Special article
REC Interv Cardiol. 2019;2:108-119
Requirements and sustainability of primary PCI programs in Spain for the management of patients with STEMI. SEC, AEEC, and SEMES consensus document
Requisitos y sostenibilidad de los programas de ICP primaria en España en el IAMCEST. Documento de consenso de SEC, AEEC y SEMES
a Área de Enfermedades del Corazón, Hospital Universitario de Bellvitge, IDIBELL, Universidad de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain b Servicio de Cardiología, Hospital Universitario de León, León, Spain c Servicio de Cardiología, Hospital Clínico Universitario de Santiago, Santiago de Compostela, A Coruña, Spain d Servicio de Cardiología, Hospital Universitario de Salamanca, Salamanca, Spain e Servicio de Cardiología, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain f Servicio de Cardiología, Hospital Galdakao-Usansolo, Galdakao, Vizcaya, Spain g Servicio de Cardiología, Hospital Universitario La Paz, IDIPAZ, Madrid, Spain h Servicio de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain i Servicio de Cardiología, Hospital Álvaro Cunqueiro, Vigo, Pontevedra, Spain j Servicio de Cardiología, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain k Servicio de Cardiología, Hospital Universitario Virgen de la Victoria, Málaga, Spain l SUMMA 112, Madrid, Universidad Alfonso X el Sabio, Villanueva de la Cañada, Madrid, Spain m Servicio de Cardiología, Hospital do Salnés, Vilagarcía de Arousa, Pontevedra, Spain n Urgencias Sanitarias de Galicia 061, Santiago de Compostela, A Coruña, Spain o Servicio de Cardiología, Hospital Universitario 12 de Octubre, Madrid, Spain p Servicio de Cardiología, Hospital Universitario Reina Sofía, Córdoba, Spain
ABSTRACT
Transcatheter aortic valve implantation (TAVI) is established as the standard of care for patients across all surgical risk profiles, with expanding indications in younger and lower-risk populations. A substantial proportion of patients eligible for TAVI have preexisting cardiac implantable electronic devices (CIED). Temporary transvenous right ventricular (RV) pacing is routinely used during TAVI to facilitate procedural safety but carries inherent risks, including RV perforation, cardiac tamponade, and lead dislodgement. Alternative pacing strategies, such as left ventricular pacing via the valve delivery guidewire, have been proposed to reduce procedural complications. In patients with a preexisting CIED, leveraging the implanted device for procedural pacing represents a rational and potentially safer option. However, its adoption remains limited, largely due to unfamiliarity with device-specific programming. This article provides a detailed, step-by-step practical guide for programming rapid ventricular pacing using the most widely encountered CIED platforms: Biotronik, Medtronic, Abbott/St. Jude, Sorin, and Boston Scientific. The specific programming pathways for each manufacturer are summarized to facilitate safe, effective, and reproducible implementation during TAVI.
Keywords: TAVI. Pacing. Cardiac implantable electronic devices. Ventricular perforation.
RESUMEN
El implante percutáneo de válvula aórtica (TAVI) se ha establecido como el estándar de atención para pacientes de todos los perfiles de riesgo quirúrgico. Una proporción significativa de los candidatos a TAVI son portadores de dispositivos cardiacos implantables (DCI). La estimulación ventricular derecha transvenosa temporal es una práctica habitual, pero conlleva riesgos como perforación ventricular, taponamiento y desplazamiento del electrodo. Para reducir las complicaciones se han propuesto estrategias de estimulación alternativas, como la estimulación ventricular izquierda a través de la guía de liberación de la válvula; sin embargo, en los pacientes con un DCI preexistente, aprovechar dicho dispositivo para la estimulación durante el procedimiento representa una opción racional y potencialmente más segura. No obstante, su utilización sigue siendo limitada, principalmente debido al desconocimiento de la programación específica de cada dispositivo. Este artículo ofrece una guía práctica detallada, paso a paso, para la programación de la estimulación ventricular rápida utilizando las plataformas de DCI más comunes (Biotronik, Medtronic, Abbott/St. Jude, Sorin y Boston Scientific) y se resume las rutas de programación específicas de cada fabricante para facilitar una implementación segura, eficaz y reproducible.
Palabras clave: TAVI. Marcapasos. Dispositivos cardiacos implantables. Perforación ventricular.
Abbreviations
CIED: cardiac implantable electronic device. ICD: implantable cardioverter defibrillator. NIPS: non-invasive programmed stimulation. RV: right ventricular. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has emerged as the preferred therapeutic option across the full spectrum of surgical risk, and more recently its indications have expanded to include younger patients and those at low surgical risk.1-4 Patients undergoing TAVI are typically elderly and burdened with significant cardiovascular comorbidities, with a considerable proportion (8.7% to 23.1%) having preexisting cardiac implantable electronic devices (CIED).5-6
Temporary transvenous pacing is a well-established and integral component of TAVI, facilitating procedural success by reducing cardiac contractility during balloon aortic valvuloplasty and valve deployment. The target pacing rate varies according to the valve type: approximately 120 bpm for self-expanding valves and approximately 180 bpm for balloon-expandable valves.7 In the 2 scenarios, the objective is to mitigate the risk of micro- or macro-dislodgement or embolization during implantation.
Notwithstanding its utility, temporary right ventricular (RV) pacing is associated with potential complications, including myocardial injury, RV dysfunction, and perforation, which may culminate in cardiac tamponade.8-10 Furthermore, lead dislodgement and pacing failure have been reported among patients undergoing temporary pacing for a variety of indications, with incidence rates of approximately 4.6% and 9.5%, respectively. Although these figures are not specific to TAVI, they highlight that such complications are not rare and may result in ineffective pacing or sensing, thereby increasing the risk of critical intraoperative events, such as valve embolization or migration.11
To address these limitations, alternative pacing strategies have been proposed. Among them, left ventricular pacing via the valve delivery guidewire offers several advantages, such as elimination of the need for venous access, reduction of the risk of RV perforation, and potential reduction in procedural duration.12,13
In patients with preexisting CIED, use of the permanent device for procedural pacing appears to be a rational strategy to minimize complications.
The present article aims to address this unmet need by providing a concise and practical step-by-step guide for the use of the most widely encountered CIED programming consoles, thereby promoting safer and more efficient pacing management during TAVI.
USE OF ICD PROGRAMMING CONSOLES
This step-by-step guide for pacing patients with preexisting CIED during TAVI is derived from the routine clinical practice of our high-volume TAVI center, developed in close collaboration with our Electrophysiology Unit and in full compliance with the usage recommendations provided by our industry partners.
Biotronik (Germany)
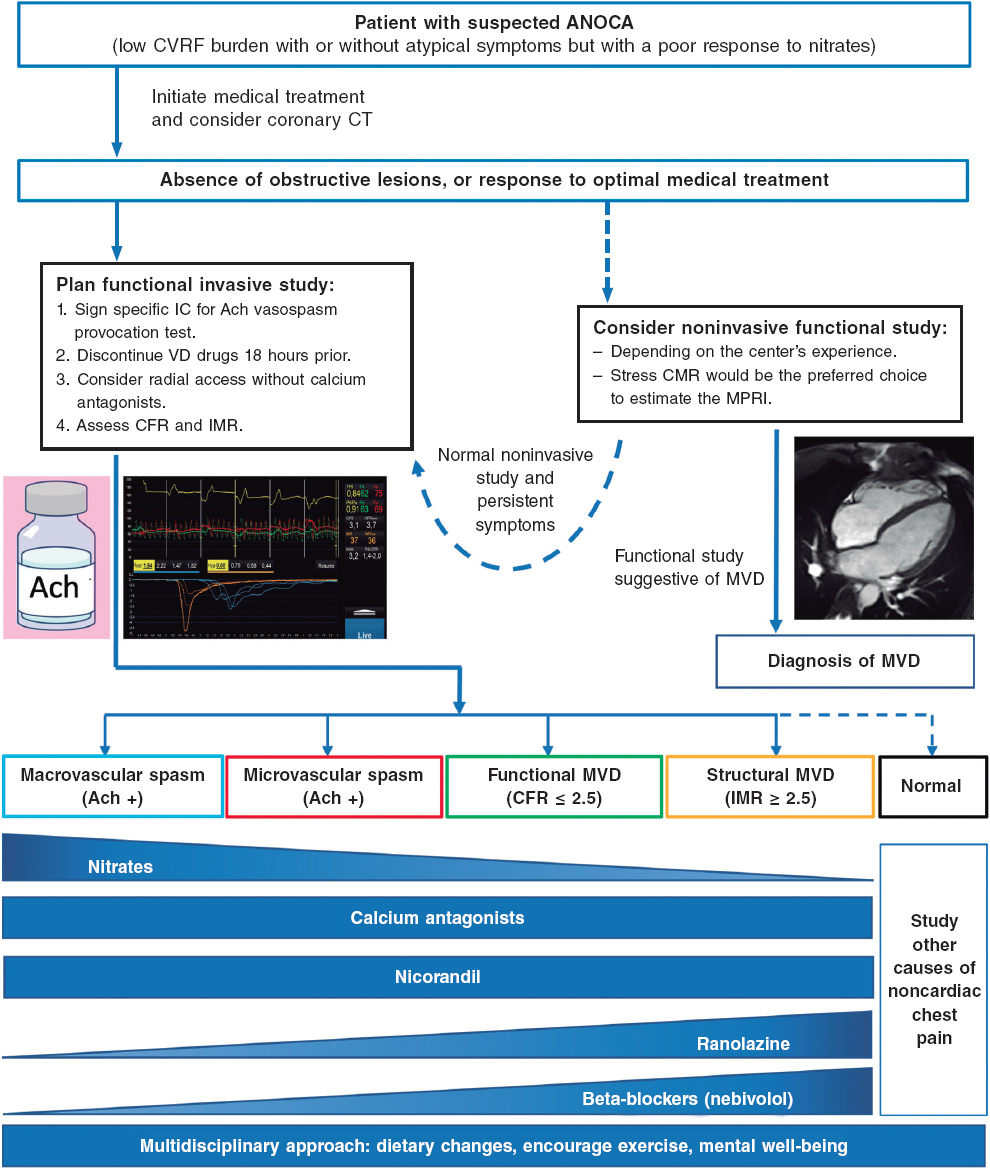
Not all pacemaker models support electrophysiological testing. However, this limitation can be overcome by using the threshold measurement section. Select “Test”, then “Pacing Threshold”, and choose “Manual” (figure 1A,B). First, select the pacing mode (eg, VVI), then set the desired pacing rate (up to 200 bpm) and ensure the output is set to the maximum (7.5V) to guarantee proper capture. Once all parameters are configured, press the “Start” button to begin stimulation, which will continue until manually stopped. If the device supports rapid pacing, such as non-Invasive programmed stimulation (NIPS), overdrive pacing can be performed using the VVI or V00 mode (figure 1C). Select the desired mode and basic rate, then press the “Start Backup Program” button.
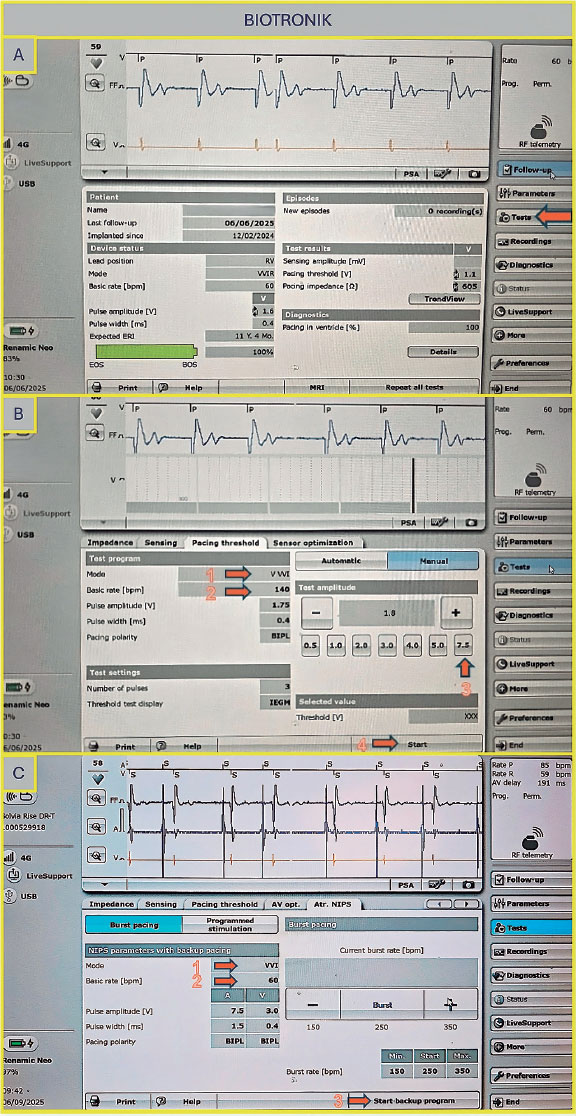
Figure 1. A: main screen of the Biotronik console. Select “Test” as indicated by the arrow to proceed to the pacing tests. B: to proceed to the pacing tests, select “Manual,” then set the following parameters: pacing mode (arrow 1), basic rate (arrow 2), amplitude (arrow 3), and press “Start” to initiate pacing (arrow 4). C: configure the NIPS parameters by selecting VVI mode (arrow 1), setting the basic rate (arrow 2), and pressing “Start” to begin pacing (arrow 3). NIPS: Non-Invasive Programmed Stimulation.
Medtronic (United States)
Once on the main screen (figure 2A), press the “Test” tab, then navigate to “Electrophysiology Study” and select either “Fixed Burst” or “Burst” (figure 2B). The interface will prompt you to choose the chamber in which pacing will be applied — select “Right Ventricular”. Adjust the interval to the desired value (as the interval decreases, the frequency increases — for example, 200 bpm corresponds to 300 ms). Once all parameters are set, press and hold the “Press and Hold” button to begin stimulation; release it to stop.
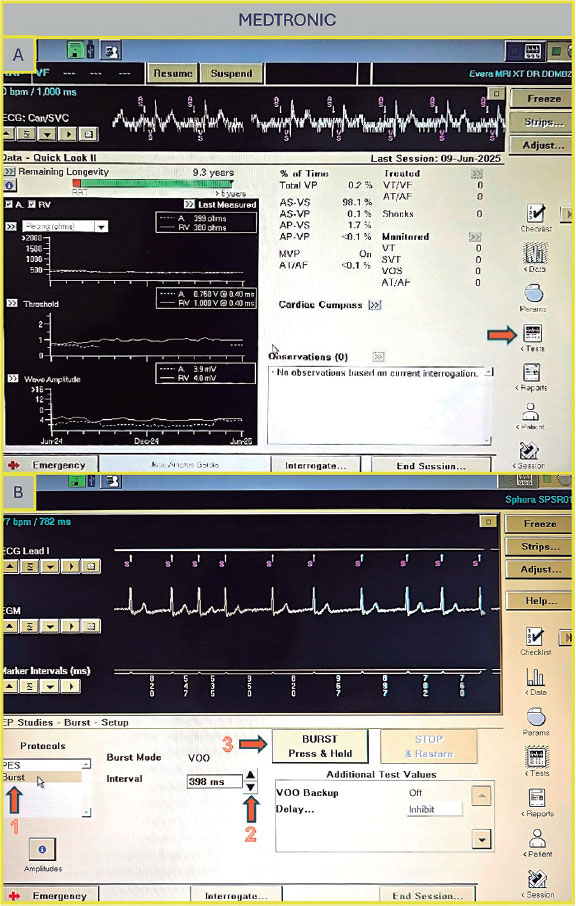
Figure 2. A: main screen of the Medtronic console. Press “Tests” (arrow) to access the electrophysiology study screen. B: select “Fixed Burst” or “Burst” (arrow 1), adjust the interval as needed (arrow 2), and press and hold the “Burst” button (arrow 3) to deliver pacing.
Abbott/St Jude (United States)
Once on the main screen (figure 3A), press “Test”. There are 2 options to perform rapid ventricular pacing:
- –NIPS (figure 3B): Click “Ventricular NIPS”, then select “Burst”, set the “Pulse Amplitude” (the output should be high to ensure capture), and adjust the pacing rate. In this case, the S1–S1 interval corresponds to the cycle length of the stimuli applied and must be set according to the desired heart rate. For example, 200 bpm corresponds to an S1–S1 interval of 300 ms.
- –Temporary pacing (figure 3C): Select the pacing mode (in this case, VVI), the desired rate (up to 170 bpm), and the maximum output to ensure capture. Once all parameters are set, press “Start Temporary” to begin pacing. Stimulation will continue until manually stopped.
Figure 3. A: main screen of the Abbott/St. Jude console. Press “Test” to proceed to the electrophysiology study screen. B: ventricular NIPS screen. Select “Burst” (arrow 1), set the amplitude (arrow 2), adjust the S1–S1 interval (arrow 3), and press the green button to deliver pacing (arrow 4). C: temporary pacing screen. Select the pacing mode (arrow 1), desired rate (arrow 2), and pulse amplitude (arrow 3), then press the “Start Temporary” button (arrow 4).
Sorin (Italy)
Select ”Tests”, then choose ”NIPS”. Under ”Mode”, select either ventricular pacing or ventricular burst, depending on the desired function. Next, set the ”Basic Period” (figure 4A,B) by choosing the corresponding rate in milliseconds (for example, 200 bpm corresponds to 300 ms). Once all parameters are set, press ”Start NIPS” to initiate the test.
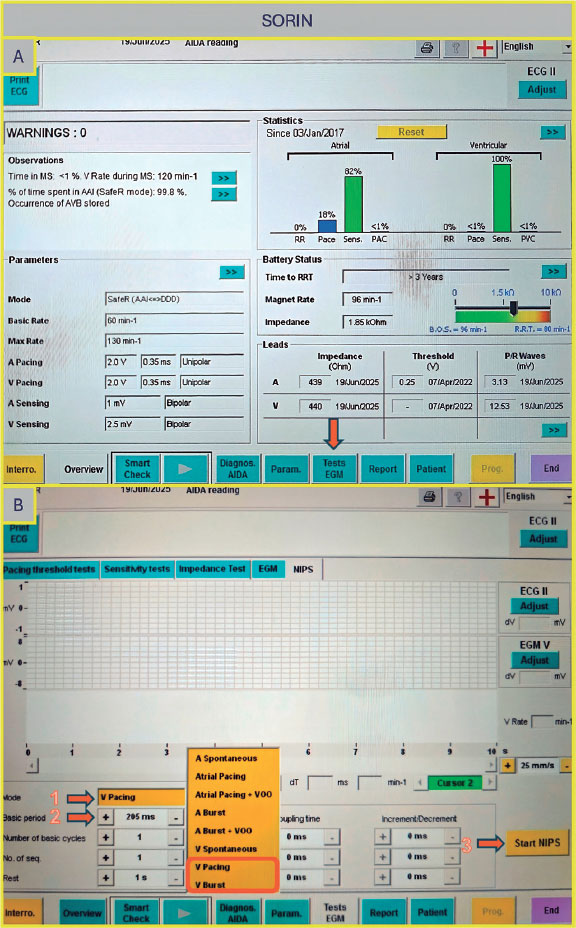
Figure 4. A: main screen of the Sorin console. Select “Tests EGM” (arrow) to access pacing options. B: “Tests EGM” screen. Select the pacing mode (arrow 1), the interval period (arrow 2), and then press the “Start NIPS” button (arrow 3).
Boston Scientific (United States)
Once on the main screen (figure 5A), select “Test”, then choose “Temp Brady” (figure 5B) From there, select VVI mode and set the desired heart rate under “Lower Rate Limit.” As in previous cases, ensure that the maximum output is selected to guarantee proper capture. After all parameters have been configured, press the ”Start” button to begin.
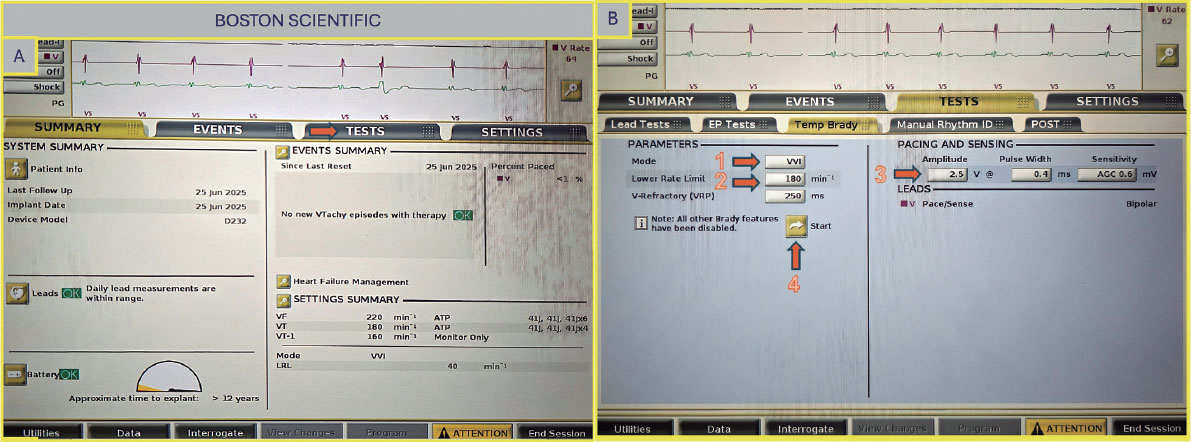
Figure 5. A: main screen of the Boston Scientific console. Select “Test” (arrow) to proceed to the pacing setup. B: select VVI mode (arrow 1), set the lower rate limit (arrow 2) and amplitude (arrow 3), then press the “Start” button to begin pacing (arrow 4).
DISCUSSION
While earlier permanent pacing devices had limitations in achieving instantaneous burst pacing —such as ramping up the ventricular rate instead of immediate increase and requiring time to reset the function—contemporary devices have largely overcome these issues.14 Most current models allow rapid burst pacing up to 180 bpm with immediate start and stop, using electrophysiology program mode settings or whenever electrophysiology program is not available (especially in some pacemakers) via a threshold test with maximum output and maximum duration. In cases where this is not possible or does not ensure adequate rapid pacing, the placement of a temporary pacing lead should be considered. Table 1 lists several of the most widely used CIED in our routine clinical practice and specifies whether they provide the capability for rapid temporary pacing or electrophysiological testing). It is always required to ensure that any changes in device programming are reversed and individually optimized; therefore, verification of the device settings at the beginning of the procedure is recommended, and any changes or modifications at the end should be avoided. In patients with implantable cardioverter defibrillator or cardiac resynchronization therapy-defibrillator devices, tachyarrhythmia therapies should be deactivated before the procedure to avoid inappropriate shocks. Of note, some temporary pacing algorithms have a programmed duration limit, so it is essential to verify in advance that high-rate temporary pacing can be maintained for the entire time required for prosthesis deployment, as this may vary depending on the device and pacing mode used.
Table 1. Characteristics of the main cardiac implantable electronic devices.
| Manufacturer | VVI-Pacemaker (EP study available yes/no) | DDD-Pacemaker (EP study available yes/no) | VVI-Defibrillator (EP study available yes/no) | DDD-Defibrillator (EP study available yes/no) | CRT-Pacemaker (EP study available yes/no) | CRT-Defibrillator (EP study available yes/no) |
|---|---|---|---|---|---|---|
| Biotronik | Ecuro SR (No) Enticos 4 SR (Yes) Edora 8 SR T (No) Evia SR T (Yes) Amvia Edge SR-T (Yes) Amvia Sky SR-T (Yes) |
Amvia Sky DR-T (No) Amvia Edge DR-T (Yes) Edora 8 DR-T (No) Evity 8 DR T (No) Ecuro DR (No) Effecta DR (Yes) Enticos 4 DR (Yes) Evia DR (Yes) Solvia Rise DR-T (no) |
Intica Neo 5 VRT (Yes) Iforia 3 VR-T (Yes) |
Iforia 3 DR-T (Yes) Inlexa 3 DR-T (Yes) Intica 5/7 (Yes) |
Edora 8 HF-T (Yes) Evia HF-T (Yes) Rivacor HF-T (Yes) Etrinsa 8 HF-T (Yes) |
Intica HF-T (Yes) Rivacor HF-T QP (Yes) |
| Boston Scientific | Essentio SR (Yes) Advantio SR (Yes) Accolade SR (Yes) |
Essentio DR (Yes) Advantio DR (Yes) Accolade DR (Yes) Ingenio (Yes) |
Charisma EL ICD (Yes) Inogen VR (Yes) Punctua NE ICD (Yes) Vigilant EL VR (Yes) Resonate VR (Yes) Autogen VR (Yes) Energen VR (Yes) |
Vigilant EL DR (Yes) Resonate (Yes) Energen (Yes) |
Intua (Yes) Visionist CRT-P (Yes) Valitude CRT-P (Yes) Invive (Yes) Accolate CRT-P (Yes) Incepta CRT-P (Yes) Essentio CRT-P (Yes) Resonate X4 CRT-P (Yes) |
Charisma CRT-D (Yes) Inogen CRT-D (Yes) Origen CRT-D (Yes) |
| Medtronic | Micra VR (No) Attesta SR (Yes) Sphera SR (Yes) Advisa SR (Yes) Ensura SR (Yes) Azure SR (Yes) |
Azure DR (Yes) Adapta DR (Yes) Advisa DR (Yes) Attesta DR (Yes) Ensura DR (Yes) Sphera DR (Yes) Sensia DR (Yes) Relia DR (Yes) |
Evera S VR (Yes) Maximo II VR (Yes/No) Protecta VR (Yes) Visia AF (Yes) Cobalt XT (Yes) |
Evera DR (Yes) Protecta DR (Yes) |
Serena CRT-P (Yes) Percepta CRT-P (Yes) |
Brava CRT-D (Yes) Compia CRT-D (Yes) Cobalt CRT-D (Yes) Claria CRT-D (Yes) |
| Sorin | Reply SR (Yes) Kora SR (Yes) |
Reply DR (Yes) Kora DR (Yes) Vega DR (Yes) |
Resiliant (Yes) Platinum SR (Yes) Intensia VR (Yes) Paradym SR (Yes) |
Platinum DR (Yes) Intensia DR (Yes) Paradym DR (Yes) |
Platinum CRT-P (Yes) Kora CRT-P (Yes) Luna CRT-P (Yes) Resiliant CRT-P (Yes) |
Platinum CRT-D (Yes) Paradym CRT-D (Yes) |
| Abbott/ St. Jude | Endurity Core (Yes) Assurity SR (Yes) Zephyr XL SR (Yes) |
Assurity DR (Yes) Accent DR (Yes) Endurity (Yes) Sustain XL DR (No) Verity ADx XL DR (No) |
Ellipse VR (Yes) Assura VR (Yes) Fortify Assura SR (Yes) Ellipse VR (Yes) Gallant VR (Yes) Neutrino (Yes) Entrant (Yes) |
Ellipse DR (Yes) Assura DR (Yes) Fortify Assura DR (Yes) Ellipse DR (Yes) Gallant DR (Yes) Neutrino DR (Yes) |
Quadra Allure CRT-P (Yes) Endurity CRT-P (Yes) Entrant CRT-P (Yes) Gallant CRT-P (Yes) |
Quadra Assura CRT-D (Yes) Unify Assura (Yes) CRT-D Fortify Assura CRT-D (Yes) Ellipse HF CRT-D (Yes) Entrant HF CRT-D (Yes) |
|
CRT, cardiac resynchronization therapy; CRT-P, cardiac resynchronization therapy pacemaker; CRT-D, cardiac resynchronization therapy defibrillator; DDD, dual-chamber pacing, dual-chamber sensing, and dual response to sensing; DR, dual chamber-rate; EP, electrophysiological; HF, heart failure; ICD, implantable cardioverter-defibrillators; SR, single rate; VVI, ventricular pacing, ventricular sensing, and inhibited response to a sensed event. |
||||||
Given the high ventricular rates required during this process and the deactivation of therapies in patients with implantable cardioverter defibrillator or cardiac resynchronization therapy-defibrillator devices, these maneuvers should be undertaken in a controlled environment with continuous monitoring, immediate availability of cardiopulmonary resuscitation, and access to external defibrillation equipment.
