ABSTRACT
Introduction and objectives: After the results of several randomized trials, routine thrombus aspiration (TA) has remained out of the spotlight after not improving the prognosis of patients with ST-segment elevation myocardial infarction and even increasing their complications. The goal here was to assess the impact of selective TA during primary percutaneous coronary intervention (pPCI), its safety and clinical benefits at 1-year follow-up.
Methods: The TAPER registry (efficacy and safety of selective Thrombus Aspiration in Real clinical Practice) retrospectively included patients with ST-segment elevation myocardial infarction treated with pPCI. The clinical and procedural characteristics and the composite endpoint of cardiovascular mortality, non-fatal myocardial infarction, stent thrombosis, target lesion revascularization or stroke were evaluated after at 1-year follow-up.
Results: 687 patients (76.9% males, 64 ± 12 years) were analyzed. The TA was performed in 40.3% of cases (in 89.9% as the initial strategy and in 10.1% as the bailout strategy) and it was successful in 93.8% of them. The most important predictor of TA use was a higher initial Thrombolysis in Myocardial Infarction (TIMI) thrombus grade (OR, 3.2; 95%CI, 2.5-3.9; P < .0001). TA achieved a significant improvement of TIMI-flow (2.4 points) and a significant reduction of the TIMI thrombus grade (2.6 points). At 1-year follow-up, no stroke was observed in the TA-group and the rate of the composite endpoint (cardiovascular mortality, non-fatal myocardial infarction, stent thrombosis, target lesion revascularization or stroke) was similar in both groups (TA-group 8% vs non-TA-group 5.7%; P = .24).
Conclusions: Selective TA is frequently used in the current clinical practice with a high success rate and a low rate of associated complications. It significantly reduces thrombotic burden and improves coronary flow. At 1-year follow-up, a similar rate of adverse events was observed regardless of the use of TA.
Keywords: Thrombus aspiration. Primary PCI. STEMI.
RESUMEN
Introducción y objetivos: Tras los resultados de varios estudios aleatorizados, la tromboaspiración (TA) sistemática ha sido relegada a un segundo plano por no mejorar el pronóstico de los pacientes con infarto agudo de miocardio con elevación del segmento ST e incluso aumentar sus complicaciones. El objetivo de este trabajo fue evaluar el impacto de la TA selectiva durante la angioplastia primaria (ICPp), su seguridad y sus beneficios clínicos tras 1 año de seguimiento.
Métodos: El registro TAPER (eficacia y seguridad de la tromboaspiración selectiva en la práctica clínica real) incluyó retrospectivamente pacientes con infarto de miocardio con elevación del segmento ST tratados con ICPp. Se evaluaron las características clínicas y de los procedimientos, así como la presentación del evento combinado de muerte cardiovascular, infarto de miocardio no fatal, trombosis de stent, necesidad de revascularización de la lesión tratada o ictus tras 1 año de seguimiento.
Resultados: Se analizaron 687 pacientes (76,9% varones, 64 ± 12 años). La TA se realizó en el 40,3% de los casos (89,9% como estrategia inicial y 10,1% como rescate) y fue exitosa en el 93,8%. El predictor más importante de uso de TA fue un alto grado de trombo inicial según la escala TIMI (Thrombolysis in Myocardial Infarction) (odds ratio = 3,2; intervalo de confianza del 95%, 2,5-3,9; p < 0,0001). La TA consiguió una mejora significativa del flujo de 2,4 puntos en la escala TIMI de flujo y una reducción significativa del grado de trombo de 2,6 puntos en la escala TIMI de trombo. En 1 año de seguimiento no se observó ningún ictus en el grupo de TA y la tasa del evento combinado fue similar en ambos grupos (grupo de TA 8% y grupo de no-TA 5,7%; p = 0,24).
Conclusiones: La TA selectiva se usa con frecuencia en la práctica clínica actual, con una alta tasa de éxito y pocas complicaciones asociadas. La TA selectiva reduce significativamente la carga de trombo y mejora el flujo coronario. Tras 1 año de seguimiento, se observó una tasa similar de eventos adversos en los pacientes a quienes se realizó ICPp con independencia del uso de TA.
Palabras clave: Tromboaspiracion. Angioplastia primaria. IAMCEST.
Abreviaturas: Abbreviations pPCI: primary percutaneous coronary intervention. TA: thrombus aspiration.
