ABSTRACT
Introduction and objectives: Percutaneous pulmonary valve implantation is currently a common procedure in patients with congenital heart disease with a dysfunctional right ventricular outflow tract. Until April 2022, there were only balloon-expandable valves available in Europe, which did not cover the needs of the different anatomies of the right ventricular outflow tract. Since that date we have available the self-expandible Venus P-valve (Venus MedTech, China). We present the initial experience with this new percutaneous pulmonary valve in our center.
Methods: Description of the valve implants with the new self-expandible valve performed between September and November 2022.
Results: Eight valve implants have been performed, all successful and without severe complications during the procedure. All patients had severe pulmonary regurgitation with a dilated right ventricle and severe dilatation of the pulmonary trunk and were not good candidates for percutaneous balloon-expandable valves. Five patients had a tetralogy of Fallot. In 7 patients, the implant was performed through the femoral vein and in one through jugular access. As a safety measure, all valves were implanted through a DrySeal sheath (Gore, W.L. Gore & Associates, Inc., United States). The mean hospital stay was 3-day.
Conclusions: Valve implantation with the new self-expandible Venus P-valve was, in our preliminary experience, a safe and feasible procedure, allowing us to treat very dilated right outflow tracts, not suitable for the current balloon-expandable valves.
Keywords: Percutaneous valve implantation. Venus P-valve. Tetralogy of Fallot. Pulmonary regurgitation. Pulmonary valve. Congenital heart disease.
RESUMEN
Introducción y objetivos: El implante percutÁneo de vÁlvula pulmonar es, actualmente, un procedimiento habitual en pacientes con cardiopatías congénitas con un tracto de salida del ventrículo derecho disfuncionante. Hasta abril de 2022, en Europa solo estaban disponibles las vÁlvulas expandibles con balón, que no cubrían las necesidades de las distintas anatomías del tracto de salida derecho. Desde esa fecha estÁ disponible la vÁlvula autoexpandible Venus P ( Venus MedTech, China). Presentamos la experiencia inicial en nuestro centro con esta nueva vÁlvula pulmonar para implante percutÁneo.
Métodos: Descripción de los implantes valvulares con la nueva vÁlvula autoexpandible realizados entre septiembre y noviembre de 2022.
Resultados: Se han realizado 8 implantes valvulares, todos con éxito y sin complicaciones graves durante el procedimiento. Todos los pacientes presentaban insuficiencia pulmonar grave con repercusión sobre el ventrículo derecho y dilatación del tronco pulmonar, y no eran buenos candidatos para las vÁlvulas expandibles con balón. Cinco pacientes tenían una tetralogía de Fallot de base. En 7 pacientes el implante se llevó a cabo por vía femoral y en 1 por vía yugular. Como medida de seguridad, en todos los pacientes el implante se hizo a través de una vaina DrySeal (Gore, W.L. Gore & Associates, Inc., Estados Unidos). La media de tiempo de ingreso fue de 3 días.
Conclusiones: El implante de la nueva vÁlvula autoexpandible Venus P fue, en nuestra experiencia preliminar, un procedimiento seguro y factible, que permite valvular tractos de salida derechos muy dilatados con contraindicación para las actuales vÁlvulas expandibles con balón.
Palabras clave: Implante percutÁneo valvular. VÁlvula pulmonar Venus P. Tetralogía de Fallot. Insuficiencia pulmonar. VÁlvula pulmonar. Cardiopatías congénitas.
Abbreviations
CT: computed tomography. LMCA: left main coronary artery. MRI: magnetic resonance imaging. PAT: pulmonary arterial trunk. RV: right ventricle. RVOT: right ventricular outflow tract.
