ABSTRACT
The management of chronic total coronary occlusions (CTO) is still today one of the greatest challenges of cardiology. The complexity of the angioplasty procedure of a CTO added to its controversial clinical benefits has generated certain skepticism in the community of cardiologists when developing coronary deocclusion programs at the catheterization laboratory. However, the evidence from observational studies indicates that if the intervention is successful it can significantly increase the patient’s quality of life, improve the left ventricular function, reduce the need for a subsequent CABG, and possibly improve survival. Several factors must be taken into consideration in the selection of patients elective for an intervention, including the extent of ischemia surrounding the occlusion, the myocardial viability, the coronary location of the CTO, and the chances of being successful with the procedure. This review provides a general description of the anatomy and histopathology of the CTOs, the evidence surrounding the clinical benefit of these procedures, the use of useful scoring systems to assess more objectively the probability of success, and a summary of the latest techniques available today to perform this procedure.
Keywords: Coronary artery disease. Chronic total coronary occlusion. Percutaneous coronary intervention. Stable ischemic heart disease.
RESUMEN
El tratamiento de la oclusión total coronaria (OTC) sigue siendo uno de los grandes retos de la cardiología. La complejidad del procedimiento de angioplastia de una OTC, unida a cierta controversia en cuanto al beneficio clínico, han generado resistencias en la comunidad de cardiólogos para desarrollar programas de intervención coronaria en los laboratorios de hemodinámica. Sin embargo, la evidencia proveniente de estudios observacionales indica que el intervencionismo con éxito puede aumentar de manera significativa la calidad de vida del paciente, mejorar la función ventricular izquierda, reducir la necesidad de una posterior cirugía y, posiblemente, prolongar la supervivencia. Deben tenerse en cuenta varios factores en la selección de los pacientes para el intervencionismo, como la extensión de la isquemia que rodea a la oclusión, la viabilidad miocárdica, la ubicación coronaria de la OTC y la probabilidad de éxito del procedimiento. Esta revisión proporciona una descripción general de la anatomía y de la histopatología de las OTC, la evidencia sobre el beneficio clínico del intervencionismo, el uso de sistemas de puntuación que pueden ser útiles para evaluar de forma más objetiva la probabilidad de éxito, y un resumen de las técnicas actuales para la realización del procedimiento.
Palabras clave: Enfermedad arterial coronaria. Oclusión total crónica. Intervención coronaria percutánea. Cardiopatía isquémica estable
Abbreviations: CABG: coronary artery bypass graft. CAD: coronary artery disease. CTO: chronic total coronary occlusion. DLM: dual lumen micro-catheter. IVUS: intravascular ultrasound. MACE: major adverse cardiovascular event. PCI: percutaneous coronary intervention.
INTRODUCTION
Treating patients with chronic total coronary occlusions (CTO) is one of the toughest challenges in the management of coronary artery disease (CAD). Nowadays, the indications for prescribing percutaneous coronary interventions (PCI) in patients with CTOs and the possible impact of revascularization on final prognosis are controversial. No wonder that many interventional cardiologists try to avoid these potentially expensive and long procedures with a significant exposure to radiation. The complexity and lack of familiarity with the new devices and techniques often leads to failed attempts and abandoning this intervention prematurely. However, there are techniques currently available with high rates of success.
That is why in this review we urge the community of interventional cardiologists to promote clinical excellence by teaching coronary disocclusion through learning curves and promoting research and technological advances in this field.1
DEFINITION, EPIDEMIOLOGY, AND CLINICAL SIGNS
Definition
The current consensus establishes the definition of a true CTO as the presence of TIMI (Thrombolysis In Myocardial Infarction) grade-0 flow in the occluded segment with an estimate duration of over 3 months. The duration of an occlusion is difficult to determine with absolute certainty. That is why it is often established after carefully assessing the clinical history and the heart disease symptoms over the previous 3 months.
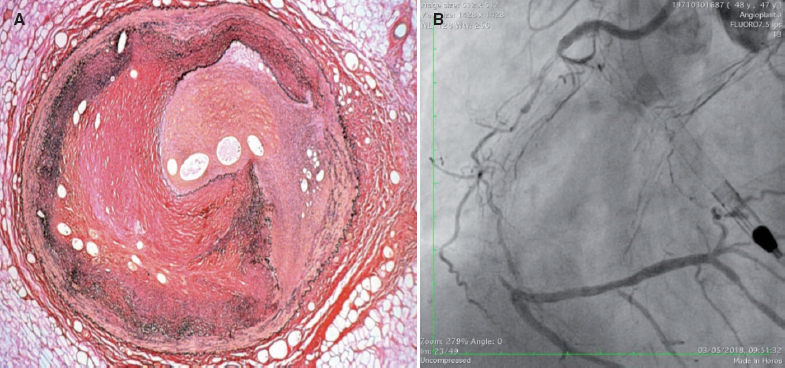
An important aspect is the process of neovascularization that happens all through the occlusive lesion and on the vessel wall. Neoangiogenesis grows with the time of occlusion. In CTO durations < 1 year, the formation of new blood capillaries is predominantly adventitial. In CTO durations > 1 year, there is usually a rich network of new blood vessels running through the adventitial layer of the vessel wall towards the intima forming the bridging collaterals. The process of new vascular formation can promote the formation of relatively long capillary blood vessels called microchannels that run through the body of the occlusion partially recanalizing the distal lumen (figure 1A). Their presence is important because an angioplasty guidewire with hydrophilic coating can run through them and reach the distal lumen. Also, microchannels can connect to the vasa vasorum of the adventitial layer creating an extraluminal collateral pathway to the distal lumen with the typical appearance of caput medusae (figure 1B). This is typical of complex CTOs of long duration. In general, the toughness and density of the fibrocalcific material and the complexity of the CTO are related to the duration of the occlusion.2
Figure 1. A: presence of microchannels inside the vessel lumen surrounded by significant fibrosis. B: typical caput medusae extraluminal neovascularization.
