ABSTRACT
Introduction and objectives: Several studies have shown that reduced (< 50%) left ventricular ejection fraction (LVEF) is an independent risk factor for cardiovascular events and mortality in patients with severe aortic stenosis (AS) undergoing valve replacement. Although patients with preserved LVEF (> 50%) have a better prognosis, there is a group with supranormal LVEF (≥ 70%) whose prognosis seems to differ due to their characteristics. The aim of this study was to evaluate outcomes after transcatheter aortic valve implantation (TAVI) in patients with severe AS and supranormal LVEF.
Methods: We performed a retrospective cohort study that included 1160 patients undergoing TAVI between 2007 and 2021 at Hospital Clínico San Carlos (Madrid, Spain). The patients were classified according to preoperative LVEF into reduced (< 50%), normal (50% to 69%), and supranormal (≥ 70%). Clinical, echocardiographic variables, and the following outcomes were compared: death from any cause at 30 days and at 1 year, death from cardiovascular causes at 1 year, and rehospitalization due to cardiovascular causes at 1 year.
Results: Of the 1160 patients with severe AS who underwent TAVI during the study period, 276 (23.8%) had reduced LVEF, 702 (60.5%) had normal LVEF, and 182 (15.7%) had supranormal LVEF. Patients with supranormal LVEF were predominantly men (82.9 ± 5.3 years) and had lower ventricular volumes, higher relative wall thickness, and concentric geometry. There were no differences in 30-day or 1-year mortality. However, rehospitalization for cardiovascular causes at 1 year was significantly higher in the supranormal LVEF group (LVEF < 50%: 29.2%; LVEF 50% to 69%: 27.4%; LVEF ≥ 70%: 34.4%; P < .043).
Conclusions: Patients with severe AS and supranormal preprocedural LVEF (≥ 70%) who underwent TAVI had a higher rate of cardiovascular rehospitalization at 1 year, with no differences in mortality.
Keywords: Supranormal ejection fraction. Severe aortic stenosis. TAVI. Rehospitalization.
RESUMEN
Introducción y objetivos: Se ha evidenciado en diversos estudios que la fracción de eyección del ventrículo izquierdo (FEVI) reducida (< 50%) es un factor de riesgo independiente de eventos y mortalidad en pacientes con estenosis aórtica (EA) grave tratados con recambio valvular. A pesar de que aquellos con FEVI conservada (> 50%) muestran mejor pronóstico, existe un grupo con FEVI supranormal (≥ 70%) que parece tener un pronóstico diferente por sus características particulares. El objetivo de este estudio fue evaluar los resultados del implante percutáneo de válvula aórtica (TAVI) en pacientes con EA grave y FEVI supranormal.
Métodos: Estudio de cohorte retrospectiva que incluyó 1.160 pacientes tratados con TAVI en 2007-2021 en el Hospital Clínico San Carlos (Madrid, España). Se clasificaron según su FEVI preoperatoria en reducida (< 50%), normal (50-69%) y supranormal (≥ 70%). Se compararon variables clínicas y ecocardiográficas, y los siguientes desenlaces: mortalidad por cualquier causa a los 30 días y al año, muerte por causa cardiovascular al año y rehospitalización por causa cardiovascular al año.
Resultados: De los 1.160 pacientes con EA grave que recibieron un TAVI durante el periodo del estudio, 276 (23,8%) se registraron con FEVI reducida, 702 (60,5%) con FEVI normal y 182 (15,7%) con FEVI supranormal. Los pacientes con FEVI supranormal eran predominantemente varones (82,9 ± 5,3 años), tenían menores volúmenes ventriculares, mayor grosor parietal relativo y geometría concéntrica. No hubo diferencias en la mortalidad a 30 días ni al año; sin embargo, la rehospitalización por causa cardiovascular al año fue significativamente superior en el grupo de FEVI supranormal (FEVI < 50%, 9,2%; FEVI 50-69%, 27,4%; FEVI ≥ 70%, 34,4%; p < 0,043).
Conclusiones: Los pacientes con EA grave tratados con TAVI que presentaban FEVI supranormal (≥ 70%) preprocedimiento tuvieron una mayor tasa de rehospitalización por causa cardiovascular al año, sin diferencias en la mortalidad.
