Finalist case in the ACCIS 2023 Madrid course
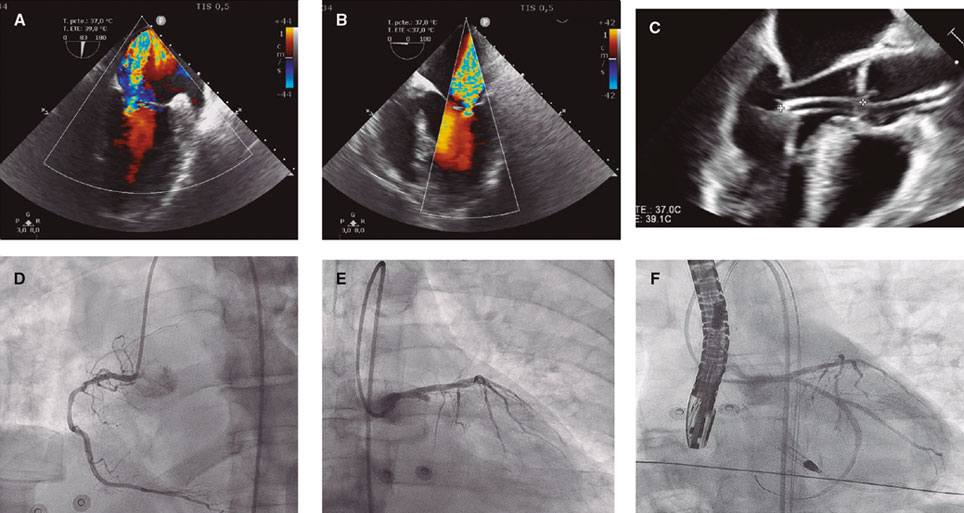
A 59-year-old man was admitted to the cardiac intensive care unit (CICU) due to evolved inferior-posterior ST-segment elevation myocardial infarction complicated by cardiogenic shock. Upon arrival, transesophageal echocardiography revealed severe mitral regurgitation (MR) secondary to posterior leaflet restriction (figure 1A,B). After rupture of papillary muscles was ruled out, the patient was transferred to the cath lab, where a 100% thrombotic lesion was observed in the proximal left circumflex artery (figure 1D-E). Due to hypotension, we decided to support the angioplasty with the Impella CP device (Abiomed, United States) (figure 1C). Flow was finally restored (figure 1F).
Figure 1.
Five days later, the patient was hemodynamically stable with Impella at P6, but developed multiple complications, including acute kidney failure, significant bleeding, and hemolysis. Three-dimensional echocardiography showed MR without changes. At this point, spontaneous improvement of MR seemed unlikely, and the risk of heart transplant or surgery was unacceptable. Finally, we decided to implant a MitraClip (Abbott, United States) supported by Impella.
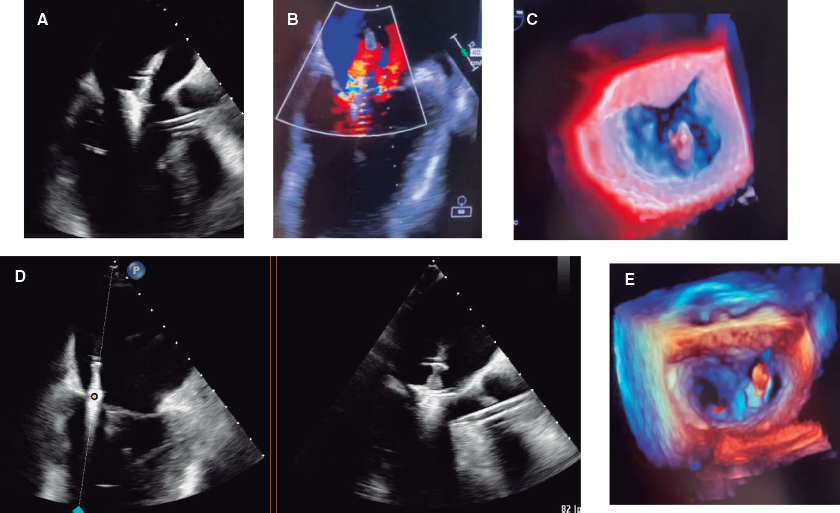
The first MitraClip NTW (Abbott, United States) was placed between P2 and A2, with significant posterior-medial regurgitation (figure 2A-C). The second MitraClip NT (Abbott, United States) was implanted nearby. Residual MR was mild (figure 2D,E, video 1 of the supplementary data).
Figure 2.
The patient was extubated after the procedure and the Impella device was removed the following day. He left the CICU 10 days later.
This case is in line with other case reports suggesting that MitraClip, supported by Impella CP, could be an effective strategy in patients with severe functional MR. In this case, hemodynamic support by Impella CP was used to complete the primary percutaneous coronary intervention during CICU admission and during edge-to-edge mitral valve repair. Informed consent was obtained from the patient to publish this manuscript.
FUNDING
None.
ETHICAL CONSIDERATIONS
Informed consent was obtained from the patient to publish this properly anonymized manuscript. Because this is a single case report, approval from ethics committee was not required and gender considerations were not applicable.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence software was used to write this manuscript.
AUTHORS’ CONTRIBUTIONS
All authors contributed to data collection, drafting, review, and approval of the manuscript.
CONFLICTS OF INTEREST
I. Pascual Calleja: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Abbot Vascular. C. Garrote Coloma: proctor for MitraClip implant, Abbot. J.M. de la Torre-Hernández: grants or contracts from Abbot, Amgen, Boston SCI; consulting fees from Medtronic, Boston SCI, Abbot; support for attending meetings from Medtronic, Abbot, Boston SCI.
J.M. de la Torre-Hernández is also editor-in-chief of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
The remaining authors have no conflicts of interest.
SUPPLEMENTARY DATA
Vídeo 1. Coroas Pascual C. DOI: 10.24875/RECICE.M23000414