Currently, temporary RV pacing remains a common clinical practice during TAVI in this patient subgroup despite growing evidence that pacing via an implanted CIED is safe and feasible and may reduce pacemaker-related complications, such as cardiac tamponade and hemorrhage vs temporary RV pacing.9,15 However, certain challenges may arise, mainly of an organizational nature. During TAVI, a manufacturer-specific CIED programmer must be available, and the operator responsible for pacing should be adequately trained on how to operate the device programming functions. Because programming options vary by manufacturer and device type, operators must have thorough and specialized knowledge. In addition, the presence of trained nurses with specific expertise is essential to support device programming and ensure procedural safety. These devices can deliver rapid ventricular pacing through anti-tachycardia pacing functions but programming them often requires adjusting more complex parameters. Our proposed strategy is simpler and more practical, especially for professionals less experienced with advanced device programming.
CONCLUSIONS
In patients undergoing TAVI with preexisting cardiac implantable electronic devices, leveraging the implanted device for procedural pacing is a feasible and potentially safer alternative to temporary RV pacing. This strategy may reduce the risk of complications such as perforation, lead dislodgement, and bleeding. However, its broader adoption is hindered by the variability in device programming interfaces and limited operator familiarity. The step-by-step instructions provided in this guide aim to facilitate the practical implementation of this approach across the most widely encountered CIED platforms, ultimately promoting safer, more efficient, and complication-free TAVI procedures.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This is a step-by-step practical guide article; therefore, ethical committee approval was deemed unnecessary. No informed consent was required. Potential gender bias has been considered and excluded.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used in the development of this paper.
AUTHORS’ CONTRIBUTIONS
F. Pensotti and L.J. Garnacho elaborated the manuscript under the supervision of I.J. Amat-Santos that revised and drafted the manuscript. All the remaining authors conducted a critical review. All authors approved the final version.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite the fact that a substantial proportion of patients undergoing TAVI have a preexisting CIED, temporary pacing through these devices is rarely used. Instead, operators often prefer temporary RV pacing, which, however, carries inherent risks.
WHAT DOES THIS STUDY ADD?
- The step-by-step instructions of this guide are designed to help implement this approach on the most common CIED platforms, thereby ensuring safer, more efficient, and complication-free TAVI.
REFERENCES
1. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1):a randomised controlled trial. Lancet. 2015;385:2477-2484.
2. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374: 1609-1620.
3. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023;389: 1949-1960.
4. Forrest JK, Deeb GM, Yakubov SJ, et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J Am Coll Cardiol. 2023;82: 2163-2165.
5. Tichelbäcker T, Bergau L, Puls M, et al. Insights into permanent pacemaker implantation following TAVR in a real-world cohort. PLoS One. 2018; 13:0204503.
6. Wasim D, Ali AM, Bleie Ø, et al. Prevalence and predictors of permanent pacemaker implantation in patients with aortic stenosis undergoing transcatheter aortic valve implantation:a prospective cohort study. BMJ Open. 2025;15:093073.
7. Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of Balloon-Expandable vs Self-expandable Valves in Patients Undergoing Transcatheter Aortic Valve Replacement:The CHOICE Randomized Clinical Trial. JAMA. 2014;311:1503.
8. Axell RG, White PA, Giblett JP, et al. Rapid Pacing–Induced Right Ventricular Dysfunction Is Evident After Balloon-Expandable Transfemoral Aortic Valve Replacement. J Am Coll Cardiol. 2017;69:903-904.
9. Feldt K, Dalén M, Meduri CU, et al. Reducing cardiac tamponade caused by temporary pacemaker perforation in transcatheter aortic valve replacement. Int J Cardiol. 2023;377:26-32.
10. Barbash IM, Dvir D, Ben-Dor I, et al. Prevalence and Effect of Myocardial Injury After Transcatheter Aortic Valve Replacement. Am J Cardiol. 2013; 111:1337-1343.
11. Tjong FVY, de Ruijter UW, Beurskens NEG, et al. A comprehensive scoping review on transvenous temporary pacing therapy. Neth Heart J. 2019;27: 462-473.
12. Hilling?Smith R, Cockburn J, Dooley M, et al Rapid pacing using the 0.035?in. Retrograde left ventricular support wire in 208 cases of transcatheter aortic valve implantation and balloon aortic valvuloplasty. Cathet Cardio Intervent. 2017;89:783-786.
13. Savvoulidis P, Mechery A, Lawton E, et al. Comparison of left ventricular with right ventricular rapid pacing on tamponade during TAVI. Int J Cardiol. 2022;360:46-52.
14. DAS MK, Dandamudi G, Steiner HA. Modern pacemakers:hope or hype?Pacing Clin Electrophysiol. 2009;32:1207-1221.
15. Haum M, Steffen J, Sadoni S, et al. Pacing Using Cardiac Implantable Electric Device During TAVR:10-Year Experience of a High-Volume Center. JACC Cardiovasc Interv. 2024;17:1020-1028.
ABSTRACT
The gender gap in interventional subspecialties is largely due to concerns about occupational radiation exposure. The belief that it is not possible to continue working in cath labs during pregnancy is perceived by many female physicians as a barrier to develop their career or fulfill their motherhood wishes. Many physicians are unaware of the doses of ionizing radiation that are harmful for the fetus, which is the dose received by women who continue to work in cath labs throughout their pregnancies, or do not know the existing regulations. The Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC), the Heart Rhythm Association of the Spanish Society of Cardiology (ARC-SEC), the Spanish Society of Vascular and Interventional Radiology (SERVEI), the Spanish Society of Neuroradiology (SENR), the Spanish Society of Medical Radiology (SERAM), and the Society of the Spanish Group of Interventional Neuroradiology (GeNI) consider it necessary to draft this informative document and joint position paper to provide female physicians with the necessary knowledge to make fully informed decisions on whether to choose an interventional subspecialty or work exposed to ionizing radiation during their pregnancy.
Keywords: Ionizing radiation. Occupational radiation. Pregnancy. Interventional subspecialty. Female physicians.
RESUMEN
La exposición a radiaciones ionizantes subyace en la brecha de género existente en subespecialidades intervencionistas. La creencia de que no es posible continuar trabajando en la sala durante el embarazo es percibida como un impedimento para el desarrollo profesional o para llevar a cabo los deseos genésicos. Muchas profesionales desconocen qué dosis de radiaciones ionizantes son deletéreas para el feto, cuál es la dosis recibida si se mantiene la actividad en la sala durante el embarazo y cuál es la normativa vigente. Desde la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC), la Asociación del Ritmo Cardiaco de la Sociedad Española de Cardiología (ARC-SEC), la Sociedad Española de Radiología Vascular e Intervencionista (SERVEI), la Sociedad Española de Neurorradiología (SENR), la Sociedad Española de Radiología Médica (SERAM) y la Sociedad del Grupo Español de Neurorradiología Intervencionista (GeNI) se considera necesario este documento informativo y de consenso, para proporcionar a las profesionales el conocimiento necesario para tomar decisiones plenamente informadas en cuanto a la elección o no de una subespecialidad intervencionista y la decisión de mantener o no una actividad con exposición a radiaciones ionizantes durante el embarazo.
Palabras clave: Radiación ionizante. Radiación ocupacional. Embarazo. Subespecialidad intervencionista. Profesionales mujeres.
Abbreviations
CSN: Spanish Nuclear Safety Council. EURATOM: European Atomic Energy Community. IR: ionizing radiation. RD: Royal Decree. RP: radiological protection.
INTRODUCTION
The percentage of women in subspecialties with exposure to ionizing radiation (IR) is significantly lower compared with men1-5 in a society where women largely outnumber men in medical schools. Only 25% of the members from the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC)6,7 are women, 28% of the members of the Spanish Society of Vascular and Interventional Radiology (SERVEI),8 and 34.5% of professionals accredited as electrophysiology specialists.9 Similarly, in other medical societies, such as the Society of the Spanish Working Group of Interventional Neuroradiology (GeNI), the percentage of women is even lower, currently at 22%.
One of the reasons for this gender gap is the belief that professional activity is incompatible with IR exposure during pregnancies.10-12 There is a lack of knowledge about the IR doses that have deleterious effects on the fetus, the dose received by a pregnant worker who remains active in the cath lab, and the current legislation on this issue. Furthermore, there is no homogeneity in occupational hazard prevention and radiological protection (RP) services across various health care centers when issuing recommendations on whether women are fit to continue working in an environment with IR exposure once pregnancy has been declared. Thus, in some centers, pregnant women can keep on working with IR exposure, while in others they are removed from their positions. On the other hand, women who decide to avoid working in an environment with IR exposure during pregnancy and change positions within their departments during gestation also face problems, as their career development and remuneration are altered during that period. In fact, they sometimes find little support when trying to come back to their pre-pregnancy job. The confusion, lack of information, and absence of unanimous criteria when determining the professional occupation of women during this period influence their decision to rule out training in these subspecialties and lead to their professional development being diminished or slowed down.
Therefore, ACI-SEC, the Heart Rhythm Association of the Spanish Society of Cardiology (ARC-SEC), the Spanish Society of Vascular and Interventional Radiology (SERVEI), the Spanish Society of Neuroradiology (SENR), the Spanish Society of Medical Radiology (SERAM), and the GeNI Society have deemed it necessary to prepare this document, which is not only informative but also a consensus document on those IR doses that have demonstrated deleterious effects on the fetus, the average dosimetry received by a worker who remains active in the cath lab during pregnancy, what the Spanish current legislation actually says, and what advice and recommendations are considered reasonable for exposed professionals, in accordance with available scientific evidence and current regulations.
The objective of this document is to provide professionals with the necessary knowledge to make a fully informed decision on whether to choose an interventional subspecialty and continue with their job during gestation.
BIOLOGICAL EFFECTS OF IONIZING RADIATION ON THE FETUS
IR interferes with cell multiplication, especially in tissues with a high replication rate.13 Exposure to IR during the fetal period can lead to intrauterine growth restriction, malformations, tumors, and even fetal death. The risk depends on the magnitude and temporal distribution of the exposure, as well as the gestational age at which it occurs. Table 1 illustrates the IR-induced tissue reactions in the embryo or fetus, according to the gestational period, and the radiation threshold value.
Table 1. Tissue reaction to ionizing radiation in the embryo or fetus according to gestational period and threshold value
| Gestational period | Effect | Estimated radiation threshold |
|---|---|---|
| Pre-implantation (0-2 weeks post-conception) | Embryo death or no effect | 50-100 mGy |
| Organogenesis (2-8 weeks) | Congenital malformations (skeletal, eyes, genitals) | 200 mGy |
| Growth retardation | 200-250 mGy | |
| 8-15 weeks | Severe intellectual disability (high risk of occurrence) | 60-310 mGy |
| Decrease in intellectual quotient | 20 IQ points/100 mGy | |
| Microcephaly | 200 mGy | |
| 16-25 weeks | Severe intellectual disability (low risk of occurrence) | 250-280 mGy |
|
Adapted with permission from Cheney et al.21. |
||
Radiation exposure, measured by the magnitude “absorbed dose” or kerma, is defined as the radiation energy received by an organ or tissue per unit mass and measured in milligray (mGy). The same absorbed radiation dose can cause a different biological effect depending on the ionizing agent emitting it, hence the term “equivalent dose,” which is the absorbed dose corrected by a weighting factor measured in millisievert (mSv). Since the radiation weighting factor for X-rays is 1, in this case, both the absorbed and the equivalent doses are numerically equal, with 1 mGy of absorbed dose being equal to 1 mSv of equivalent dose.14
IR can cause deleterious effects through deterministic and stochastic effects. The former have dose thresholds with intensity being proportional to the magnitude of the radiation. They are constant, reproducible, and related to moderate-to-high doses due to direct damage to multiple cell lines. The term “tissue reaction”15 is recommended as it better reflects the mechanism of damage and the dose-response relationship. The latter have no dose threshold, are caused by random damage to cellular genetic material, and show as growth and multiplication changes. Although a linear risk-exposure relationship with no minimum threshold is assumed, there is greater uncertainty surrounding this relationship at low dose levels (< 0.1 Gy).15 Their severity is independent of exposure.
General effects of radiation during the embryonic and fetal period
Evidence of these effects in humans comes from longitudinal studies of survivors of nuclear disasters, pregnant women exposed to medical or occupational radiation, and case-control studies of childhood leukemias and tumors.16 The only experimental studies have been conducted with animals.13 The magnitude of exposure is the main determinant of damage. Thus, irradiation at moderate-to-high doses can cause abortions, malformations, intellectual disability, and intrauterine growth restriction;17,18 at low doses, results are inconsistent. Damage varies depending on the stage of pregnancy. Within the first few weeks of gestation, there is greater radiosensitivity; each organ has critical weeks that are consistent with its organogenesis.19 As the fetus matures, the damage progressively decreases.18 Dose fractionation causes less damage than a single dose of the same intensity but shorter duration.18,20
Risk of fetal damage at moderate doses
Moderate doses are those between 100 mGy and 1 Gy. These have been recorded mainly in nuclear disasters and medical procedures, such as radiotherapy. These doses are not reached in the cath lab setting. Up to the 4th gestational week, doses of 0.1-0.2 Gy can be lethal.13,18 Doses > 2 Gy at any stage of pregnancy are associated with fetal death.21 This effect is an “all or nothing” phenomenon, where a sufficiently intense aggression can cause the death of the embryo or be completely repaired, given the high capacity for repair and differentiation of pluripotent cells.17 The threshold for the appearance of malformations stands between 1 Gy and 2 Gy,14 highlighting alterations in brain development, ocular, musculoskeletal, and genital systems. The most sensitive period corresponds to gestational weeks 8-25, followed by weeks 16-25. Fetal susceptibility decreases significantly after the 26th gestational week.20,22 Doses > 200 mGy have been associated with intrauterine growth restriction, low birth weight, shorter height, and decreased head circumference.20 Moreover, intellectual disability17 is associated with high doses of radiation. The central nervous system is especially radiosensitive during gestational weeks 8-25, specifically weeks 8-15, a period known as the “cortical sensitivity window”.13 Doses > 100 mGy are related to a decrease in intellectual quotient and risk of severe intellectual disability. This effect has been attributed to direct cell death and neuronal migration alterations. The high plasticity and redundancy of brain tissue could be the cause of the absence of effects below these doses. Doses < 100 mGy have not shown an association with fetal alterations or abortions.23
Risk of fetal damage at low doses
Low doses are those < 100 mSv, especially < 50 mSv. These doses can be reached in therapeutic procedures, while the doses involved in occupational exposures, living in regions of widespread environmental radiation, or frequently taking transatlantic flights are negligible. The appearance of tumors throughout life is their main stochastic effect, lacking safe minimum values.18,24 However, confirming this effect and estimating its magnitude present great methodological difficulties due to its low incidence rate, high latency, and low number of irradiated pregnant women.15 For this reason, results have been disparate.
Follow-up until 2012 of cohorts exposed to nuclear bombings found a higher mortality rate due to solid tumors only from adulthood in daughters of women exposed to this radiation, with an estimated mean dose received by these pregnant women of 123 mGy.25 This finding is only partially consistent with studies conducted in Europe after the Chernobyl accident, which only found a possible increase in thyroid tumors in uteri exposed to radioactive iodine release.26 Nonetheless, there was not an increased risk of childhood leukemia.27 In other nuclear accidents, studies of populations living close to nuclear facilities or nuclear test sites have not found any changes in the incidence rate of childhood cancer.15 Studies on occupational exposures have not demonstrated an increased risk of cancer after in utero exposures in the nuclear industry28 or medical radiology.29
Regarding the exposure of pregnant women to radiological tests, most are case-control studies. One of the main sources was the Oxford Survey of Childhood Cancer, from which the first studies emerged linking in utero exposure to childhood cancer.24 This research allowed for estimating a first absolute excess risk of cancer mortality of 500-650/10 000 people/year/Gy, updated to a relative risk excess of 51%/10 mGy for leukemia and 46%/10 mGy for other solid tumors.16 In 1982, data from these studies were used to estimate the probabilities of childhood cancer and malformations according to the exposure level (table 2). These values are the most widely used to this date.30
Table 2. Increased risk of malformations and childhood cancer according to received dose
| Embryo or fetus dose (mSv) | Absence of malformations (%) | Absence of childhood cancer (%) | Absence of malformations or childhood cancer (%) |
|---|---|---|---|
| 0 | 96.00 | 99.93 | 95.93 |
| 0.5 | 95.99 | 99.92 | 95.92 |
| 1 | 95.99 | 99.921 | 95.92 |
| 5 | 95.99 | 99.89 | 95.88 |
| 10 | 95.98 | 99.84 | 95.83 |
|
Adapted with permission from Wagner et al.30. The table was prepared assuming a dose increase above existing environmental radiation. |
|||
A review of the main studies found an association between in utero irradiation and leukemias or solid tumors, mainly in older cohorts.31 However, longitudinal studies with cohorts of pregnant women who underwent radiodiagnostic tests did not yield significant findings.32
Experimental studies with mice have found an increase in ovarian cancer with single doses of 0.25 Gy,33 and lymphoma with single doses of 0.18 Gy.34 Other studies, however, have not found changes to the incidence rate with doses of 2-3 Gy, except when these doses were administered after birth.35
EUROPEAN AND SPANISH LEGISLATION
The exposure of workers to IR is regulated by both national and international agencies. The European Atomic Energy Community (EURATOM) is a European public organization that coordinates nuclear energy research programs and develops regulations. These regulations are based on guidelines from the International Atomic Energy Agency, which, in turn, draws upon recommendations from the International Commission on Radiological Protection. The latter is an autonomous organization composed of experts in radiation protection. The European Union EURATOM treaty sets out the RP legislation that member states must adopt and transpose into their own national laws.
In Spain, the only competent organization in matters of nuclear safety and RP is the Consejo de Seguridad Nuclear, the Spanish Nuclear Safety Council (CSN), an independent entity of the Spanish General State Administration, which regulates the operation of nuclear and radioactive facilities, proposing regulations and standards. The instructions from the CSN become binding after publication in the Spanish Boletín Oficial del Estado (Official State Bulletin).
In 2013, the International Commission on Radiological Protection developed radiological protection (RP) guidelines for cardiology. These guidelines were subsequently adopted by EURATOM in Directive 2013/59.36 These guidelines and Article 10 of the EURATOM Directive state that pregnancy does not imply the exclusion of women from their job, but rather that working conditions must be carefully evaluated to ensure the safe limit of 1 mSv for the fetus throughout the pregnancy. This 1 mSv limit is established because it is considered that the protection of the fetus should be comparable to that of any person, who should not receive > 1 mSv in 1 year due to activities derived from the operation of nuclear and radioactive facilities. The transposition of European regulations in Spain is embodied in the form of Royal Decrees (RD). The RD applicable to the occupational exposure of pregnant workers are RD 298/200937 and RD 1029/2022.38 The former incorporated Annex VIII, which details a list of agents to which pregnant workers should not be at risk of exposure, including IR. This RD specifies that pregnant workers may not carry out activities that pose a risk of exposure to these agents if, according to the conclusions obtained from risk assessment, this may endanger their health or that of the fetus. Article 12 of RD 1029/2022 literally transposes Article 10 of the EURATOM Directive, establishing the same limit of 1 mSv during pregnancy. This RD repeals previous RD 783/2001 and 413/1997 on the protection of workers at risk of IR exposure, as well as all norms of equal or lower rank that contradict or oppose the provisions of this RD.
In 2016, the CSN approved the document “Protection of pregnant workers exposed to IR in the health care setting”39 specifying that, “as a general rule, the condition of pregnancy of an exposed professional does not imply her automatic withdrawal from work; what is necessary is to review her working conditions to comply with current regulations,” and that “pregnant workers may not carry out activities that involve a risk of exposure to IR if, according to the conclusions obtained in a risk assessment, there may be danger to their safety, their health, that of the child, or that of the fetus.” Furthermore, the document states that “from the moment a pregnant woman communicates her condition, the protection of the fetus must be comparable to that of the rest of the population. Therefore, the equivalent dose to the fetus must be as low as reasonably achievable (ALARA criteria),13 so that it is unlikely to exceed 1 mSv, at least from the communication of her condition until the end of her pregnancy.” Although this dose of 1 mSv is established for 1 cm depth, the actual depth at which the fetus is located is greater, and dose attenuation occurs due to the abdominal wall and the uterus. According to some models, the dose received by the fetus is 0.27% of that measured by the surface dosimeter during the first trimester, 0.23% during the second, and 0.17% during the third trimester. Thus, the CSN establishes that, in practice, the limit during pregnancy is 2 mSv on the abdominal dosimeter, or an equivalent dose received by the fetus of 1 mSv.14,40,41
MEANS OF PROTECTION VS IONIZING RADIATION
Exposure to IR requires RP measures for both patients and the health care personnel.42 Pregnant professionals must implement extraordinary measures.
Use of personal protective equipment
Lead aprons are a primary barrier that protects the organs most sensitive to radiation. They are designed to absorb and scatter radiation, significantly reducing the dose received by personnel. A lead apron, composed of a vest and skirt, with a lead thickness of 0.25 mm, is sufficient during gestation, as the overlap of the 2 layers of the skirt on the anterior abdominal wall provides protection equivalent to 0.5 mm, thus attenuating 98% of scattered radiation, which is the main source of radiation. As the abdomen grows, a larger apron should be used to ensure the double layer on the anterior abdominal wall. There are specific aprons for pregnant women that add an extra layer to the double layer of the skirt on the anterior abdominal wall. During the first trimester, 0.5 mm lead gonad shields can be internally attached to the lead skirt14,43,44. Although adding additional skirts or aprons would minimally reduce radiation, it could lead to musculoskeletal problems. Both during gestation and lactation, complete breast protection must be ensured, as breast tissue is particularly radiosensitive; for this, aprons of the appropriate size for each worker should be used, avoiding uncovered areas in the axillary region that could expose the breasts.
Training and continuous education in RP
Health care personnel must be aware of the risks and available protective measures. Working as far as possible from the radiation source, raising the table as much as possible, and being as efficient as possible with emitted radiation doses are basic measures in any circumstance. If feasible, pregnant workers should avoid participating in long procedures, due to both IR exposure and prolonged static standing. Similarly, since the embryo or fetus is more radiosensitive within the first gestational weeks, it is reasonable to abstain from participating in examinations with IR exposure during the first trimester.
The design of the work environment is critical. Cath labs must be equipped with lead barriers and protective screens to minimize exposure and reduce the amount of scattered radiation. They are an indispensable protective tool during pregnancy, as they attenuate 99% of scattered radiation and reduce overall radiation exposure by 50%-75%.43,45,46
Finally, monitoring radiation exposure is an essential measure. Personal dosimeters allow continuous monitoring of the received radiation dose and record cumulative exposure. They ensure that doses remain within the limits established by regulations and help identify risk situations that require corrective measures. The history of each worker’s cumulative exposure helps identify trends. In the case of pregnant women, there are dosimeters with real-time readings45,47 that allow confirming that the permitted dosimetry is not exceeded, both monthly and at any given time. The personal dosimeter must be changed monthly and worn in the appropriate place; for the abdominal dosimeter, it will be under the lead apron to record the equivalent dose received beneath it. Incorrect use of the dosimeter prevents RP services from evaluating the dose the worker receives in her usual work and, consequently, whether she can continue working during pregnancy.