Another key anatomic component of CTOs is collateral circulation that supplies blood flow to the occluded territory. It can be epicardial or intramyocardial and it originates at the homolateral or contralateral coronary territory. When it is present before the CTO occurs, it supplies enough blood flow to maintain the viability of the myocardium irrigated by the occluded artery. However, this is often insufficient to prevent the appearance of exercise-induced angina pectoris or ischemia. Collateral circulation does not require myocardial viability to develop. That is why it is important to emphasize that the disocclusion of a CTO should not be based on the presence, amount or quality of collateral circulation.3,4
Epidemiology
According to data obtained from the HLBI Dynamic Registry5 between 1997 and 1999, CTOs are more prevalent in the right coronary artery and less prevalent in the circumflex artery. In this series, the percentage of patients who underwent PCIs due to their CTOs was 15.6%. According to data from the EuroCTO Club, in 28 283 patients, 12% of the PCIs were performed on CTOs. The prevalence of the CTOs reported ranges from 16% to 50% in patients with clinically significant CAD but in general it is around 18% to 20%.6,7
Clinical signs
Patients with CTOs have a more unfavorable profile of cardiovascular risk compared to those without CTOs.6 The clinical presentation of a CTO is varied: stable angina, silent ischemia, ischemia- related heart failure, early-onset angina or as an incidental finding in patients undergoing primary PCIs due to acute occlusion in a different culprit vessel.
In stable CAD, the goal of revascularizing a CTO is to improve symptoms and prognosis by assessing the presence of symptoms, viability or ischemia. For this reason, in asymptomatic patients with CTOs, the ischemic load should be assessed before considering a PCI.8 In patients with confirmed prior myocardial infarction and segmental contraction abnormalities, it is advisable to conduct a non-invasive study before the treatment to establish the presence of ischemia or viability in the territory of the occluded artery.
BENEFIT OF CORONARY INTERVENTIONS WHEN TREATING CHRONIC TOTAL CORONARY OCCLUSIONS
Several studies have documented that the successful treatment of a CTO leads to the clinical improvement of angina pectoris9, the normalization of functional tests, an improved left ventricular function, and less coronary revascularization surgeries.10,11 It has also been reported that the recanalization of a CTO contributes to the electric stabilization of the myocardial segment and improves clinical tolerance to future coronary events in the non-occluded territory.12
Yet despite this, many patients with single-vessel CAD chronically occluded are only treated with drugs, regardless of the severity of the symptoms and level of ischemia. The presence of a CTO in patients with multivessel disease is a classic indication for surgery. In the randomized SYNTAX trial13 (surgery versus PCI in multivessel disease), 27% of the patients of each group had at least 1 CTO and in general they were more complex patients and with higher SYNTAX scores. Occluded vessels were revascularized in only 68.1% of the patients randomized to surgery, and the rate of success of percutaneous revascularization in patients undergoing PCI was 49.4%. This lead to complete revascularization in 49.6% of surgical cases and 35.8% of PCI cases. This means that the strongest reason for having a SYNTAX score > 32 —the presence of a CTO— is not necessarily indicative that this CTO should be surgically revascularized as it actually happened in 31.9% of the cases. Patients with incomplete revascularizations had a significantly larger number of the composite endpoints of death, infarction or stroke.
Regarding the benefit of interventional procedures, the clinical evidence from randomized clinical trials and observational studies varies. Overall, randomized trials have limitations such as slow recruitment rates that give rise to insufficient and inadequately powered samples with a high rate of crossing among the study groups. Similarly, patients are subject to selection bias since it is unacceptable to randomize patients with a great ischemic load who may benefit from interventional procedures. For this reason, the most favorable data on interventional procedures come from observational studies that have become an essential tool in the overall assessment of the benefits of revascularization.
Randomized clinical trials
The EuroCTO trial proved that interventional procedures on a CTO improve health, the frequency of angina, the level of physical limitations, and the quality of life of patients with stable angina.14
However, the EXPLORE trial15 showed no differences in the left ventricular function in patients with ST-segment elevation myocardial infarction undergoing early interventional procedures of their CTOs compared to the optimal medical treatment. The DECISION- CTO16 showed similar rates of death, infarction, stroke or target lesion revascularization at 3 years in groups undergoing interventional procedures and receiving drugs in patients with acute coronary syndrome or stable angina.15,16
The recently published REVASC trial17 did not show any improvement either in the regional myocardial function at 6 months assessed though MRI in the CTOs of patients consecutively treated with PCI compared to the optimal medical treatment. Nevertheless, the annual rate of major adverse cardiovascular events (MACE) was significantly lower in the PCI group.17
Table 1 shows the results of randomized clinical trials on most relevant CTOs.