INTRODUCTION
Primary percutaneous coronary intervention (pPCI) is the preferred treatment for the management of ST-segment elevation myocardial infarction.1 However, one of its limitations is the possibility of distal embolization of thrombus and failure to restore flow at the microvascular level, which is associated with a significantly higher mortality rate.2 Thrombus aspiration (TA) was thought to be a simple method to remove thrombus before stent deployment, thereby reducing distal embolization and improving outcomes.3
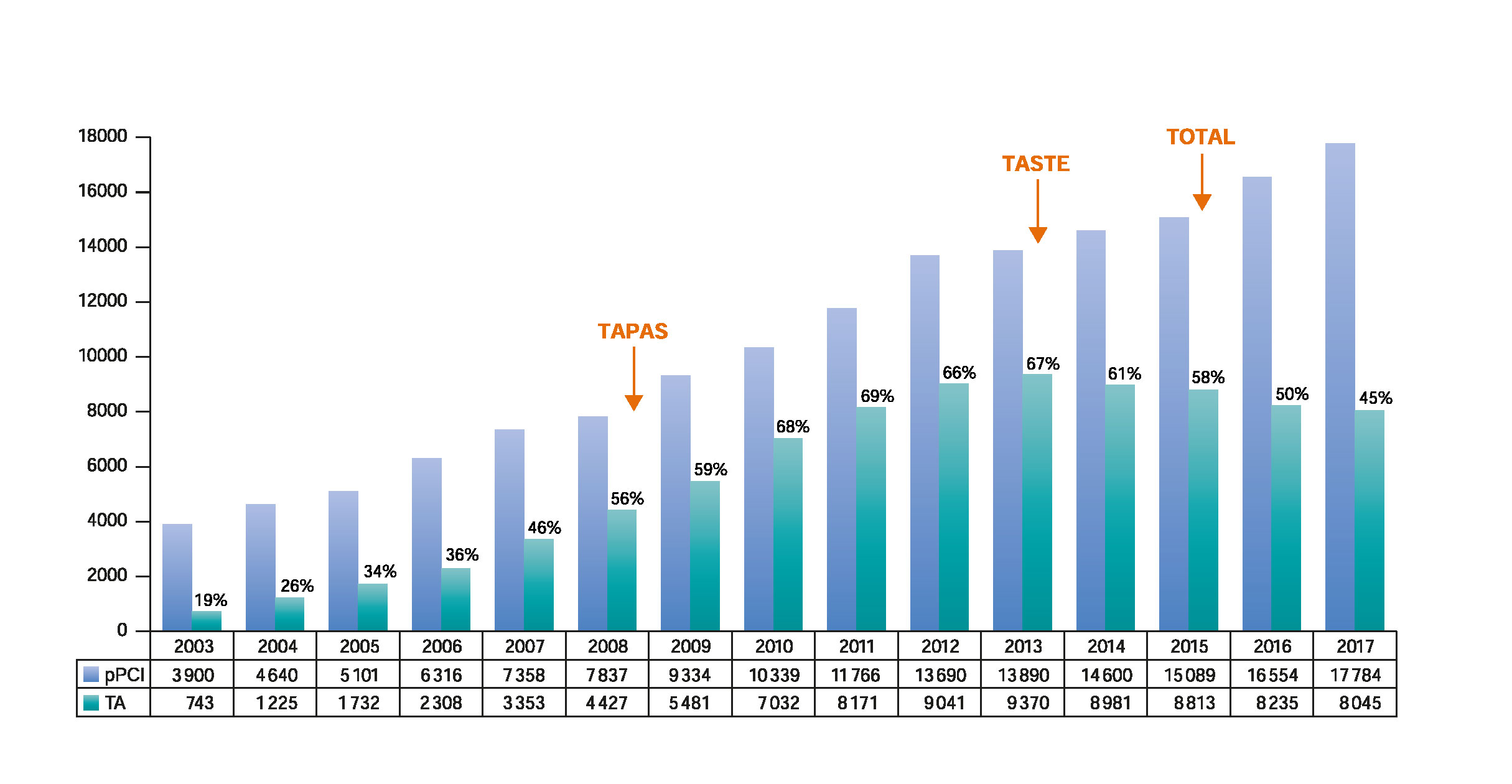
After the promising results of the TAPAS trial,4,5 TA was included in the routine practice and was probably overused.6 However, the results from the TASTE8 and TOTAL9 clinical trials have brought uncertainty to the clinical benefits of TA. Additionally, possible harm from an increased risk of stroke has been suggested.9 Subsequently, guidelines have downgraded the indication for routine TA from IIa10-12 to III,13,14 resulting in a progressive reduction in the use of TA (figure 1).4,7,9,15
Figure 1. Evolution of pPCI and TA over the last 15 years: Evolution of primary percutaneous coronary intervention and TA in Spain over the last 15 years15 in relation to the publication of the main TA trials.4,7,9 pPCI, primary percutaneous coronary intervention; TA, thrombus aspiration.
In addition to the fact that the above-mentioned clinical trials may not reflect the actual clinical practice,6 we should be consider that these recommendations apply for routine TA and not for selective TA, where the operator performs the technique in cases where the expected benefit is higher. Although selective TA may be more indicative of the common practice, we do not have actual data on its application. For this reason, we designed the TAPER registry (efficacy and safety of selective Thrombus Aspiration in Real clinical Practice) in an attempt to analyze the procedural advantages of selective TA during pPCI, its safety and clinical benefit at 1-year of follow-up.
METHODS
Patients and study design
The TAPER registry retrospectively included patients with ST-segment elevation myocardial infarction treated with pPCI in 4 high-volume centres of different countries (A, B, C, D) on a 24/7 program. These centers serve communities of 615 000, 400 000, 450 000, and 350 000 people, respectively.
Consecutive patients with ST-segment elevation myocardial infarction who were referred to undergo pPCI within 12 hours after symptoms onset in the period between January 2015 and December 2016 were included. Those who had received fibrinolytic therapy were not eligible.