BACKGROUND RADIATION, OTHER DAILY LIFE RADIATIONS, AND OCCUPATIONAL DOSE
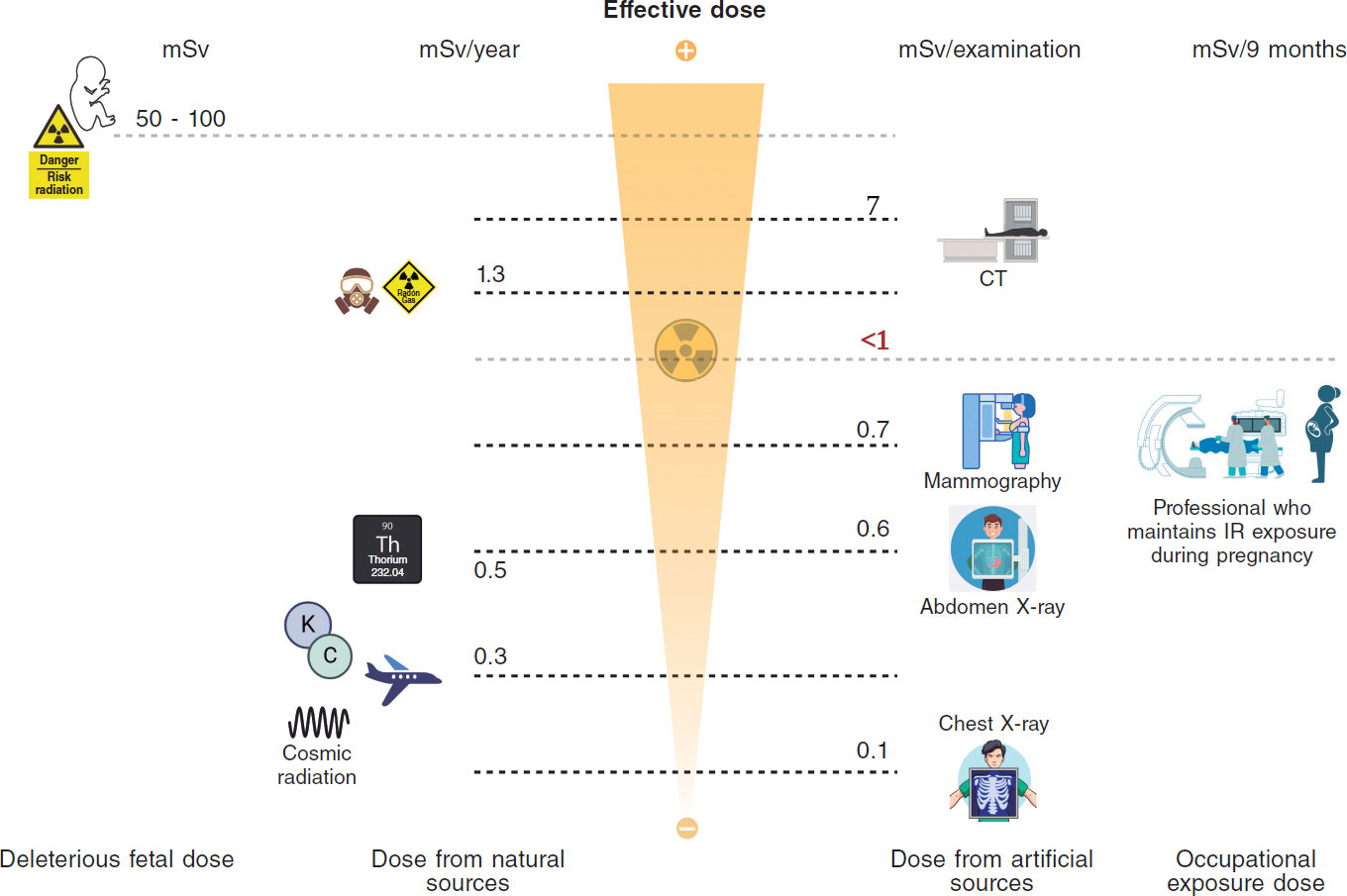
Understanding the doses received by the population allows for putting occupational doses into perspective. The average annual exposure to background radiation ranges between 1 mSv and 2.3 mSv;14 this radiation comes from radon in the air, cosmic radiation from space, and natural radioactivity in soil and building materials. Radon is a radioactive gas emitted by soil and rocks that accounts for 50% of a person’s annual radiation dose, approximately 1.3 mSv.48 Radioactive materials from the Earth’s crust, such as uranium, thorium, and potassium, represent an average annual exposure of 0.5 mSv. Cosmic radiation, from high-energy particles from outer space that penetrate the atmosphere, represents an average annual exposure of about 0.3 mSv at sea level. On a long-haul flight, the average radiation is about 0.003-0.0097 mSv/h.49 Internal radiation from radioactive isotopes in food and water, such as potassium-40 and carbon-14, generates an average absorbed radiation dose of 0.3 mSv per year.50,51 Background radiation varies depending on the area where one lives; for example, in some places in Galicia (Spain), 1.45 mSv of annual environmental radiation is reached.52 Considering these data and the dosimetric information of workers who have maintained their activity in the cath lab during pregnancy, the occupational dose received during pregnancy can be even lower than the dose received as a result of background radiation.25-27,53,54 Similarly, a round-trip transatlantic flight can expose a person to 0.1 mSv due to cosmic radiation at high altitude. This IR dose is similar to that of a chest X-ray and greater than the doses received by most interventional women who keep working in the cath lab throughout pregnancy45 (figure 1).
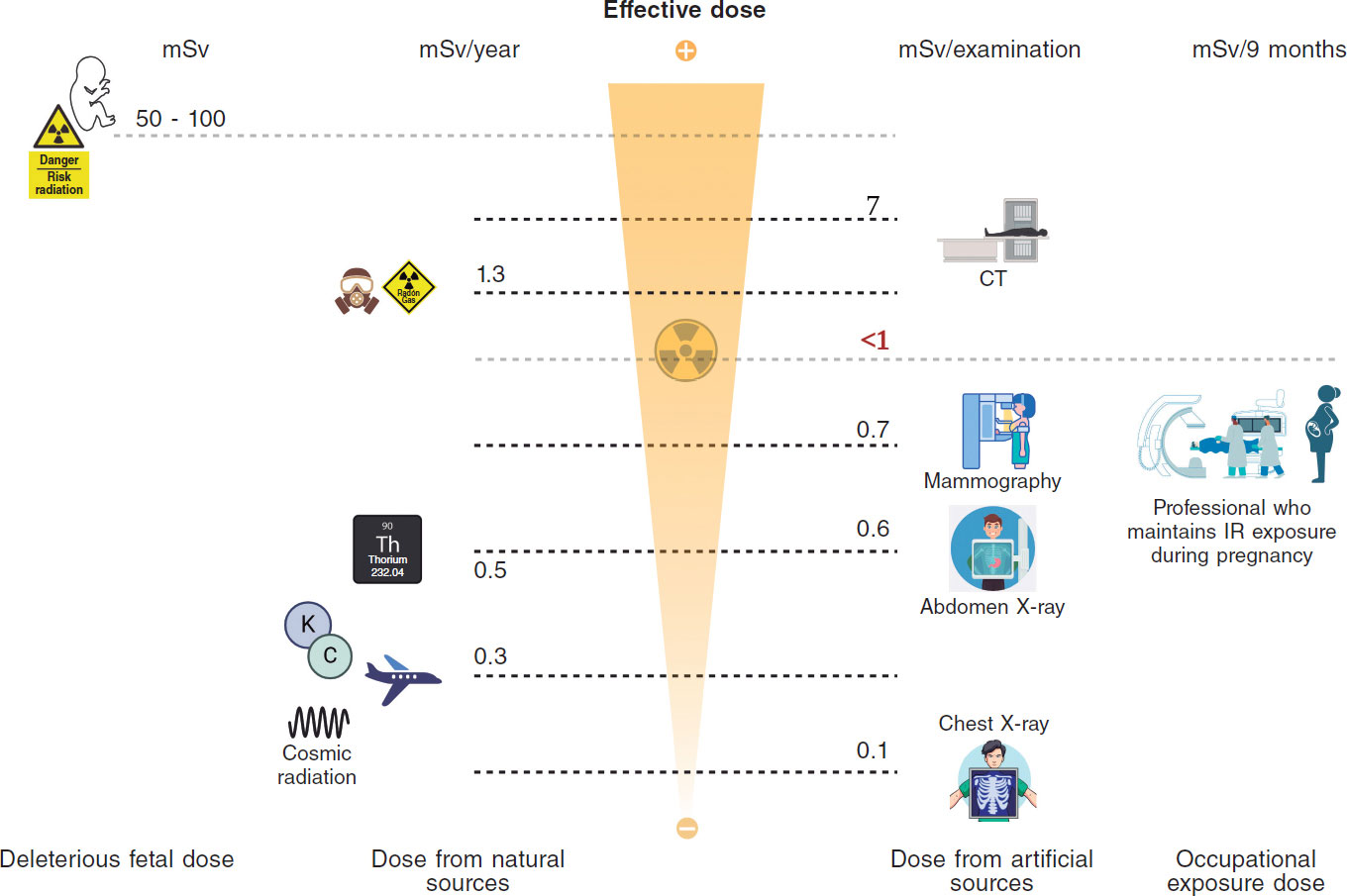
Figure 1. Central illustration. Comparison between ionizing radiation doses causing stochastic or deterministic effects in the fetus, doses due to natural or medical radiation sources, and occupational doses.14 (Created with BioRender.) CT, computed tomography; IR, ionizing radiation; Rx, X-rays.
Regarding occupational doses, the periodic reports issued by the CSN provide information on the dose received by workers exposed to IR in Spain. According to these reports, in 2023, 127 234 workers in Spain were dosimetrically controlled,55 of whom 96% received < 1 mSv, with 0.6 mSv/year being the average dose received by workers in medical radiodiagnostic centers. Even so, to make an informed decision, we need to know the doses received by interventional women who continue working in the cath lab during pregnancy. Table 3 illustrates the doses reported through questionnaires or direct interviews with women who continued working in the cath lab during pregnancy; although this information is very limited due to the small number of exposed pregnant workers, the doses registered on abdominal dosimeters are < 1 mSv in all cases.12,43,45
Table 3. Published data on occupational doses of pregnant workers with ionizing radiation exposure
| Country | No. of pregnancies/No. of interventional workers | Protection used | Dose received during gestation | Outcome |
|---|---|---|---|---|
| Spain | 15 pregnancies / 11 interventional cardiologists or electrophysiologists | Standard vest + skirt (7/15) Extra skirt or additional gonadal protection (8/15) | Background dose (8/15) 0.2 mSv (2/15) < 1 mSv (3/15) Not reported (2/15) | 14/15 normal gestations 1/15 placental insufficiency |
| France | 8 pregnancies / 5 interventional cardiologists or electrophysiologists | Standard vest + skirt (5/8) Additional mobile protective screen (3/8) | Background dose (7/8) 0.2 mSv (1/8) | 8/8 normal gestations |
| New Zealand | Isolated cases of pregnancies in interventional cardiologists and electrophysiologists | Information not available in the study | Monthly monitoring described with abdominal dosimeter clearly below the dose threshold | All gestations reported as normal |
| Australia | 21 pregnancies / 11 interventional cardiologists or electrophysiologists | 2 pregnancies left the cath lab for 6-9 weeks | Monthly monitoring described with abdominal dosimeter clearly below the dose threshold | Outcomes reported as comparable to those of the non-exposed population |
However, the dose received by a worker depends on the number and type of procedures performed, whether she is the primary operator, the patient’s body mass, the fluoroscopy-cine time, technical aspects of the X-ray machine, and the radiation protection measures adopted. Therefore, if the worker decides to keep on working in the cath lab during pregnancy, the received dose can be reduced by modifying usual activity, keeping it as low as possible, below the maximum permitted by the current legislation. Regarding on-call duties, when deciding whether to continue performing them during gestation, the worker should take into consideration that most procedures performed during these will be therapeutic, and some of them can be prolonged, leading to greater exposure and long periods of standing.
DISCUSSION
One of the duties of scientific societies is to respond to the problems and concerns of the professionals they represent promoting initiatives that reduce gender bias in the workplace. According to surveys and published works, occupational exposure to IR is one of the reasons that lead to a gender bias in subspecialties such as interventional cardiology, electrophysiology, or interventional radiology.1,56 The belief that pregnant workers cannot perform activities with occupational IR exposure causes professionals to perceive difficulties in these subspecialties in reconciling professional development with motherhood, which leads them to self-exclude. However, considering the review of doses that have demonstrated biological effects on the fetus25-27,53,54, there is no risk of abortion with received doses < 50 mSv, nor risk of deterministic effects, such as malformations or intellectual disability with doses < 60 mSv.18,23 We should remember that the rate of early spontaneous abortion in pregnancies known to women is 10%-20%,57 which is a multifactorial event. Regarding stochastic effects, there is no evidence either of a higher risk with doses < 50 mSv.15,27
Occupational exposure to IR during pregnancy is regulated in Europe by directives that take into consideration the recommendations of the International Commission on Radiological Protection. In Spain, the regulations governing the dose that the fetus can receive due to the mother’s occupational activity during pregnancy are included in several RD and have been developed following the recommendations made by the CSN.37-39 The fetus is considered a member of the general population and, like anyone else, cannot receive > 1 mSv/year. This postulate is one of the most conservative ones, since the International Commission on Radiological Protection establishes this limit of 1 mSv for the fetus, and the National Council on Radiation Protection and Measurements establishes a threshold limit for the fetus of 5 mSv.58 This is so because, in the United States, employers prioritize the rights of pregnant workers from an anti-discrimination perspective, while European legislation policies prioritize fetal safety. Australia and Israel set the bar at 1 mSv throughout pregnancy too, and Japan sets a threshold limit of 2 mSv.45
Both deterministic and stochastic effects present a threshold for onset several orders of magnitude higher than occupational doses. Furthermore, they are very far from the doses received by professionals who have kept on working in the lab during pregnancy, since in none of the published works has the received dose been > 1 mSv.45 Table 2 illustrates the spontaneous risk of congenital malformations in newborns (4%) and the spontaneous probability of a child developing cancer in childhood (0.07%). The increase in the probability that the fetus will have a malformation at birth or develop cancer in childhood if it receives 1 mSv during gestation would be 0.008% above the spontaneous risk, that is, an extremely low increase in risk.30
In Europe, Austria, Hungary, Italy, Portugal, and Romania have not transposed the EURATOM directive into their own legislations and do not allow women to work in the lab during pregnancy.45 Furthermore, in Spain, the occupational risk prevention and RP services of some centers, in full compliance with Annex VIII of the 2009 RD,37 declare the worker “unfit” to keep on working during pregnancy. However, this measure would not be justified for several reasons. Firstly, because the 2009 RD specifies that a pregnant worker may not carry out activities that pose a risk of exposure if, according to the conclusions obtained from the risk assessment, this may endanger her safety or health or that of the fetus, which is not the case if the dose threshold of 1 mSv is respected. Secondly, because the 2022 RD establishes that the working conditions of pregnant women shall be such that the equivalent dose to the fetus should be as low as reasonably achievable, in such a way that the dose does not exceed 1 mSv, at least since the worker communicated her condition until the end of pregnancy, which implies that it is possible to continue working during pregnancy if said limit is not exceeded. A third reason would be that, as stated in the health surveillance guide for occupational risk prevention prepared by the Spanish Ministry of Health, Consumer and Social Welfare, the preferred way to communicate the results of health surveillance, from an occupational risk prevention perspective, is in the form of preventive recommendations.59 This document only considers that the qualification of “unfit” should be applied when there is a high probability of harm to the health of the worker or to any third parties and workplace adaptation is not possible, recommending in all other cases the qualification of “fit with adaptation measures”.59 As we have seen, in the case of occupational exposure to IR, the probability of harm to the health of third parties (fetus) is not high, since occupational doses are < 1 mSv. Moreover, workplace adaptation is possible by providing the worker with an abdominal dosimeter, even with real-time reading, to closely monitor the dose received, thus increasing protection with a specific lead apron for pregnant women or with gonadal shields, and even excluding the pregnant woman from procedures associated with high IR doses, while preserving her usual activity in the cath lab. Therefore, the qualification that should be given to workers exposed to IR when they become pregnant is “fit with adaptation measures”.59
Of note that declaring a professional “unfit” to perform her job during pregnancy removes her from her usual work environment interrupting her working projects and procedures in which she is involved. Furthermore, sometimes after pregnancy and maternity leave, women are not easily reintegrated into the professional projects they were developing in their departments before gestation. All this constitutes a situation of workplace inequality, a slowdown in professional development, and a loss of opportunities compared with their male colleagues, an unjustified prejudice if we adhere to the scientific evidence and current Spanish legislation.
Therefore, greater efforts must be made by scientific societies, the CSN, and the Spanish Ministry of Health to promote the dissemination of knowledge about the deleterious effects of IR, the doses that cause them, and the doses to which pregnant women with occupational exposure are exposed. Initiatives are needed to recognize, make visible, and solve the difficulties these professionals face. It’s also crucial to engage officials at the Spanish Ministry of Labor, as they oversee occupational risk prevention services. The goal is to raise awareness about the need for clearer regulations regarding pregnant professionals in laboratory settings. This would eliminate any ambiguity about their legal permission to continue working, provided the radiological protection department approves based on their dosimetry history.
In any case, when making a decision in this regard, we should remember that information on the dose received by workers who kept working in the cath lab during pregnancy comes from a small number of interventional cardiologists and electrophysiologists, and that information comes only from surveys and observational data. Of note, there are few studies on the health of the offspring of women who have maintained activity with IR exposure during pregnancy, and the available data are not consistent due to the small number of irradiated women and the low incidence and high latency of the effects. Similarly, the value of 1 mSv should not be considered a strict regulatory threshold; what should be attempted is that, once the worker has notified the pregnancy, her working conditions are such that it is unlikely that the fetus will receive a dose > 1 mSv during the remainder of the pregnancy.
The decision to keep on working in the cath lab and performing on-call duties during pregnancy must be made freely by the worker after consulting with her health care professionals, and based on scientific information, her dosimetric history, her physical and emotional state, and the correct use of RP measures. This choice can have both physical (exposure to unwanted doses of ionizing radiation or musculoskeletal discomfort from wearing a lead apron) and emotional implications. Of note, 10%-20% of pregnancies end in spontaneous abortion, due to mostly multifactorial and often unknown causes, not necessarily attributable to occupational radiation exposure per se. This situation can lead to a feeling of “retrospective guilt,” as can the possible appearance of medical problems in the fetus or child, even if there is no scientific evidence linking said condition to occupational exposure. Therefore, it is a reality that making the decision involves a great silent emotional burden and a significant internal conflict and must be free from external pressures and accompanied by adequate emotional support for all pregnant women, whatever their decision.
Since the current legislation allow pregnant workers to continue in their workplace as long as dose limits are respected, while contemplating the possibility of requesting a workplace adaptation without radiation exposure, it is essential to preserve each woman’s freedom of decision, preventing her from being removed from the cath lab against her will and ensuring, in all cases, a safe and understanding working environment. For those who choose not to be exposed to radiation during gestation, it is crucial to facilitate not only workplace adaptation but also full reintegration into their previous job and projects after maternity leave, including training in techniques that may have been incorporated during their absence.
Promoting this flexibility and institutional support is key to preventing sex discrimination and attracting and retaining female talent in interventional subspecialties where women are still a minority. Occupational radiation should not be perceived as a factor incompatible with pregnancy per se, but rather as a manageable circumstance based on autonomy, knowledge, fetal safety, and respect.
CONCLUSIONS
The fear of IR effects on the fetus is one of the reasons many female workers dismiss training in an interventional subspecialty. However, both the European directive and current Spanish law state that pregnant women should not be excluded from their jobs, provided the fetus is protected like any other individual and its maximum occupational radiation dose during gestation does not exceed 1 mSv.
The doses received by professionals who maintain their activity with IR exposure during pregnancy do not exceed the 1 mSv limit in any of the published studies, placing them at an order of magnitude 50 or 100 times lower than those that have demonstrated deleterious effects on the fetus. Abdominal dosimeters allow close monitoring of the doses received by workers with occupational IR exposure, ensuring that the limit established by regulations is not exceeded. Therefore, according to the CSN, any worker with occupational IR exposure, under working conditions where it is improbable that the fetus will receive a dose > 1 mSv, can feel safe in her workplace as long as she responsibly abides by the recommendations of the RP department and correctly uses the abdominal dosimeter.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
Since this is not an experimental work, but a review and opinion article, it has been deemed unnecessary to consider international recommendations on clinical research or submit it to the ethical committees of the centers of the corresponding authors. Since this is not a study conducted with patients informed consent was deemed unnecessary as well. An analysis of possible sex and gender biases is not applicable because it focuses only on female workers and occupational exposure to IR during pregnancy.
AUTHORS’ CONTRIBUTIONS
M. Velázquez Martín, S. Lojo Lendoiro, B. Cid Álvarez, and T. Bastante Valiente contributed to the conception and design of the study. M. Velázquez Martín, S. Lojo Lendoiro, Nina Soto, T. Bastante Valiente, and B. Cid Álvarez contributed to the drafting of the text. All authors critically reviewed the intellectual content of this manuscript and gave their final approval to the version published. Similarly, all authors take full responsibility for all aspects of the article and commit themselves to investigating and resolving any issues related to the accuracy and veracity of any part of the work.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Manzo-Silberman S, Piccaluga E, Radu M, et al. Radiation protection measures and sex distribution in European interventional catheterisation laboratories. EuroIntervention. 2020;16: 80-82.
2. Abdulsalam N, Gillis AM, Rzeszut AK, et al. Gender Differences in the Pursuit of Cardiac Electrophysiology Training in North America. J Am Coll Cardiol. 2021;78: 898-909.
3. Wang TY, Grines C, Ortega R, Dai D, Jacobs AK, Skelding KA, et al. Women in interventional cardiology: Update in percutaneous coronary intervention practice patterns and outcomes of female operators from the National Cardiovascular Data Registry®. Catheter Cardiovasc Interv. 2016;87: 663-668.
4. Burgess S, Shaw E, Ellenberger K, Thomas L, Grines C, Zaman S. Women in Medicine: Addressing the Gender Gap in Interventional Cardiology. J Am Coll Cardiol. 2018;72: 2663-2667.
5. Tamirisa KP. Women and cardiac electrophysiology as a career path. Heart Rhythm Case Rep. 2023;9: 267.
6. Cid Álvarez AB. Mujer, cardiología y subespecialidades intervencionistas. REC: CardioClinics. 2022;58: 70-71.
7. Bastante T, Arzamendi D, Martín-Moreiras J, et al. Spanish cardiac catheterization and coronary intervention registry. 33rd official report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2023). Rev Esp Cardiol. 2024;77: 936-946.
8. Lojo Lendoiro S, Moreno Sánchez T. Radiación ocupacional y embarazo: realidad o desinformación. Revisión en la literatura y actualización según guías clínicas vigentes. Radiologia. 2022;64: 128-135.
9. Portwood Cl. Reasons and resolutions for gender inequality among cardiologists and cardiology trainees. Br J Cardiol. 2023;30: 13.
10. Capranzano P, Kunadian V, Mauri J, et al. Motivations for and barriers to choosing an interventional cardiology career path: results from the EAPCI Women Committee worldwide survey. EuroIntervention. 2016;12: 53-59.
11. Buchanan G, Ortega R, Chieffo A, Mehran R, Gilard M, Morice MC. Why stronger radiation safety measures are essential for the modern workforce. A perspective from EAPCI Women and Women as One. EuroIntervention. 2020;16: 24-25.
12. Adeliño R, Malaczynska-Rajpold K, Perrotta L, et al. Occupational radiation exposure of electrophysiology staff with reproductive potential and during pregnancy: an EHRA survey. Europace. 2023;25: euad216.
13. Valentin J. Biological effects after prenatal irradiation (embryo and fetus): ICRP Publication 90 Approved by the Commission in October 2002. Ann ICRP. 2003;33: 1-206.
14. Saada M, Sanchez-Jimenez E, Roguin A. Risk of ionizing radiation in pregnancy: just a myth or a real concern?Europace. 2022;25: 270-276.
15. National Council on Radiation Protection and Measurements. Report No. 174 –Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance (2013). Bethesda, MD: NCRP;January 30, 2018. Available at: https://ncrponline.org/shop/reports/report-no-174-preconception-and-prenatal-radiation-exposure-health-effects-and-protective-guidance-2013/. Accessed 13 Jul 2024.
16. Wakeford R, Bithell JF. A review of the types of childhood cancer associated with a medical X-ray examination of the pregnant mother. Int J Radiat Biol. 2021;97: 571-592.
17. National Research Council (US) Committee on the Biological Effects of Ionizing Radiation (BEIR V). Health Effects of Exposure to Low Levels of Ionizing Radiation: Beir V. Washington (DC): National Academies Press (US);1990.
18. Brent RL. Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol. 2009;200: 4-24.
19. De Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF, Caruso A. Radiation effects on development. Birth Defects Res C Embryo Today: 2007;81: 177-182.
20. Valentin J, ed. Pregnancy and Medical Radiation. Oxford: Pergamon Press;2000.
21. Cheney AE, Vincent LL, McCabe JM, Kearney KE. Pregnancy in the Cardiac Catheterization Laboratory: A Safe and Feasible Endeavor. Circ Cardiovasc Interv. 2021;14: e009636.
22. Ikenoue T, Ikeda T, Ibara S, Otake M, Schull WJ. Effects of environmental factors on perinatal outcome: neurological development in cases of intrauterine growth retardation and school performance of children perinatally exposed to ionizing radiation. Environ Health Perspect. 1993;101(Suppl 2): 53-57.
23. Mainprize JG, Yaffe MJ, Chawla T, Glanc P. Effects of ionizing radiation exposure during pregnancy. Abdom Radiol (NY). 2023;48: 1564-1578.
24. Stewart A, Webb J, Giles D, Hewitt D. Malignant disease in childhood and diagnostic irradiation in utero. Lancet. 1956;268: 447.
25. Sugiyama H, Misumi M, Sakata R, Brenner AV, Utada M, Ozasa K. Mortality among individuals exposed to atomic bomb radiation in utero: 1950–2012. Eur J Epidemiol. 2021;36: 415-428.
26. Hatch M, Brenner AV, Cahoon EK, et al. Thyroid Cancer and Benign Nodules After Exposure In Utero to Fallout From Chernobyl. J Clin Endocrinol Metab. 2018;104: 41-48.
27. Parkin DM, Clayton D, Black RJ, et al. Childhood leukaemia in Europe after Chernobyl: 5 year follow-up. Br J Cancer. 1996;73: 1006-1012.
28. Roman E, Doyle P, Ansell P, Bull D, Beral V. Health of children born to medical radiographers. Occup Environ Med. 1996;53: 73-79.
29. Johnson KJ, Alexander BH, Doody MM, et al. Childhood cancer in the offspring born in 1921–1984 to US radiologic technologists. Br J Cancer. 2008;99: 545-550.
30. Wagner LK, Hayman LA. Pregnancy and women radiologists. Radiology. 1982;145: 559-562.
31. Little MP, Wakeford R, Bouffler SD, et al. Cancer risks among studies of medical diagnostic radiation exposure in early life without quantitative estimates of dose. Sci Total Environ. 2022;832: 154723.
32. Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70: 130-139.
33. Uma Devi P, Hossain M. Induction of solid tumours in the Swiss albino mouse by low-dose foetal irradiation. Int J Radiat Biol. 2000;76: 95-99.
34. Benjamin SA, Lee AC, Angleton GM, Saunders WJ, Keefe TJ, Mallinckrodt CH. Mortality in beagles irradiated during prenatal and postnatal development. II. Contribution of benign and malignant neoplasia. Radiat Res. 1998;150: 330-348.
35. Ellender M, Harrison JD, Kozlowski R, Szłuin´ska M, Bouffler SD, Cox R. In utero and neonatal sensitivity of ApcMin/+mice to radiation-induced intestinal neoplasia. Int J Radiat Biol. 2006;82: 141-151.