Table 1. Results of randomized clinical trials on the most relevant chronic total coronary occlusions
| DECISION-CTO16 | EuroCTO14 | EXPLORE15 | REVASC17 | |
|---|---|---|---|---|
| Patients | 834 | 407 | 304 | 205 |
| Study period | 2010-2016 | 2012-2015 | 2007-2015 | 2007-2015 |
| Comparison | CTO PCI vs OMT | CTO PCI vs OMT | CTO PCI vs OMT in STEMI | CTO PCI vs OMT |
| Primary endpoint | Death, infarction, stroke, TVR | Clinical state and quality of life | LVEF, LVEDV | Segmental thickening CTO territory through MRI. MACE: NS |
| Rate of crossing (%) | 18.1 | 7.3 | ||
| J-CTO score | 2.2 ± 1.2 | 1.82 ± 1.07 | 2 ± 1 | 2 ± 1 |
| Rate of success (%) | 91.1 | 86.3 | 73 | 99 |
| Follow-up | 3 years | 12 months | 4 months | 12 months |
| MACE (%) | 19.0 (OMT) vs 21.4 (PCI); P = NS | 6.7 (OMT) vs 5.2 (PCI); P = NS | 5.4 (OMT) vs 2.6 (PCI); P = NS | 16.3 (OMT) vs 5.9 (PCI); P = .02 |
| Conclusion | No improvement in primary endpoint | Improvement in clinical signs and quality of life | No improvement in LVEF or LVEDV | No improvement in segmental thickening |
|
CTO, chronic total coronary occlusions; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event; MRI, magnetic resonance imaging; NS, not significant; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TVR, target vessel revascularization. |
||||
Observational studies
Several observational studies have compared the results of PCI and medical treatment. Tomasello et al.18 examined the long-term results of 1777 patients with CTOs from the Italian CTO registry based on the treatment strategy: PCI (43.7%), medical treatment (46.5%) or surgery (9.8%). At the 1-year follow-up, medical treatment was associated with a higher rate of MACE (7.6% vs 1.7%; P < .001), cardiovascular mortality (4.4% vs 1.5%; P = .002), acute myocardial infarction (2.9% vs 1.1%), and rehospitalization (4.4% vs 2.3%; P = .04). Jang et al.19 compared the long-term results of different treatment strategies in 738 patients with, at least, 1 occlusive lesion and well-developed collaterals. The 42-month mean follow-up showed that the incidence of cardiovascular mortality (hazard ratio [HR], 0.27; 95% confidence interval [95%CI], 0.09-0.80; P = .029) and MACE (HR, 0.44; 95%CI, 0.23-0.82; P = .01) in patients who underwent coronary revascularizations was lower.
Several small observational studies have explored the possible effect the PCI has on CTOs through several secondary endpoints like depression, the capacity of performing physical activities, and the risk of ventricular arrhythmias with favorable results for the PCI.12,20,21
Similarly, other studies have compared successfully recanalized CTOs with failed ones. A meta-analysis of 25 studies compared successful (71%) with failed procedures (29%) performed from 1990 through 2014 in 28 486 patients. The 3.11-year mean follow-up showed that success was associated with a lower mortality rate (odds ratio [OR], 0.52; 95%CI, 0.43-0.63), less residual angina (OR, 0.38; 95%CI, 0.24-0.60), lower risk of stroke (OR, 0.72; 95%CI, 0.60-0.88), and less need for further coronary revascularization surgeries (OR, 0.18; 95%CI, 0.14-0.22).22 Tsai et al.23 examined the interventional attempts on the CTOs of 2394 patients from 79 centers between 2007 and 2013. The success of the PCI was associated with a lower risk-adjusted mortality rate and referral to coronary revascularization at 2 years.
The OPEN CTO registry24 used the Seattle Angina Questionnaire in 1000 consecutive patients undergoing a hybrid strategy. According to the questionnaire, at the 1-month follow-up the quality of life improved (from 49.4 ± 0.9 to 75.0 ± 0.7; P < .01) with a simultaneous reduction of symptoms.
It should be mentioned that the location of the CTO in the coronary tree can be important for patient’s survival. In a study of 2608 patients, the PCI of the CTO benefited the survival of patients with occlusions in their anterior descending artery only (88.9% vs 80.2%; P < .001).25
Lastly, a meta-analysis that studied these 4 randomized trials and 3 observational studies found no differences in the primary composite endpoint studied (cardiovascular mortality, myocardial infarction and coronary reinterventions). The independent analysis of each component showed that the interventional procedure had better results on cardiovascular mortality (OR, 0.52; 95%CI, 0.33-0.81; P < .01) basically at the expense of the favorable results of observational studies.26
FACTORS IMPACTING SUCCESS
Added to clinical factors such as the extent of ischemia surrounding the occlusion, myocardial viability, and the location of the CTO, the probability of success of the procedure when recanalizing an occlusion should also be taken into consideration.
Prerequisites to perform interventional procedures on CTOs
To optimize the chances of success and overcome the differences in the rates of success achieved by different registries (54% to 80%) and experienced centers (85% to 90%), new machines and techniques should probably be developed, as well as training, continuing medical education programs, and live-case demonstrations.27 The best case-scenario would be that each center training interventional cardiologists implemented CTO disocclusion programs to provide enough theoretical knowledge for the right selection of patients and CTOs; as well as having practical experience to increase the chances of success and avoid the most common mistakes.28
Predictors of success and failure
Numerous predictors of success and failure have been reported in the recanalization of CTOs although, in general, there is wide consensus among the studies.
A meta-analysis reviewed the angiographic and demographic predictors of clinical and technical success.29 Among the demographic variables, it has been shown that prior infarctions and PCIs, coronary revascularization surgeries, strokes, and peripheral vascular disease are associated with a reduction of at least 20% of success probability. The angiographic variables associated with lower chances of success were the presence of bridging collaterals, moderate-to-severe calcifications, vessel angulations > 45º, vessel tortuosity, presence of blunt stumps, ostial occlusive lesions, and CTOs in vessels other than the anterior descending artery.29
Scoring systems in the interventional procedures of a CTO
Over the last few years numerous scoring systems have been developed to predict the chances of technical success in disocclusion procedures.
Scoring systems are considered very useful for several reasons: a) they quantify the chances of success and complications; b) they optimize the selection of cases; c) they study and plan how the CTO should be accessed; and d) they contribute to standardize the complexity of the lesions and compare the results.30
The J-CTO scoring system31 assigns 1 point to every independent predictor of crossing the occlusive lesion within 30 minutes after starting the procedure. The total value was used to develop a model to categorize all lesions into 4 groups depending on the difficulty of the procedure: easy (score = 0), intermediate (score = 1), difficult (score = 2) or extremely difficult (score = 3-5). In our own opinion, high J-CTO scores do not mean that we should not perform an interventional procedure but that the patient should be referred to an experienced center for revascularization surgery.31
The ORA scoring system is more appropriate for experienced interventional cardiologists used to hybrid and retrograde procedures.32 The CL33 is more suitable for operators who use the antegrade access only, and the PROGRESS CTO system34 is suitable for hybrid procedures of disocclusion.