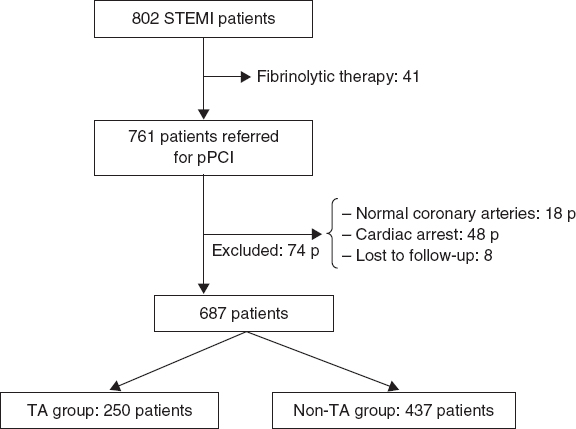
We excluded those patients who did not have an evident culprit coronary lesion, those who presented with cardiac arrest and those who were lost to follow-up. Patients with contraindications to antiplatelet therapy were also excluded (figure 2).
Figure 2. Study flowchart. P, patients; pPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TA, thrombus aspiration.
The TA group was defined as those patients in whom the TA was performed as an initial strategy and non-TA group as those patients in whom the TA was not performed or it was performed as a bailout strategy after balloon dilatation or stent implantation.
Both the clinical and procedural characteristics were analyzed and a combined endpoint of cardiovascular mortality, non-fatal myocardial infarction related to the treated lesion, stent thrombosis, target lesion revascularization or stroke was evaluated at 1-year follow-up.
Study procedures
Patients received antiplatelet and anticoagulant treatment according to the clinical practice guidelines.16 The addition of IIb/IIIa glycoprotein inhibitors was left to the discretion of the operator. The use of TA and other technical details of the pPCI were left at the discretion of the interventional cardiologist. TA was performed using a standard technique.9
Angiographic assessment
The angiographic analysis was performed by 4 experienced interventional cardiologists. After defining the culprit lesion in the initial coronary angiogram, the distal flow of the culprit vessel was assessed using the Thrombolysis in Myocardial Infarction (TIMI) grade score.17 Once the culprit lesion had been crossed with a coronary guidewire, the thrombotic burden was defined according to the TIMI-thrombus scale.18 Both the TIMI-flow scale and the TIMI-thrombus scale were reassessed after the TA. The presence of no-reflow phenomenon and thrombus distal embolization were also evaluated.
Follow-up and clinical endpoints definitions
The follow-up of the patients was carried out through telephone calls and in-hospital clinical records of the visits to the cardiology department after the initial admission.
The occurrence of major acute cardiovascular events (MACE) [cardiovascular mortality, myocardial infarction related to the treated lesion, stent thrombosis or need for revascularization of the treated lesion or stroke] at 1-year follow-up was established as the primary endpoint. The secondary endpoints were the independent analysis of each individual event of the composite endpoint.
All deaths were considered cardiac unless another specific cause was documented. Myocardial infarction was defined following the actual recommendations19 and only those related to the treated lesion, whether periprocedural or at follow-up, were taken into consideration. Target lesion revascularization or stent thrombosis was defined according to the Academic Research Consortium criteria.20
The angiographic success was defined as final TIMI 3 distal flow with less than 20% of vessel stenosis and no immediate mechanical complications. TA was considered successful if an improvement of TIMI-flow ≥ 1 grades or a reduction of TIMI-thrombus scale ≥ 1 grades were achieved, without any immediate complications related to the technique.
Statistical analysis
Quantitative variables following a normal distribution were expressed as mean ± standard deviation. Those that did not follow were described by the median [range]. Qualitative variables were expressed as absolute and relative frequencies of their categories.
P levels < .05 were considered statistically significant and the 95% confidence interval (95%CI) of the target analysis variables was estimated. When it comes to the bivariate analysis, the Student t test or the non-parametric Mann-Whitney U test were used for mean comparison purposes and the chi-square test or Fisher’s exact test were used to compare qualitative variables.
For the multivariate analysis, logistic regression was used. Variables were considered as potential predictors of risk in the multivariate model when they showed a statistically significant association in the univariate analysis. The SPSS statistical package software version 20 (Armonk, NY: IBM Corp), was used for calculations.
RESULTS
Out of the 761 patients initially screened, 74 were excluded (18 patients did not have any evident culprit coronary lesions, 48 patients presented with cardiac arrest, and 8 patients were lost to follow-up). The remaining 687 patients (64.1 ± 12.2 years; 76.9% male) were finally analyzed. The baseline characteristics are shown on table 1.
Table 1. Baseline characteristics
| TA group N = 250 | Non-TA group N = 437 | P | |
|---|---|---|---|
| Age (y) | 63.6 ± 12.6 | 64.4 ± 12.1 | .46 |
| Male | 208 (83.2%) | 320 (73.2%) | .003 |
| BMI | 27.2 ± 6.4 | 26.6 ± 5.8 | .23 |
| Current smoker | 105 (42%) | 140 (32%) | .012 |
| Diabetes mellitus | 44 (17.6%) | 80 (18.3%) | .86 |
| Dyslipidemia | 74 (29.6%) | 103 (23.6%) | .07 |
| Hypertension | 114 (45.6%) | 195 (44.6%) | .68 |
| LVEF | 48.7 ± 10.7 | 49.7 ± 10.4 | .27 |
| Previous PCI | 27 (10.8%) | 37 (8.5%) | .29 |
| Previous CABG | 3 (1.2%) | 5 (1.1%) | .93 |
| Chronic kidney disease | 13 (5.2%) | 13 (2.9%) | .14 |
|
BMI, body mass index; CABG, coronary artery bypass grafting; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention; TA, thrombus aspiration. Data are expressed as no. (%) or mean ± standard deviation. |
|||
Procedural characteristics
In the overall cohort, the culprit lesion was more frequently located at the left anterior descending coronary artery (45.6%), followed by the right coronary artery (36.9%). Forty-eitgh-point-one per cent of patients had multivessel disease. The initial TIMI-flow was 0-1 in 72.7% of cases and the TIMI-thrombus grade was ≥ 3 in 61.6% of the cases.