Palabras clave: Fracción de eyección supranormal. Estenosis aórtica grave. TAVI. Rehospitalización.
Abbreviations AS: aortic stenosis. LVEF: left ventricular ejection fraction. LVOT: left ventricular outflow tract. RPT: relative parietal thickness. TAVI: transcatheter aortic valve implantation. VTI: velocity time integral.
INTRODUCTION
Aortic stenosis (AS) is the second most common valvular heart disease, affecting 12% of people older than 75 years.1,2 Without treatment, the survival rate for symptomatic severe AS is less than 3 years.3 Transcatheter aortic valve implantation (TAVI) is recommended for symptomatic patients and for asymptomatic patients with a reduced left ventricular ejection fraction (LVEF) of less than 50%.4
Reduced LVEF is recognized as an independent risk factor for events and mortality in patients with severe AS.5 However, the prognosis of severe AS in patients with preserved LVEF (> 50%) remains uncertain, especially in the presence of markers of subclinical myocardial injury, such as hypertrophy and fibrosis.6 Among these patients, those with a supranormal LVEF (≥ 70%) may have a worse prognosis after TAVI due to specific ventricular geometry and functional characteristics.7
The aim of this study was to evaluate the prognosis of patients with supranormal LVEF (≥ 70%) undergoing TAVI and study their echocardiographic and clinical characteristics.
METHODS
We conducted a retrospective cohort study of patients with severe AS who underwent TAVI at Hospital Clínico San Carlos in Madrid, Spain, between June 2007 and December 2021. Severe AS was defined according to current guideline criteria: mean gradient > 40 mmHg, peak velocity > 4 m/s, aortic valve area < 1 cm², or indexed aortic valve area < 0.6 cm²/m². The decision to perform TAVI was made by a multidisciplinary medical-surgical team. Patients were categorized into 3 groups based on their preprocedural LVEF, as assessed by echocardiography: reduced (< 50%), normal (50%-69%), and supranormal (≥ 70%). Clinical data were collected from medical records. Patients were excluded if they did not survive the procedure, had previous cardiac valve surgery, had cardiomyopathy unrelated to valvular disease, had a life expectancy of less than 1 year, or had missing data in their preprocedural echocardiographic study or clinical follow-up.
The clinical endpoints used to evaluate the prognosis of patients with supranormal LVEF (≥ 70%) undergoing TAVI were all-cause mortality at 30 days and 1 year, cardiovascular mortality at 1 year, and cardiovascular-related rehospitalization at 1 year. We also assessed their correlation with echocardiographic and clinical characteristics.
The study was conducted in accordance with the principles of the Declaration of Helsinki by the World Medical Association and received approval from the ethics committee of Hospital Clínico San Carlos in Madrid, Spain. Since the study was retrospective and posed no risk to patients, informed consent was not required. All data were handled with the utmost confidentiality by the researchers.
Echocardiography
Two-dimensional Doppler echocardiography was performed using the available equipment and following clinical practice guidelines.8 Measurements included septal thickness, posterior wall thickness, end-diastolic diameter, and left ventricular outflow tract (LVOT) diameter in the parasternal long-axis view. Peak and mean valvular gradients were assessed using continuous Doppler in multiple windows to obtain the highest velocity. The velocity-time integral (VTI) was measured with pulsed Doppler by placing the sample volume just before the aortic valve annulus. The aortic valve area was then calculated using the continuity equation:
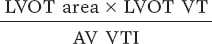
Ventricular volumes and LVEF were calculated using the biplane Simpson method. The left ventricular (LV) mass was calculated using the Devereux formula and indexed to body surface area. Relative parietal thickness (RPT) was calculated using the following formula:
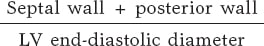
The indexed stroke volume was obtained using the following formula:
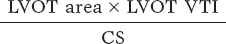
Statistical analysis
The statistical analysis was conducted using available commercial software (IBM SPSS 28.0). Normally distributed continuous variables are expressed as the mean and standard deviation, with a 95% confidence interval (95%CI). Categorical variables are expressed as absolute numbers and percentages. The Student t-test was used to compare variables with a normal distribution. Analysis of variance and the Tukey post hoc test were used to compare means, while the chi-square test was used to compare prevalences among the 3 groups. A univariable logistic regression analysis was applied to evaluate predictors of hospitalization and mortality. P values < .05 were considered statistically significant.