Table 2 shows some of the most common scoring systems used today.
Table 2. Most common scoring systems used today
| Variable | J-CTO31 | ORA32 | CL33 | PROGRESS34 |
|---|---|---|---|---|
| No. of cases | 494 | 1073 | 1657 | 781 |
| Primary endpoint | Guidewire crossing < 30 min | Technical success | Technical success | Technical success |
| Age, years | – | + (≥ 75) | – | – |
| Prior coronary revascularization surgery | – | – | + | – |
| Prior failure | + | – | – | – |
| Proximal capsule | + (blunt) | + (ostial) | + (blunt) | + (ambiguous) |
| Tortuosity | + (> 45° intralesional) | – | – | + (moderate/proximal) |
| Calcification | + | – | + (serious) | – |
| Lesion length | + (≥ 20 mm) | – | + (≥ 20 mm) | – |
| Target vessel | – | – | + (if target vessel different from anterior descending artery) | + (if the target vessel is the circumflex artery) |
| Collaterals | – | + (Rentrop < 2) | – | + (non-accessible) |
| Other | – | – | Prior infarction | – |
Diagnostic study of a CTO
The rate of success is associated with a good diagnostic study to determine the vessel architecture in the occlusion region. It is important to locate the occlusion proximal edge and see if there are microchannels or proximity collaterals, but we do not need to use more than 15 images per second; however, at times it is necessary to increase the volume and pressure of contrast injection. The catheters should be perfectly placed inside the coronary ostia to avoid losing contrast through the aorta. Contralateral injections are also crucial (sometimes collateral circulation is homolateral) to see the occlusion final edge, the anatomy of the distal bed, make correct assessments of the collaterals, and determine whether the retrograde interventional procedure is possible.
Table 3 shows the basic projections for the right assessment of occluded segments (estimate values).
Table 3. Best projections to see different occluded segments in coronary arteries
| Artery | Anterior descending | Anterior descending | Circumflex | Right coronary | Right coronary |
|---|---|---|---|---|---|
| Segment | Middle | Ostium | Proximal-medial | Proximal-medial | Distal |
| Projection | AP 0°, cranial 30º-40° | LAO 30°, caudate 30º | AP 0°, caudate 30º-40º | LAO 90° | AP 0°, cranial 30º-40°, LAO 30°, cranial 30° |
|
AP, anteroposterior; LAO, left anterior oblique. |
|||||
There is a special situation when the anterior descending artery receives collateral circulation through Viuessens’ arterial ring35 where the right conus artery may anastomose with the left conus artery that exits the proximal or medial segment of the anterior descending artery. There are times when this conus artery exits an independent ostium of the right coronary artery and needs to be cannulated using a mammary artery catheter with a specifically curved tip or a hockey stick design. It is advisable to perform a coronary CT scan to evaluate the architecture of the occlusion in patients undergoing aortocoronary revascularization surgery, with high J-CTO scores or aorto-ostial occlusions.
TECHNIQUE AND METHOD OF INTERVENTIONAL PROCEDURE
General aspects
Although there is a tendency to perform minimalist interventional procedures, when the values of the scoring scales are high it is recommended to following these patterns:
– Antegrade access. It is advisable to use 8-Fr guide catheters preferably with maximum internal lumen. Extra back up curves should be used in the left coronary artery. In right coronary artery occlusions, the 8-Fr Amplatz Left 1 guidewire with side holes should be used. These catheters should be used with both hands with a Teflon coated 0.035-8’’ in guidewire inside to avoid sudden moves and prevent ostial dissections. Depending on the anatomy of the left coronary artery, there are times when a 3.5 Judkins Left catheter should be used. When treating the right coronary artery, a 3.5-4 multipurpose Judkins Left (vertical exits) or an Amplatz Left 2 (elongated aortas) catheter should be used.
– Retrograde access. When only injecting contrast, radial access with a 5-Fr or 6-Fr guide catheter can be used. It is advisable to use a guide catheter to insert an angioplasty guidewire inside the coronary artery and stabilize the catheter; this improves the quality of contrast injections and prevents problems due to possible ostial dissections. If collaterals are eligible for an intervention, the 7-Fr or 8-Fr extra back-up guide catheters can be used in the left coronary artery and the Amplatz Left 1 in the right coronary artery. Contralateral guide catheters should not have lateral holes to avoid losing contrast towards the aorta.
Hybrid algorithm and Asia-Pacific algorithm
Until the arrival of the hybrid algorithm36 there were no defined guidelines on how to access a CTO. This algorithm promotes a dual coronary injection for careful anatomy assessment and to determine the best strategy to treat the CTO by using the antegrade and retrograde accesses, dissection, and re-entry. When the level of difficulty of the CTO is low, the rate of success is high with the antegrade access. With more difficult anatomies, conventional techniques have a lower rate of success and it is necessary to use the retrograde access or specific techniques of dissection and re-entry. This algorithm assesses the presence of ambiguous stumps, lengths > 20 mm, and the quality of the distal bed to make decisions about the access. The rate of success of this algorithm is 87%; antegrade access, 52%; retrograde access, 27%; and dissection and re-entry, 21%.