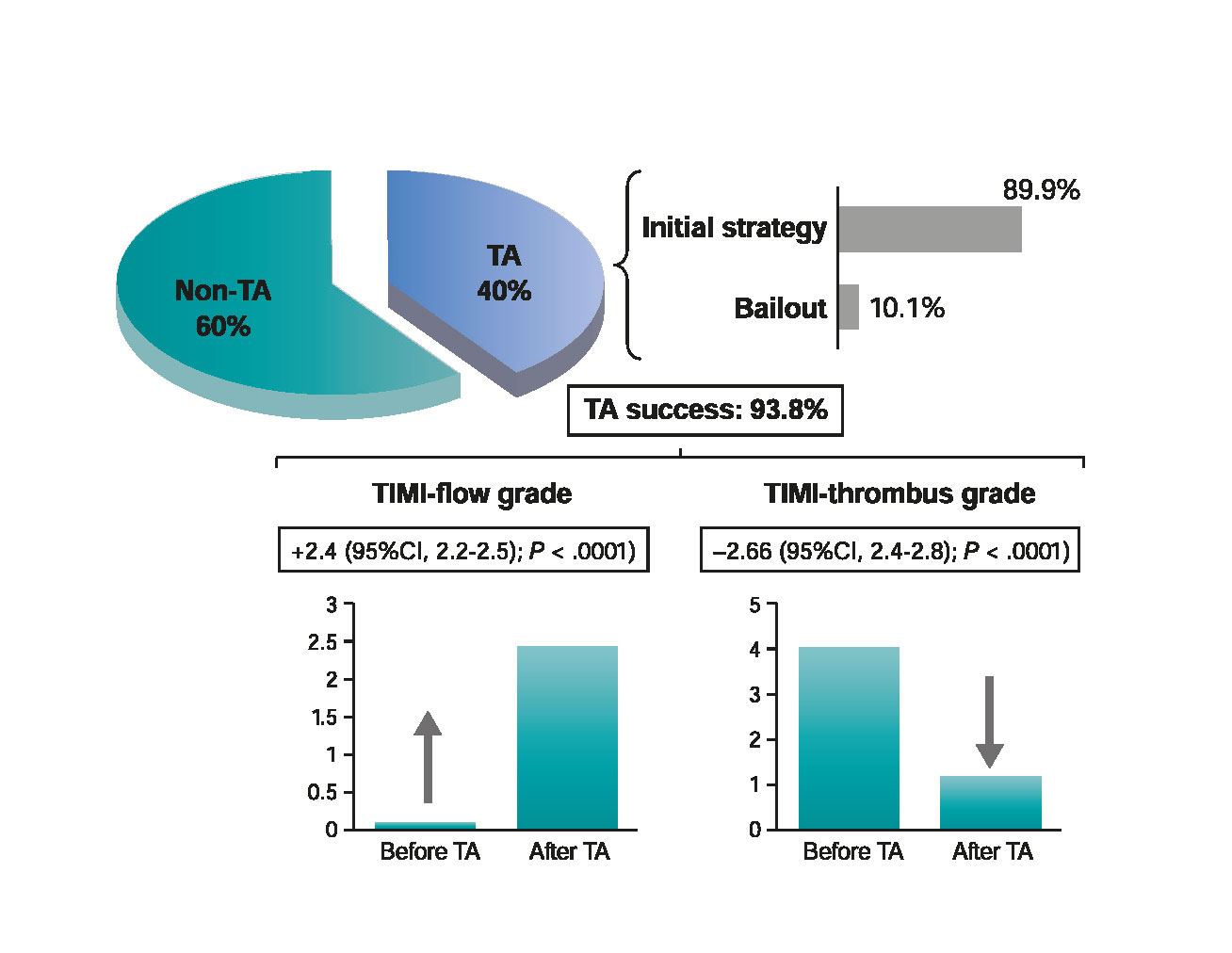
The TA was performed in 40.3% of cases. In 89.9%, the TA was the initial strategy after crossing the culprit lesion with the coronary guidewire, whereas in 10.1% of the cases it was performed as a bailout strategy (figure 3). Procedural characteristics are shown on table 2.