INTRODUCTION
Currently, transcatheter pulmonary valve implantation is a common procedure in patients with congenital heart disease and dysfunctional right ventricular outflow tract (RVOT).1 Transcatheter balloon-expandable pulmonary valves (Melody by Medtronic Inc., United States, and Sapien by Edwards Lifescience, United States, both with CE marking) have an indication for conduit, native tract, and prosthetic valve implantation.1,3 However, a large number of patients—most with tetralogy of Fallot—who require pulmonary valve implantation are treated with transannular repair or post-commissurotomy pulmonary regurgitation or valvuloplasty for pulmonary valve stenosis. These patients have very pulsatile outflow tracts with larger sizes compared to the ones of current balloon-expandable valves (22 mm and 29 mm for Melody and Edwards, respectively).4 Not even the 32 mm Myval device (Meril Life Sciences Pvt. Ltd., India) without an indication for pulmonary valve implantation would be adequate for the largest RVOTs out there.
Back in April 2022, the self-expanding Venus P-valve (Venus MedTech, China) achieved the CE marking, and became an actual alternative for the largest native tracts.
This is our initial experience with this new transcatheter pulmonary valve at our center and in our country.
METHODS
Valve description
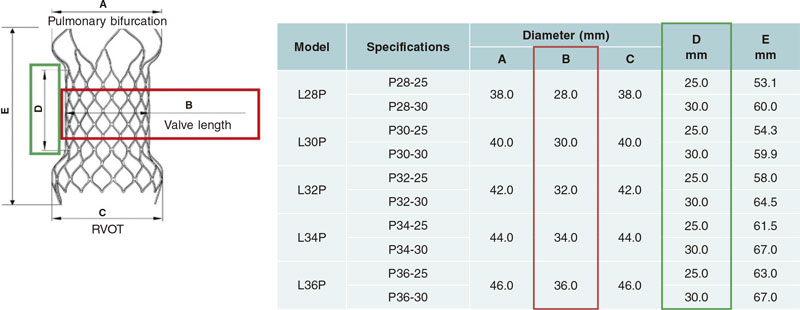
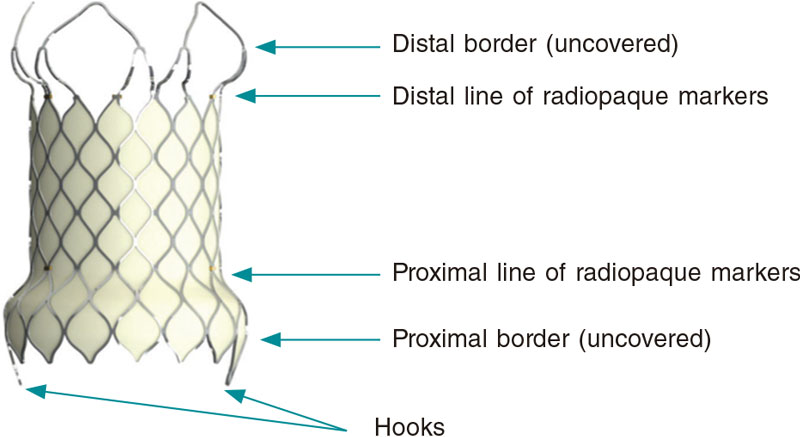
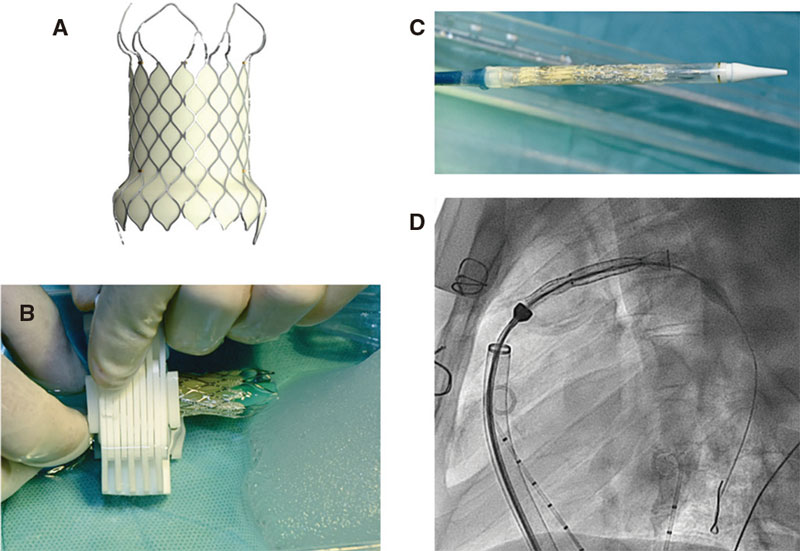
The structure of the Venus-P valve consists of a nitinol stent. Both the leaflets, and the stent coverage are made of porcine pericardium. Nitinol provides the stent with some sort of shape memory so it can adapt to the pulmonary arterial trunk (PAT) without compressing neighboring structures. This valve is available in sizes from 28 mm to 36 mm in diameter with 2 mm increases (figure 1). It can be used with the largest caliber native tracts.5 The Venus P-valve stent has a diabolo-shaped configuration and adds 10 mm to the borders of the central region (figure 1). It is wider in its borders because it has been designed for tubular PAT implantation without distal or pulmonary artery stenosis. Both the central region and the proximal border are covered with porcine pericardium to prevent paravalvular leak. The distal border remains uncovered to avoid occluding the pulmonary arteries (figure 2).6 The stent has radiopaque marks in the distal border of its tubular region and in its proximal border both indicative of the degree of the porcine valve implantation. The valve crimping system on its delivery system is performed under ice water. In these conditions, nitinol becomes softer and can be crimped onto the delivery system (figure 3). The valve is then fixed to the delivery system through 2 small hooks (figure 2). Once the valve has been attached and its size reduced, it is covered with the delivery sheath capsule in such a way that the valve enters the patient fully covered (figure 3 and figure 4) [22-Fr sheaths in 28 mm and 30 mm valves, and 24-Fr sheaths in the largest ones (> 30 mm)]. Once in the pulmonary tree and in the desired location, the delivery sheath capsule will be retracted so the valve can regain its diabolo-shaped configuration when entering blood flow at 36 ºC to 37 °C temperature.
Figure 1. Selection of different valvular sizes with the dimensions of the different parts of the valve. RV, right ventricle.
Figure 2. Image of the Venus P-valve with its typical diabolo-shaped configuration, and coverage of the entire valve leaving both the distal border and the radiopaque markers uncovered.
Figure 3. A: Venus P-valve prior to crimping. B: valve crimping onto the delivery system inside a frozen physiological saline solution with a specific crimping system. C: crimped valve inside the delivery system while covered by a capsule. D: fluoroscopy showing the valve in the delivery system inside the capsule already inserted into the patient’s pulmonary artery.
Procedural description
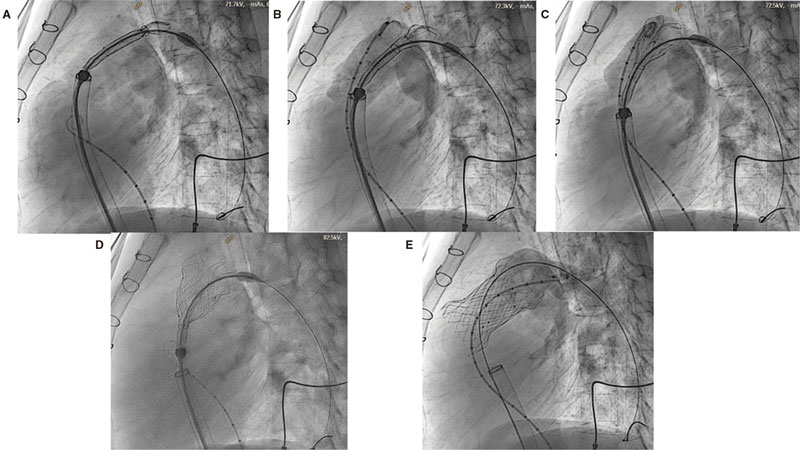
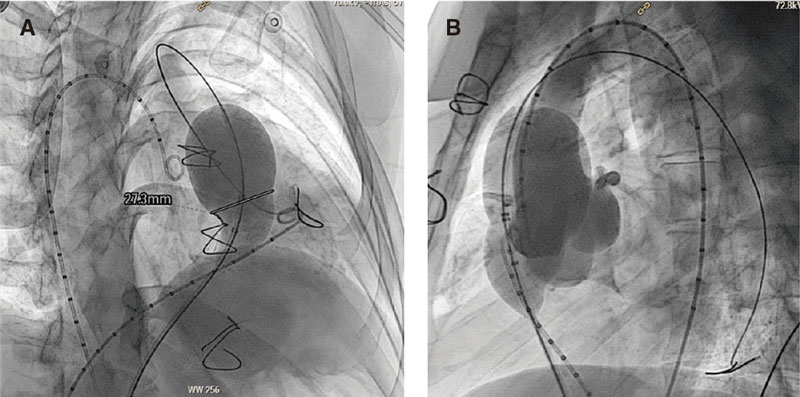
Cardiac catheterization was performed under general anesthesia while the patient remained intubated and with heparinization at 100 U/kg. Two femoral veins where cannulated, 1 for diagnostic catheterization, cutting, and advance of the valve delivery system, and the other one to perform the follow-up angiography with a pigtail catheter in the RVOT during valve implantation (figure 4). The coronary arteries of all the patients were interrogated through aortograms or selective coronary angiographies with the same 34 mm cutting balloon (Sizing Balloon, AGA Medical Corp., United States) inflated in the RVOT to discard the risk of coronary compression during the procedure (figure 5B). Similarly, the RVOT was cut with the same 34 mm balloon to see if the anatomy was viable and choose the right valve diameter and length. Cutting was considered occlusive when aortic pressure dropped during balloon inflation and the lack of right ventricular (RV) flow to the pulmonary arteries was angiographically confirmed through an intracoronary injection of contrast into the RV during the balloon peak inflation rate (figure 5A).
Figure 4. Valve implantation process. A-D: slow capsule removal so the valve can adapt to the left ventricular outflow tract (LVOT). Angiography monitorization with the pigtail catheter placed in the LVOT. E: final outcomes after valve implantation.