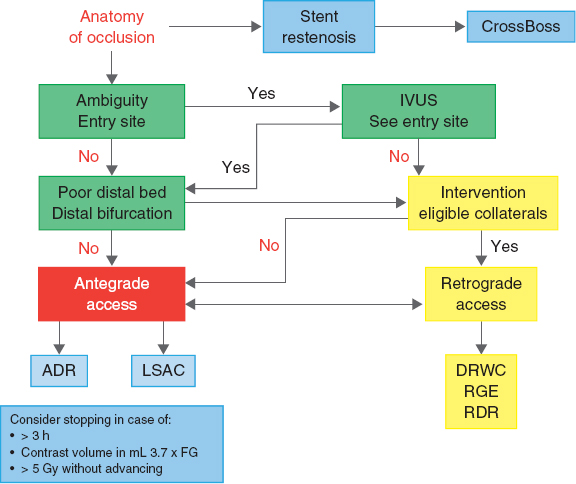
The Asia-Pacific algorithm37 integrates all techniques of coronary interventions on CTOs and establishes the strategy based on the anatomical findings. It also assesses the need to terminate a procedure (figure 2).
Figure 2. Modified algorithm (Asia-Pacific) to approach occlusions. ADR, antegrade dissection/re-entry; DRWC, direct retrograde wire crossing; GF, glomerular filtration; IVUS, intravascular ultrasound; LSAC, limited subintimal antegrade access; RDR, retrograde dissection/re-entry; RGE, retrograde guidewire escalation.
The Asia-Pacific algorithm takes into consideration the vessel architecture in the occlusion region; that is why a good bilateral injection is required to see the coronary arteries, even a coronary CT scan when necessary. Three basic parameters of the coronary anatomy are established (lengths > 20 mm do not determine the approach):
-
The ambiguity of the entry site.
-
The characteristics of the distal bed with respect to the occlusion site, assessing the quality of the distal bed and whether the CTO ends in a large bifurcation.
-
The presence of collaterals suitable for the retrograde access.
This algorithm also takes into consideration other factors like vessel tortuosity in the occlusion site, calcification, prior failed attempts, possible microchannels, and regions of reference (stents, calcium, contrast) to decide the strategy.
Antegrade access
Intravascular ultrasound
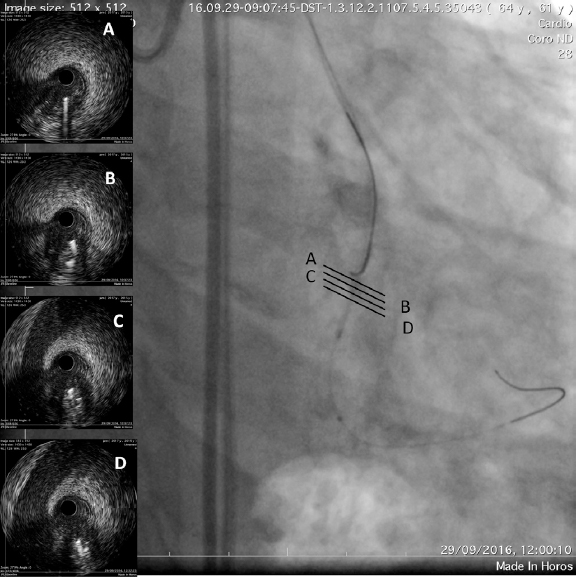
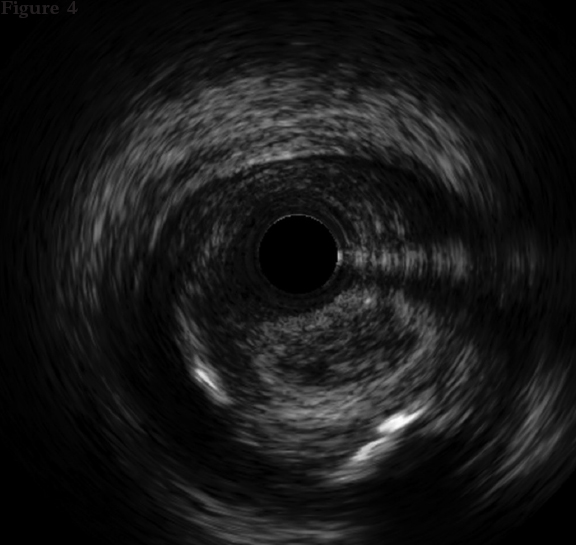
The intravascular ultrasound (IVUS) is useful when the entry site is ambiguous and there are no references to position the guidewire. A lateral branch is required to place the guidewire and IVUS. In these cases, the Slipstream technique38 is very useful. It consists in placing a dual-lumen micro-catheter (DLM) behind the IVUS above the branch guidewire. This increases tremendously the strength exerted with the guidewire that exits the DML lateral port providing better torque and grip (figure 3). The IVUS shows where the occlusion of the vessel is in order to navigate the guidewire (of high-gram, directivity, and torque-response) towards that point (figure 4).
Figure 3. The occluded vessel is at the 6 o’clock position approximately (A) as the guidewire is moving towards the occlusion (B, C and D).
Figure 4. The catheter of intravascular ultrasound in the subintimal space is between the 9 and 12 o’clock positions and true lumen between the 3 and 9 o’clock positions.
Guidewire escalation
It is essential to use a microcatheter that navigates well. It should be flexible enough and it should not condition the direction of the guidewire inside the vessel architecture.
Chosing the guidewire is at the operator’s discretion. It is advisable to check the occlusion proximal edge since the degree of toughness of the plaque is unknown and can be variable. Starting with high-gram or high penetration power or polymer-coated guidewires can make us lose perception of the true characteristics of the CTO. The main problem is when the guidewire advances towards the subintimal space limiting the possibilities of successful recanalization; this happens because the plaque is tougher than the guidewire, which conditions the selection of higher-gram guidewires.
Dual-lumen microcatheters
The actual way of re-entry from the subintimal space to the distal true lumen is using a DLM. If a guidewire advances towards the subintimal space it is essential to stop moving it and not inject contrast to avoid the formation of hematomas that would limit distal re-entry. It should be used in such a way to allow the insertion of another higher-gram guidewire through the lateral port to penetrate the occlusion, increase the strength of the guidewire, and reach the distal bed.
Like Tanaka et al.39 explain, this technique requires the 3D control of the guidewire inside the vessel architecture through specific turns depending on the arterial segment we are treating. The 90°-180° rotation turns are shown on table 4.