Table 2. Angiographic and procedural characteristics
| TA group n = 250 | Non-TA group n = 437 | P | |
|---|---|---|---|
| Culprit artery | .01 | ||
| LM | 5 (2%) | 2 (0.5%) | |
| LAD | 98 (39.2%) | 209 (47.8%) | |
| LCx | 30 (12%) | 71 (16.2%) | |
| RCA | 114 (45.6%) | 148 (33.8%) | |
| Other | 3 (1.2%) | 1 (0.2%) | |
| Multivessel disease | 107 (42.8%) | 221 (50.6%) | .08 |
| P2Y12 inhibitor | < .0001 | ||
| Clopidogrel | 185 (74%) | 272 (62.2%) | |
| Prasugrel | 15 (6%) | 29 (6.6%) | |
| Ticagrelor | 37 (14.8%) | 119 (27.2%) | |
| Anticoagulation | .69 | ||
| UFH | 245 (96%) | 433 (99%) | |
| Bivalirudin | 2 (0.8%) | 2 (0.45%) | |
| Enoxaparin | 0 (0%) | 1 (0.22%) | |
| Glycoprotein IIb/IIIa inhibitor | .13 | ||
| Abciximab | 91 (36.4%) | 120 (27.5%) | |
| Eptifibatide | 15 (6%) | 18 (4.1%) | |
| Ventricular assist device | 11 (4.4%) | 12 (2.7%) | .24 |
| Initial TIMI-flow | 0.3 ± 0.8 | 1.1 ± 1.3 | < .0001 |
| Initial TIMI-flow 0-1 | 228 (91.2%) | 271 (62%) | < .0001 |
| Initial TIMI-thrombus grade | 4.8 ± 0.9 | 2.5 ± 1.4 | < .0001 |
| Initial TIMI-thrombus grade ≥ 3 | 233 (93.2%) | 191 (43.7%) | < .0001 |
| Initial stent thrombosis (as culprit lesion) | 14 (5.6%) | 7 (1.6%) | .004 |
| Bifurcation (at the culprit lesion) | 62 (24.8%) | 108 (24.7%) | .8 |
| DTB time (minutes) | 101 ± 55 | 102 ± 83 | .8 |
| TA device | |||
| Medtronic Export | 134 (53.6%) | NA | |
| Terumo Eliminate | 96 (38.4%) | NA | |
| Hexacath Recover | 20 (8%) | NA | |
| Direct stenting | 178 (71.2%) | 144 (32.9%) | < .0001 |
| Type of stent | .04 | ||
| Bare metal | 80 (32%) | 108 (24.7%) | |
| Drug-eluting | 170 (68%) | 329 (75.3%) | |
| Stent length (mm) | 29 ± 13.8 | 27.8 ± 14.8 | .29 |
| Stent diameter (mm) | 3.3 ± 0.7 | 3.4 ± 2.2 | .8 |
| Post-dilatation | 43 (17.2%) | 82 (18.8%) | .47 |
| No reflow | 24 (9.6%) | 31 (7.1%) | .24 |
| Distal embolization | 4 (1.6%) | 7 (1.6%) | .97 |
| Angiographic success | 238 (95.2%) | 404 (92.4%) | .16 |
|
DTB, door-to-balloon time; LAD, left anterior descending coronary artery; LCx, left circumflex artery; LM, left main coronary artery; RCA, right coronary artery; TA, thrombus aspiration; TIMI, Thrombolysis in Myocardial Infarction. Data are expressed as no. (%) or mean ± standard deviation. |
|||
Figure 3. Selective TA performance and beneficial effects during primary percutaneous coronary intervention. Percentage of cases in which TA was used (as an initial or bailout strategy) and TA success rate by improving TIMI-flow or TIMI-thrombus grade. TA, thrombus aspiration; TIMI, Thrombolysis in Myocardial Infarction.
Predictors of use of thrombus aspiration
There were significant differences in the use of TA rates among the different centers (A = 63.7%; B = 32.9%; C = 16.9%; D = 15.7%; P < .0001). The TA was more frequently used as the initial strategy in male patients (40.9% vs 26.4%; P = .003), in current smokers (47.7% vs 36.1%; P = .012), and when the culprit lesion was the thrombosis of a former stent (66.7% vs 36%; P = .004). The rate of TA was also different when it comes to the culprit artery (left anterior descending coronary artery, 31.9%; left circumflex coronary artery, 29.7%; right coronary artery, 43.5%; P = .01). Also, the patients from the non-TA group were treated more often with ticagrelor or prasugrel compared to clopidogrel (P < .0001) and received more frequently drug-eluting stents (TA group, 68% vs non-TA group, 75.3%; P = .04). In the patients from the TA-group, the initial TIMI-flow was significantly lower (0.3 ± 0.8 vs 1.1 ± 1.3; P < .0001) and the initial TIMI-thrombus grade was higher (4.3 ± 0.9 vs 2.5 ± 1.4; P < .0001).
In the multivariate analysis, we included those variables that showed a statistically significant association with TA in the univariate analysis: gender, current smoking habit, culprit artery, P2Y12 inhibitor, initial TIMI-flow, initial TIMI-thrombus grade, initial stent thrombosis (as culprit lesion), center and type of stent. The strongest independent predictor for the use of TA as the initial strategy was a higher initial TIMI-thrombus grade (odds ratio [OR], 3.2; 95%CI, 2.5-3.9; P < .0001). The performance of the pPCI in center A (OR, 20.7; 95%CI, 10-42.5; P < .0001) or B (OR, 3.3; 95%CI, 1.4-7.5; P = .005) was also an independent predictor of TA (compared to center D; the center where the TA was less frequently used). Culprit lesions located at the right coronary artery [OR, 2; 95%CI, 1.008-3.9; P = .047] were also identified as predictors for the use of TA as the initial strategy.