RESULTS
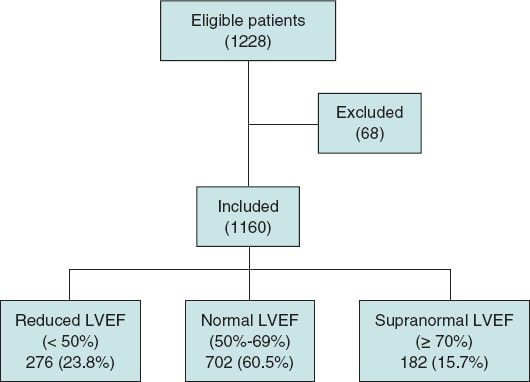
Of the 1228 patients who underwent TAVI during the study period, 1160 were included in the analysis. Among these, 276 patients (23.7%) had a reduced LVEF (< 50%), 702 patients (60.5%) had a normal LVEF (50%-69%), and 182 patients (15.6%) had a supranormal LVEF (≥ 70%). Sixty-eight patients were excluded based on the following criteria: 23 due to death during the procedure, 15 with previous cardiac valve surgery, 6 with cardiomyopathy unrelated to valvular disease, 18 with a life expectancy of less than 1 year, and 6 with missing data in the preprocedural echocardiographic study or clinical follow-up (figure 1).
Figure 1. Flow chart illustrating the patients included and excluded from the study, the final sample analyzed, and its distribution among the 3 study groups. LVEF, left ventricular ejection fraction.
The baseline characteristics of the study population are shown in table 1. The mean age was 82.2 ± 5.8 years and was slightly lower in the reduced LVEF group than in the other 2 groups. Male sex was more common in the group with LVEF ≥ 70% (P < .005). Patients with LVEF < 50% had a higher prevalence of prior myocardial infarction, coronary artery disease, and revascularization, along with a higher EUROSCORE II (22.5 [14.7-32.0]; P < .001). This group also more frequently required the intervention as an emergency procedure (P < .001).
Table 1. Patients’ baseline characteristics
| Characteristics | LVEF < 50% (n = 276) | LVEF 50%-69% (n = 702) | LVEF ≥ 70% (n = 182) | P value |
|---|---|---|---|---|
| Age (years) | 81.6 ± 6.3 | 82.2 ± 5.9 | 82.9 ± 5.3 | < .050 |
| Male sex | 38.1% | 58.2% | 68.3% | < .001 |
| Hypertension | 80.7% | 82.9% | 86.0% | .363 |
| Diabetes mellitus | 41.7% | 35.6% | 33.9% | .182 |
| Body mass index | 27.1 ± 4.4 | 28.4 ± 5.2 | 27.7 ± 5.1 | < .002 |
| Hyperlipidemia | 56.9% | 59.8% | 56.0% | .254 |
| Previous PTA | 30.6% | 19.4% | 16.8% | < .001 |
| Previous CABG | 9.6% | 4.5% | 3.3% | < .002 |
| Previous infarction | 20.6% | 9.1% | 7.6% | < .001 |
| Coronary artery disease | 45.6% | 32.7% | 34.7% | < .002 |
| Left main coronary artery disease | 5.6% | 3.4% | 1.8% | .222 |
| Incomplete revascularization | 20.7% | 30.4% | 35.3% | .174 |
| COPD | 16.7% | 15.1% | 14.5% | .714 |
| Smoking | 37.2% | 41.7% | 14.4% | .034 |
| Atrial fibrillation | 38.6% | 37.8% | 42.1% | .570 |
| Glomerular filtration | 61.2 (46.0-77.9) | 63.1 (46.8-79.4) | 60.9 (45.5-75.2) | .311 |
| Cancer | 16.0% | 15.5% | 18.7% | .725 |
| EuroSCORE II | 22.5 (14.7-32.0) | 14.3 (7.4-18.0) | 11.8 (8.9-18.9) | < .001 |
| Dyspnea | 87.5% | 87.5% | 91.7% | .289 |
| Emergency procedure | 33.9% | 17.5% | 14.1% | < .001 |
| Valve-in-valve | 3.6% | 3.3% | 2.7% | .881 |
| Post-TAVI outcome | ||||
| Peak gradient (mmHg) | 18.3 ± 7.3 | 19.3 ± 8.9 | 19.4 ± 8.7 | .223 |
| Mean gradient (mmHg) | 9.3 ± 3.8 | 9.9 ± 4.8 | 10.0 ± 5.7 | .229 |
|
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; PTA, percutaneous transluminal angioplasty; TAVI, transcatheter aortic valve implantation. |
||||
Echocardiographic data