36. Directiva 2013/59/Euratom del Consejo, de 5 de diciembre de 2013, por la que se establecen normas de seguridad básicas para la protección contra los peligros derivados de la exposición a radiaciones ionizantes, y se derogan las Directivas 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom y 2003/122/Euratom. Available at: https://www.boe.es/doue/2014/013/L00001-00073.pdf. Accessed 24 Nov 2024.
37. Boletín Oficial del Estado. Disposiciones generales. Ministerio de la Presidencia. 7 de marzo de 2009, núm. 57, sec. I. Pág. 23288. Available at: https://www.boe.es/boe/dias/2009/03/07/pdfs/BOE-A-2009-3905.pdf. Accessed 13 Jul 2024.
38. Boletín Oficial del Estado. Disposiciones generales. Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática. 21 de diciembre de 2022, núm. 305, sec. I. Pág. 178672. Real Decreto 1029/2022, de 20 de diciembre, por el que se aprueba el Reglamento sobre protección de la salud contra los riesgos derivados de la exposición a las radiaciones ionizantes. Available at: https://www.boe.es/eli/es/rd/2022/12/20/1029/dof/spa/pdf. Accessed 24 Nov 2024.
39. Consejo de Seguridad Nuclear. Protección de las trabajadoras gestantes expuestas a radiaciones ionizantes en el ámbito sanitario. Available at: https://www.csn.es/documents/10182/914805/Protecci%C3%B3n%20de%20las%20trabajadoras%20gestantes%20expuestas%20a%20radiaciones%20ionizantes%20en%20el%20%C3%A1mbito%20sanitario%20(Actualizaci%C3%B3n%202024). Accessed 13 Jul 2024.
40. Consejo de Seguridad Nuclear. Requisitos técnicos-administrativos para los servicios de dosimetría personal. Available at: https://www.csn.es/documents/10182/896572/GS+07-01+Revisi%C3%B3n+1+-+Requisitos+t%C3%A9cnico-administrativos+para+los+servicios+de+dosimetr%C3%ADa+personal+(Febrero+2006)/dfe4292b-7792-45fc-ba67-b16302e19c64?version=1.4. Accessed 13 Jul 2024.
41. Damilakis J, Perisinakis K, Theocharopoulos N, et al. Anticipation of Radiation Dose to the Conceptus from Occupational Exposure of Pregnant Staff During Fluoroscopically Guided Electrophysiological Procedures. J Cardiovasc Electrophysiol. 2005;16: 773-780.
42. Vu CT, Elder DH. Pregnancy and the Working Interventional Radiologist. Semin Intervent Radiol. 2013;30: 403-407.
43. Velázquez M, Pombo M, UnzuéL, Bastante T, Mejía E, Albarrán A. Radiation Exposure to the Pregnant Interventional Cardiologist. Does It Really Pose a Risk to the Fetus?Rev Esp Cardiol. 2017;70: 606-608.
44. Marx MV. Baby on Board: Managing Occupational Radiation Exposure During Pregnancy. Tech Vasc Interv Radiol. 2018;21: 32-36.
45. Manzo-Silberman S, Velázquez M, Burgess S, et al. Radiation protection for healthcare professionals working in catheterisation laboratories during pregnancy: a statement of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the European Heart Rhythm Association (EHRA), the European Association of Cardiovascular Imaging (EACVI), the ESC Regulatory Affairs Committee and Women as One. EuroIntervention. 2023;19: 53-62.
46. Miller DL, VañóE, Bartal G, et al. Occupational Radiation Protection in Interventional Radiology: A Joint Guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2010;33: 230-239.
47. Poudel S, Weir L, Dowling D, Medich DC. Changes in Occupational Radiation Exposures after Incorporation of a Real-time Dosimetry System in the Interventional Radiology Suite. Health Physics. 2016;111: S166.
48. Consejo de Seguridad Nuclear. Mapa del potencial de radón en España. Available at: https://www.csn.es/mapa-del-potencial-de-radon-en-espana. Accessed 13 Oct 2024.
49. Bottollier-Depois JF, Chau Q, Bouisset P, Kerlau G, Plawinski L, Lebaron-Jacobs L. Assessing exposure to cosmic radiation on board aircraft. Adv Space Res. 2003;32: 59-66.
50. Ortega García JA, Ferrís i Tortajada J, OrtíMartín A, et al. Contaminantes medio-ambientales en la alimentación. An Esp Pediatr. 2002;56: 69-76.
51. Diario Oficial de las Comunidades Europeas. 22/07/1989. N.ºL 211/1. Reglamento (EURATOM) N.º2218/89 del Consejo de 18 de julio de 1989. Available at: https://www.boe.es/doue/1989/211/L00001-00003.pdf. Accessed 13 Oct 2024.
52. Consejo de Seguridad Nuclear. Mapa de radiación gamma natural en España (MARNA) MAPA. Available at: https://www.csn.es/mapa-de-radiacion-gamma-natural-marna-mapa. Accessed 4 Jan 2025.
53. Hatch M, Brenner A, Bogdanova T, et al. A Screening Study of Thyroid Cancer and Other Thyroid Diseases among Individuals Exposed in Utero to Iodine-131 from Chernobyl Fallout. J Clin Endocrinol Metab. 2009;94: 899-906.
54. Stewart A, Barber R. Survey of childhood malignancies. Public Health Rep (1896). 1962;77: 129-139.
55. Consejo de Seguridad Nuclear. Informe del Consejo de Seguridad Nuclear al Congreso de los Diputados y al Senado. Año 2023. Available at: https://www.calameo.com/read/006700665a458974bd45d. Accessed 8 Apr 2025.
56. Bernelli C, Cerrato E, Ortega R, et al. Gender Issues in Italian Catheterization Laboratories: The Gender-CATH Study. J Am Heart Assoc. 2021;10: e017537.
57. Benson LS, Holt SK, Gore JL, et al. Early Pregnancy Loss Management in the Emergency Department vs Outpatient Setting. JAMA Network Open. 2023;6: e232639.
58. National Council on Radiation Protection and Measurements. NCRP Report No. 174, Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance. Bethesda, MD: NCRP;June 1, 2015. Available at: https://ncrponline.org/publications/reports/ncrp-report-174/. Accessed 13 Jul 2024.
59. Ministerio de Sanidad, Consumo y Bienestar Social. Vigilancia de la salud para la prevención de riesgos laborables. Guía básica y general de orienta-ción. Available at: https://www.sanidad.gob.es/ciudadanos/saludAmbLaboral/docs/guiavigisalud.pdf. Accessed 19 Jul 2024.
* Corresponding author.
E-mail addresses: ; (M. Velázquez Martín).
@maitevelazquezm;
@shci_sec;
@SERVEISoc;
@saralojo86;
@Geni_NRI;
@ritmo_SEC;
@SERAM_RX;
@SENR_ORG
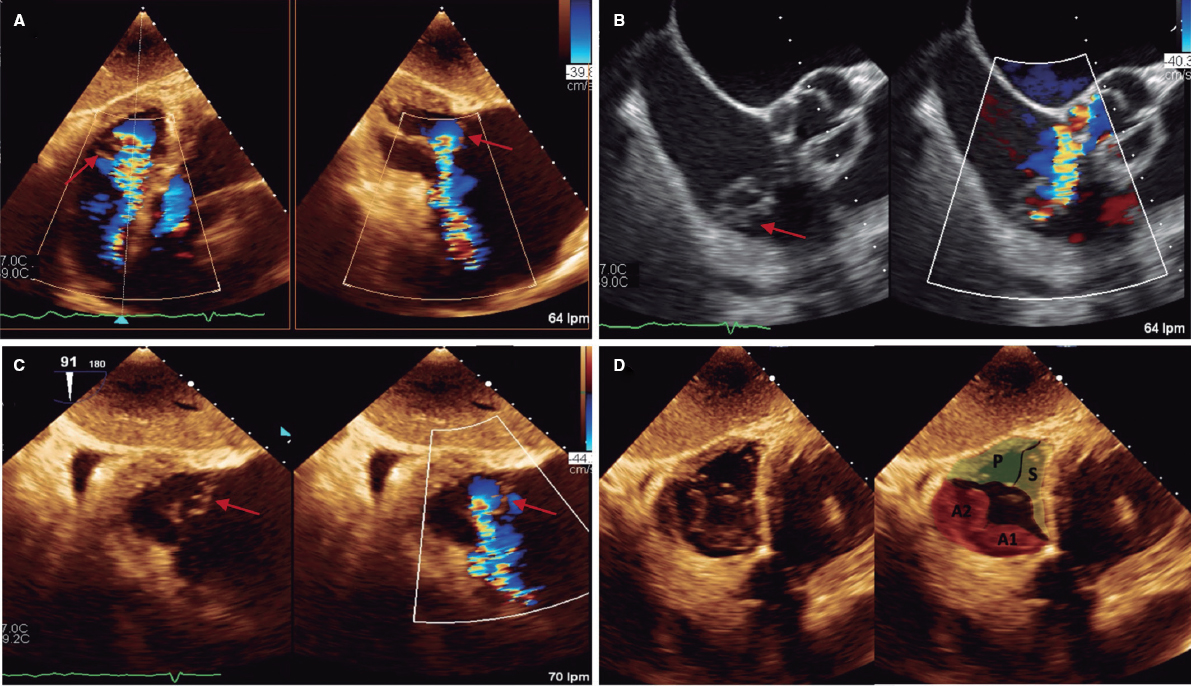
ABSTRACT
The approach to patients with acute mitral regurgitation poses a therapeutic challenge. These patients have a very high morbidity and mortality rate, thus requiring a multidisciplinary approach. This document presents the position of 3 associations involved in the management of these patients: the Ischemic Heart Disease and Acute Cardiovascular Care Association, the Interventional Cardiology Association, and the Cardiac Imaging Association. The document discusses aspects related to patient selection and care, technical features of the edge-to-edge procedure from both the interventional and imaging unit perspectives, and the outcomes of this process. The results of mitral repair and/or replacement surgery, which is the first-line treatment option to consider in these patients, have not been included as they exceed the scope of the aims of the document.
Keywords: Mitral regurgitation. Acute myocardial infarction. Left ventricular ejection fraction. Papillary muscle rupture. Transcatheter edge-to-edge mitral valve repair.
RESUMEN
El tratamiento de los pacientes con insuficiencia mitral aguda supone un reto terapéutico. Estos pacientes tienen una morbimortalidad muy elevada, que requiere un abordaje multidisciplinario. El presente documento recoge el posicionamiento de tres asociaciones implicadas en el tratamiento de estos pacientes: la Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares, la Asociación de Cardiología Intervencionista y la Asociación de Imagen Cardiaca. Incluye aspectos relacionados con la selección y los cuidados del paciente, los aspectos técnicos del tratamiento de borde a borde desde el punto de vista intervencionista y de la imagen cardiaca, y los resultados de este proceso. No se han incluido los resultados de la cirugía de reparación o sustitución mitral, que es la primera opción terapéutica a considerar en estos pacientes, por exceder los objetivos del documento.
Palabras clave: Insuficiencia mitral. Infarto agudo de miocardio. Fracción de eyección del ventrículo izquierdo. Rotura del músculo papilar. Tratamiento de reparación percutánea de borde a borde.
Abbreviations LV: left ventricle. MR: mitral regurgitation. PMR: papillary muscle rupture. TEER: transcatheter edge-to-edge repair.
PATIENT SELECTION, OPTIMAL TIMING, AND MANAGEMENT IN THE CARDIAC INTENSIVE CARE UNIT
In severe acute mitral regurgitation (MR), the sharp increase in left ventricular (LV) end-diastolic volume leads to a rapid rise in LV and left atrial end-diastolic pressure. This ultimately results in marked pulmonary congestion and the development of acute pulmonary edema.1 Concurrently, the large volume of regurgitation reduces forward flow and cardiac output. Patients with pre-existing MR and normal ventricles have better hemodynamic tolerance; conversely, those with with associated ischemia and ventricular dysfunction experience clinical worsening.1,2
The etiology of acute MR can be divided into 2 groups (table 1): ischemic and nonischemic. Ischemic causes include acute ischemia of the papillary muscle, its rupture in the context of acute myocardial infarction, ventricular remodeling, and increased leaflet traction and tethering. Nonischemic causes encompass chordal rupture in myxomatous valve disease and complications from interventional cardiology procedures. Other causes include endocarditis, trauma, and dynamic MR due to anterior systolic motion of the mitral valve in patients with hypertrophic or stress-induced cardiomyopathy.2,3
Table 1. Etiology, pathophysiology, and clinical presentation of acute mitral regurgitation
| Etiology | Pathophysiology | Clinical presentation | Treatment |
|---|---|---|---|
| Papillary muscle ischemia | Increase in left ventricular end-diastolic pressure | Acute heart failure/acute pulmonary edema | Diuretics |
| Infarction-related papillary muscle rupture | Increase in left atrial pressure | Flash acute pulmonary edema | Inotropes (dobutamine, milrinone) |
| Ruptured chordae tendineae | Increase in pulmonary capillary wedge pressure | Cardiogenic shock | Vasodilators (nitroprusside) / vasopressors |
| Anterior systolic motion (obstructive hypertrophic cardiomyopathy, tako-tsubo syndrome) | Decreased output due to reduced antegrade flow | Revascularization | |
| Dilated cardiomyopathy - secondary mitral regurgitation | Mechanical circulatory support (intra-aortic balloon pump, extracorporeal membrane oxygenator, Impella) | ||
| Endocarditis | Edge-to-edge repair | ||
| Trauma | Surgery | ||
| Perioperative complication |
Patients with acute MR are usually symptomatic. The clinical presentation varies depending on the mechanism, speed of onset, presence of prior MR, and ventricular function. Flash pulmonary edema can occur in patients with dynamic MR and normal ventricular function, often due to increased afterload. In these patients, blood pressure may remain normal or be elevated.1-4 The most severe form of severe acute MR is cardiogenic shock, which is. It commonly arises in patients with LV systolic dysfunction but can also develop in those with preserved ventricular function and sudden onset of MR due to papillary muscle rupture (PMR). In intermediate stages, patients may have acute pulmonary edema and maintained blood pressure without progressing to shock.3,5
The primary objective of treatment should be clinical and hemodynamic stabilization (figure 1). These patients should be promptly transferred to a tertiary referral center with specialized acute/intensive cardiac care units, cath labs, and cardiac surgery units. High-dose intravenous loop diuretics are the cornerstone of medical treatment, preferably administered in continuous infusion. Inotropic agents are recommended in patients with LV systolic dysfunction. In patients with normal or elevated blood pressure, intravenous vasodilators—mainly nitroprusside or nitroglycerin—are recommended because they reduce LV afterload and thereby mitigate MR severity.6-8 The use of vasopressors is reserved to patients in cardiogenic shock with hypotension and persistent hypoperfusion despite inotropic therapy. Because these drugs increase afterload and may exacerbate MR, they should be administered at the lowest effective dose to maintain adequate tissue perfusion pressure.1,7,8

Figure 1. Treatment algorithm for acute mitral regurgitation. ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenator; IABP, intra-aortic balloon pump; RxTx, chest X-ray; TEE, transesophageal echocardiography.
Noninvasive mechanical ventilation can be beneficial in patients experiencing flash pulmonary edema, commonly associated with hypertension. Positive pressure ventilation improves ventilation-perfusion matching, reduces alveolar edema, decreases dead space, and enhances pulmonary blood flow distribution. However, patients in cardiogenic shock due to severe acute MR require early orotracheal intubation and mechanical ventilation to achieve adequate stabilization, reduce adrenergic stimulation, and ensure effective oxygenation.8
Continuous and accurate monitoring of electrocardiographic, hemodynamic, and gasometric parameters is essential. This includes placing an arterial line for invasive arterial monitoring, establishing central venous access in patients with cardiogenic shock, measuring central venous pressure, continuously quantifying urine output, and performing gasometric checks at intervals tailored to the patient’s clinical status. If there is inadequate response to diuretics, early initiation of continuous renal replacement therapy is recommended to promptly reduce pulmonary congestion.9,10
If initial pharmacological treatment fails and clinical and hemodynamic deterioration persists within the first 12 to 24 hours, consideration should be given to initiating mechanical circulatory support.11 In such cases, consulting the center’s shock team is recommended to collectively determine the most appropriate treatment sequence and select the device to be used. This decision should weigh 4 key factors: a) patient-related factors and comorbidities; b) the underlying cause and mechanism of MR, and ventricular function; c) the patient’s hemodynamic status and severity of shock; and d) the center’s experience. A detailed approach to circulatory support is beyond the scope of this document. Briefly, intra-aortic balloon pump may be useful in patients with myocardial infarction-induced MR in preshock conditions (stage B of the SCAI [Society for Cardiovascular Angiography and Interventions] classification, when the patient is hypotensive or tachycardic but maintains adequate tissue perfusion), or in early shock stages (stage C of the SCAI, when inotropes, vasopressors, or mechanical support are needed to maintain systemic perfusion).12,13 In more advanced shock stages (stage D of the SCAI classification, when there is no response to measures established in the previous stage), and especially in the presence of PMR, the preferred device is peripheral venoarterial extracorporeal membrane oxygenation with or without LV unloading using an intra-aortic balloon pump or the Impella device (Abiomed, United States), with special caution required in cases involving PMR.13,14 In patients with ischemic MR in the context of myocardial infarction due to papillary muscle ischemia or ischemic dilated cardiomyopathy, or LV dysfunction with preserved right ventricular function, Impella can be highly effective as it allows direct LV unloading, thus reducing LV end-diastolic pressures and MR while enhancing cardiac output11 (figures 1 and 2 of the supplementary data). A key aspect to be considered is early initiation of mechanical circulatory support in patients with an indication to anticipate and prevent the onset of established multiple organ failure.

Figure 2. Optimal timing of mitral valve repair therapy.
Coronary revascularization is strongly recommended when MR is associated with acute ischemia.15 In the context of acute myocardial infarction and percutaneous revascularization, the severity of MR may vary from the acute phase near angioplasty to the most chronic stage. Nevertheless, the persistence of significant MR adversely affects patients’ short- and mid-term prognosis.16 Definitive treatment requires mitral valve replacement or repair.
Currently, the optimal timing for performing percutaneous coronary interventions in the mitral valve remains under debate. The timing varies based on the underlying cause, ventricular function, and the patient’s clinical status and any comorbidities. When the clinical and hemodynamic situation allows, a deferred implant is preferable. However, this is not always possible, and sometimes acute treatment of MR with edge-to-edge repair is necessary to stabilize the patient.
Thus, in patients with flash pulmonary edema and normal LV function, in whom MR is usually associated with hypertension, and who respond well to medical treatment with oxygen therapy/noninvasive mechanical ventilation and diuretics, as well as in unstable patients with adequate treatment response, repair should be deferred. This deferred repair should occur after resolution of the acute heart failure, when the patient is in a state of euvolemia, and the diuretic dose has been adjusted.
In some patients with MR-related heart failure who cannot discontinue intravenous diuretic therapy, urgent repair within the first 72 hours should be considered. In the most severe cases—such as patients with MR in cardiogenic shock and inadequate response to treatment, and persistence of refractory shock—the feasibility of emergent mitral valve repair within the next 24 hours should be evaluated. Alternatives such as heart transplantation should also be considered (figure 2). In these more unstable patients, transcatheter repair can alter the severity spectrum, even with partial reductions in MR severity, facilitating the transition to a more stable condition that allows definitive treatment.3
ROLE OF IMAGING MODALITIES IN THE QUANTITATIVE ASSESSMENT OF ACUTE MITRAL REGURGITATION
A high level of suspicion is required to identify patients with significant acute-onset MR. Transthoracic echocardiography can be performed at the bedside, including in the emergency room, and should be the initial imaging method for evaluating acute dyspnea. Echocardiography is the preferred imaging modality to identify the underlying mechanism of MR and rule out other causes of a new systolic murmur in this clinical setting. Transesophageal echocardiography is often necessary to confirm the diagnosis, assess the severity of MR, and determine the treatment strategy, including identifying suitable candidates for edge-to-edge mitral valve repair (TEER) (figure 3).

Figure 3. Eligibility assessment for transcatheter edge-to-edge mitral valve repair. ECMO, extracorporeal membrane oxygenator; LVAD, left ventricular assist device; MR, mitral regurgitation; TEER, transcatheter edge-to-edge repair.
Echocardiographic assessment should carefully evaluate the left ventricle (including ejection fraction, dimensions, and wall motion abnormalities), mitral valve anatomy (annulus, leaflets, chordae tendineae, and papillary muscles), and determine the etiology, mechanism, and severity of MR. Quantifying MR requires an integrated approach using qualitative, semiquantitative, and quantitative parameters as per current guidelines.17,18 Color Doppler often shows markedly eccentric flow, which can underestimate MR severity. The vena contracta width and continuous-wave Doppler signal density are simple techniques to quickly assess significant MR. The velocity-time integral curve in continuous-wave Doppler typically has a triangular shape due to rapid late systolic deceleration, indicating an abrupt increase in left atrial pressure, known as a “v-wave”. Ischemic MR is more pronounced in early and late systole due to opposing traction forces (systolic LV contraction). The severity of MR correlates with its holosystolic duration. However, some Doppler parameters may better evaluate chronic rather than acute MR. Hypotension and elevated left atrial pressure lead to a low transmitral gradient and reduced MR jet velocity on color Doppler, potentially underestimating or failing to detect MR. Anatomical features like flail leaflets, PMR, or a hyperdynamic left ventricle in pulmonary edema or cardiogenic shock should confirm the diagnosis, even when color Doppler does not show a large MR jet.
Echocardiography often reveals the underlying cause of acute MR. Among older patients, a frequent cause is chordal rupture associated with fibroelastic degeneration. Ischemic MR, resulting from leaflet tethering, is characterized by wall motion abnormalities in the region supplied by the culprit coronary artery, leading to leaflet tethering. This type of acute ischemic MR may occur during active or reversible myocardial ischemia and can resolve following ischemia treatment, highlighting the importance of reassessment postrevascularization.19
Acute MR due to LV remodeling occurs when the normal spatial relationship between the mitral valve apparatus and the left ventricle is distorted. Adverse remodeling of the left ventricle, characterized by dilation and shape change, causes one or both mitral leaflets to move apically and radially away from the ventricular center, driven outward by the displacement of papillary muscles secondary to remodeling. This pattern is most clearly observed in apical 3- and 4-chamber views.20 The leaflets are typically normal in the acute phase, but a remodeling process with increased thickness has been described during follow-up.21 The mitral annulus may also be dilated, a feature more commonly seen in nonacute MR cases. While both regional and global remodeling can lead to MR, the specific location of the remodeling is critical. Inferolateral myocardial infarctions are more likely to be associated with significant MR than anterior myocardial infarctions.19 The differences between regional and global remodeling typically result in different tethering patterns. Patients with symmetrical tethering exhibit central jets, and those with asymmetrical tethering, eccentric jets.
The most severe form of acute MR is PMR. Common 2-dimensional echocardiographic features include a flail mitral leaflet with severed chordae or a papillary muscle head moving freely within the left heart. Due to differences in coronary vascular anatomy, posteromedial PMR is more common than anterolateral PMR. New-onset leaflet prolapse during the acute phase of myocardial infarction may indicate imminent PMR requiring careful attention. LV function often becomes hyperdynamic due to a sudden decrease in afterload, whereas regional wall motion abnormalities may be subtle or overlooked. Color Doppler assessment typically shows eccentric MR, which can lead to- underestimation of its severity.
TRANSCATHETER INTERVENTION IN ACUTE MITRAL REGURGITATION
To date, surgical treatment remains the primary approach for acute MR, despite the selective nature of patients in surgical studies and the limitations of observational evidence. In the SHOCK Trial Registry, only 38% of postmyocardial infarction acute MR patients complicated by cardiogenic shock underwent mitral valve surgery, with a mortality rate of 40% in these cases.22 Similarly, a study examining evaluated the presence of PMR in a large cohort of patients with MR found that only 57.5% underwent surgical treatment,23 a decision influenced by the patients’ age, comorbidities, and clinical stability. This group of patients had a 36% mortality rate. Even among those who underwent surgery, outcomes were suboptimal due to early mortality, high transfusion rates, renal insufficiency, and prolonged mechanical ventilation.24
Therefore, developing less invasive approaches to address MR in this context, where patients often have a high surgical risk, is crucial to potentially expand the number of patients benefiting from MR correction.
Transcatheter techniques for treating MR have seen significant advancements in recent years. Among all available devices, transcatheter edge-to-edge repair (TEER) with the MitraClip system (Abbott Vascular, USA) is the most widely used and has accumulated extensive clinical experience. TEER with MitraClip has proven to be a safe and effective method for reducing MR in high-surgical-risk patients, and for improving symptoms, quality of life, and prognosis in those with functional and degenerative MR.25-28 In the randomized CLASP IID trial,29 the PASCAL Precision system (Edwards Lifesciences, United States) has also demonstrated safe and effective performance compared with MitraClip in patients with degenerative MR. Similarly, registry data have shown no significant differences with MitraClip in secondary MR.30
However, while most TEER procedures are performed in stable patients with advanced functional status and chronic MR, patients with acute MR are underrepresented in the literature. Acute MR represents a significant unmet need where the use of transcatheter interventions has grown significantly in recent years.
Increasing evidence supports the safety and efficacy profile of TEER in patients who develop severe symptomatic acute functional MR. The EREMMI group (European registry of MitraClip in acute MR following an acute myocardial infarction) has published the largest series to date on this topic. The first article—published in 2020—revealed the European experience with MitraClip in this context.31 The study included 44 patients with a mean age of 70 years and high surgical risk (median EuroSCORE II of 15.1%) from 2016 through 2018. Notably, the median time from acute myocardial infarction diagnosis to MitraClip intervention was 18 days, and from MR onset to treatment was 12.5 days, indicating insufficient stabilization with medical management alone. Patients were markedly symptomatic, with 63.6% classified as New York Heart Association (NYHA) class IV at the time of the procedure. In this series, technical success reached 86.6%. During follow-up, the 30-day mortality rate was 9.1%, a figure deemed acceptable considering that surgery for acute ischemic MR has the highest mortality rate among all surgical procedures performed for acute MR.32 At 6 months, MR ≤ 2+ was reported in 72.5%, with 75.9% of surviving patients achieving NYHA functional class I-II.
Subsequently, the researchers examined the role of TEER in treating acute severe MR in a cohort of 93 patients with cardiogenic shock.33 Technical success was high, and although 30-day mortality was higher among those in cardiogenic shock, the difference compared with nonshock patients was not statistically significant (10% vs 2.3%; P = 0.212). Conversely, mortality rates were markedly low in nonshock patients, even in a population at very high risk, highlighting the beneficial hemodynamic impact of percutaneous MR correction. Therefore, provided the TEER team has ample experience, cardiogenic shock should not preclude consideration of this therapeutic approach. These findings, together with recent insights into the efficacy of TEER in patients with shock,34-36 should position this therapy as a viable strategy due to its safety and efficacy.