Figure 5. Right ventricular outflow tract cutting with a 34 mm cutting balloon. A: simultaneous angiography in the right ventricle showing the complete occlusion of the RVOT by the balloon that shows a 27 mm notch at stenosis level. B: coronary artery interrogation during cutting balloon inflation without coronary compromise.
Although the imaging modalities performed while planning (computed tomography scan [CT] with or without magnetic resonance imaging (MRI)] inform us on the most appropriate valvular size for each patient, size was picked based on angiography measurements (30° lateral and 30°cranial right anterior oblique), and on the diameter and location of the notch seen in the balloon during occlusive cutting. Balloon cutting allows us to assess the compliance of the PAT, something that is merely suggested on the MRI. Therefore, this is an essential step that should be made before selecting the size of the valve. We select a 2 mm-to-4 mm larger valve compared to the waist of the cutting balloon with a length that should leave the distal border at pulmonary bifurcation level, and the diabolo proximal border at RV level.
The Venus P-valve is implanted without the need for previous stenting to create a landing zone. A Lunderquist high-support guidewire is placed (Cook Medical, Denmark) preferably in the left pulmonary artery. In the presence of stenosis, hypoplasia or unfavorable angle, the guidewire is placed in the right pulmonary artery. The delivery system is advanced through a 65 cm 26-Fr or 24-Fr Dryseal sheath (Gore, W.L. Gore & Associates, Inc., United States) for valves > 30 mm or < 30 mm in diameter, respectively. Once the Dryseal sheath is in position into the selected pulmonary artery, the Venus P-valve delivery system is advanced. Afterwards, the Dryseal sheath is retracted and the correct position of the capsule into the pulmonary artery is verified. Then, the valve is slowly uncovered by withdrawing the capsule (with a clockwise twist of the wheel of the delivery system). As it gradually enters the patient’s bloodstream, the valve regains its normal diabolo-shaped configuration. When its most distal border has been partially adapted to the pulmonary branch, the whole system is then smoothly removed to the PAT and the valve is slowly uncovered while checking—through pigtail catheter injections into the RVOT—that the valve is in position (figure 4). The valve has 2 lines of radiopaque markers to guide implantation. The distal marks that indicate the distal border of the stent tubular region will remain at bifurcation level. The proximal ones should remain at PAT narrowest region or native annulus level (in the location of the notch seen during balloon cutting), thus reducing the risk of embolization. The valve proximal region—10 mm larger than the central one—will remain, in most cases, in the infundibulum of the RV.