Table 4. Move and direction of angioplasty guidewires
| Artery | Clockwise | Counterclockwise |
|---|---|---|
| Left main coronary artery | Towards the circumflex artery | Towards the anterior descending artery |
| Anterior descending artery | Towards diagonal branches | Towards septal branches |
| Circumflex artery | Towards the main circumflex artery | Towards marginal branches |
| Right coronary artery | Towards the right coronary and posterolateral branches | Towards the acute marginal and posterior descending branches |
Dissection and re-entry with specific devices
Inside the hybrid algorithm36 when the CTOs show ambiguous stumps, lengths > 20 mm, and it is impossible to use the retrograde access, the dissection/re-entry technique that can be used. It is done using a specific device to achieve an easy and effective re-entry of subintimal space into the true lumen using the Stingray LP balloon with its specific guidewire (Boston Scientific, Nattick, Massachusetts, United States).
Until recently, this dissection/re-entry technique was conventionally performed using the CrossBoss and Stingray catheters. Nowadays there are times than the CrossBoss catheter is not even necessary. The 135-cm Corsair (ASAHI Intec, Nagoya, Japan) or Turnpike (Teleflex Inc, Wayne, PA, United States) catheters can be used to advance the guidewire with the microcatheter to a point where it cannot advance anymore. Once it has reached this point in the subintimal position, it is changed for the Stingray LP balloon that is inflated at 4 atm in the subintimal space. Through the 2 lateral ports situated at 180º from one another, the distal true lumen is re-entered with a rigid guidewire (Stingray wire, Hornet 14, Confianza Pro 12) using the stick and swap or multiple fenestration techniques. Then distal canalization occurs using a polymeric Pilot 200 (Abbott Santa Clara, CA, United States) or Gladius Mongo guidewire (ASAHI Intecc, Nagoya, Japan).
Retrograde access
Retrograde access depends on the distal region histological characteristics since the degree of toughness is lower compared to the proximal region40 because it is not exposed to the system direct arterial pressure.
Retrograde access routes
The native collateral channels or coronary artery bypass grafting are the access routes. Collateral branches are located in the septal, epicardial or intramyocardial regions. The most important limitation when crossing them is vessel tortuosity. A collateral branch can be approached if it can be crossed without causing perforations. Werner’s classification41 is the most widely used today: CC0, no continuous connection between donor and receiver; CC1, threadlike continuous connections (estimate diameter of 0.3 mm); and CC2, side-branch like connection along the entire route (estimate diameter of 0.4 mm).
There are added risks when crossing a collateral: tears, dissections, and occlusions. However, the septal branches (usually more numerous) have the lowest risk and are used 68% of the times;42 if they tear, the tear can be contained or fistulized to the ventricular cavity. There are invisible branches that can be easily crossed. The best projection to study them is the right anterior oblique branch at 0º or 30° of cranial angulation. Usually the septal branches go from the anterior descending to the posterior descending artery. It is easier to cross from the anterior descending to the posterior descending artery using the septal branch exit angulation. Sometimes very proximal septal branches connect to the posterolateral artery, and the distal ones connect to the branches that run through the right ventricular free wall.
Epicardial branches connect the anterior descending to the posterior descending artery at apical level, from the posterolateral to the obtuse marginal artery or from the obtuse marginal to the diagonal branches. Crossing them brings the added risk of tear with pericardial tamponade as it happens with the collaterals of the atrioventricular region.
Intramyocardial collaterals are found between the obtuse marginal and the posterolateral artery, and between the obtuse marginal and the posterior descending artery.
The preferred catheters are the Corsair, Turnpike, Mamba Flex (Boston Scientific Natick, Massachusetts, United States) or the Teleport (OrbusNeich, Hong Kong) built with several internal meshes to facilitate fracture-free rotations. The guidewire of choice to cross a collateral is the SION or SION black (ASAHI). In cases of major collateral tortuosity, the guidewire of choice is the SUOH03 (ASAHI).
Guidewire escalation
Retrograde access allows advancing towards the CTO with the same guidewire that passed the collateral except if it was with a SUOH03. Once the CTO has been reached, it is possible to study the toughness of the plaque and establish guidewire escalation.41 In 40% of the cases direct recanalization is possible with guidewire escalation: sometimes using a more rigid guidewire for penetration control or quick rotations, other times using polymeric guidewires to easily glide though the plaque.
Dissection and re-entry: knuckle, R-CART
In 60% of the cases when direct recanalization is not possible, interventional procedures are required to connect proximal and distal regions.
When vessel architecture is unknown, polymer-coated guidewires with knuckle wiring are required to shorten the occlusion with subintimal advancement. Sometimes the antegrade access is required when the proximal entry site or vessel architecture are unknown. Similarly, in cases of severe calcifications, these polymer- coated guidewires with or without knuckle wiring are advanced inside the occluded segment through the subintimal space by excluding calcium and advancing towards the proximal region. If one of the guidewires is in dissection and cannot be redirected, a DLM (Sasuke, ASAHI) can be used through the retrograde access to canalize a different region of the occluded segment.
When the guidewires cannot be connected, the R-CART (reverse controlled antegrade and retrograde subintimal tracking) is necessary.42 First, it is advisable to use this technique with IVUS through the antegrade access to see the position of the antegrade guidewire and the size of the balloon that will be used for antegrade dilatation. The antegrade segment is dilated with a balloon and the retrograde guidewire is oriented towards it; when it is near, the balloon is deflated to enter the dilated space through the balloon to connect both lumens. Using an extension of the guide catheter like the Trapliner balloon (Teleflex) may help. When both spaces are connected through interplaque or subintimal passage, a 300 cm-guidewire is externalized to end the procedure. After finishing the intervention, possible damage to the donor main vessel (dissection) and collaterals (tear) should be verified.