Patients with LVEF ≥ 70% had smaller LV end-diastolic and end-systolic volumes, and greater septal wall thickness and RPT than the other 2 groups. In this group, left ventricular mass index (LVMI) was 126.3 ± 32.8 g/m², reflecting a predominant phenotype of concentric hypertrophy and remodelling. A similar pattern was observed in patients with normal LVEF (50%-69%), although this group had a larger end-diastolic LV volume (table 2). In contrast, patients with LVEF < 50% had a greater LV mass, with an LVMI of 147.6 ± 40.2 g/m² (P < .001), a low RWT (< 0.42), and an elevated end-diastolic volume, indicating a predominant phenotype of eccentric hypertrophy. In addition, this group had a lower indexed stroke volume (32.5 ± 11.8; P < .001).
Table 2. Patients’ baseline characteristics
| Characteristics | LVEF < 50% | LVEF 50%-69% | LVEF ≥ 70% | P value |
|---|---|---|---|---|
| RPT | 0.48 (0.41-0.58) | 0.57 (0.50-0.65) | 0.60 (0.52-0.69) | < .001 |
| Indexed LVESV (mL/m²) | 31 (25-39) | 38 (31-45) | 39 (31-49) | < .001 |
| Indexed LVEDV (mL/m²) | 63 (48-80) | 48 (38-59) | 45 (35-56) | < .001 |
| LVMI (g/m²) | 147.6 ± 40.2 | 128.8 ± 34.2 | 126.3 ± 32.8 | < .001 |
| IVS (mm) | 12.1 ± 2.6 | 13.6 ± 2.4 | 14.1 ± 2.7 | < .001 |
| Indexed stroke volume (mL/m²) | 32.5 ± 11.8 | 38 ± 11.5 | 40 ± 11.6 | < .001 |
|
IVS, interventricular septum; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVMI, left ventricular mass index; RPT, relative parietal thickness. |
||||
Perioperative clinical endpoints
There were no significant differences among the 3 groups regarding intra- and postoperative mortality.
Clinical endpoints at follow-up
During the 1-year follow-up, 164 patients (14.13%) died, with no significant differences among the 3 groups (LVEF < 50%, 14.6%; LVEF 50%-69%, 12.6%; LVEF ≥ 70%, 12.7%; P < .736). However, significant differences were found in the rate of cardiovascular rehospitalization at 1 year, with higher rates in the supranormal LVEF group (LVEF ≥ 70%, 34.4%; LVEF < 50%, 29.2%; LVEF 50%- 69%, 27.4%; P < .043). Clinical endpoints are shown in table 3.
| Variables | LVEF < 50% (n = 276) | LVEF 50%-69% (n = 702) | LVEF ≥ 70% (n = 182) | P value |
|---|---|---|---|---|
| Perioperative | ||||
| Intraoperative mortality | 0.4% | 1.4% | 0.6% | .345 |
| Postoperative mortality | 2.8% | 3.7% | 4.3% | .676 |
| Follow-up | ||||
| All-cause mortality at 30 days | 2.4% | 3.9% | 5.0% | .359 |
| Cardiovascular mortality at 1 year | 12.8% | 9.6% | 15.2% | .370 |
| All-cause mortality at 1 year | 14.6% | 12.6% | 12.7% | .736 |
| Cardiovascular rehospitalization at 1 year | 29.2% | 27.4% | 34.4% | < .043 |
|
LVEF, left ventricular ejection fraction. |
||||
Univariable regression analysis
In patients with supranormal LVEF, coronary artery disease and increased interventricular septal thickness were predictors of cardiovascular hospitalization at 1 year (table 4). In this group, indexed LV end-diastolic volume and a history of coronary artery disease were predictors of all-cause mortality at 1 year (table 5). In the general population, no predictors of 1 year mortality were identified, except for age (table 6).