Procedure should be performed through a Dryseal sheath to facilitate the maneuverability of the valve delivery system and guarantee safe valve recapture should repositioning be required. The valve can be recaptured until half of its structure has been uncovered.
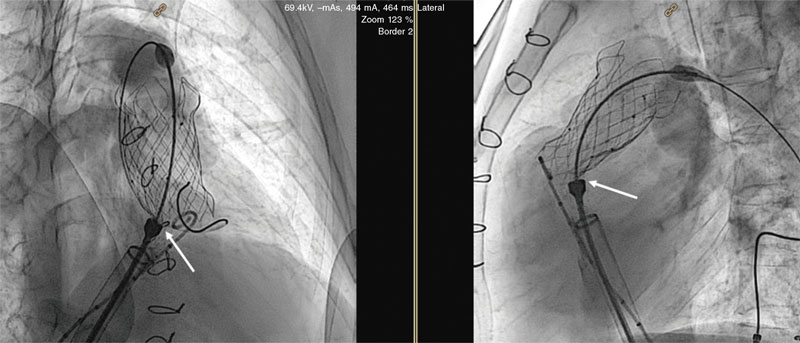
An aortogram was performed at the end of all procedures (preferably 30° caudal and 20º left anterior oblique) to confirm the lack of coronary artery compression (figure 6).
Figure 6. Final aortogram for coronary artery assessment. Arrows are indicative of the trajectory of the left anterior descending coronary artery.
Patient selection
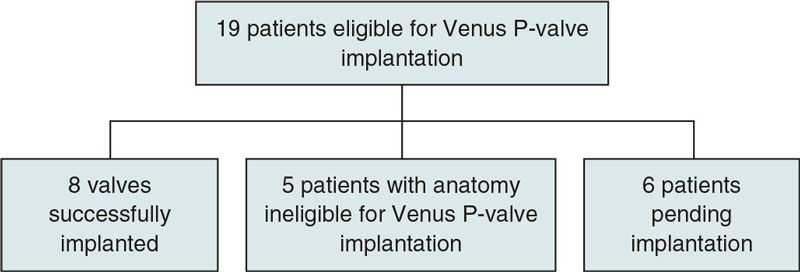
A previous study through diagnostic catheterization or MRI with or without CT scan was conducted to see whether the anatomy of potentially eligible patients with valve implantation criteria according to the clinical practice guidelines published by the ESC7,8 was ripe for Venus P-valve implantation (figure 7). CT or MRI acquired images were analyzed by Venus Medtech image technicians who video-called our hospital heart team to discuss the convenience of valve implantation and proper valvular size that best suited the patient’s RVOT size. However, the final size was not decided until balloon cutting was used during cardiac catheterization.
Figure 7. Flowchart of patients included in the study.
This first imaging study discarded 5 patients with unsuitable anatomies for this kind of valve: 2 patients with stents and pulmonary artery stenoses, 2 patients with larger RVOTs compared to the sizes recommended for this kind of valve, and 1 patient with pyramid-shaped right ventricular outflow tract.
Study description
This was a prospective study of the first patients treated with Venus P-valve implantation conducted at our center from September 20th through November 4th, 2022. These are the very first implantations of this type of valve ever performed in our country.
Inclusion criteria
This study included patients with dysfunctional native RVOTs and an indication for pulmonary valve implantation in whom diagnostic catheterization and cutting test allowed such procedure.