COMPLICATIONS
The rate of complications is a little higher compared to the general interventional procedure:42 Q-wave myocardial infarction, 2.5% vs 0.02%; urgent revascularization surgery, 0.1% vs 0.03%; stroke, 0.01% vs 0.04%; death, 0.2%-0.9% vs 0.14%; and perforation, 2%-4.8% vs 0.38%.
The most common extracardiac complications are vascular complications (2%) and contrast-induced nephropathy (3.8%). Radiation-induced lesions may appear weeks or months after catheterization and are often misdiagnosed and misreported in studies.43
Coronary cardiac complications are perforations of main, distal or collateral vessels that may lead to cardiac tamponade, donor vessel acute occlusion, dissection, aerial embolization, and device entrapment.
STENTS AND DUAL ANTIPLATELET THERAPY
CTOs are lesions with higher risk of restenosis and thrombosis: they are longer, more calcified, more tortuous, and require longer stents and even overlapping stents. Since the arrival of drug-eluting stents, restenosis, revascularizations, and thrombosis reduced significantly compared to the use of bare metal stents.44
The CIBELES study45 showed that the late loss of everolimus-eluting stents at 9 months was 0.13 ± 0.69 mm promoting better results for this kind of patients. Lee et al.46 analyzed a consecutive series of 539 CTOs including everolimus-eluting stents (n = 313) and zotarolimus-eluting stents (n = 226), and obtained a composite event rate (death, infarction or target vessel revascularization) of 12.2% at the 3.3-year mean follow-up.
Antiplatelet therapy should not differ from the pattern established by the underlying coronary disease. If the risk of bleeding is low, a 1-year course of dual antiplatelet therapy plus acetylsalicylic acid and clopidrogel should be prescribed.
LINGERING CONTROVERSIES
There is great variability among patients undergoing interventional procedures on their CTOs: with angina, asymptomatic, ventricular dysfunction or positive ischemia detection tests. It is still under discussion how to define objectively in a definitive clinical trial what patients would benefit in terms of event-free survival. Future challenges include to define symptomatology, ischemia, viability for every patient, and to standardize a highly successful interventional technique easily reproducible regardless of the operator. Another important aspect is to determine how the results of the angioplasty actually impact the preservation of microcirculation.
INNOVATIVE TECHNIQUES
Innovation in this field is based on 2 strategies: devices and 3D anatomic reconstruction techniques. The angioplasty guidewire is a key element here. Success will come from a technology using multiple coils and hydrophilic coatings to facilitate tactile sensation, and oriented directivity. There are new devices available that apply energy to the angioplasty guidewires using radiofrequency, with very promising results.
The same way as when driving we turn the steering wheel left and right when a curve is ahead, we could open a coronary artery with specific devices by moving precisely in 3 dimensions inside the vessel architecture to enter and exit the distal lumen quickly and safely. This could be done with software tools added to the angiography and coronary CT scan.
CONCLUSIONS
The evidence available favors performing interventional procedures on CTOs because they clinically improve angina, ventricular function when there is viability, and increase event-free survival. The rate of success of experienced operators is close to 90% with few complications, which benefits all patients. Performing interventional procedures on coronary arteries makes the interventional cardiologist be more prepared to face daily routine interventions and benefits all patients undergoing PCIs.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Abbott JD, Kip KE, Vlachos HA, et al. Recent trends in the percutaneous treatment of chronic total coronary occlusions. Am J Cardiol. 2006;97: 1691-1696.
2. Dimitri GS, Vasilis AT, Lambros KM. Chronic Total Coronary occlusions:a review of their special features and the existing techiniques of percutaneous treatment. Hellenic J Cardiol. 2003;44:136-142.
3. Werner GS, Figulla HR. Direct assessment of coronary steal and associated changes of collateral hemodynamics in chronic total coronary occlusions. Circulation. 2002;106:435-440.
4. Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J. 2006;27:2406-2412.
5. Cohen HA, Williams DO, Holmes DR, et al. Impact of age on procedural and 1-year outcome in percutaneous transluminal coronary angioplasty:the NHLBI Dynamic Registry. Am Heart J. 2003;146:513-519.
6. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions:the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
7. Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalization of Chronic Total Occlusions:consensus document from the EuroCTO Club. EuroIntervention. 2007;3:30-43.
8. Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden:results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008; 117:1283-1291.
9. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization:Results from the FlowCardia's Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ Cardiovasc Qual Outcomes. 2010;3:284-290.
10. Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes:a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:95-107.
11. Werner GS, Betge S, Kuthe F, Figulla HR, Betge S, Kuthe F. Delayed recovery of left ventricular function after recanalization of a chronic coronary occlusion. Catheter Cardiovasc Interv. 2003;60:491-495.
12. Nombela-Franco L, Mitroi CD, Fernández Lozano I, et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention:impact of chronic total coronary occlusion (VACTO Primary Study). Circ Arrhythm Electrophysiol. 2012;5:147-154.
13. Serruys PW, Morice MC, Kappetein AP, et al.;for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360: 961-972.
14. Werner GS, Martin-Yuste V, Hildick-Smith D, et al. A randomized multicenter trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39: 2484-2493.
15. Henriques JP, Hoebers LP, Ramunddal T, et al.;EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI:the EXPLORE trial. J Am Coll Cardiol. 2016;68: 1622-1632.
16. Park S. Drug-eluting stent implantation versus optimal medical treatment in patients with chronic total occlusion (DECISION-CTO). En:American College of Cardiology's 66th Annual Scientific Session &Expo;2017 Mar 18;Washington, DC. Available online: http://www.acc.org/latest-in-cardiology/clinical-trials/2017/03/17/08/40/decision-cto. Accessed 31 May 2019.
17. Mashayekhi K, Nührenberg TG, Toma A, et al. A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion:The REVASC trial. JACC Cardiovasc Interv. 2018;11:1982-1991.