Table 4. Supranormal left ventricular ejection fraction and predictors of cardiovascular hospitalization at 1 year
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.077 | 0.991-1.169 | .080 |  |
| Hypertension | 1.687 | 0.546-5.213 | .364 | |
| Diabetes mellitus | 1.846 | 0.767-4.440 | .171 | |
| Body mass index | 1.012 | 0.933-1.099 | .770 | |
| Coronary artery disease | 0.327 | 0.137-0.780 | .012 | |
| Smoking | 1.796 | 0.650-4.965 | .259 | |
| EuroSCORE II | 1.046 | 0.998-1.096 | .060 | |
| RPT | 1.004 | 0.041-24.392 | .998 | |
| Indexed LVEDV | 0.979 | 0.949-1.010 | .188 | |
| IVS | 0.965 | 0.933-0.998 | .036 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
Table 5. Supranormal left ventricular ejection fraction and predictors of 1-year mortality
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.180 | 0.976-1.426 | .087 |  |
| Hypertension | 2.181 | 0.167-28.575 | .552 | |
| Diabetes mellitus | 0.875 | 0.154-4.968 | .154 | |
| Body mass index | 1.004 | 0.796-1.265 | .976 | |
| Coronary artery disease | 3.372 | 0.612-18.575 | .012 | |
| Smoking | 7.453 | 0.691-61.024 | .259 | |
| EuroSCORE II | 0.921 | 0.831-1.022 | .120 | |
| RPT | 0.011 | 0.000-154.979 | .998 | |
| Indexed LVEDV | 1.094 | 1.018-1.177 | .015 | |
| IVS | 1.004 | 0.943-1.068 | .912 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
Table 6. Predictors of 1-year mortality in the general population
| Characteristics | HR | 95%CI | P value | HR |
|---|---|---|---|---|
| Age | 1.070 | 1.002-1.143 | .043 |  |
| Hypertension | 1.268 | 0.545-2.947 | .582 | |
| Diabetes mellitus | 1.458 | 0.764-2.784 | .253 | |
| Body mass index | 0.949 | 0.882-1.020 | .152 | |
| Coronary artery disease | 1.593 | 0.867-2.929 | .134 | |
| Smoking | 1.794 | 0.899-3.581 | .097 | |
| EuroSCORE II | 1.046 | 0.973-1.033 | .868 | |
| RPT | 0.252 | 0.022-2.836 | .264 | |
| Indexed LVEDV | 0.986 | 0.967-1.006 | .188 | |
| IVS | 1.000 | 0.974-1.027 | .036 | |
| 1.0 | ||||
|
95%CI, 95% confidence interval; HR, hazard ratio; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; RPT, relative parietal thickness. |
||||
DISCUSSION
This study demonstrates that LVEF is an important prognostic factor in patients with severe AS treated with TAVI. While no differences in mortality were observed at 1 month or 1 year, patients with supranormal LVEF (≥ 70%) had a higher rate of rehospitalization at 1 year than those with reduced (< 50%) or normal (50%-69%) LVEF.
LVEF has been widely recognized in the literature as a prognostic factor in various clinical contexts. A study by Wehner et al.9 reported that an LVEF of 60% to 65% is associated with the best prognosis, while patients with LVEF ≥ 70% have a 5-year mortality rate similar to those with reduced LVEF. A study by Gu et al.,10 found higher mortality and hospitalization rates at 5 years in patients hospitalized for heart failure with LVEF > 65% than in those with normal LVEF.
In patients with AS undergoing TAVI, the OCEAN-TAVI registry found that LVEF > 65% was an independent predictor of death and rehospitalization at the 3-year follow-up (hazard ratio [HR], 1.16; 95%CI, 1.02-1.31; P = .023).¹¹ There were no significant differences in mortality among the study groups, except for the rehospitalization rate. It remains to be elucidated whether longer-term follow-up could also detect differences in mortality.