Angiographic results after thrombus aspiration
When TA was performed as initial strategy, a significant improvement of TIMI-flow (2.4; 95%CI, 2.2-2.5; P < .0001) and a significant reduction of TIMI-thrombus grade [2.6; 95%CI, 2.4-2.8; P < .0001] were observed (figure 3). There were no significant differences between both groups in the occurrence of no-reflow phenomenon or distal embolization. The rate of direct stenting was twice as frequent in the TA group. Similarly, the rate of procedural success was high and similar in both groups (TA group, 95.2% vs non-TA group, 92.4%; P = .16) (table 2).
Adverse events at follow-up
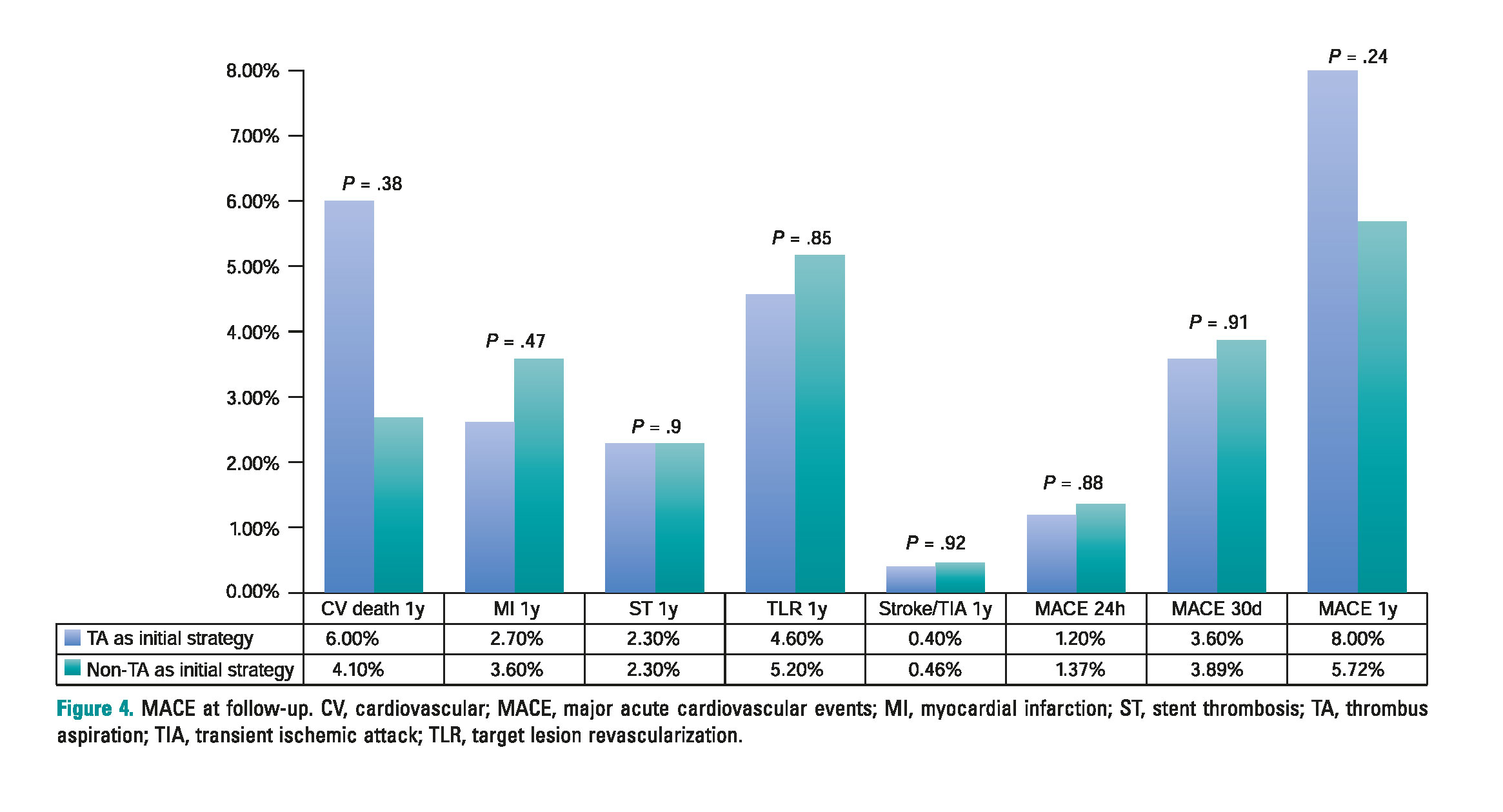
After a 1-year follow-up, there were no significant differences in terms of the overall rate of MACE between both groups (TA group, 8% vs non-TA group, 5.7%; P = .24). Also, no differences were seen in any of the individual adverse events: cardiovascular mortality (TA group, 5.2% vs non-TA group, 3.9%, P = .38), myocardial infarction (TA group, 2.4% vs non-TA group, 3.4%; P = .47), stent thrombo- sis (TA group, 2.4% vs non-TA group, 2.3%, P = .9) or target lesion revascularization (TA group, 4.4% vs non-TA group, 4.8%; P = .85). The incidence of cerebral ischemic events was similar in both groups (TA group, 0.4% vs non-TA group, 0.46%; P = .92). One patient only was diagnosed with transient ischemic attack in the TA group that occurred > 30 days after the pPCI. None of the patients of this group suffered a stroke during follow-up. In the non-TA group, two pati- ents suffered a stroke (one was a ischemic stroke 24 hours after the pPCI and the other one was a hemorrhagic stroke that occurred 3 months after the procedure in a patient treated with triple therapy due to atrial fibrillation). There were no differences in the rate of MACE during the first 24 hours after pPCI (TA group, 1.2% vs non-TA group, 1.4%; P = .88) or at the 1-month follow-up (TA group, 3.6% vs non-TA group, 3.9%; P = .9) (figure 4).