Variables
Demographic and anthropometric data were collected, as well as imaging modality and procedural data to conduct a descriptive analysis of our own experience.
Definitions
Complications were categorized as minor or major. The later were death, potentially life-threatening adverse events, and events requiring surgery (embolization, myocardial perforation, vascular rupture, residual PR, hemolysis, valvular lesion). The former were complications that resolve spontaneously or subside with clinical treatment without potentially fatal outcomes (vascular access problems, fever, neuroapraxias, etc.). Implantation was considered successful in the absence of major complications 24 hours after the procedure.
RESULTS
A total of 8 Venus P-valves were implanted in 8 patients at our center from September 20th through November 4th, 2022. The underlying conditions were tetralogy of Fallot (5 patients), pulmonary atresia with ventricular septal defect (1 patient), atrial septal defect with pulmonary stenosis (1 patient), and ventricular septal defect and pulmonary artery banding (1 patient) who required RVOT dilatation in its configuration. All the patients had a transannular patch. Also, all patients had severe pulmonary regurgitation with RV repercussion and PAT dilatation, and were ineligible for transcatheter balloon-expandable valve implantation due to the size of their RVOTs. table 1 shows a overall description of the patients.
Table 1. Overall description of the sample
| Patient | Sex | Age (years) |
Weight (kg) |
RV (mlLm2) |
PAT (mm) MRI |
PAT (mm) CT |
PAT (mm) angiography |
Cutting balloon (mm) |
Valvular size |
|---|---|---|---|---|---|---|---|---|---|
| 1 | W | 41 | 56 | 122 | 28 | NA | 26 | 26 | 30-25 |
| 2 | M | 34 | 62 | 164 | 28 | 27 | 28 | 28 | 32-25 |
| 3 | W | 25 | 66 | NA | NA | NA | 28 | 28 | 32-25 |
| 4 | M | 33 | 90 | NA | NA | 33 | 31 | 32 | 36-25 |
| 5 | M | 34 | 68 | 134 | 31 | NA | 26 | 27 | 34-25 |
| 6 | M | 17 | 63 | 173 | 29 | 31 | 31 | 31 | 34-25 |
| 7 | M | 45 | 68 | NA | NA | 34 | 26 | 32 | 34-30 |
| 8 | W | 43 | 42 | 130 | 25 | NA | 24 | 24 | 28-25 |
|
CT, computed tomography scan; M, man; MRI, magnetic resonance imaging; NA, not available; PAT, pulmonary arterial trunk; RV, right ventricle; W, woman. |
|||||||||
All patients had dilated PATs as seen on the CT scan or MRI—meaning they were suboptimal candidates for balloon-expandable valve implantation—and tubular PATs without stenoses in, at least, 1 pulmonary artery.
The valves were successfully and uneventfully implanted in all the patients with optimal valvular competence immediately after implantation. A total of 7 implantations were performed via femoral access, and 1 via right jugular vein due to bilateral femoral venous thrombosis. High-support guidewires were placed in the left pulmonary artery (6 cases), and right pulmonary artery (2 cases, 1 due to moderate left pulmonary artery stenosis, and the other one because it was a jugular access). In 7 and 1 cases, respectively, 26-Fr and 24-Fr Dryseal sheaths were used through which implantation occurred. The length of the valves implanted was 25 mm (n = 7) and 30 mm (n = 1).
The median fluoroscopy time was 34 min (interquartile range, 32-37), and the mean radiation dose, 307 mGy/m2 (standard deviation, 64.4). We saw an adequate correlation between the CT and MRI measurements of the PAT and the angiography measurements and the cutting balloon.
No severe complications were reported in any of the cases. In 1 case, while the valve was being deployed, 1 of the proximal hooks got trapped in the delivery system due to incomplete removal of the capsule covering the valve. It, however, resolved uneventfully (figure 8). A total of 3 patients had mild thoracic pain 24 hours after implantation not showing elevated troponin levels or ECG abnormalities. Also, 1 patient complained of right scapula pain. One of the patients had common ventricular extrasystole that started right after valve implantation and subsided spontaneously within the next 48 hours not requiring any therapy at discharge. One patient broke a fever 48 hours after implantation without elevation of acute phase reactants.