In patients with AS undergoing surgical interventions, LVEF is a recognized prognostic marker. In a study by Dahl et al.,¹² reduced LVEF (< 50%) was a clear predictor of 5-year risk. The study revealed that patients with supranormal LVEF experienced longer hospital stays, increased need for mechanical ventilation, a higher incidence of hemodialysis, and a greater rate of rehospitalization. This latter finding is consistent with the findings of the present study. There is no clear explanation for these results, but they may be related to the persistence of myocardial hypertrophy or diastolic dysfunction following the intervention.13
According to previous studies, increased (> 80 mL/m²) and reduced (< 55 mL/m²) ventricular volumes are risk factors to consider in patients with severe AS.14,15 In this analysis, in the subgroup of patients with supranormal LVEF, indexed LV end-diastolic volume was a predictor of 1-year mortality (HR, 1.094; 95%CI, 1.018-1.177; P < .015). A low indexed stroke volume has also been associated with worse prognosis in patients with AS, both with reduced and preserved LVEF.16 Patients with preserved LVEF may have a low stroke volume when the ventricular cavity is small and they have restrictive physiology that limits the stroke volume, even with a supranormal ejection fraction.17 In most studies, these patients have a worse prognosis, with a higher mortality risk and less event-free time.18,19
Supranormal LVEF represents a new phenotype in patients with preserved LVEF (> 50%), with distinctive clinical and hemodynamic characteristics. There is no universal agreement on the exact LVEF value to define supranormal. According to the American College of Cardiology, a LVEF ≥ 70% is considered supranormal,20 while other groups set this threshold at ≥ 65%. For this study, LVEF ≥ 70% was used as the reference to better highlight clinical and echocardiographic differences among the study groups, which likely influenced the prevalence observed in the population.
In a study by Wehner et al.,9 which reviewed 403 977 echocardiograms from 203 135 patients without prespecified diagnoses, an LVEF ≥ 70% was found in 3% (13 553) of participants. In the present study of patients with severe AS, 15% had LVEFs ≥ 70%. Other studies, such as the OCEAN-TAVI registry,¹¹ reported a higher percentage of patients with supranormal LVEF and AS (47%), likely because they used a lower cutoff for supranormal LVEF (≥ 65%). These findings suggest that severe AS is associated with a higher-than-normal LVEF, likely due to left ventricular (LV) remodelling and concentric hypertrophy resulting from elevated afterload.21-24 In our study, the LVMI was elevated in most patients, regardless of LVEF. Patients with normal and supranormal LVEF predominantly exhibited concentric geometry, characterized by a reduced LV cavity and increased septal thickness. In contrast, patients with reduced LVEF showed predominantly eccentric geometry with a dilated LV.
Finally, our results suggest that while widely used risk scales like EuroSCORE II remain valid, echocardiographic factors should also be considered when determining the timing and type of intervention.25
Limitations
This retrospective, observational study was conducted at a single center. All patients underwent TAVI, and there was no comparison with those treated with surgical valve replacement. The medical and pharmacological treatment were not specified, which is an important omission given recent advancements in heart failure management. In addition, a 1-year follow-up may be too short to detect differences in mortality between the groups and a longer-term follow-up might reveal differences.
CONCLUSIONS
LVEF remains an important prognostic factor in decision-making for patients with severe AS. In this study, patients with reduced (< 50%), normal (50%-69%), or supranormal (≥ 70%) preprocedural LVEF who underwent TAVI showed no differences in 1-year mortality. However, those with supranormal LVEF (≥ 70%) had a higher rate of cardiovascular-related rehospitalization at 1 year, suggesting that this subgroup may have unfavorable factors, such as significant diastolic dysfunction. Further research is needed to investigate and confirm these findings.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
This study adhered to the Declaration of Helsinki and received approval from the ethics committee of Hospital Clínico San Carlos in Madrid, Spain. As the study was retrospective and posed no risk to patients, informed consent was not required. All information was handled with strict confidentiality by the researchers. Consecutive patients were recruited during the study period without sampling or randomization, so sex or gender biases were not considered in the analysis
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence tools were used during the performance of the study.