When comparing patients with a left ventricular ejection fraction above or below 35%, the study found no significant differences in either in-hospital mortality or at 1 year (11% vs 7%, P = .51, and 19% vs 12%, P = .49), nor in the 3-month rehospitalization rate. Therefore, the positive effect of transcatehter treatment is maintained in patients with lower ejection fractions.37
Finally, the most extensive analysis of the group compared 3 strategies for the management of MR early after infarction: conservative management, surgical intervention, and TEER.38 The series included involved 471 patients, with 266 managed conservatively and 205 undergoing intervention (106 surgically and 99 with TEER). Consistent with prior research, medically managed patients experienced the highest mortality rates, twice that of the intervention groups. Notably, surgical correction resulted in poorer outcomes compared with MitraClip, with hospital mortality exceeding twice that at 1 year, largely driven by higher in-hospital mortality (16% vs 6%; P = .03). This trend was independent of the patients’ surgical risk profiles.
In the context of PMR, the largest series treated with TEER has been reported.39 The study included 23 patients, with a mean age of 68 years, and 56% were male. All were deemed ineligible for surgery due to high surgical risk. Nearly 90% were in cardiogenic shock, with 17 receiving mechanical circulatory support (11 with intra-aortic balloon pump, 2 with Impella, and 4 with venoarterial extracorporeal membrane oxygenation). Immediate success after the intervention was achieved in 87% of the patients, resulting in rapid hemodynamic improvement. Hospital mortality was 30%, which, while still high, was deemed acceptable given that these patients had no surgical options and faced poor prognoses with medical management alone. Importantly, 5 discharged patients underwent successful surgical mitral valve replacement during follow-up, highlighting the importance of stabilizing patients before considering deferred surgical interventions
In this scenario, guidelines and recommendations40-42 advise transcatheter therapy only in selected high-risk patients who are unsuitable for surgery. However, due to the difficulty of decision-making, limitations in offering surgery more broadly, and the complexity of managing patients in cardiogenic shock, most patients should be evaluated by a shock team to consider various therapeutic options, including percutaneous interventions (figure 1).
There are several potential advantages to the trancatheter approach in the management of acute MR. These patients often show significant clinical deterioration, primarily due to the development of MR affecting a small and noncompliant left atrium. This leads to markedly elevated pulmonary pressures and a low effective ejection volume, which are the main physiological factors causing the disease. TEER induces almost immediate hemodynamic improvement by reducing MR. This decreases pressures in the left chambers and pulmonary artery, increases cardiac output, and facilitates faster recovery with minimal tissue damage.43 Furthermore, TEER does not rule out scheduled cardiac surgery in the event of device failure or recurrent MR. Indeed, the role of TEER as a bridge to lower-risk surgery is appealing. In patients with poor progress, heart transplantation remains a viable option.
While outcomes with TEER in this condition are promising, evidence is currently limited to retrospective observational analyses of small patient populations. There may be selection bias among patients treated with TEER, as only those who responded well to medical therapy and cardiac support likely underwent the intervention. Long-term clinical and echocardiographic follow-up is also sparse. In additional, nearly all studies have included patients treated before 2020, before the introduction of newer generations of devices with independent capture capabilities or larger sizes, potentially limiting the effectiveness of TEER.
To provide more robust information on the appropriateness of this treatment for acute MR, ideally, prospective registries and a well-designed, executed randomized trial should be developed.
Currently, 2 very early-phase trials are underway that could shed light in this scenario. The international multicenter trial EMCAMI (Early Transcatheter Mitral Valve Repair After Myocardial Infarction; ClinicalTrials.gov: NCT06282042) was designed to prospectively evaluate the role of early treatment with MitraClip edge-to-edge repair vs conservative conventional treatment in acute MR occurring within 90 days of acute myocardial infarction, focusing on mortality and heart failure readmissions. The MINOS trial (Transcatheter Mitral Valve Repair for Inotrope Dependent Cardiogenic Shock; ClinicalTrials.gov: NCT05298124) will assess these treatment strategies in patients with cardiogenic shock and acute MR.
Technical and organizational considerations
The use of TEER in acute MR poses technical and organizational challenges, with several important considerations.
The left atrium is usually small and noncompliant. Therefore, transseptal puncture and positioning to achieve sufficient height above the valve can be complex and requires experience. Likewise, puncturing outside the fossa ovalis may be required. Systems allowing radiofrequency puncture for a precise entry point may be recommended for accurate placement.44
The complexity of valvular anatomy, especially in cases of primary MR in which large, wide gaps and commissural jets are common, suggests the use of the new features of the MitraClip G4 or PASCAL Ace devices,45,46 which allow independent leaflet capture and optimization to improve outcomes. With these new generation devices, most cases are technically feasible. For primary MR due to posterior medial prolapse, stabilizing the papillary muscle and controlling additional movement typically occurs after deploying multiple devices, preventing further tissue tears. Care must be taken to avoid device interference with the muscle and prevent additional damage or complete rupture in cases of partial tears.
Clinical deterioration can be rapid in some patients, raising the question of whether specialized mitral valve teams should be prepared to perform emergency treatment. If patients are too unstable for transfer, these teams may even need to travel to centers lacking such capabilities. In this context, teams should aim to initiate treatment within 24 hours of clinical deterioration for primary MR and patients in cardiogenic shock, and as promptly as feasible in other patients. These treatments should be considered within the framework of a “shock code”, a concept still under development in many regions, and organized based on available resources.
CONCLUSIONS
Patients with acute MR require a multidisciplinary approach both for their diagnostic assessment and in decision-making about treatment strategy. TEER is an effective treatment option for acute MR, either as a definitive treatment or as a bridge to a more stable scenario for other treatments, with a high procedural success rate and improved patient prognosis in centers experienced with the technique. Proper patient selection, meticulous anatomical evaluation, and choosing the optimal timing for implantation are key to treatment success.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the writing of the text and its critical review, and approved the article final version.
CONFLICTS INTEREST
None declared.
REFERENCES
1. Bernard S, Deferm S, Bertrand PB. Acute valvular emergencies. Eur Heart J Acute Cardiovasc Care. 2022;11:653-665.
2. Boudoulas KD, Triposkiadis F, Koenig S, et al. Acute mitral regurgitation with and without acute heart failure. Heart Fail Rev. 2023;28:1201-1209.
3. Shuvy M, Maisano F, Strauss BH. Transcatheter Mitral Edge-to-Edge Repair for Treatment of Acute Mitral Regurgitation.Can J Cardiol. 2023;39:1382-1389.
4. Stout KK, Verrier ED. Acute valvular regurgitation. Circulation. 2009;119:3232-3241.
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease:a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:72-227.
6. Harmon L, Boccalandro F. Cardiogenic shock secondary to severe acute ischemic mitral regurgitation managed with an Impella 2.5 percutaneous left ventricular assist device. Catheter Cardiovasc Interv. 2012;79:1129-1134.
7. Akodad M, Schurtz G, Adda J, Leclercq F, Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis. 2019;112:773-780.
8. Diepen S, Katz JN, Albert NM, et al.;American Heart Association Council on Clinical Cardiology;Council on Cardiovascular and Stroke Nursing;Council on Quality of Care and Outcomes Research;and Mission:Lifeline. Contemporary management of cardiogenic shock:a scientific statement from the American Heart Association. Circulation. 2017;136:232-268.
9. Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315-1341.
10. Saxena A, Garan AR, Kapur NK, et al. Value of hemodynamic monitoring in patients with cardiogenic shock undergoing mechanical circulatory support. Circulation. 2020;141:1184-1197.
11. Vandenbriele C, Balthazar T, Wilson J, et al. Left Impella®-device as bridge from cardiogenic shock with acute, severe mitral regurgitation to MitraClip®-procedure:a new option for critically ill patients. Eur Heart J Acute Cardiovasc Care. 2021;10:415-421.
12. Kettner J, Sramko M, Holek M, Pirk J, Kautzner J. Utility of intra-aortic balloon pump support for ventricular septal rupture and acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol. 2013;112:1709-1713.
13. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock:This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29-37.
14. Martínez-Sellés M, Hernández-Pérez FJ, Uribarri A, et al. Cardiogenic shock code 2023. Expert document for a multidisciplinary organization that allows quality care. Rev Esp Cardiol. 2023;76:261-269.
15. Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation:a 20-year experience. Circulation. 2014;129:2547-2556.
16. Nishino S, Watanabe N, Kimura T, et al. The Course of Ischemic Mitral Regurgitation in Acute Myocardial Infarction After Primary Percutaneous Coronary Intervention:From Emergency Room to Long-Term Follow-Up. Circ Cardiovasc Imaging. 2016;9:004841.
17. Lancellotti P, Pibarot P, Chambers J, et al. Multi-modality imaging assessment of native valvular regurgitation:an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23:E171-232.
18. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J Am Soc Echocardiogr. 2017;30:303-371.
19. Watanabe N. Acute mitral regurgitation. Heart. 2019;105:671-677.
20. Kimura T, Roger VL, Watanabe N, et al. The unique mechanism of functional mitral regurgitation in acute myocardial infarction:a prospective dynamic 4D quantitative echocardiographic study. Eur Heart J Cardiovasc Imaging. 2019;20:396-406.
21. Nishino S, Watanabe N, Gi T, Kuriyama N, Shibata Y, Asada Y. Longitudinal Evaluation of Mitral Valve Leaflet Remodeling After Acute Myocardial Infarction:Serial Quantitation of Valve Geometry Using Real-Time 3-Dimensional Echocardiography. Circ Cardiovasc Imaging. 2020;13:E011396.
22. Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction:A report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36(3 Suppl. A):1104-1109.
23. Bhardwaj B, Sidhu G, Balla S, et al. Outcomes and Hospital Utilization in Patients With Papillary Muscle Rupture Associated With Acute Myocardial Infarction. Am J Cardiol. 2020:125:1020-1025.
24. Kilic A, Sultan I, Chu D, Wang Y, Gleason TG. Mitral Valve Surgery for Papillary Muscle Rupture:Outcomes in 1342 Patients From The Society of Thoracic Surgeons Database. Ann Thorac Surg. 2020;110:1975-1981.
25. Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge Repair:In-hospital results and 1-year follow-up of 628 patients of the 2011-2012 pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875-884.
26. Mack MJ, Lindenfeld JA, Abraham WT, et al. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients With Heart Failure. J Am Coll Cardiol. 2021;77:1029-1040.
27. Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol. 2013;62:317-328.
28. Stone GW, Abraham WT, Lindenfeld J, et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N Engl J Med. 2023;388:2037-2048.
29. Lim DS, Smith RL, Gillam LD, et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc Interv. 2022;15:2523-2536.
30. Haschemi J, Haurand JM, Bönner F, Kelm M, Westenfeld R, Horn P. PASCAL vs MitraClip for Mitral Valve Transcatheter Edge-to-Edge Repair:A Single-Center Real-World Experience. JACC Cardiovasc Interv. 2022;15:1002-1004.
31. Estévez-Loureiro R, Adamo M, Arzamendi D, et al. Transcatheter mitral valve repair in patients with acute myocardial infarction:Insights from the European Registry of MitraClip in Acute Mitral Regurgitation following an acute myocardial infarction (EREMMI). EuroIntervention. 2020;15:1248-1250.
32. Lorusso R, Gelsomino S, De Cicco G, Beghi C, et al. Mitral valve surgery in emergency for severe acute regurgitation:analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg. 2008;33:573-582.
33. Estévez-Loureiro R, Shuvy M, Taramasso M, et al. Use of MitraClip for mitral valve repair in patients with acute mitral regurgitation following acute myocardial infarction:Effect of cardiogenic shock on outcomes (IREMMI Registry). Catheter Cardiovasc Interv. 2021;97:1259-1267.
34. Tang GHL, Estevez-Loureiro R, Yu Y, Prillinger JB, Zaid S, Psotka MA. Survival following edge-to-edge transcatheter mitral valve repair in patients with cardiogenic shock:A nationwide analysis. J Am Heart Assoc. 2021;10:019882.
35. Simard T, Vemulapalli S, Jung RG, et al. Transcatheter Edge-to-Edge Mitral Valve Repair in Patients With Severe Mitral Regurgitation and Cardiogenic Shock. J Am Coll Cardiol. 2022;80:2072-2084.
36. Martínez Gómez E, Mclernie A, Tirado-Conte G, et al. Percutaneous valve repair with Mitraclip device in hemodynamically unstable patients:A systematic review. Catheter Cardiovasc Interv. 2021;98:E627-625
37. Haberman D, Estévez-Loureiro R, Benito-González T, et al. Safety and Feasibility of MitraClip Implantation in Patients with Acute Mitral Regurgitation after Recent Myocardial Infarction and Severe Left Ventricle Dysfunction. J Clin Med. 2021;10:1819.
38. Haberman D, Estévez-Loureiro R, Benito-González T, et al. Conservative, surgical, and percutaneous treatment for mitral regurgitation shortly after acute myocardial infarction. Eur Heart J. 2022;43:641-650.
39. So C, Kang G, Lee J, et al. Transcatheter Edge-to-Edge Repair for Acute Mitral Regurgitation With Cardiogenic Shock Secondary to Mechanical Complication. Cardiovasc Revasc Med. 2022;45:44-50.
40. Damluji AA, Van Diepen S, Katz JN, et al. Mechanical Complications of Acute Myocardial Infarction:A Scientific Statement From the American Heart Association. Circulation. 2021;144:E16-35.
41. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60:727-800.
42. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease:Executive Summary:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450-500.
43. Siegel RJ, Biner S, Rafique AM, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658-1665.
44. Sharma SP, Nalamasu R, Gopinathannair R, Vasamreddy C, Lakkireddy D. Transseptal Puncture:Devices, Techniques, and Considerations for Specific Interventions. Curr Cardiol Rep. 2019;21:52.
45. Hausleiter J, Lim DS, Gillam LD, et al. Transcatheter Edge-to-Edge Repair in Patients With Anatomically Complex Degenerative Mitral Regurgitation. J Am Coll Cardiol. 2023;81:431-442.
46. Chakravarty T, Makar M, Patel D, et al. Transcatheter Edge-to-Edge Mitral Valve Repair With the MitraClip G4 System. JACC Cardiovasc Interv. 2020;13:2402-2414.
* Corresponding author.
E-mail address: (A. Viana-Tejedor).
@Ana_Viana_T;
@belcid7;
@RodrigoEstevez1;
@DrFerreraD;
@PabloJ;
@manuelbarreirop
ABSTRACT
Computed tomography is a noninvasive imaging technique with high spatial resolution, providing excellent definition of calcium and intravascular space through the use of contrast media. This imaging modality allows both highly accurate measurements and virtual simulations for preprocedural planning in coronary and structural heart disease interventions. Computed tomography is currently the gold standard technique for patient selection and preprocedural planning in numerous scenarios, such as transcatheter aortic valve implantation, left atrial appendage occlusion, transcatheter mitral valve repair, and transcatheter tricuspid valve repair. This article reviews the role of computed tomography in transcatheter coronary and structural heart disease interventions.
Keywords: Computed tomography. Structural heart disease interventions. TAVR. LAAO. TMVR.
RESUMEN
La tomografía computarizada es una técnica no invasiva, de gran resolución espacial, con excelente definición del calcio y del espacio intravascular al emplear medios de contraste, que brinda la posibilidad de realizar tanto mediciones como simulaciones virtuales de intervencionismo coronario y estructural. Se ha establecido como la técnica de referencia en la selección de pacientes y la planificación de procedimientos de intervencionismo transcatéter coronario y estructural en diferentes escenarios (implante percutáneo de válvula aórtica, cierre percutáneo de orejuela izquierda, reemplazo de válvula mitral transcatéter y reemplazo de válvula tricúspide transcatéter). El presente trabajo revisa el papel de la tomografía computarizada en el intervencionismo cardiaco coronario y estructural.
Palabras clave: Tomografía computarizada. Intervencionismo estructural. TAVI. LAAO. TMVR.
Abbreviations CT: computed tomography. ECG: electrocardiogram. LAAO: left atrial appendage occlusion. LVOT: left ventricular outflow tract. TAVI: transcatheter aortic valve implantation. TMVR: transcatheter mitral valve replacement.
INTRODUCTION
Coronary and structural heart disease interventions have traditionally relied on fluoroscopy and transesophageal echocardiography as the imaging modalities of choice, especially for intraprocedural monitoring. Imaging-based patient selection has also usually relied on echocardiography. However, the technological and knowledge advancements made in recent years have led to the incorporation of new imaging modalities—particularly computed tomography (CT) and, to a lesser extent, magnetic resonance—into the field of structural heart interventions.
Currently, CT is the imaging modality of choice before structural heart interventions in a wide range of procedures, as well as the screening technique for coronary artery disease, and even for planning coronary interventions.
This review examines the applications and indications of cardiac CT in transcatheter coronary and structural heart disease interventions.
GENERAL FEATURES OF CARDIAC COMPUTED TOMOGRAPHY
Cardiac CT is an optimal technique for evaluating patients prior to a structural heart intervention. This modality offers contrast-enhanced noninvasive imaging with excellent definition of calcium and intravascular space, submillimeter isotropic spatial resolution, and acceptable temporal resolution.
Like invasive coronary angiography, cardiac CT uses an X-ray source to create the image. Modern machines feature an O-shaped gantry ring with the X-ray tube positioned opposite a ring of detectors. The emitted radiation beam is attenuated and absorbed depending on tissue densities, with the captured energy reconstructed to form a medical image.
When acquiring tomographic images of heart structures and coronary arteries, it is important to consider their small-caliber, with each structure moving independently in all 3 spatial axes. Therefore, the equipment must be technically capable of producing conclusive studies. Table 1 outlines key technical parameters of CT generated images.
Table 1. Main basic concepts of computed tomography
| Concept | Definition |
|---|---|
| Spatial resolution | The ability to visualize 2 separate points that are very close together. Depends on the size of the detectors; in modern CT scanners, it is < 1 mm. |
| Isotropism | Image composed of voxels with a similar size in all 3 spatial planes. Allows for image reformatting while minimizing the loss of resolution. |
| Temporal resolution | The shortest time required by the CT scanner to acquire an image. Depends on the gantry rotation speed and the acquisition method. |
A cardiac CT scan should employ the ECG-gated technique to compensate for cardiac motion, with the study conducted during breath-holding to minimize respiratory movements. Acquisitions can cover the entire cardiac cycle or a preselected phase. Acquisition of the entire cardiac cycle (called “retrospective” in scanners with < 16 cm z-axis coverage) offers the advantage of allowing reconstruction of all phases, as well as functional assessments (volumes, ejection fraction, leaflet motion) and 4D reconstructions. However, this method requires higher radiation doses. This can be partially mitigated through retrospective acquisitions with dose modulation, acquiring high-quality images in 1 or more predefined phases while capturing the rest at lower quality, thereby reducing radiation exposure.1
Technological advances and the wider availability of CT scanners with cardiac acquisition software have allowed this imaging modality to be established as a standard in various structural interventional procedures. While it is widely acknowledged that the minimum equipment required includes an ECG-gated 64-slice CT scanner, the latest models offer superior image quality, decreased radiation exposure, and reduced contrast use. The latest generation of CT scanners follow various development paths: a) wide-detector CT scanners increase the scanned distance per heartbeat by incorporating more detectors; some scanners have more than 300 detectors, enabling cardiac coverage in a single heartbeat; b) high-pitch dual-source CT scanners use 2 radiation sources at a 90° offset and a high speed table to markedly enhance temporal resolution); c) spectral CT scanners use detectors with differing sensitivities or various energy levels from the emitter to capture images at different energy spectra, allowing a certain degree of tissue characterization; and d) photon-counting CT scanners eliminate the need for intermediate photoluminescent detectors, thus enhancing spatial resolution to 0.2 mm.
In addition to the CT scanner, an at least dual-phase injector is required to allow high flow (4-7 mL/s), a contrast agent with an iodine concentration around 350 mg/mL (ideally iso-osmolar), and a digital processing and image storage system in DICOM format (Digital Imaging and Communication in Medicine).
Preparing patients for a cardiac CT is essential to ensure high-quality diagnostic tests. Prior to the procedure, patients must provide informed consent and undergo an assessment to rule out any contraindications. A peripheral venous line is usually established in the right antecubital fossa (18-20 G). Patients are usually placed in the supine position with their arms raised above their heads. ECG electrodes are applied, ensuring excellent trace quality. It is important to explain and practice the breath-holding technique required during the scan with the patient, as well as to monitor ECG-quality during the breath-hold.
Depending on the indication of the study, if the patient’s heart rate is high or the rhythm is irregular, premedication may be necessary, with the most common choice being IV beta-blockers. In studies that require assessing the coronary lumen, sublingual nitroglycerin is usually also administered. When performing a cardiac CT prior to structural intervention, it is important to remember that severe symptomatic aortic or mitral stenosis is a contraindication for nitroglycerin use. Beta-blockers should be administered with caution, under the supervision of qualified personnel, ensuring that advanced cardiopulmonary resuscitation can be performed if necessary.
APPLICATION TO STRUCTURAL HEART INTERVENTIONS
Coronary computed tomography angiography (CCTA) provides a detailed anatomical assessment of the coronary tree, including its origin and course, detects the presence of atherosclerotic lesions, quantifies affected segments, and determines the severity of stenosis and atherosclerotic burden. CCTA is the standard imaging modality to assess symptomatic patients and can be considered in selected high-risk asymptomatic patients. It has a sensitivity of 97% and a specificity of 78% when taking invasive coronary angiography in a population with a pretest probability of 56% as a reference. While CCTA has the highest sensitivity compared with other invasive imaging modalities, functional imaging techniques such as stress magnetic resonance (80%), stress echocardiography (82%), and positron emission tomography (85%) have superior specificity.2 Despite its lower specificity, the CT-based anatomical strategy has been proven to be noninferior in terms of prognosis compared with the ischemia test-based functional strategy (PROMISE trial).3
Due to its high negative predictive value, CT is recommended by clinical practice guidelines as a first-line imaging modality to rule out obstructive coronary artery disease in low-to-intermediate risk symptomatic patients.4 Table 2 outlines the main indications for CCTA in various clinical scenarios.
Table 2. Current indications for computed tomography of coronary arteries and measurement of coronary artery calcium based on the European Society of Cardiology clinical practice guidelines
| Acute symptoms | Degree of recommendation | Level of evidence | Year | Ref. |
|---|---|---|---|---|
| Suspected acute coronary syndrome, normal or uncertain range troponins, normal electrocardiogram, and no recurrence of pain; may be considered as part of the initial diagnostic evaluation | IIA | A | 2023 | 5 |
| Systematic use in patients with suspected acute coronary syndrome | III | B | 2023 | 5 |
| Stable symptoms | Degree of recommendation | Level of evidence | Year | Ref. |
| Symptomatic patient with suspected coronary artery disease that cannot be clinically ruled out | I | B | 2019 | 4 |
| Risk stratification in patients with suspected or newly diagnosed coronary artery disease | I | B | 2019 | 4 |
| Patients with suspected vasospastic angina to study underlying coronary artery disease | I | C | 2019 | 4 |
| Screening for coronary artery disease in hemodynamically stable patients with aortic vegetations requiring cardiac surgery | I | B | 2023 | 6 |
| Patients with a low-to-intermediate probability of coronary artery disease and a previous equivocal noninvasive stress test | IIA | C | 2021 | 7 |
| Alternative to invasive coronary angiography prior to valvular cardiac surgery in patients with a low probability of coronary artery disease | IIA | C | 2021 | 8 |
| Patients with suspected cardiomyopathy for screening of coronary artery disease, or coronary anomalies that may be causing the cardiomyopathy | IIA | C | 2023 | 9 |
| Intermediate-to-high risk patients with prior nonemergency, noncardiac surgery: a) low-to-intermediate probability of coronary artery disease and suspected chronic or acute coronary syndrome without enzyme mobilization; b) patients ineligible for noninvasive functional tests | IIA | C | 2022 | 10 |
| Coronary computed tomography angiography is not recommended for the routine follow-up of patients with established coronary artery disease | III | C | 2019 | 4 |
| Asymptomatic | Degree of recommendation | Level of evidence | Year | Ref. |
| Calcium scoring as a risk modifier in asymptomatic patients with moderate cardiovascular risk | IIB | B | 2019 | 4 |
| Selected individuals with no history of coronary artery disease, high cardiovascular risk (SCORE > 10%, strong family history, familial hypercholesterolemia) and desire to start an intensive exercise program | IIB | B | 2021 | 11 |
| High cardiovascular risk (diabetes mellitus, family history, or previous test suggesting coronary artery disease) | IIB | C | 2019 | 4 |
| Asymptomatic adults (> 40 years) with diabetes mellitus | IIB | B | 2019 | 4 |
| Asymptomatic nondiabetic low-risk adults | III | C | 2019 | 4 |
Technological advances and the incorporation of new imaging modalities, such as stress CT perfusion and fractional flow reserve CT (FFRCT) have increased specificity rates to 85% to 87%.12 This enhances the positive predictive value of the imaging modality and allows meticulous evaluation of intermediate-to-high risk patients.
Landmark studies have been published on the prognosis of patients evaluated using CT. The SCOT-HEART trial13 demonstrated a reduction in cardiovascular deaths and nonfatal myocardial infarctions at the 5-year follow-up with a CT-guided strategy with outcome-based treatment adjustment compared with a conventional management strategy. On the other hand, the DISCHARGE trial14 showed a similar risk of major cardiovascular events during follow-up in patients with intermediate probability and stable chest pain randomized to CT vs invasive coronary angiography, with a lower rate of complications in the noninvasive imaging modality group. These studies support CT as a first-line imaging modality to rule out coronary artery disease, establish preventive treatment in patients with nonobstructive coronary artery disease, stratify patients with obstructive coronary artery disease, and offer an alternative to invasive coronary angiography in a wide range of patients.
In patients with a history of coronary artery disease, CCTA can be used to assess coronary artery bypass graft surgery, verify the patency of coronary stents in specific cases (proximal segments and stents > 3.0 mm), and assess chronic total occlusions prior to percutaneous coronary revascularization. In the BYPASS-CTCA trial,15 which randomized patients with prior surgical coronary revascularization to undergo CT-based anatomical assessment and invasive coronary angiography, or isolated invasive coronary angiography, shorter procedures and fewer episodes of contrast-induced nephropathy were observed in patients with noninvasive assessment of coronary artery bypass grafts.