Figure 4. MACE at follow-up. CV, cardiovascular; MACE, major acute cardiovascular events; MI, myocardial infarction; ST, stent thrombosis; TA, thrombus aspiration; TIA, transient ischemic attack; TLR, target lesion revascularization.
DISCUSSION
The main findings of this study are: a) TA is frequently used during pPCI (40.3%), mainly as an initial strategy, with significant differences between the different centers; b) A higher initial TIMI-thrombus grade is the most important predictor for the use of TA; c) TA has a high technical success rate, leading to a significant reduction of the thrombus burden and an improvement of TIMI-flow, facilitating pPCI by allowing more frequently direct stenting; d) TA was not associated with higher rates of cerebrovascular events; and e) The TA was not associated with any differences in the occurrence of MACE during acute phase or at the 1-year follow-up.
The present study analyzes the efficacy and safety of selective TA in the real clinical practice. And this is remarkable for 2 reasons: the most important TA studies4,7,9 assessed the routine use of this technique. Performing routine TAs during pPCI is not the standard in clinical practice, where TA is selectively performed in scenarios where it is expected that this technique will be more effective. Also, some of these trials may have a non-negligible sample selection bias6,7,9 that may not reflect the actual clinical practice. In our study, the average rate of TA use was around 40.3%. This rate was similar in other nationwide registries.15 The most important predictor for the use of TA was a high initial thrombotic burden.
A key finding of this registry is that TA is effective when it comes to facilitating the pPCI. Unlike other studies, we specifically described thrombotic burden reductions and coronary flow improvements after the TA which, in our opinion, are the most representative effects of the utility of this technique. TA success was achieved in 93.8% of the cases. Since this was not a randomized study, it is not easy to analyze the reduction of no-reflow or the rate of distal embolization with TA. This is so because while trying to reflect real practice TA was used at the discretion of the operator and consequently the TA-group had a higher initial thrombus grade (approximately twice as much) compared to the non-TA group (table 2). It is precisely in patients with a higher thrombotic burden where we can expect higher no-reflow or distal embolization rates. However, probably due to this initial TA that allowed significant reductions of the TIMI-thrombus grade, there were no differences in the rates of no-reflow or distal embolization with the non-TA group that had a lower initial thrombus grade.
Also, patients who underwent TA as an initial strategy had a more than two-fold increase in the rate of direct-stenting compared to those treated conventionally. Beyond the potential economic benefit, direct stenting could be associated with clinical benefits.21
As our study suggests it is possible that the greatest benefit of TA occurs when performed selectively in patients with a higher thrombotic burden. This idea has been suggested in a meta-analysis including the most important TA studies.3 These results must be interpreted with caution since a significant percentage of patients who underwent routine TA did not have significant thrombotic loads. In the TAPAS trial, angiographic thrombi were not observed in 51.4% of cases,4 which also happened in 35% of patients included in the TASTE trial.7 The TOTAL study showed that in up to 90% of patients the thrombus scale ≥ 3.9 Nevertheless, the thrombotic burden was assessed before crossing the culprit lesion with the coronary guidewire which probably lead to overestimating the TIMI-thrombus scale18 as 65% of patients showed TIMI 0 flow. In our own opinion this limits the conclusions drawn from this trial and subsequent subanalyses.22
Beyond the effectiveness of TA, our results support the safety of the technique. The TOTAL study9 described a slight increase in the rate of TA-related strokes. This fact was not consistent with previous trials and ignited and ongoing controversy that still goes on. In our study, there were no significant differences in the stroke rate between both groups. These data are similar to those from the TASTE7,8 or INFUSE-AMI trial.23 On this regard, TA-related strokes would initially be of ischemic nature, appear during the procedure, and would be evident during the first 24 hours. It is unlikely that hemorrhagic or ischemic strokes occurring > 24 hours after the procedure would have anything to do with this technique.24 In the TOTAL trial,9 the rate of ischemic strokes during the first 48 hours after the procedure was low and did not significantly differ between arms. Also, in the on-treatment and per-protocol analyses the rate of all-cause stroke at 30 days was no significantly different between groups.
Similar to the TA trials most recently published,7,9 we did not find a significant prognostic benefit associated with TA during the acute phase or at the 1-year follow-up. Despite having repeatedly demonstrated that TA improves reperfusion parameters,4,9 the lack of an association with any prognostic benefits somehow makes sense. First because it is unlikely that a technical tool, designed to facilitate pPCI, can reduce mortality. Secondly, because the low rate of events described (reported in our cohort as in previous studies) make it difficult for an individual therapy to prove significant reductions of mortality rate. Finally, because mortality depends on many more factors that were not analyzed in our study or in these trials.25
Limitations
This is a retrospective, observational study, with the natural limitations of this design. The exclusion of patients who had a cardiac arrest or underwent bailout PCIs may be indicative of selection bias.