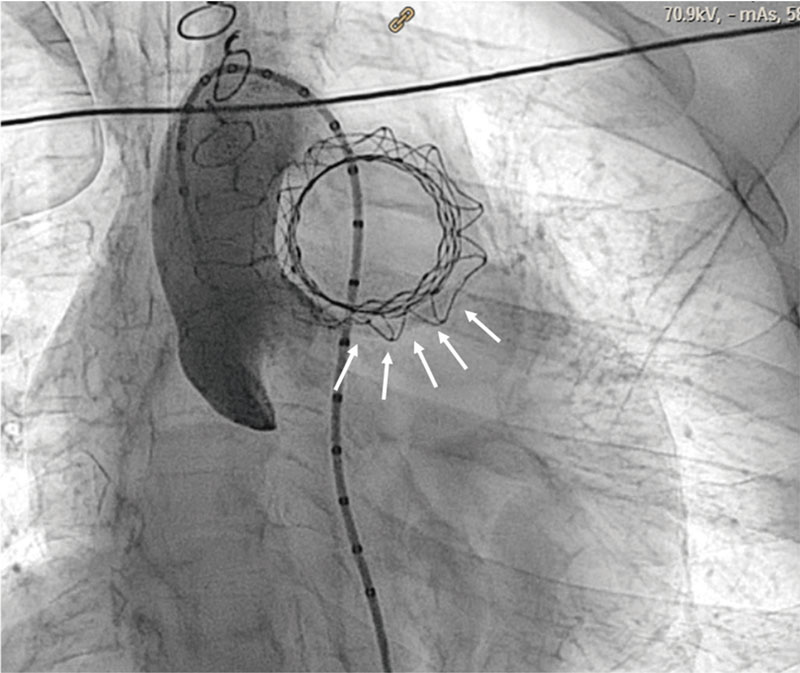
Figure 8. Arrows indicate, in 2 different projections, that 1 of the attachment hooks is still trapped in the delivery system preventing full valve deployment.
The mean length of stay was 3 days (range 2-4). All patients were discharged on acetylsalicylic acid.
DISCUSSION
Transcatheter pulmonary valve implantation is, currently, a procedure widely performed in the cath labs of congenital coronary care units. Up until now, the valves available—with sizes up to 29 mm—left a significant number of patients without transcatheter therapeutic options available. In some cases, off-label techniques were used (several stents implanted in the PAT to reduce its caliber, stent landing in the left pulmonary artery, etc.) or larger sized balloon-expandable valve implantation with an indication for the aorta (32 mm Myval valve), but not approved for the RVOT.9
Among the main advantages of the Venus P-valve is the possibility or performing one-stage diagnostic catheterizations and valve implantations since previous stenting is not required to create a landing zone (as it was the case with the Melody and Edwards valves in several native tracts). In the most dilated pulmonary arterial trunks (> 24 mm-to-26 mm) one-stage stenting is the gold standard. Then, wait for, at least, 6 weeks until endothelization occurs to minimize the risk of embolization while the valve is being implanted, which requires a second catheterization to implant the valve. Another advantage of the Venus P-valve is how easy it is to use. It is a short procedure with 34 min fluoroscopy times in our cases (shorter to the times reported in our series of native RVOTs and other valves between 40 min and 50 min).
The flexibility of the valve allows PAT adaptation without exerting radial strength on adjacent structures, which is a plus for cases of coronary RVOT-related anatomies.