CCTA should adhere to the recommendations established by the Society of Cardiovascular Computed Tomography.16 There are different image representation formats (axial, multiplanar reformatting, maximum intensity projection, curved multiplanar reformatting, or volumetric reconstruction), each with complementary uses. CCTA reading begins by assessing its quality, identifying potential artifacts, and visualizing the origin, course, and coronary dominance. The following are general principles for interpretation: a) cross-sectional systematic review of each coronary segment from multiple planes; b) vigilance for possible artifacts; c) evaluation of lesion morphology and composition; and d) grading lesion severity using high-resolution images in longitudinal and cross-sectional views of the vessel lumen. Following the modified distribution of the American Heart Association, coronary arteries are divided into 18 coronary segments. Identified lesions are listed based on the affected segment, the nature of the lesion (noncalcified, partially calcified, or calcified), and degree of resulting stenosis: normal (no lesion or stenosis), minimal (< 25% lumen reduction), mild (25%-49%), moderate (50%-69%), severe (70%-99%), or occlusion (> 99%).
Detailed analysis of the CT image enables the selection of a plan for transcatheter intervention and the materials to be used, and potentially reduces procedural length and complexity. This can be particularly useful when optimizing the fluoroscopy angle based on CT analysis in complex or bifurcated coronary artery lesions, as well as when performing complex cardiac catheterizations in patients with percutaneous aortic valve prostheses.17
The overall complexity of coronary artery disease can be represented by indices such as the coronary calcium score, or the number of segments with some degree of coronary artery disease, but several specific scales are available. Among these, the most widely used are the CAD-RADSTM (Coronary Artery Disease Reporting and Data System)18 and its updated version, the CAD-RADSTM 2.0,19 which incorporates parameters of perfusion and plaque complexity. Other more specific scales include the CT-SYNTAX20 scale, which combines CT-based anatomical information with clinical data from the SYNTAX scale, and the Functional CT-SYNTAX21 and Functional FFRCT22 scales, which add incorporate FFRCT-based functional information. These scales help refine the decision between surgical and percutaneous revascularization strategies, with promising initial results.23 Their prognostic validation in different scenarios, and their implementation in clinical practice, may represent a paradigm shift in the performance of invasive diagnostic imaging studies in stable patients.
In patients with chronic total coronary occlusions, preprocedural CT analysis allows estimation of the probability of success of percutaneous coronary revascularization; several prognostic scales have been developed for this purpose, such as the J-CTO,24 the CT-RECTOR,25 and the KCCT26 (table 3). The parameters analyzed include the extent of calcification, vascular tortuosity, the morphology of the occlusion stump, the presence of multiple occlusions, and the length of the lesion.
Table 3. Prediction scales for the success and complications associated with the revascularization of chronic total occlusions by computed tomography
| Score | Variables (points) | Classification |
|---|---|---|
| J-CTO | Tapered (0) vs blunt end (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (≥ 3) |
| No calcification (0) vs some calcification (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| Occlusion length < 20 mm (0) vs ≥ 20 mm (1) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| CT-RECTOR | < 2 occlusions (0) vs ≥ 2 complete interruptions (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (≥ 3) |
| Tapered (0) vs blunt end (1) | ||
| < 50% calcification of vessel perimeter on short axis (0) vs ≥ 50% calcification at some point of the occlusion (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| Duration of chronic total coronary occlusion < 12 months (0) vs ≥ 12 months (1) | ||
| KCCT | Tapered (0) vs blunt end (1) | Easy (0) Intermediate (1) Difficult (2) Very difficult (3) Extremely difficult (≥ 4) |
| No adjacent collateral branches (0) vs with collateral branches (1) | ||
| Occlusion length < 15 mm (0) vs ≥ 15 mm (1) | ||
| Occlusion angle ≤ 45° (0) vs > 45° (1) | ||
| Vessel calcification on the short axis < 180° of perimeter or < 50% of area (0) vs ≥ 180° of perimeter and ≥ 50% of area (1) vs complete central calcification of 360° of perimeter and 100% of area (2) | ||
| No previous failed revascularization attempts (0) vs with previous attempts (1) | ||
| Duration of chronic total coronary occlusion < 12 months (0) vs ≥ 12 months (1) |
APPLICATION TO STRUCTURAL HEART INTERVENTIONS
Transcatheter aortic valve implantation
After echocardiographic diagnosis of severe aortic stenosis, CT is the imaging modality of choice for a comprehensive assessment of patients eligible for transcatheter aortic valve implantation (TAVI).27 In a single scan, CT can evaluate vascular access, verify the degree of aortic stenosis and valve morphology, measure the aortic annulus, assess the risk of coronary occlusion, and determine the optimal fluoroscopy angles, among other aspects. In addition, in a high percentage of cases, CT facilitates the screening of proximal obstructive coronary artery disease and assessment of extracardiac findings.28
Preprocedural assessment for TAVI includes: a) an optional noncontrast acquisition to quantify aortic valve calcium; b) ECG-gated acquisition in the systolic phase, at least in the region of the aortic valve complex; and c) depending on the speed and coverage of the equipment used, 1 or more acquisitions for iliofemoral access, without the need for ECG-gated synchronization in this region. The study requires the injection of contrast medium (50-90 mL, with a flow rate of 3-5 mL/s, subject to variations based on the equipment used and the patient’s body surface area).28
The main aspects that should appear in the CT report prior to performing TAVI are listed in table 4.
Table 4. Main features that need to be included in the computed tomography report prior to transcatheter aortic valve implantation or percutaneous left atrial appendage occlusion
| Transcatheter aortic valve implantation | |
|---|---|
| Aortic annulus | Measurement in systolic phase |
| Area and perimeter | |
| Major and minor diameters, | |
| Optimal fluoroscopy view | |
| Calcium and valve | Presence, morphology, and extent of calcium |
| Valvular morphology | |
| Aorta and accesses | Height of the origin of coronary arteries |
| Minimum luminal diameter of each vascular segment | |
| Description of calcifications and vascular disease | |
| Others | Coronary anatomy |
| Extracardiac findings | |
| Percutaneous left atrial appendage occlusion | |
| Thrombus | Screening for arterial/venous filling defect |
| Morphology and landing zone | Describe the morphology and presence of proximal lobes |
| Measure the landing zone, maximum diameter | |
| Measure the depth and length of the appendage | |
| Optimal fluoroscopy view | |
| Others | Anatomy of the interatrial septum |
| Anatomy of the pulmonary veins | |
| Describe if there is pericardial effusion | |
Currently, there are 2 general designs of transcatheter aortic valve prostheses: balloon-expandable and self-expanding. Balloon-expandable TAVIs use radial force along with balloon inflation to fit their circular design to the oval shape of the aortic annulus. In contrast, self-expanding TAVIs expand on their own, due to nitinol memory, to fit over the annulus. In addition to technical and design differences, it is important to note that the sizing algorithms for these devices are not interchangeable. Sizing of balloon-expandable prostheses is based on the area of the aortic annulus, while that of self-expandig valves is based on the perimeter.
All the assessments necessary before TAVI are illustrated in Figure 2.
Figure 1. Computed tomography allows the study of coronary arteries to rule out the presence of coronary artery disease (A, normal coronary arteries), or to establish the severity and location of obstructive coronary disease (B, severe lesion in the proximal left anterior descending coronary artery [LAD] and chronic total occlusion in the mid and distal regions of the right coronary artery [RCA]). The functionality of the lesions can be assessed using computer simulation (C, fractional flow reserve computed tomography [FFRCT], severe lesion in the mid LAD and distal left circumflex artery [LCx]).
Figure 2. Preassessment for transcatheter aortic valve implantation using computed tomography and 3mensio CT analysis software: aortic annulus (A), aortic valvular calcium (B), left ventricular outflow tract (C), diameters of the Valsalva sinuses (D), height of the right coronary artery origin (E), height of the sinotubular junction (F), 3-cusp coplanar view (G), cusp-overlap view (H), and transfemoral accesses (I).
It is important to understand and analyze the anatomy of the aortic valve complex, which comprises the left ventricular outflow tract (LVOT), the Valsalva sinuses, the fibrous triangles between the aortic leaflets, and the leaflets themselves. A key measurement is the correct assessment of the plane of the aortic annulus, defined as the virtual plane aligned with the lowest insertion point of each aortic cusp or nadir. This involves determining the major and minor diameters, area, and perimeter of the aortic annulus. These measurements guide the selection of TAVI size. The aortic annulus undergoes changes in size and shape throughout the cardiac cycle, with mesosystole (30-35% R-R) often being the optimal time for measurement (larger size and reduced ellipticity).29 Specialized software is available to automate these measurements and simulate the implant procedure, streamlining workflow and reducing inter- and intra-observer variability.
The landing zone for the prosthesis includes the aortic cusps, the aortic annulus, and the LVOT. Severe calcification in the LVOT and aortic valve increases the risk of subsequent periprosthetic regurgitation, while large nodular calcifications may pose a higher risk of aortic annulus rupture, especially with balloon-expandable prostheses.30 It is essential to describe the location and extent of calcification in the aortic valve and the first 5 to 7 mm of the LVOT, as this area serves as the sealing zone for most available TAVIs. The morphology and degree of calcification of the aortic valve should be systematically reported, with particular attention to the presence of bulky calcification or partial fusion of the aortic commissures.28
The perpendicular height from the plane of the aortic annulus to the origin of the coronary arteries must be evaluated. Although absolute cutoff values have not been established, a coronary artery origin height of < 12 mm and sinuses of Valsalva < 30 mm are associated with a higher risk of TAVI-related coronary occlusion.31
The report should also include the optimal CT projections for valve deployment. Identifying these projections reduces radiation dose, contrast, and procedure duration.29 Angulation should be reported to obtain a coplanar projection (3 cusps), aligning the cusps, and the angulation for obtaining an overlapping projection (cusp-overlap), with the left and right cusps overlapped. This plane deploys the LVOT and allows better control of implant depth during valve deployment, especially with self-expanding valves.32
CT allows assessment of vascular access in a single study, providing excellent resolution and detailed delineation of the presence and extent of calcifications. Vascular complications increase the morbidity and mortality associated with TAVI. Factors associated with the occurrence of vascular complications include the sheath-to-femoral artery ratio, the presence of moderate to severe calcification, and vascular tortuosity.33 The report should include details on the minimum luminal diameters, the extent, distribution, and severity of calcification, as well as the presence or absence of vascular disease in all vascular segments between the aortic valve and the left and right common femoral arteries at the level of the femoral head.28 If femoral accesses are deemed unsuitable, alternative accesses can be considered, with the most common being axillary/subclavian, carotid, transcaval, and transapical accesses.
Special attention should be paid to the bicuspid aortic valve, given its lower success rate in procedures and higher rates of periprosthetic regurgitation, albeit with similar clinical outcomes.34 It is essential to determine the type of bicuspid valve (whether sinus fusion, 2 sinuses, or forme fruste),35 presence of a raphe, calcium distribution, annulus size and eccentricity, as well as the origin and height of the coronary arteries. Measuring the aortic annulus can be particularly complex in 2-sinus bicuspid valves, requiring specific methodology.28 The aortic annulus is defined as the virtual plane aligned with the lowest insertion point of the anterior/lateral cusp. Starting from this point, counterclockwise rotation to the lowest insertion point of the posterior/medial cusp is performed. Measurements should be taken at the line perpendicular to these 2 points, centered at the point where the smallest cross-sectional area is reached (as improper angulation can lead to inaccurate size estimation). The major and minor diameters, area, and perimeter of the aortic annulus are then determined. Algorithms have been developed for prosthesis size selection based on aortic annulus size, considering raphe length, calcium volume, and distribution (CASPER, calcium algorithm sizing for bicuspid evaluation with raphe).36 Additionally, a method (LIRA, level of implantation at the raphe) has been proposed by delineating the perimeter of the bicuspid valve opening,37 although its superiority over conventional measurements remains unclear.38
A variant of TAVI is the valve-in-valve implant, in which a percutaneous prosthesis is placed over a dysfunctional bioprosthesis. CT plays a key role in prosthesis size selection, especially when the model or size of the implanted prosthesis is unknown, but also in stratifying the risk of coronary occlusion. Among the main parameters for determining the risk of coronary obstruction are the level reached by the prosthesis cusps relative to the origin of the coronary arteries and the sinotubular junction, risk associated with the proximity of the valve to the sinotubular junction, < 2 mm distance from the virtual TAVI to the sinotubular junction, < 4 mm distance from the virtual TAVI to the origin of the coronary arteries, a prior supra-annular or supracoronary prosthesis, a surgical prosthesis with leaflets implanted outside the annulus (Mitroflow or Trifecta type), a prior implant in a high position, and the presence of moderate or severe commissural misalignment.39,40
After the TAVI procedure, CT allows assessment of the position and geometry of the prosthesis, as well as the thickness and mobility of the prosthetic leaflets. Following TAVI, a CT scan may be performed if prosthetic dysfunction or degeneration is identified by echocardiography, suspected thrombosis, infectious endocarditis, or periprosthetic regurgitation requiring anatomical assessment. The phenomenon of thickening with hypoattenuation and reduced mobility in the prosthetic leaflets has been described, which is associated with subclinical thrombosis and resolves with anticoagulation therapy. This finding has been associated with a higher but nonsignificant tendency for embolic events, and consequently there is no consensus or established indication for systematic performance of CT after TAVI. Its occurrence is more common in valve-in-valve, balloon-expandable prostheses, and larger prostheses, as well as those with eccentric expansion due to bicuspid valves, for example.41
Lastly, there is the option of using CT scans to resolve diagnostic uncertainties regarding the severity of aortic stenosis. Assessing aortic valve calcium can be especially helpful in patients with low-flow, low-gradient aortic stenosis and preserved ejection fraction. Agatston scores ≥ 2000 in men and ≥ 1200 in women indicate severe degenerative aortic stenosis, while scores < 1600 in men and < 800 in women suggest the absence of severe degenerative stenosis.8
Percutaneous left atrial appendage occlusion
Percutaneous closure of the left atrial appendage (LAAO) is an alternative to oral anticoagulation in patients with atrial fibrillation and a contraindication to oral anticoagulation. The traditional technique used for patient selection is transesophageal echocardiography (TEE) to rule out the presence of thrombus in the appendage and to take measurements for device selection. Three-dimensional measurements (3D-TEE, CT) have consistently been shown to be more accurate in selecting device size than 2D-TEE. Therefore, CT is an alternative technique in patient selection, as it allows visualization of the presence of thrombus and evaluation of the anatomy and size of the appendage, as well as the interatrial septum.42
CT evaluation of LAAO should be performed with ECG-gated acquisition, ideally in the telesystolic phase (when the left atrial appendage is maximally expanded), and a second acquisition should be performed in the venous phase, 60 to 90 seconds after contrast administration, to assess the presence or absence of thrombus in the left atrial appendage.43 The main features that should be included in a CT report for LAAO are listed in table 4. If the quality allows, it is advisable to perform an assessment of coronary anatomy.
The morphology of the left atrial appendage is highly variable and complex. Several devices for LAAO have been marketed, with the most commonly used being lobe and disc devices. Measurement of the landing zone is performed using multiplanar reformatting from 2-chamber and coronal planes. In the case of lobe devices, the landing zone extends from the circumflex artery to a point located 10 to 20 mm inside the ligament of Marshall.
The morphology of the left atrial appendage is highly variable and complex. Different devices for LAAO have been commercialized, with the most commonly used being lobe and disc devices. Measurement of the deployment zone is performed using multiplanar reformatting from two-chamber and coronal planes. In the case of lobe devices, the deployment zone extends from the circumflex artery to a point located 10-20 mm inside the ligament of Marshall. The depth is determined from the landing zone to the most distal end of the appendage. With disc devices, the landing zone is located 10 to 12 mm inside the ostium of the appendage, covering the course of the circumflex artery at its lower end. The depth in this type of device is defined from the ostium to the opposite wall of the appendage.43 It is also important to assess the anatomy of adjacent structures, especially the ligament of Marshall, to assess the feasibility of fully covering it with a disc device and to avoid thrombus formation during follow-up,44 as well as the anatomical characteristics of the pulmonary artery in relation to the left atrial appendage.45
Specific software has been designed to automate these measurements and simulate the implantation process (figure 3). Utilizing simulation software through computing enhances device selection and procedural outcomes.46
Figure 3. Planning for percutaneous left atrial appendage occlusion using computed tomography and 3mensio CT analysis software: identification of the left atrial appendage ostium (A and B), left atrial appendage morphology (C), measurement of the landing zone (D, longitudinal and cross-sectional views), simulation of the occluder device (E, longitudinal and cross-sectional views), simulation of the fluoroscopy view and position of the transseptal puncture (F), and simulation of the occluder device in fluoroscopy (G).
After LAAO, it is recommended to perform an imaging test 45 to 60 days postimplantation to verify the stability and positioning of the device, to search for residual leaks, and to rule out the presence of device-related thrombus. The most commonly used techniques are TEE and CT. CT allows better visualization of the position and deployment of the device, has equal thrombus detection capability, and has higher sensitivity in detecting residual contrast passage. The latter may be due to device malapposition, the presence of a peridevice leak, or the patency of the covering tissue.47 The clinical relevance of residual leaks, as well as the importance of their size, are not entirely clear.48
Transcatheter mitral valve replacement
Within transcatheter mitral valve intervention, there are options for repair and replacement. Edge-to-edge repair techniques are clinically established, with patient selection and procedural monitoring conducted via TEE. In contrast, for various valve replacement techniques, CT is indispensable. CT with ECG-gated acquisition is required to cover and reconstruct the entire cardiac cycle after contrast administration with adequate opacification of at least the left chambers, and ideally the right chambers, as well as to enhance visualization of the anatomy and its relationships. Detailed recommendations for acquisition and optimization have been published.49 CT allows evaluation of mitral annulus size and shape, selection of prosthesis type and size for implantation, virtual simulation of implantation, assessment of resulting neo-TSVI, selection of optimal fluoroscopy angles, and planning of vascular access (transseptal or transapical).49 (figure 4). Specific measurements for each device are determined by the manufacturer.
Figure 4. Several steps in the planning of transcatheter mitral valve replacement using computed tomography and 3mensio CT analysis software in 2 patients with valve-in-MAC (A-C) and native valve (D-F): delineation and measurement of the mitral annulus (A and D), evaluation of the distance from the virtual valve to the interventricular septum (D and E), and measurement of the neo-left ventricular outflow tract (C and F).
Transcatheter mitral valve replacement (TMVR) has been described for native valve, prior surgical annuloplasty (valve-in-ring), dysfunctional bioprosthetic valve (valve-in-valve), and severely calcified native mitral annulus (valve-in-MAC).50 CT is particularly useful to select prosthesis size and assess embolic risk in valve-in-MAC procedures by evaluating the thickness of the mitral annular calcium, its extension around the posterior perimeter or mitral trigones, and the damage to the mitral leaflets.51
The main complication to avoid during TMVR planning is LVOT obstruction after the procedure. The neo-LVOT refers to the distance or area between the lower edge of the virtual implant and the interventricular septum. The main predictors of neo-LVOT obstruction are detailed in table 5.52 The neo-LVOT area should be assessed in meso-telesystole (40%-50% R-R; the smallest area during the cardiac cycle), with obstruction risk increasing as the neo-LVOT area decreases: < 170 mm² indicates very high risk, 170 to 190 mm² indicates high risk, 190 to 220 mm² indicates acceptable risk, and > 220 mm² indicates low risk. In selected high-risk cases, techniques such as laceration of the anterior mitral leaflet (LAMPOON) or interventricular septal ablation (alcohol septal ablation) can be employed to enlarge the neo-LVOT area.53
Table 5. Predictors of left ventricular outflow tract obstruction in transcatheter mitral valve replacement
| Obstruction predictors | Obstruction risk limit |
|---|---|
| Area of the neo-LVOT | < 1.9 cm² |
| Area of the neo-LVOT skirt | < 1.5 cm² |
| Sizes of the anterior mitral leaflet | > 25 mm |
| Protruding interventricular septum | Thickness > 15 mm |
| Distance between the mitral annulus and the interventricular septum | < 17.8 mm |
| Acute aortomitral angle | < 110° |
| Small left ventricle | End-diastolic diameter < 48 mm |
| Left ventricular hypertrophy | Indexed myocardial mass > 105 g/m² |
|
LVOT, left ventricular outflow tract. |
|
Transcatheter tricuspid valve replacement
Transcatheter procedures for the tricuspid valve mainly include edge-to-edge repair, annuloplasty, and both orthotopic and heterotopic valve replacement (valve prostheses in the venae cavae).
The acquisition process is similar to that of pre-TMVR CT (ECG-gated covering and reconstructing the entire cardiac cycle following contrast administration). However, it is optimized for contrast in the right heart chambers using triphasic injection protocols (a mixture of contrast and saline at different concentrations). Detailed recommendations for acquisition and optimization have been published.49 CT imaging allows assessment of the tricuspid annulus geometry and size throughout the cardiac cycle, the morphology and mobility of the tricuspid leaflets, the position and relationship of the right coronary artery to the tricuspid annulus, right ventricular volume and ejection fraction, the optimal fluoroscopy angle, and vascular access54 (figure 5).
Figure 5. Evaluation of the tricuspid valve using computed tomography and 3mensio CT analysis software: measurement of the tricuspid annulus (A), simulation of the fluoroscopy view (B), simulation of the percutaneous annuloplasty anchors relative to the right coronary artery (C), distance from the tricuspid annulus to the anterior papillary muscle (D), distance from the tricuspid annulus to the roof of the coronary sinus, the inferior vena cava, and the roof of the right atrium (E), distance from the tricuspid annulus to the right ventricular free wall and apex (F), curved multiplanar reconstruction of the superior (G) and inferior venae cavae, and femoral accesses (H).
CT imaging can also aid in assessing the position and relationship of pacing leads with the tricuspid leaflets in selected cases of edge-to-edge repair. However, its main role lies in patient selection and planning of annuloplasty and valve replacement procedures, in which it is the imaging modality of choice. In annuloplasty, CT imaging facilitates device sizing, allows certain possibilities to be ruled out via simulation of the interaction of anchoring systems and the course of the right coronary artery, and evaluates tricuspid leaflet tenting to assess potential residual regurgitation postprocedure.54 In heterotopic replacement, CT enables sizing of the superior and inferior vena cava at different levels, assesses the anatomy and location of the suprahepatic veins, and determines the size of the right atrium, all of which determine the type and size of the device to be implanted.55 Finally, in orthotopic replacement, the selection criteria largely depend on the chosen device; however, it is generally necessary to evaluate the annulus size, distance to the anterior papillary muscle or free wall of the right ventricle, the confluence position of the vena cavae, and the angles between these and the tricuspid annulus, as well as the access route.56
Other procedures
Paravalvular leak closure
CT has shown good diagnostic performance in detecting aortic and mitral paravalvular leaks, allowing definition of the number, location, shape, and size of the defects.57 CT is especially useful in assessing infective endocarditis-related complications,58 as well as for planning and supporting the closure of paravalvular leaks in the aortic position.59 In addition, CT-based simulation prior to procedures can predict the occurrence of paravalvular leaks.60
Congenital heart diseases
Magnetic resonance imaging is the technique of choice in the diagnosis, evaluation, and follow-up of congenital heart diseases due to its ability to acquire any imaging geometry and perform anatomical and functional assessment, tissue characterization, and flow analysis, as well as the absence of radiation in a generally young population. CT is reserved for selected patients and cases.
Either CT or magnetic resonance can be used for patient selection, device choice, and sizing prior to intervention in congenital heart diseases. CT offers higher spatial resolution, enabling more precise delineation of calcification areas and proper sizing of prostheses. The use of CT or magnetic resonance is essential before transcatheter pulmonary valve replacement and percutaneous treatment of aortic coarctation. CT may also prove useful in cases of patent ductus arteriosus and complex fistulas. However, CT has lower added value in the closure of septal defects, such as atrial or ventricular septal defects.61 Nevertheless, in postmyocardial infarction ventricular septal defects, CT can be highly useful for sizing the defect and assessing their morphology, extent, and borders, given the often intricate and complex nature of these defects, which hampers accurate evaluation by echocardiography.62
CT-fluoroscopy image fusion during structural heart interventions
The anatomical information and preprocedural planning can be integrated into procedural monitoring. Using specific software and a workstation, cardiac structures are semiautomatically segmented and coregistered with the patient’s anatomy on the cath lab treatment table from 2 fluoroscopy projections. After coregistration, all CT information can be integrated into the procedure, allowing for expanded visibility, improved understanding of anatomical relationships, placement of markers or trajectories, and planning of optimal fluoroscopy angles.63 However, these are static non-ECG- or respiratory-gated images (figure 6).
Figure 6. Examples of computed tomography and fluoroscopy image fusion in various procedures using Heart Navigator (Philips): transcatheter aortic valve replacement (A), left atrial appendage occlusion (B), transcatheter valve-in-MAC mitral valve replacement (C), valve implantation in the superior (D) and inferior venae cavae (E), and closure of mitral paravalvular leak (F).
CT-fluoroscopy image fusion has been shown to reduce procedural length, contrast volume, and radiation exposure in TAVI and LAAO procedures, as well as a decreased need for intraprocedural device size adjustments in LAAO. The application and utility of CT- fluoroscopy image fusion have been reported in various procedures and have been shown to be particularly advantageous in complex interventions such as TMVR, transcatheter tricuspid valve replacement, transcaval TAVI, and paravalvular leak closure.64
CONCLUSIONS
CT is a high spatial resolution noninvasive imaging modality, providing excellent delineation of calcium and intravascular space using contrast media. The technique offers the possibility of performing measurements and virtual simulations for both coronary and structural interventions. CT has been established as the gold standard for patient selection and procedural planning in various scenarios of transcatheter coronary and structural interventions (such as TAVI, LAAO, TMVR, and transcatheter tricuspid valve replacement).
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONS
The manuscript was drafted by M. Barreiro-Pérez and I. Cruz-González and thoroughly reviewed and approved by all authors. Berenice Caneiro Queija and M. Barreiro-Pérez made corrections and editorial changes, and responded the reviewers.
CONFLICTS OF INTEREST
M. Barreiro-Pérez has received payments for presentations or educational activities from Abbott Vascular, Edwards Lifesciences, Venus MedTech, Lifetech, and Cardiovalve. I. Cruz-González has received payments for presentations or educational activities from Abbott Vascular and Boston Scientific. R. Estévez Loureiro has received payments for presentations or educational activities from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Venus MedTech.
REFERENCES
1. Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography:A report of the society of Cardiovascular Computed Tomography Guidelines Committee:Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10:435-449.
2. Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina:a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39:3322-3330.
3. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
4. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
5. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826.
6. Delgado V, Ajmone Marsan N, De Waha S, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023;44:3948-4042.
7. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726.
8. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
9. Arbelo E, Protonotarios A, Gimeno JR, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503-3626.
10. Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022;43:3826-3924.
11. Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17-96.
12. Pontone G, Baggiano A, Andreini D, et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease:The PERFECTION Study. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1487-1497.
13. Newby DE, Adamson PD, Berry C, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924-933.
14. Maurovich-Horvat P, Bosserdt M, Kofoed KF, et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N Engl J Med. 2022;386:1591-1602.
15. Jones DA, Beirne AM, Kelham M, et al. Computed Tomography Cardiac Angiography Before Invasive Coronary Angiography in Patients With Previous Bypass Surgery:The BYPASS-CTCA Trial. Circulation. 2023;148:1371-1380.
16. Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography:A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342-358.
17. Andreini D, Collet C, Leipsic J, et al. Pre-procedural planning of coronary revascularization by cardiac computed tomography:An expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2022;16:558-572.
18. Cury RC, Abbara S, Achenbach S, et al. CAD-RADSTM Coronary Artery Disease –Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269-281.
19. Cury RC, Leipsic J, Abbara S, et al. CAD-RADSTM 2.0 –2022 Coronary Artery Disease-Reporting and Data System:An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2022;16:536-557.
20. Papadopoulou SL, Girasis C, Dharampal A, et al. CT-SYNTAX score:a feasibility and reproducibility study. JACC Cardiovasc Imaging. 2013;6:413-415.
21. Collet C, Miyazaki Y, Ryan N, et al. Fractional Flow Reserve Derived From Computed Tomographic Angiography in Patients With Multivessel CAD. J Am Coll Cardiol. 2018;71:2756-2769.
22. Gabara L, Hinton J, Kira M, et al. Derivation and validation of a novel functional FFRCT score incorporating the burden of coronary stenosis severity and flow impairment to predict clinical events. J Cardiovasc Comput Tomogr. 2024;18:33-42.
23. Kageyama S, Serruys PW, Kotoku N, et al. Coronary computed tomography angiography-based SYNTAX score for comprehensive assessment of advanced coronary artery disease. J Cardiovasc Comput Tomogr. 2024;18:120-136.
24. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes:the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213-221.
25. Opolski MP, Achenbach S, Schuhbäck A, et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion:insights from the CT-RECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc Interv. 2015;8:257-267.
26. Yu CW, Lee HJ, Suh J, et al. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion:Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Imaging. 2017;10:e005800.
27. Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis:A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313-1346.
28. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR):An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging. 2019;12:1-24.
29. Francone M, Budde RPJ, Bremerich J, et al. CT and MR imaging prior to transcatheter aortic valve implantation:standardisation of scanning protocols, measurements and reporting —a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. 2020;30:2627-2650.
30. Hahn RT, Kodali S, Tuzcu EM, et al. Echocardiographic imaging of procedural complications during balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2015;8:288-318.
31. Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation:insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562.
32. Kim WK, Toggweiler S, Renker M, et al. Comparison of 3-Cusp Coplanar and 2-Cusp Overlap Views for the Implantation of a Self-Expanding Transcatheter Heart Valve. JACC Cardiovasc Interv. 2023;16:1422-1424.
33. Toggweiler S, Gurvitch R, Leipsic J, et al. Percutaneous aortic valve replacement:vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113-118.
34. Jilaihawi H, Chen M, Webb J, et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc Imaging. 2016;9:1145-1158.
35. Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg. 2021;60:448-476.
36. Petronio AS, Angelillis M, De Backer O, et al. Bicuspid aortic valve sizing for transcatheter aortic valve implantation:Development and validation of an algorithm based on multi-slice computed tomography. J Cardiovasc Comput Tomogr. 2020;14:452-461.
37. Iannopollo G, Romano V, Buzzatti N, et al. Supra-annular sizing of transcatheter aortic valve prostheses in raphe-type bicuspid aortic valve disease:the LIRA method. Int J Cardiol. 2020;317:144-151.
38. Weir-McCall JR, Attinger-Toller A, Blanke P, et al. Annular versus supra-annular sizing for transcatheter aortic valve replacement in bicuspid aortic valve disease. J Cardiovasc Comput Tomogr. 2020;14:407-413.
39. Tang GHL, Komatsu I, Tzemach L, et al. Risk of coronary obstruction and the need to perform BASILICA:the VIVID classification. EuroIntervention. 2020;16:E757-E759.
40. Tarantini G, Sathananthan J, Fabris T, et al. Transcatheter Aortic Valve Replacement in Failed Transcatheter Bioprosthetic Valves. JACC Cardiovasc Interv. 2022;15:1777-1793.
41. Jilaihawi H, Asch FM, Manasse E, et al. Systematic CT Methodology for the Evaluation of Subclinical Leaflet Thrombosis. JACC Cardiovasc Imaging. 2017;10:461-470.
42. Eng MH, Wang DD, Greenbaum AB, et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018;92:401-407.
43. Korsholm K, Berti S, Iriart X, et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2020;13:277-292.
44. Cepas-Guillén P, Flores-Umanzor E, Leduc N, et al. Impact of Device Implant Depth After Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2023;16:2139-2149.
45. Halkin A, Cohen C, Rosso R, et al. Left atrial appendage and pulmonary artery anatomic relationship by cardiac-gated computed tomography:Implications for late pulmonary artery perforation by left atrial appendage closure devices. Heart Rhythm. 2016;13:2064-2069.
46. De Backer O, Iriart X, Kefer J, et al. Impact of Computational Modeling on Transcatheter Left Atrial Appendage Closure Efficiency and Outcomes. JACC Cardiovasc Interv. 2023;16:655-666.
47. Agudelo V, Millán X, Li CH, et al. Prevalence, mechanisms and impact of residual patency and device-related thrombosis following left atrial appendage occlusion:a computed tomography analysis. EuroIntervention. 2021;17:E944-E952.
48. Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. 2015;16:1198-1206.
49. Pulerwitz TC, Khalique OK, Leb J, et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc Imaging. 2020;13:836-850.
50. Babaliaros VC, Lederman RJ, Gleason PT, et al. The Art of SAPIEN 3 Transcatheter Mitral Valve Replacement in Valve-in-Ring and Valve-in-Mitral-Annular-Calcification Procedures. JACC Cardiovasc Interv. 2021;14:2195-2214.
51. Guerrero M, Wang DD, Pursnani A, et al. A Cardiac Computed Tomography-Based Score to Categorize Mitral Annular Calcification Severity and Predict Valve Embolization. JACC Cardiovasc Imaging. 2020;13:1945-1957.
52. Yoon SH, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:182-193.
53. Barreiro-Perez M, Caneiro-Queija B, Puga L, et al. Imaging in Transcatheter Mitral Valve Replacement:State-of-Art Review. J Clin Med. 2021;10:5973.
54. Hell MM, Emrich T, Kreidel F, et al. Computed tomography imaging needs for novel transcatheter tricuspid valve repair and replacement therapies. Eur Heart J Cardiovasc Imaging. 2021;22:601-610.
55. Lopes BBC, Hashimoto G, Bapat VN, Sorajja P, Scherer MD, Cavalcante JL. Cardiac Computed Tomography and Magnetic Resonance Imaging of the Tricuspid Valve:Preprocedural Planning and Postprocedural Follow-up. Interv Cardiol Clin. 2022;11:27-40.
56. Barreiro-Pérez M, González-Ferreiro R, Caneiro-Queija B, et al. Transcatheter Tricuspid Valve Replacement:Illustrative Case Reports and Review of State-of-Art. J Clin Med. 2023;12:1371.
57. Koo HJ, Lee JY, Kim GH, et al. Paravalvular leakage in patients with prosthetic heart valves:cardiac computed tomography findings and clinical features. Eur Heart J Cardiovasc Imaging. 2018;19:1419-1427.
58. Entrikin DW, Gupta P, Kon ND, Carr JJ. Imaging of infective endocarditis with cardiac CT angiography. J Cardiovasc Comput Tomogr. 2012;6:399-405.
59. Lesser JR, Han BK, Newell M, Schwartz RS, Pedersen W, Sorajja P. Use of cardiac CT angiography to assist in the diagnosis and treatment of aortic prosthetic paravalvular leak:a practical guide. J Cardiovasc Comput Tomogr. 2015;9:159-164.
60. Morris MF, Pena A, Kalya A, et al. Predicting paravalvular leak after transcatheter mitral valve replacement using commercially available software modeling. J Cardiovasc Comput Tomogr. 2020;14:495-499.
61. Siripornpitak S, Goo HW. CT and MRI for Repaired Complex Adult Congenital Heart Diseases. Korean J Radiol. 2021;22:308-323.
62. Arzamendi D, Li CH, Serra A. Cardiac Computed Tomography-guided Closure of Ventricular Septal Defect Secondary to Myocardial Infarction. Rev Esp Cardiol. 2015;68:626.
63. Hussain MA, Nabi F. Complex Structural Interventions:The Role of Computed Tomography, Fluoroscopy, and Fusion Imaging. Methodist Debakey Cardiovasc J. 2017;13:98-105.
64. Barreiro-Perez M, Cruz-Gonzalez I, Moreno-Samos JC, Barahona MF, Sanchez PL. Cardiovascular Structural Interventions —Echo/Computed Tomography-Fluoroscopy Fusion Imaging Atlas. Circ J. 2018;82:2206-2207.
* Corresponding author.
E-mail address: (M. Barreiro-Pérez).
@manuelbarreirop; @icruzgonzalez; @RodrigoEstvez1; @CHPedroLi; @che_parada; @lvaroRodriperez; @b_caneiro
ABSTRACT
A substantial number of patients undergoing coronary angiography for angina or ischemia in noninvasive tests have coronary arteries without lesions or with nonsignificant stenosis. Many of these patients have nonobstructive myocardial ischemia (INOCA/ANOCA), which is an entity with prognostic importance that significantly affects patients’ quality of life. The absence of a proper diagnosis leads to inappropriate medical treatment, repeat diagnostic tests, and greater use of social and health resources. An adequate diagnostic strategy is required for individualized treatment that improves symptoms and quality of life. In this document from the SEC-Clinical Cardiology Association, SEC Interventional Cardiology Association, SEC-Ischemic Heart Disease and Acute Cardiac Care Association, and SEC-Cardiovascular Imaging Association of the Spanish Society of Cardiology, we provide simple and practical algorithms, with the aim of facilitating the early diagnosis and most appropriate treatment for patients with ANOCA.
Keywords: ANOCA. INOCA. Microvascular dysfunction. Vasospastic angina.
RESUMEN
Un número importante de aquellos pacientes en quienes se realiza coronariografía por angina o isquemia presentan en pruebas no invasivas arterias coronarias sin lesiones o con estenosis no significativas. Muchos de estos pacientes tienen isquemia miocárdica de causa no obstructiva (INOCA/ANOCA), una condición con importancia pronóstica que afecta de manera considerable la calidad de vida. La ausencia de un diagnóstico que haga posible un tratamiento médico efectivo acarrea la repetición de pruebas diagnósticas y un mayor uso de recursos sociosanitarios. Es necesaria una estrategia diagnóstica adecuada para poder realizar un tratamiento personalizado, que mejore los síntomas y la calidad de vida. En este documento de la SEC-Asociación de Cardiología Clínica, SEC Asociación de Cardiología Intervencionista, SEC-Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares, y SEC-Asociación de Imagen Cardiaca, se establecen unos algoritmos sencillos y prácticos con el objetivo de facilitar el diagnóstico precoz y el tratamiento más adecuado de los pacientes con ANOCA.
Palabras clave: ANOCA. INOCA. Disfunción microvascular. Angina vasoespástica.
Abbreviations ANOCA: angina with nonobstructive coronary arteries. CFR: coronary flow reserve. IMR: index of microcirculatory resistance. INOCA: ischemia with nonobstructive coronary artery disease. PET: positron emission tomography. SEC: Sociedad Española de Cardiología.
INTRODUCTION
Angina pectoris affects more than 100 million persons worldwide.1-5 According to the OFRECE study, the prevalence of angina in Spain is around 2.6%, indicating that there are more than 270 000 affected individuals.4 A significant number of stable patients referred for coronary angiography due to angina or a positive ischemia test do not have obstructive coronary artery disease.1 Many of these patients have ANOCA (angina with nonobstructive coronary arteries), or INOCA (ischemia with nonobstructive coronary artery disease) of nonobstructive origin. These 2 entities are manifestations of the same disease, which is why the recommendations provided by this document are applicable to both.
Angina pectoris is more prevalent among women (50%-70%) than men (30%-50%), although its true prevalence remains unknown.1-5 In these patients, angina or ischemia is produced by coronary vascular dysfunction due to vasomotor disorders of the epicardial vessels or arterioles, and/or coronary microvascular dysfunction.6-8
An important point is that, currently, angina pectoris is significantly underdiagnosed, and consequently many patients suffer its consequences without receiving potentially effective treatment. The reasons for this lack of diagnosis and treatment are various. First, there is the inertia associated with the paradigm that has dominated the diagnostic approach to patients with angina for decades focused on identifying coronary artery stenosis rather than vasomotor or coronary microvascular disorders. Additionally, patients with angina without coronary artery stenosis have generally been considered low-risk patients with poor response to conventional antianginal medical therapy.9 Second, and partly related to the previous point, many noninvasive techniques are based on identifying the regional ischemia that is characteristic of coronary artery stenosis (dysregulated contraction or isotope uptake during exertion or stress), making them less sensitive and specific for the detection of nonobstructive ischemia. Third, most cardiologists have not had access to the invasive techniques that provide objective evidence of vascular dysfunction in their patients. These intracoronary techniques have been considered the sole domain of interventional cardiologists, who do not usually play a key role in the management and follow-up of patients with INOCA. These barriers prevent the valuable information provided by invasive techniques from being used in the clinical management of these patients. Finally, patients with ANOCA/INOCA often have extracardiac diseases and conditions that require a multidisciplinary approach, complicating follow-up for specialized cardiologists.
In 2019, the European Society of Cardiology guidelines on the diagnosis and management of patients with chronic coronary syndrome represented a significant advance in the recognition of microvascular angina and the value of specific diagnostic techniques. Therefore, in the diagnostic approach in patients with suspected coronary microvascular angina, the guidelines indicate that coronary flow reserve (CFR) and microcirculatory resistance should be measured through pressure-guided techniques in patients with persistent symptoms but angiographically normal coronary arteries, or moderate stenosis and a normal fractional flow reserve (recommendation IIaB). Even the remaining recommendations, such as the administration of intracoronary acetylcholine during coronary angiography, or the use of transthoracic Doppler echocardiography of the anterior descending artery, cardiac magnetic resonance (CMR), or positron emission tomography (PET) for the noninvasive evaluation of CFR, have a lower level of recommendation (IIbB). In patients with suspected vasospastic angina, the guidelines recommend intracoronary provocation testing to identify coronary artery spasm (recommendation IIaB).10
However, over the past few years, numerous studies have been conducted in patients with ANOCA to assess the efficacy profile of new invasive diagnostic tests for their specific diagnosis, as well as randomized clinical trials assessing symptomatic improvement with individualized therapies. These trials consistently suggest that individualized and multidisciplinary approaches to these patients help to relieve symptoms, reduce the number of medical visits and prescribed therapies, and lower the costs associated with this syndrome.11-13
OBJECTIVES OF THIS DOCUMENT
This document is endorsed by the Clinical Cardiology Association, and the Interventional Cardiology Association, Ischemic Heart Disease and Acute Cardiac Care Association, and Cardiovascular Imaging Association of the Spanish Society of Cardiology (SEC) and aims to:
-
Review the various causes of ANOCA syndrome and current methods for its diagnosis and individualized treatment.
-
Propose a diagnostic and treatment algorithm for the approach to these patients in compliance with the clinical practice guidelines of the European Society of Cardiology on the management chronic coronary syndrome and the latest evidence.
-
Encourage various health care entities to create multidisciplinary pathways for the diagnosis, treatment, and targeted follow-up of these patients.
This document was drafted based on the interpretation of the latest scientific evidence, with an eminently practical focus so that the recommendations can be effectively applied in our setting. Each Association of the SEC provided scientific evidence and their view of their respective fields. Afterward, through consensus, they all created a single document including practical recommendations. The selection of the members that would eventually draft the document was left to the presidents of these Associations and was based on their clinical experience and expertise in the field.
IMPORTANCE OF ANOCA IN ROUTINE CLINICAL PRACTICE
While it has been acknowledged for decades that angina without coronary artery lesions could constitute a separate nosological entity (initially called syndrome X), routine clinical practice has paid little attention to affected patients, primarily due to the widespread notion that their prognosis is good.14 However, numerous subsequent studies in which the diagnosis of ANOCA was based on objective evidence of coronary vascular dysfunction, unlike that of syndrome X, consistently showed that nonobstructive ischemia has a significant prognostic impact. The risk of adverse coronary events in these patients is largely determined by factors such as plaque burden, demonstration of myocardial ischemia, microvascular dysfunction, and the presence of vasospasm or coronary endothelial dysfunction. For example, a study of 917 women with signs or symptoms of myocardial ischemia showed that the composite endpoint of myocardial infarction or cardiac death occurred in 6.5% of women without coronary artery disease, 12.8% of those with nonobstructive atherosclerosis, and 25.9% of those with obstructive coronary artery disease at 10 years of follow-up (figure 1).15 A meta-analysis of 54 studies and 35 039 patients confirmed an increased risk of nonfatal myocardial infarction and death, with an incidence rate of 0.98 per 100 person-years in patients with ANOCA at 5 years of follow-up. The risk was higher in individuals with confirmed ischemia (vs those without ischemia) and patients with nonobstructive coronary artery disease (vs those with normal coronary arteries).16
Figure 1. Risk of myocardial infarction or cardiovascular death at 10 years of follow-up in a cohort of women.15 ANOCA/INOCA, angina/ischemia with nonobstructive coronary arteries; CAD, coronary artery disease.
Similarly, even in the presence of angiographically normal coronary arteries, microvascular dysfunction demonstrated by a reduced CFR has proven to be a powerful determinant of the risk of death and myocardial infarction in these patients.17 Additionally, more cardiovascular complications, including stroke and heart failure,18 have also been reported in these individuals, along with a higher prevalence of small vessel cerebral disease.19 In conclusion, patients with coronary microvascular dysfunction, identified by an impaired CFR, have a higher risk of major cardiovascular events.20
Intracoronary acetylcholine provocation testing also allows coronary risk stratification. An abnormal response to intracoronary acetylcholine indicates vasomotor disorders due to endothelial dysfunction or smooth muscle cell hyperreactivity. In addition to causing vasospastic angina, coronary vasomotor disorders are associated with a higher long-term risk of cardiovascular events in patients with angina, especially when associated with increased coronary microcirculation.13,21 Even moderate vasoconstrictor responses to acetylcholine can be predictive of a worse prognosis in this context.20
Additionally, patients with ANOCA often show persistent symptoms, partly due to the lack of an early diagnosis, thus leading to treatment delay. This is associated with a higher number of unnecessary diagnostic tests to rule out obstructive coronary artery disease, visits to the emergency room, hospital admissions, anxiety, impaired quality of life, episodes of sick leave, and higher direct and indirect health care costs.16,22,23
Diagnosing INOCA is essential to provide effective therapies to control angina symptoms. The CorMicA trial (Coronary microvascular angina) included 151 patients with ANOCA who underwent cardiac catheterization and invasive functional assessment (CFR determination, index of microcirculatory resistance, and fractional flow reserve) followed by acetylcholine vasoreactivity testing.11 The patients were randomized to reveal their specific endotype, which would guide treatment based on the results (intervention group), vs standard treatment, which would be administered blind to the test results (control group). Targeted therapy was individualized based on the endotypes documented in the invasive study (vasospastic angina: smoking cessation, long-acting calcium channel blockers, long-acting nitrates, and lifestyle changes; microvascular angina: beta-blockers, lifestyle changes, possible angiotensin-converting enzyme inhibitors and statins; noncardiac chest pain: withdrawal of antianginal treatment). Targeted therapy was significantly associated with an improved angina-related quality of life at 6 months (measured using the Seattle Angina Questionnaire), disease perception, and treatment satisfaction, although no differences were reported in the risk of major adverse cardiovascular events. More antianginal drugs were prescribed in the intervention group (87.8% vs 48.7%; P < .001). While these results are very interesting, it is important to note that this was a single study with a limited number of patients.
ENDOTYPES OF PATIENTS WITH ANOCA
The specific causes of ANOCA are not yet fully described, and are likely multifactorial in most patients. Figure 2 illustrates the specific causes discovered so far and the pathophysiological mechanisms involved in their genesis. Of note, specific diagnostic techniques often do not allow us to differentiate among the various pathophysiological mechanisms. In fact, in many patients, these mechanisms overlap. Four pathophysiological mechanisms causing ANOCA have been described to date:
Figure 2. Possible results of an invasive functional study in a patient with ANOCA. Specific causes discovered to date with the pathophysiological mechanisms involved in their genesis. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; IMR, index of microcirculatory resistance. (Figure self-developed from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kunadian et al.,34 and Hokimoto et al.35.)
-
Microvascular dysfunction due to structural changes to the microcirculation. The density of microvessels in patients with hypertensive cardiomyopathy is lower than that in patients without this condition.24 Remodeling of the coronary microcirculation has also been described, including arteriolar medial layer hypertrophy and induration in patients with hypertension, added to other cardiovascular risk factors, vascular infiltration by amyloid in cardiac amyloidosis, and reduced luminal caliber due to extrinsic compression in cases of ventricular hypertrophy or increased left intraventricular pressure.3,7,25 These changes reduce microcirculatory conductance, resulting in increased microvascular resistances (index of microcirculatory resistance [IMR] ≥ 25). Elevated IMR values are associated with older age and left ventricular hypertrophy, with no clear difference between the sexes.26,27
-
Functional microvascular disease. An increase in resting coronary blood flow, leading to reduced CFR levels has been reported, especially in women with few risk factors and no objectively observable structural heart disease.28 Although coronary flow is usually preserved at maximum hyperemia, many of these patients have a low exercise capacity. These patients may have an imbalance in oxygen availability (due to increased demand), with endothelial involvement being the main mechanism (due to increased nitric oxide synthesis).29 In addition, these patients tend to have a greater number of associated ischemic abnormalities in organs such as the kidneys, retina, and central nervous system, suggesting systemic involvement.30
-
Microvascular dysfunction due to microcirculatory spasm. Microvascular dysfunction due to vasospasm is more common in women with cardiovascular risk factors, with endothelial dysfunction likely playing a significant role. It is a common finding in larger and medium-sized arterioles and manifests as paradoxical vasoconstriction in response to increased myocardial oxygen demand, which becomes apparent after intracoronary of acetylcholine administration.3,7,19,31
-
Epicardial spasm. Epicardial spasm is not usually associated with traditional risk factors, except for smoking. This type of vasospasm is believed to be caused by 2 main mechanisms: endothelial dysfunction and smooth muscle cell hyperreactivity. These 2 mechanisms respond differently to stimuli from the autonomic nervous system, depending on whether the stimuli are from the sympathetic system (such as exercise or a cold stimulation test), or whether the stimuli are from the parasympathetic system and provoke an exacerbated response (eg, nocturnal spasms).19,32
CLINICAL CHARACTERISTICS OF PATIENTS WITH ANOCA
The first step in identifying patients with ANOCA is diagnostic suspicion. Patients with microvascular angina often report angina-like chest pain, typically on exertion, but it can also occur at rest. ANOCA is more common in women, and affected individuals generally show poor response to short-acting nitrates. In some cases, instead of angina, patients may have angina equivalents such as exertional dyspnea or atypical symptoms such as nausea, vomiting, dizziness, or fatigue. In microvascular spasm, which is also more common in women, unstable angina can occur with a variable response to nitrates.1-3
Regarding angina due to coronary vasomotor disorders, the spectrum and clinical signs of these disorders are much more varied than the pattern of Prinzmetal’s angina, which is a highly specific case of vasomotor disorder caused by an occlusive spasm of an epicardial vessel. However, this disorder is not representative of much more common substrates such as nonocclusive diffuse spasm and arteriolar or microvascular spasm. For example, in vasomotor disorders due to endothelial dysfunction, the dominant symptom is exertional angina, whereas in vasomotor disorders triggered by smooth muscle cell hyperreactivity of coronary vessels (such as in Prinzmetal’s angina), angina tends to occur at rest or becomes unstable, especially at night. Nevertheless, it can also be associated with exertional chest pain and be triggered by specific stimuli such as stress, cold, or an increase in vasoconstrictor humoral factors. Angina can also be associated with other conditions such as migraines or Raynaud’s phenomenon. Some anticancer drugs, such as 5-fluorouracil and capecitabine, among others, are known to be associated with vasospastic angina.33 Similarly, the initial clinical manifestation of epicardial spasm can be myocardial infarction with nonobstructive coronary arteries (MINOCA).19 This condition is often associated with smoking, unlike other traditional risk factors such as hypertension, diabetes mellitus, and dyslipidemia.19,32
NONINVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
The diagnostic approach to patients with ANOCA falls within the diagnostic process of chronic coronary syndrome as recommended by the current clinical practice guidelines and is initially noninvasive.10 However, it is important to note that the available scientific evidence—sometimes scarce—has already been analyzed, and consequently some statements are based not only on clinical trials but also on consensus among the authors of the document.
After angina is suspected, the patient should be referred to the cardiology unit for basic symptom examination, including an electrocardiogram, echocardiogram, a complete blood count, and clinical response to initial antianginal treatment. A noninvasive strategy is advised for most patients with nonlimiting symptoms and a low or intermediate pretest risk of obstructive coronary artery. This strategy involves noninvasive imaging modalities, including functional studies, based on surrogates of myocardial blood flow and CFR, and/or anatomical studies, mainly coronary computed tomography.3 The diagnostic tests performed will depend, among other factors, on the patient’s exercise tolerance and the availability and experience of each center (figure 3).1,3,7,34,35
Figure 3. Diagnostic approach to patients with suspected ANOCA or INOCA. Ach, acetylcholine; ANOCA, angina with nonobstructive coronary arteries; IC, informed consent; CFR, coronary flow reserve; CT, computed tomography; CVRF, cardiovascular risk factors; IMR, index of microcirculatory resistance; INOCA, ischemia with nonobstructive coronary arteries; MPRI, myocardial perfusion reserve index; MRI, magnetic resonance imaging; MVD, microvascular dysfunction; VD, vasodilator. (Figure self-developed from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35.)