The quantification of thrombotic load according to the previously validated TIMI-thrombus scale may not be accurate due to the design of this tool. We have maintained this classification because it is the most widely used in this setting. However, the degree of thrombus before the TA was assessed after crossing the culprit lesion with the angioplasty guidewire in order to reduce the number of cases with initial TIMI-flow = 0 in which it is impossible to assess the initial TIMI-thrombus grade.
We decided to define TA success when achieving improvements of TIMI-flow grade ≥ 1 or reductions of TIMI-thrombus scale grades ≥ 1 without any immediate complications associated with the technique. However, other parameters of microvascular reperfusion such as the ST-segment elevation resolution or the myocardial blush grade were not measured.
The angiographic coronary flow data are not described after the alternative strategy to perform TA in the non-TA group. This can be a limitation since the immediate results cannot be compared to the TA group. Also, the angiographic analysis was not conducted by an independent core-lab that would have added validity to the results.
The high heterogeneity of the operators involved may have influenced the results seen due to their individual preferences in relation to TA. However, we believe that this may play a favorable role in the external validation of our findings.
CONCLUSIONS
Despite being recently discredited, TA is frequently used in current clinical practice during the pPCI, basically as an initial strategy. A higher initial TIMI-thrombus grade is the most important predictor for the use of selective TA. Selective TA facilitate pPCI by reducing thrombotic burden and improving coronary flow. Selective TA is not associated with with a reduction of MACE neither during the acute phase or at the 1-year follow-up. There is no association of TA with a higher stroke rate.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- Following the results of several randomized trials, routine TA has remained out of the spotlight after not improving the prognosis of patients with ST-segment elevation myocardial infarction and even increasing their complications.
- Although selective TA may reflect better common practice, we do not have enough data on its implementation.
WHAT DOES THIS STUDY ADD?
- Selective TA is still frequently used in current clinical practice during pPCI, mainly as an initial strategy.
- A higher initial TIMI-thrombus grade is the most important predictor for the use of TA.
- It facilitates pPCI by reducing thrombotic burden and improving coronary flow.
- Selective TA is not associated with a reduction of MACE at 1-year follow-up. There is no association between TA and a higher stroke rate.
REFERENCES
1. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
2. Henriques JP, Zijlstra F, Ottervanger JP, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23:1112-1117.
3. Jolly SS, James S, Dzavik V, et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction:An Individual Patient Meta-Analysis:Thrombectomy Trialists Collaboration. Circulation. 2017;135:143-152.
4. Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567.
5. Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS):a 1-year follow-up study. Lancet. 2008;371:1915-1920.
6. Jurado-Roman A, Sanchez-Perez I, Lozano-Ruiz-Poveda F. Letter by Jurado-Roman et al Regarding Article, “Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction:An Individual Patient Meta-Analysis:Thrombectomy Trialists Collaboration“. Circulation. 2017;135:e1099-e1100.
7. Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
8. Lagerqvist B, Frobert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111-1120.
9. Jolly SS, Cairns JA, Yusuf S, et al.;for the TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389-1398.
10. Wijns W, Kolh P, Danchin N, et al.;Task Force on Myocardial Revascularization of the European Society (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555.
11. O'Gara PT, Kushner FG, Ascheim DD, et al.;American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-425.
12. Levine GN, Bates ER, Blankenship JC, et al.;American College of Cardiology Foundation;American Heart Association Task Force on Practice Guidelines;Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44-122.
13. Neumann FJ, Sousa-Uva M, Ahlsson A, et al.;Group ESCSD. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
14. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction:An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2016;67:1235-1250.
15. Cid Álvarez AB, Rodríguez Leor O, Moreno R, Pérez de Prado A. Spanish Cardiac Catheterization and Coronary Intervention Registry. 27th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2017). Rev Esp Cardiol. 2018;71:1036-1046.
16. Windecker S, Kolh P, Alfonso F, et al.;Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization:The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619.
17. Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I:A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142-154.
18. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B-14B.
19. Thygesen K, Alpert JS, Jaffe AS, et al.;ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551-2567.
20. Cutlip DE, Windecker S, Mehran R, et al. Academic Research Consortium. Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344-2351.
21. Mahmoud KD, Jolly SS, James S, et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention:Thrombectomy Trialists Collaboration. Eur Heart J. 2018;39:2472-2479.
22. Jolly SS, Cairns JA, Lavi S, et al.;TOTAL Investigators. Thrombus Aspiration in Patients With High Thrombus Burden in the TOTAL Trial. J Am Coll Cardiol. 2018;72:1589-1596.
23. Stone GW, Maehara A, Witzenbichler B, et al.;INFUSE-AMI Investigators. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction:the INFUSE-AMI randomized trial. JAMA. 2012;307:1817-1826.
24. Sabate M, Brugaletta S. Thrombectomy and Stroke:Guilty or Innocent Bystander?J Am Coll Cardiol. 2018;72:1597-1599.
25. Spitzer E, Heg D, Stefanini GG, et al. Aspiration Thrombectomy for Treatment of ST-segment Elevation Myocardial Infarction:a Meta-analysis of 26 Randomized Trials in 11,943 Patients. Rev Esp Cardiol. 2015;68:746-752.
Corresponding author: Unidad de Cardiología Intervencionista, Hospital General Universitario de Ciudad Real, Avda. del Obispo Rafael Torija s/n, 13005 Ciudad Real, Spain.
E-mail addresses: alfonsojuradoroman@gmail.com (A. Jurado-Román).