18. Tomasello SD, Boukhris M, Giubilato S, et al. Management strategies in patients affected by chronic total occlusions:results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36:3189-3198.
19. Jang WJ, Yang JH, Choi SH, et al. Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Interv. 2015;8:271-279.
20. Bruckel JT, Jaffer FA, O'Brien C, Stone L, Pomerantsev E, Yeh RW. Angina severity, depression, and response to percutaneous revascularization in patients with chronic total occlusion of coronary arteries. J Invasive Cardiol. 2016;28:44-51
21. Abdullah SM, Hastings JL, Amsavelu S, et al. Percutaneous coronary intervention of coronary chronic total occlusions improves peak oxygen uptake during cardiopulmonary exercise testing. J Invasive Cardiol. 2017;29:83-91.
22. Christakopoulos GE, Christopoulos G, Carlino M, et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115:1367-1375.
23. Tsai TT, Stanislawski MA, Shunk KA, et al. Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions:insights from the VA CART program. JACC Cardiovasc Interv. 2017;10:866-875.
24. Sapontis J, Salisbury AC, Yeh RW, et al. Early Procedural and Health Status Outcomes After Chronic Total Occlusion Angioplasty. A Report From the OPEN-CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017; 10:1523-1534.
25. Safley DM, House JA, Marso SP, Grantham JA, Rutherford BD. Improvement in survival following successful percutaneous coronary intervention of coronary chronic total occlusions:variability by target vessel. JACC Cardiovasc Interv. 2008;3:295-302.
26. Iannaccone M, D'ascenzo F, Piazza F, et al. Optimal medical therapy vs. coronary revascularization for patients presenting with chronic total occlusion:A meta.analysis of randomized controlled trials and propensity score adjusted studies. Catheter Cardiovasc Interv. 2019;93:E320-E325.
27. Brilakis ES, Vo MN. How to develop a successful chronic total occlusion percutaneous coronary intervention program. Cardiovasc Revasc Med. 2016; 17:3-4.
28. Sharma V, Jadhav ST, Harcombe AA, et al. Impact of proctoring on success rates for percutaneous revascularization of coronary chronic total occlusions. Open Heart. 2015;2:e000228.
29. Wang N, Fulcher J, Abeysuriya N, Adams L, Lal S. Predictors of successful chronic total occlusion percutaneous coronary interventions:a systematic review and meta-analysis. Heart. 2018;104:517-524.
30. Karatasakis A, Danek BA, Brilakis ES. Scoring systems for chronic total occlusion percutaneous coronary intervention:if you fail to prepare you are preparing to fail. J Thorac Dis. 2016;8:E1096-E1099.
31. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes:the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011; 4:213-221.
32. Galassi AR, Boukhris M, Azzarelli S, et al. Percutaneous Coronary Revascularization for Chronic Total Occlusions:A Novel Predictive Score of Technical Failure Using Advanced Technologies. JACC Cardiovasc Interv. 2016;9:911-922.
33. Alessandrino G, Chevalier B, Lefèvre T, et al. A Clinical and Angiographic Scoring System to Predict the Probability of Successful First-Attempt Percutaneous Coronary Intervention in Patients With Total Chronic Coronary Occlusion. JACC Cardiovasc Interv. 2015;8:1540-1548.
34. Christopoulos G, Kandzari DE, Yeh RW, et al. Development and Validation of a Novel Scoring System for Predicting Technical Success of Chronic Total Occlusion Percutaneous Coronary Interventions:The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9:1-9.
35. de Agustín JA, Marcos-Alberca P, Hernández-Antolín R, et al. Collateral circulation from the conus coronary artery to the anterior descending coronary artery:assessment using multislice coronary computed tomography. Rev Esp Cardiol. 2010;63:347-351.
36. Tajti P, Karmpaliotis D, Alaswad K, et al. The Hybrid Approach to Chronic Total Occlusion Percutaneous Coronary Intervention Update From the PROGRESS CTO Registry. J Am Coll Cardiol Interv. 2018;11:1325-1335.
37. Harding SA, Wu EB, Lo S, et al. A New Algorithm for Crossing Chronic Total Occlusions From the Asia Pacific Chronic Total Occlusion Club. JACC Cardiovasc Interv. 2017;10:2135-2143.
38. Kinoshita Y, Fujiwara H, Suzuki T. “Slipstream technique“—New concept of intravascular ultrasound guided wiring technique with double lumen catheter in the treatment of coronary total occlusions. J Cardiol Cases. 2017;16:52-55.
39. Tanaka T, Okamura A, Iwakura K, et al. Efficacy and Feasibility of the 3-Dimensional Wiring Technique for Chronic Total Occlusion Percutaneous Coronary Intervention First Report of Outcomes of the 3-Dimensional Wiring Technique. JACC Cardiovasc Interv. 2019;12:545-555.
40. Danek BA, Karatasakis A, Karmpaliotis D, et al. Use of antegrade dissection re-entry in coronary chronic total occlusion percutaneous coronary intervention in a contemporary multicenter registry. Int J Cardiol. 2016;214: 428-437.
41. Werner GS, Ferrari M, Heinke S, et al. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107:1972-1977.
42. Brilakis ES, Grantham JA, Thompson CA, et al. The retrograde approach to coronary artery chronic total occlusions:a practical approach. Catheter Cardiovasc Interv. 2012;79:3-19.
43. Patel VG, Brayton KM, Tamayo A. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions:a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128-136.
44. Colmenarez HJ, Escaned J, Fernández C, et al. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization:a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1854-1866.
45. Moreno R, Garcia E, Teles R, et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions:results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circ cardiovasc Interv. 2013;6:21-28.
46. Lee PH, Cho MS, Lee SW, et al. Everolimus- versus zotarolimus-eluting stent following percutaneous coronary chronic total occlusion intervention. In J Cardiol. 2017;241:128-132.