AUTHORS’ CONTRIBUTIONS
E. Martínez Gómez, X. Solar, D. Faria, L. Nombela Franco, and J.A. de Agustín contributed to the conception and design, data acquisition, analysis, and interpretation of the study. E. Martínez Gómez, X. Solar, D. Faria, L. Nombela Franco, P. Jiménez Quevedo, G. Tirado, E. Pozo Osinalde, C. Olmos Blanco, P. Mahía Casado, P. Marcos Alberca, M. Luaces, J.J. Gómez de Diego, L. Collado Yurrita, A. Fernández-Ortiz, J. Pérez-Villacastín, and J.A. de Agustín contributed to the drafting of the article or its critical revision. All authors approved the final version of the article.
CONFLICTS OF INTEREST
None declared.
WHAT IS KNOWN ABOUT THE TOPIC?
- LVEF is a highly significant prognostic marker in cardiology. Paradoxically, studies have shown that patients with a supranormal LVEF have a worse prognosis in some scenarios, such as AS.
WHAT DOES THIS STUDY ADD?
- This study shows that patients undergoing TAVI with supranormal LVEF (≥ 70%) have higher rehospitalization rates at 1 year than those with reduced (< 50%) or normal (50%-69%) LVEF.
REFERENCES
1. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly:disease prevalence and number of candidates for transcatheter aortic valve replacement:a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002-1012.
2. D'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people:the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515-3522.
3. Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61-67.
4. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
5. Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8:e002917.
6. Bing R, Cavalcante JL, Everett RJ, et al. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:283-296.
7. Shah S, Segar MW, Kondamudi N, et al. Supranormal Left Ventricular Ejection Fraction, Stroke Volume, and Cardiovascular Risk:Findings From Population-Based Cohort Studies. JACC Heart Fail. 2022;10:583-594.
8. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
9. Wehner GJ, Jing L, Haggerty CM, et al. Routinely reported ejection fraction and mortality in clinical practice:where does the nadir of risk lie?Eur Heart J. 2020;41:1249-1257.
10. Gu J, Ke JH, Wang Y, Wang CQ, Zhang JF. Characteristics, prognosis, and treatment response in HFpEF patients with high vs. normal ejection fraction. Front Cardiovasc Med. 2022;9:944441.
11. Imamura T, Hida Y, Ueno H, et al. Clinical Implication of Supra-Normal Left Ventricular Ejection Fraction in Patients Undergoing Transcatheter Aortic Valve replacement. J Clin Med. 2023;12:7429.
12. Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8:e002917.
13. Mariage JL, Bulpa P, Michaux I, et al. Impact of myocardial hypertrophy and preoperative left ventricular ejection fraction on post operative complications after aortic valve replacement for aortic stenosis. Chest. 2005;128:268S.
14. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling concepts and clinical implications:a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569-582.
15. Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure overloaded human heart:structural deterioration and compensatory mechanisms. Circulation. 2003;107:984-991.
16. Kwak S, Everett RJ, Treibel TA, et al. Markers of Myocardial Damage Predict Mortality in Patients With Aortic Stenosis. J Am Coll Cardiol. 2021;78:545-558.
17. Severino P, Maestrini V, Mariani MV, et al. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail Rev. 2020;25:9-17.
18. Ito S, Nkomo VT, Orsinelli DA, et al. Impact of Stroke Volume Index and Left Ventricular Ejection Fraction on Mortality After Aortic Valve Replacement. Mayo Clin Proc. 2020;95:69-76.
19. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction:implications for diagnosis and treatment. Eur Heart J. 2010;31:281-289.
20. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
21. Forrest L, Rocheleau G, Bafna S, et al. Genetic and phenotypic profiling of supranormal ejection fraction reveals decreased survival and underdiagnosed heart failure. Eur J Heart Fail. 2022;24:2118-2127.
22. Dumesnil JG, Shoucri RM. Effect of the geometry of the left ventricle on the calculation of ejection fraction. Circulation. 1982;65:91-98.
23. González Gómez A, Fernández Golfín C, Monteagudo JM, et al. Severe aortic stenosis patients with preserved ejection fraction according to flow and gradient classification:Prevalence and outcomes. Int J Cardiol. 2017;248:211-215.
24. Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856-2864.
25. Strachinaru M, Van Mieghem NM. Low-gradient severe aortic stenosis with preserved ejection fraction:how fast should we act?Int J Cardiovasc Imaging. 2021;37:3177-3180.