The short 25 mm valve was used in 7 cases since these patients’ PAT anatomy allowed such length. With longer length, less flexibility for the valve and higher risk of long-term fractures. Up until today, a rate of fractures between 11% and 27% has been reported without associated complications or loss of valvular competence associated with the fractures.5,10
We should pay special attention to the complete removal of the capsule at the end of valve deployment because, if removal is incomplete as it happened with our case (figure 8), hooks can’t be released, and the valve does not get deployed with the corresponding risk of RV embolization.11 To make sure that the system has been released, the hooks should be checked in, at least, 2 different views or projections.6
The diabolo-shaped configuration makes this valve unsuitable for all RVOT anatomies. In cases of distal stenosis at PAT level or at the origin of pulmonary arteries this valve is ill-advised because such stenoses would be limiting the opening of the valve distal border. Regarding this shape—wider in its borders—the onset of transient ventricular extrasystole due to contact with the infundibulum proximal border has been reported. However, to this date, no arrhythmias have ever been reported requiring ablation or cardioversion. In our own experience, only 1 patient had frequent extrasystoles, yet no sustained ventricular arrhythmias were ever reported.
The clinical trial (NCT 02846753) conducted to obtain the CE marking revealed the presence of isolated endocarditis (incidence rate of 1.2%). When long-term follow-up results become available, the actual rates of endocarditis, fractures, and valvular dysfunction will be assessed.
Limitations
The short follow-up of patients is this study main limitation. We’ve been closely monitoring these patients including the use of Holter monitor and imaging modalities (echocardiography, CT scan, and MRI) to properly assess the mid- and long-term evolution of valve implantation. Another limitation is the lack of a control surgical group, which means that comparison can only be made with a historic cohort.
CONCLUSIONS
In conclusion, valve implantation with the new self-expanding Venus P-valve was, in our own preliminary experience, a safe and feasible procedure for valve implantation in very dilated RVOTs with a contraindication for the current balloon-expandable valves with CE marking.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the management and follow-up of the patients, data mining, and approved the manuscript final version for publication. M. Álvarez-Fuente, and M.J. del Cerro designed the study, analyzed data, and drafted the manuscript. HernÁndez, and I. García OrmazÁbal also drafted the manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THIS TOPIC?
- Transcatheter pulmonary valve implantation is a common procedure in patients with congenital heart diseases. However, the current valves available are not suitable for all anatomical variants or sizes of the pulmonary arterial trunk.
- New self-expanding valves with larger diameters to solve this problem are currently in the pipeline.
WHAT DOES THIS STUDY ADD?
- The early experience with the new self-expanding pulmonary Venus P-valve has been satisfactory in the first 8 implantations performed at our center.
- It allowed transcatheter studies of patients whose anatomies would have required surgery.
- This new valve allows one-stage implantations and is easy to use.
REFERENCES
1. McElhinney DB, Hellenbrand WE, Zahm EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122:507-516.
2. Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403-1405.
3. Boone RH, Webb JG, Horlick E, et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheter Cardiovasc Interv. 2010;75:286-294.
4. Schievano S, Coats L, Migliavacca F, et al. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson. 2007;9:687-695.
5. Sivakumar K, Sagar P, Qureshi S, et al. Outcomes of Venus P-valve for dysfunctional right ventricular outflow tracts from Indian Venus P-valve database. Ann Pediatr Cardiol. 2021;14:281-292.
6. Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus p-valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017;25:76-81.
7. Alvarez-Fuente M, Garrido-Lestache E, Fernandez-Pineda L, et al. Timing of Pulmonary Valve Replacement: How Much Can the Right Ventricle Dilate Before it Looses Its Remodeling Potential? Pediatr Cardiol. 2016;37:601-605.
8. Baumgartner H, De Backer J, Babu-Narayan SV, et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563-645.
9. Rodríguez Ogando A, Ballesteros F, Martínez JLZ. Pulmonary percutaneous valve implantation in large native right ventricular outflow tract with 32 mm Myval transcatheter heart valve. Catheter Cardiovasc Interv. 2022;99:E38-E42.
10. Morgan G, Prachasilchai P, Promphan W, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: international experience. EuroIntervention. 2019;14:1363-1370.
11. Promphan W, Prachasilchai P, Siripornpitak S, Qureshi SA, Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiol Young. 2016;26:698-710.