost-effectiveness of pharmacist care for managing hypertension in Canada. Can Pharm J (Ott). 2017;150:184-197.
43. Moran AE, Odden MC, Thanataveerat A, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372:447-455.
44. Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED):a randomised, sham-controlled trial. Lancet. 2022;399:1401-1410.
45. Mahfoud F, Mancia G, Schmieder RE, et al. Cardiovascular risk reduction after renal denervation according to time in therapeutic systolic blood pressure range. J Am Coll Cardiol. 2022;80:1871-1880.
46. Bhatt DL, Vaduganathan M, Kandzari DE, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension:final follow-up of the randomised SYMPLICITY HT-3 Trial. Lancet. 2022;400:1405-1416.