Of note, in many patients with ANOCA, noninvasive imaging modalities for detecting ischemia have low sensitivity for the diagnosis of most endotypes, especially those associated with coronary vasomotor disorders. In a registry of patients studied with noninvasive ischemia detection tests and invasive functional tests (considered the reference standard for diagnosis), only 50% of those with a low CFR showed abnormalities in the noninvasive imaging tests.36 In fact, no noninvasive stress test can reliably detect the presence of microvascular spasms or coronary endothelial dysfunction and a negative stress test does not exclude the presence of vasomotor coronary dysfunction, especially in symptomatic patients.7 The reasons for the low sensitivity of these techniques are diverse. However, an important reason is that they rely on visualizing regional differences among myocardial segments (nonuniform tracer uptake in single-photon emission computed tomography, differences in myocardial segment mobility in stress echocardiography). Given the characteristics of microvascular angina, in which ischemia can be widespread, it is difficult to find regional defects in noninvasive tests. Moreover, patients with vasospasms usually test negative in stress tests based on comparison between rest and hyperemia. Therefore, it is important to note that ANOCA should always be suspected in patients with suggestive chest pain and a normal coronary computed tomography scan, or without obstructive coronary artery disease (< 50% reduction in diameter), and in patients who test negative on noninvasive imaging modalities for ischemia detection. Currently, no imaging modality allows the direct anatomical visualization of coronary microcirculation in vivo in humans, which is why its evaluation relies on measuring parameters that reflect functional status, such as myocardial blood flow and myocardial flow reserve.7
However, certain ANOCA endotypes with low CFR and a high suspicion of microvascular angina can be diagnosed noninvasively through various imaging modalities such as PET, transthoracic Doppler echocardiography, contrast-enhanced transthoracic echocardiography, and CMR. CFR is defined as an increased flow between the resting state and maximum hyperemia. CFR values < 2 to 2.5 are considered pathological.1
PET allows determination of myocardial blood flow at rest and during hyperemia in absolute terms, which facilitates the calculation of CFR. Although PET is considered the reference noninvasive imaging modality and correlates well with invasive study (CFR < 2 is associated with a worse prognosis regardless of the severity of coronary artery disease),37 its availability is highly limited in our setting,3,38 due to its high cost and the need for specific cyclotron-produced radiation-emitting radiotracers, such as oxygen-15-labeled water, nitrogen-13-labeled ammonia, or rubidium-82, a potassium analog.
Transthoracic Doppler echocardiography allows for the measurement of baseline and hyperemic blood flow velocity (after adenosine administration) using pulsed-wave Doppler. CFR < 2.5 is considered diagnostic of microvascular dysfunction. However, this imaging modality requires highly trained personnel and can only be used in the left anterior descending coronary artery.3,39 On the other hand, contrast-enhanced transthoracic echocardiography using microbubbles allows estimation of myocardial perfusion flow based on its degree of opacification. The latter imaging modality has shown good correlation with PET, although there may be significant interobserver variability, thus requiring further validation in studies.40
Finally, CMR can determine myocardial perfusion using stress and contrast agents (gadolinium) to calculate the myocardial perfusion reserve index, which is a surrogate parameter of CFR. This imaging modality is more widely available than PET, and has less interobserver variability than echocardiographic studies, making it the most suitable imaging modality for the study microvascular dysfunction in our setting. However, CMR is still pending validation in the remaining ANOCA endotypes.3,41 Hyperemia or coronary vasodilation can be achieved through adenosine infusion, or the administration of a single bolus of regadenoson, and stress vs resting perfusion can be compared quantitatively. The diagnostic ability of stress CMR in microvascular dysfunction was demonstrated 2 decades ago.42 A myocardial perfusion reserve index < 1.84 has shown sensitivity and specificity rates of 73% and 74%, respectively, to predict abnormalities in invasive coronary physiology studies, with an area under the ROC curve of 0.78.41 A quantitative assessment of stress perfusion studies showed an even stronger correlation with invasive studies in a series of 65 patients (50 with stable angina, 46% of whom had no coronary artery lesions, and 15 healthy volunteers) to distinguish multivessel disease from microvascular dysfunction, with an area under the ROC curve of 0.94 (P < .001) for the absolute quantification of myocardial flow during stress < 1.82 mL/g/min.43 In this study, myocardial flow during stress correlated better with invasive measurements than with myocardial flow reserve. Additionally, its prognostic capability has also been demonstrated. In a series of 218 patients with angina and coronary arteries without epicardial lesions,44, a myocardial perfusion reserve index ≤ 1.47 was associated with a 3-fold higher risk of major cardiovascular events compared with patients with values > 1.47 (hazard ratio, 3.14; 95% confidence interval, 1.58-6.25; P = .001). In another series of 395 patients, myocardial perfusion reserve improved the prognostic value vs the baseline model (age, sex, and late enhancement) of the primary endpoint defined as a composite of cardiac death, nonfatal myocardial infarction, aborted sudden death, or late revascularization, at 460 days of follow-up. Moreover, this study confirmed that quantitative perfusion (defined as > 10% ischemic myocardium) was superior to qualitative perfusion (defined as perfusion defects in > 2 segments) in the assessment of ischemia.45 Rahman et al.46 also demonstrated that high-resolution CMR techniques using fully quantitative perfusion were properly accurate and outperformed visual assessment in detecting microvascular dysfunction.
Unfortunately, some of the tests that could help in the noninvasive functional diagnosis of patients with ANOCA/INOCA are not available in routine clinical practice in many centers in Spain, thus limiting the diagnostic approach in these patients.
Table 1 shows the diagnostic criteria for ANOCA, while figure 3 illustrates the complete diagnostic algorithm proposed for patients with ANOCA, specifying the initial strategy, when to schedule invasive studies, and the possible therapies based on the specific endotype.
Table 1. Diagnostic criteria for ANOCA
| Endotype | Physiopathology | Criteria | Comments |
|---|---|---|---|
| Microvascular angina | Coronary microvascular dysfunction | Myocardial ischemia symptoms | • Exertional or resting angina • Angina equivalent (exertional dyspnea) |
| Evidence of myocardial ischemia | • Positive ischemia detection test | ||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
||
| Impaired coronary microvascular function | • Adenosine test: CFR ≤ 2.0 (2.5 according to the method), IMR ≥ 25, HMR ≥ 1.9 • Microvascular spasm (spontaneous or acetylcholine test): angina, EKG changes, without epicardial spasm (lumen reduction < 90%) |
||
| Vasospastic angina | Epicardial spasm | Symptoms | • Angina, more at rest, especially nocturnal • Reduced exercise tolerance, especially in the morning • Response to nitrates and calcium antagonists |
| EKG changes | • ST-segment changes (elevation/depression) ≥ 1 mV • New negative U waves |
||
| Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
||
| Coronary spasm | • Vasoconstriction > 90% with angina and spontaneous EKG changes, or after provocation test (acetylcholine) | ||
| Preserved coronary microvascular function | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 | ||
| Mixed | Microvascular angina and epicardial spasm | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Microvascular angina | • Microvascular dysfunction • Adenosine test: CFR ≤ 2.0 (2.5 according to the method); IMR ≥ 25, HMR ≥ 1.9 |
||
| Coronary spasm | • Angina + EKG changes + epicardial vasoconstriction (> 90%) | ||
| Noncardiac chest Pain | None | Absence of obstructive coronary artery disease | • FFR > 0.80 or stenosis < 50% • Confirmed by coronary CT or coronary angiography |
| Normal functional tests | • Adenosine test: CFR > 2.0 (2.5 according to the method), IMR < 25, HMR < 1.9 • Negative acetylcholine test |
||
|
ANOCA, angina with nonobstructive coronary arteries; CFR, coronary flow reserve; CT, coronary computed tomography; EKG, electrocardiogram; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IMR, index of microvascular resistance. Table based on data from Meeder et al.,1 Perera et al.,2 Jansen et al.,3 Kunadian et al.,7 Mejia-Renteria et al.,19 Ong et al.,25 Ang and Berry,31 Kunadian et al.,34 and Hokimoto et al.35. |
|||
INVASIVE DIAGNOSTIC APPROACH IN PATIENTS WITH ANOCA
Although these are very safe procedures, there are risks involved in the invasive assessment of patients with suspected ANOCA. Therefore, it is of paramount importance that the health professionals involved should have specific training in performing and interpreting various tests. Adequate pathways should also be implemented. Currently, the use of 2 functional tests is advised, consisting of a vasospasm provocation test with intracoronary acetylcholine infusion and a microvascular function test using a pressure-temperature sensor-tipped wire at rest and during maximum pharmacological hyperemia.7,11,34,35
Vasospasm provocation testing with intracoronary acetylcholine is advised. Since the technical data sheet of acetylcholine does not include its intracoronary use, the pharmacy department of the medical center must be contacted for prior authorization. In most cases, patients must provide their prior written informed consent for the off-label use of the drug.47 This test has demonstrated high sensitivity and specificity rates (around 90% and 100%, respectively, depending on the patient’s characteristics) for diagnosing micro- and macrovascular vasospastic angina, with very few complications.47,48 Before the test is conducted, the use of long-acting vasodilator drugs should be avoided. A minimum of 18 hours without oral or topical vasodilator agents is advised to avoid false negatives. Although the use of beta-blockers may increase vasoconstriction after acetylcholine infusion, their discontinuation before the test is not advised if these drugs are deemed necessary. In procedures performed via the radial route, the use of calcium antagonists should also be avoided.47 Essentially, the test involves the infusion of increasing acetylcholine doses while simultaneously assessing the reproduction of the patient’s symptoms, changes in the 12-lead electrocardiogram, and the presence of spasms in the epicardial arteries > 90% of their baseline diameter. The Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology recently published a technical document on the performance and interpretation of this test.47
Microvascular function can be assessed using intracoronary Doppler, or pressure-temperature sensor-tipped wires. However, the only currently available guidewires are pressure-temperature sensor-tipped wires (Pressurewire X, Abbott, United States), which use the thermodilution method. Coronary thermodilution allows coronary flow values to be obtained at rest and during maximum hyperemia after the infusion of any microcirculation vasodilator agent (usually adenosine or its derivatives). These values are obtained after the infusion of 3 mL of physiological saline solution through the guide catheter and by measuring the transit time of this solution between the proximal segment of the artery and the distal segment, where the distal guidewire thermistor is located, both at rest and during maximum hyperemia. By obtaining flow data at rest and during maximum hyperemia, the CFR can be calculated, which under normal conditions should be > 2.5. CFR values ≤ 2.5 are considered diagnostic of microvascular dysfunction. Since the pressure of microcirculation perfusion (measured in the distal segment of the artery where the guidewire is located) can be obtained while performing the test during maximum hyperemia, the minimum microcirculation resistance (IMR) can be estimated. In studies performed in healthy patients, a cutoff value of 25 has been established. IMR values ≥ 25 are also indicative of microvascular dysfunction.7,34,35
There is another promising method in the invasive diagnosis of patients with ANOCA. Using the same pressure guidewire and a dedicated microcatheter (RayFlow, Hexacath, France), absolute coronary flow values (in mL/min) and absolute microcirculation resistances (in Wood units) can be obtained.49 Since these are absolute values, they partly depend on the perfusion territory of the artery and the studied segment. Currently, research is underway to develop an indexed approach using this method.50
THERAPEUTIC APPROACH IN PATIENTS WITH ANOCA
General approach
In patients with ANOCA, treatment should focus on relieving symptoms and improving the risk profile, quality of life, and prognosis. In this regard, early diagnosis, identification of the pathophysiological mechanisms involved, and early initiation of treatment tailored to the INOCA endotype are key to achieving therapeutic success.1,3,7,25,31,34,35,51-54 However, currently available studies of specific medical treatment for this condition are small, with heterogeneous methodologies and variable results, which makes it difficult to establish robust recommendations for the therapeutic management of these patients.
Lifestyle changes and control of cardiovascular risk factors
First, given the impact of cardiovascular risk factors on the development of coronary microvascular dysfunction and epicardial spasm, effective control of these risk factors is essential, including lifestyle changes (weight loss, physical exercise, smoking cessation, stress reduction), and appropriate pharmacological therapies.10 To reduce the risk of coronary vasospasm, it is important to avoid triggering factors such as smoking and the use of certain drugs (cocaine and amphetamine).10
Statins are beneficial not only due to their effect on lipid profile, but also due to their positive effect on endothelial function and in preventing the development of coronary spasms.55,56 Renin-angiotensin-aldosterone system inhibitors are beneficial to reduce blood pressure and improve endothelial function. In fact, these drugs have been reported to have positive effects on both coronary microvascular dysfunction and epicardial coronary vasospasm.55-57 The role of aspirin in patients without known cardiovascular disease is controversial.55,56 In the Japanese guidelines, aspirin is not advised in the absence of angiographically confirmed stenosis in patients with vasospasm (class IIIB indication).35
Antianginal treatment
Antianginal treatment is crucial for symptom relief. Preferential use of drugs that reduce myocardial oxygen consumption is advised in patients with a structural endotype of INOCA (microvascular dysfunction), such as beta-blockers or calcium channel blockers (ivabradine may also be considered in certain cases), along with other drugs such as ranolazine, trimetazidine, and nicorandil. On the other hand, calcium channel blockers, nitrates, nicorandil, or a combination of these, are advised in patients with a vasomotor endotype of INOCA (whether epicardial or microvascular spasm) (table 2).1,3,7,25,31,34,35,51-54
Table 2. Therapeutic approach for patients with ANOCA or INOCA
| General treatment | |||
|---|---|---|---|
| Lifestyle changes | • Mediterranean diet • Physical exercise • Weight control • Stress reduction |
||
| Cardiovascular risk factor control | • Hypertension • Dyslipidemia • Diabetes • Smoking cessation |
||
| Aspirin | • With previous CVD • Without previous CVD, its use is controversial |
||
| ACEI or ARA II | • Blood pressure reduction • Improvement in endothelial function: possible benefit in microvascular coronary dysfunction and coronary vasospasm |
||
| Statins | • Reduction in total cholesterol and LDL • Improvement in endothelial function • Possible benefit in vasospastic angina |
||
| Anti-anginal drugs | Microvascular angina | Beta-blockers | • Decreased myocardial oxygen consumption* |
| Calcium antagonists | • Decreased myocardial oxygen consumption • Vascular smooth muscle relaxation |
||
| Ranolazine | • Improvement in microvascular perfusion reserve | ||
| Trimetazidine | • Increased cellular tolerance to ischemia | ||
| Vasospastic angina | Calcium antagonists | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle |
|
| Nitrates | • Decreased myocardial oxygen consumption • Decreased coronary spasm via relaxation of vascular smooth muscle |
||
| Nicorandil | • Coronary vasodilator effect | ||
| Microvascular angina + vasospastic angina | Calcium antagonists, nitrates, ranolazine, trimetazidine, nicorandil | ||
|
* Consider the use of nebivolol due to its antioxidant properties through nitric oxide. ACEI, angiotensin-converting enzyme inhibitors; ANOCA, angina with nonobstructive coronary arteries; ARA II, angiotensin II receptor antagonists; CVD, cardiovascular disease; INOCA, ischemia with nonobstructive coronary arteries; LDL, low-density lipoproteins. Table based on data from Meeder et al.,1 Jansen et al.,3 Kunadian et al.,7 Kobayashi et al.,26 Ang and Berry,31 Kunadian et al.,34 Hokimoto et al.,35 Beltrame et al.,51 Mehta et al.,52 Seitz et al.,53 and Abouelnour et al.54. |
|||
There is some evidence on nebivolol compared with other beta-blockers, due to its potential vasodilatory effect that targets the production of nitric oxide.58 A beneficial effect of carvedilol has also been suggested by improving endothelium-dependent dilation.59 A randomized clinical trial of 81 patients demonstrated the benefit of ranolazine treatment in relieving symptoms in patients with CFR values < 2.5.60 Diltiazem treatment shows no benefits in improving symptoms, quality of life, or coronary microvascular function in the randomized EDIT-CMD trial of 73 patients with ANOCA in a 6-week course of treatment, although there was a reduction in induced epicardial vasospasms.12 Finally, there are promising potential benefits associated with drugs that have new therapeutic targets, such as cilostazol, a phosphodiesterase 3 inhibitor that targets coronary vasospasm,61 or zibotentan, a selective endothelin A antagonist with benefits on microcirculation and endothelial dysfunction,62 or fasudil, a rho-kinase enzyme inhibitor capable of reducing the IMR in patients with a positive vasospasm provocation test and elevated IMR.13
Treatment for resistant angina
The use of drugs such as low-dose tricyclic antidepressants (which modulate norepinephrine uptake and have anticholinergic effects, which can induce analgesia), or neurostimulators that block the transfer of pain at the spinal cord has been proposed in patients with resistant angina, and even coronary interventions in the case of vasospastic angina refractory to medical therapy.51
Patient follow-up
The follow-up of these patients should be coordinated between primary care physicians and cardiologists, and once symptoms are under control, follow-up should preferably be conducted in primary care units, with referrals to cardiology if there is decompensation. In addition, given the particularities of ANOCA, it is essential to inform patients about their disease and its implications. A multidisciplinary approach is necessary since other health professionals, such as psychologists, internists, and pain clinics, may sometimes be required.
Future lines of research
Finally, ongoing clinical trials are currently exploring whether intensive treatment of coronary atherosclerosis with high-intensity statins, renin-angiotensin-aldosterone system inhibitors, and low doses of aspirin improves angina and ischemia. The WARRIOR trial (NCT03417388) is studying whether such treatment improves outcomes, and the MINOCA-BAT trial (NCT03686696) is investigating whether the combined use of beta-blockers and renin-angiotensin-aldosterone system inhibitors reduces major cardiovascular clinical events.
CONCLUSIONS
Patients with suspected ANOCA exhibit a wide array of presentations that can currently be diagnosed and treated with effective individualized therapies. It is important for clinical cardiologists to become familiar with the various abnormalities in patients with ANOCA, and the currently available diagnostic and therapeutic tools. Invasive diagnostic tests constitute a new option requiring specific training for their correct performance and interpretation, as well as CMR with adenosine or regadenoson for myocardial perfusion calculation. In conclusion, specific actions need to be taken by all health centers to create diagnostic and therapeutic protocols for the management of these patients.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used in the preparation of this document.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to the conception, literature search, development, drafting, reading, and final approval of the manuscript. C. Escobar served as the consensus coordinator.
CONFLICTS OF INTEREST
J. Escaned, a recipient of the Intensification of Research Activity Project INT22/00088 from Instituto de Salud Carlos III, declared speaker’s fees for his involvement in educational activities for Abbott and Philips. C. Escobar, J.M. Gámez, and V. Barrios declared lecture fees from Menarini. The remaining authors declared no conflicts of interest whatsoever.
REFERENCES
1. Meeder JG, Hartzema-Meijer MJ, Jansen TPJ, Konst RE, Damman P, Elias-Smale SE. Outpatient Management of Patients With Angina With No Obstructive Coronary Arteries:How to Come to a Proper Diagnosis and Therapy. Front Cardiovasc Med. 2021;8:716319.
2. Perera D, Berry C, Hoole SP, et al. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease:a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart. 2022;109:88-95.
3. Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries:JACC Review Topic of the Week. J Am Coll Cardiol. 2021;78:1471-1479.
4. Alonso JJ, Muñiz J, Gómez-Doblas JC, et al. Prevalencia de angina estable en España. Resultados del estudio OFRECE. Rev Esp Cardiol. 2015;68:691-699.
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300.
6. Rahman H, Ryan M, Lumley M, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805-1816.
7. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
8. Taqueti VR. Coronary microvascular dysfunction in vasospastic angina:provocative role for the microcirculation in macrovessel disease prognosis. J Am Coll Cardiol. 2019;74:2361-2364.
9. Luu JM, Wei J, Shufelt CL, et al. Clinical Practice Variations in the Management of Ischemia With No Obstructive Coronary Artery Disease. J Am Heart Assoc. 2022;11:e022573.
10. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
11. Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina:the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855.
12. Jansen TPJ, Konst RE, de Vos A, et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA:The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc Imaging. 2022;15:1473-1484.
13. Suda A, Takahashi J, Hao K, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019;74:2350-2360.
14. Kaski JC, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA, Rosano GM. Cardiac syndrome X:clinical characteristics and left ven-tricular function:long-term follow-up study. J Am Coll Cardiol. 1995;25:807-814.
15. Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease:findings from the national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134-141.
16. Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease:a systematic review and meta-analysis. Eur Heart J. 2018;39:2135-2146.
17. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia:results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825-2832.
18. Patel S, Fung M, Liang Z, Butalia S, Anderson TJ. Temporal Trends of the Prevalence of Angina With No Obstructive Coronary Artery Disease (ANOCA). Can J Cardiol. 2023;39:63-70.
19. Mejia-Renteria H, Travieso A, Matías-Guiu JA, et al. Coronary microvascular dysfunction is associated with impaired cognitive function:the Cerebral-Coronary Connection study (C3 study). Eur Heart J. 2023;44:113-125.
20. Boerhout CKM, de Waard GA, Lee JM, et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease;from the multicentre international ILIAS registry. EuroIntervention. 2022;18:719-728.
21. Grigorian-Shamagian L, Oteo JF, Gutiérrez-Barrios A, et al. Endothelial dysfunction in patients with angina and non-obstructed coronary arteries is associated with an increased risk of mayor cardiovascular events. Results of the Spanish ENDOCOR registry. Int J Cardiol. 2023;370:18-25.
22. Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina:highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571-581.
23. Schumann CL, Mathew RC, Dean JL, et al. Functional and Economic Impact of INOCA and Influence of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:1369-1379.
24. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1-6.
25. Ong P, Camici PG, Beltrame JF, et al.;Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
26. Kobayashi Y, Fearon WF, Honda Y, et al. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433-1441.
27. Chung JH, Lee KE, Lee JM, et al. Effect of Sex Difference of Coronary Microvascular Dysfunction on Long-Term Outcomes in Deferred Lesions. JACC Cardiovasc Interv. 2020;13:1669-1679.
28. Nardone M, McCarthy M, Ardern CI, et al. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. 2022;15:e011323.
29. Rahman H, Demir OM, Khan F, et al. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538-2549.
30. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
31. Ang DTY, Berry C. What an Interventionalist Needs to Know About INOCA. Interv Cardiol. 2021;16:e32.
32. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774-1782.
33. Matsumoto T, Saito Y, Saito K, et al. Relation Between Cancer and Vasospastic Angina. Adv Ther. 2021;38:4344-4353.
34. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049-1069.
35. Hokimoto S, Kaikita K, Yasuda S, et al. JCS/CVIT/JCC 2023 Guideline Focused Update on Diagnosis and Treatment of Vasospastic Angina (Coronary Spastic Angina) and Coronary Microvascular Dysfunction. Circ J. 2023;87:879-936.
36. Lee SH, Shin D, Lee JM, et al. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J Am Heart Assoc. 2022;11:e025171.
37. Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58;740-748.
38. Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging. 2017;33:1021-1031.
39. Michelsen MM, Mygind ND, Pena A, et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction:the iPOWER study. Int J Cardiol. 2017;228:435-443.
40. Vogel R, Indermühle A, Reinhardt J, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography:algorithm and validation. J Am Coll Cardiol. 2005;45:754-762.
41. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute–sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8:e002481.
42. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948-1953.
43. Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction:Validation Against Invasive Coronary Physiology. JACC Cardiovasc Imaging. 2019;12:1958-1969.
44. Zhou W, Lee JCY, Leung ST, et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2021;14:602-611.
45. Sammut EC, Villa ADM, Di Giovine G, et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2018;11:686-694.
46. Rahman H, Scannell CM, Demir OM, et al. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:978-986.
47. Gutiérrez E, Gómez-Lara J, Escaned J, et al. Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC. REC Interv Cardiol. 2021;3:286-296.
48. Montone RA, Rinaldi R, Del Buono MG, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention. 2022;18:e666-e676.
49. Rivero F, Gutiérrez-Barrios A, Gomez-Lara J, et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution:A comparative study with index of microvascular resistance. Int J Cardiol. 2021;333:1-7.
50. de Vos A, Jansen TPJ, van't Veer M, et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc Interv. 2023;16:470-481.
51. Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ. 2021;375:e060602.
52. Mehta PK, Huang J, Levit RD, Malas W, Waheed N, Bairey Merz CN. Ischemia and no obstructive coronary arteries (INOCA):A narrative review. Atherosclerosis. 2022;363:8-21.
53. Seitz A, Martínez Pereyra V, Sechtem U, Ong P. Update on coronary artery spasm 2022 –A narrative review. Int J Cardiol. 2022;359:1-6.
54. Abouelnour A, Gori T. Vasomotor Dysfunction in Patients with Ischemia and Non-Obstructive Coronary Artery Disease:Current Diagnostic and Therapeutic Strategies. Biomedicines. 2021;9:1774.
55. Ong P, Athanasiadis A, Sechtem U. Treatment of Angina Pectoris Associated with Coronary Microvascular Dysfunction. Cardiovasc Drugs Ther. 2016;30:351-356.
56. Picard F, Sayah N, Spagnoli V, Adjedj J, Varenne O. Vasospastic angina:A literature review of current evidence. Arch Cardiovasc Dis. 2019;112:44-55.
57. Choi BG, Jeon SY, Rha SW, et al. Impact of Renin-Angiotensin System Inhibitors on Long-Term Clinical Outcomes of Patients With Coronary Artery Spasm. J Am Heart Assoc. 2016;5:e003217.
58. Erdamar H, Sen N, Tavil Y, et al. The effect of nebivolol treatment on oxidative stress and antioxidant status in patients with cardiac syndrome-X. Coron Artery Dis. 2009;20:238-244.
59. Matsuda Y, Akita H, Terashima M, et al. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753-759.
60. Rambarat CA, Elgendy IY, Handberg EM, et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction:Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019;276:8-13.
61. Shin ES, Lee JH, Yoo SY, et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart. 2014;100:1531-1536.
62. Morrow AJ, Ford TJ, Mangion K, et al. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J. 2020;229:70-80.
* Corresponding author.
@JEscaned; @AntoniCarolRuiz; @S_Raposeiras; @jmgamez3; @rfreixap; @Ana_Viana_T; @clinica_sec; @AgudosSEC
- Plaque modification techniques to treat calcified coronary lesions. Position paper from the ACI-SEC
- A brief look into Bayesian statistics in cardiology data analysis
- Renal denervation for the management of hypertension. Joint position statement from the SEH-LELHA and the ACI-SEC
- Assessment of the endothelial function and spasm provocation test performed by intracoronary infusion of acetylcholine. Technical report from the ACI-SEC
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain