To the Editor,
Anticoagulation alone has proven efficacy for the treatment of low- and low-to-intermediate- -risk acute pulmonary embolism (PE) patients.1 Nonetheless, intermediate-high and high-risk PE are associated with a considerable risk of short-term circulatory collapse, death or chronic thromboembolic pulmonary hypertension, ranging from 3% to 10%, when treated with anticoagulation alone.1 Although systemic fibrinolysis decreases this risk by 50%, this treatment significantly increases the risk of major bleeding, as seen in PEITHO trial (Fibrinolysis for patients with intermediate-risk pulmonary embolism),2 which has limited the use of systemic fibrinolysis to high-risk patients, as recommended in the current guidelines.3
This limitation has led to growing interest in catheter-directed therapies (CDT) for patients with high-risk acute PE and a contraindication or failure of systemic fibrinolysis and patients with intermediate-high risk who develop worsening hemodynamics despite anticoagulation.3 CDT allow faster resolution of perfusion defects and hemodynamic improvement without the systemic hemorrhagic effects of systemic thrombolysis.
Despite an increasing use of CDT, the clinical evidence of its benefits remains scarce, as there are no adequately powered randomized controlled trials and current studies have been limited to immediate hemodynamic improvement or imaging surrogate markers.4-6
This study aimed to assess the safety, feasibility, and mid-term effects of CDT. Between 2020 and 2022, we prospectively enrolled consecutive patients with high and intermediate-high-risk PE who underwent CDT at a single tertiary center. The selection criteria included high-risk patients with contraindicated or failed fibrinolysis and those with intermediate-high risk and worsening hemodynamics despite anticoagulation. We excluded patients with clinical onset of PE more than 2 weeks previously and/or with transit thrombus.
Right heart catheterization (RHC) and bilateral pulmonary angiography were performed through the femoral or right antecubital basilic vein before the intervention. The operators decided between in-situ fibrinolysis, mechanical thrombectomy, or both, based on thrombus burden, localization, hemodynamic status, and bleeding risk. Catheter-directed local fibrinolysis was performed using a 1 mg/h alteplase infusion for 12 hours, following a 1 mg bolus. The catheter-directed mechanical thrombectomy used the 8- and 12-Fr Indigo aspiration system (Penumbra, United States) to restore perfusion in as many branches as possible until a good angiographic result or blood aspiration of 300 to 350 mL was achieved. The follow-up protocol included an echocardiogram, computed tomography angiography scan, RHC, and pulmonary angiogram at 3 months after the CDT.
A total of 39 patients were analyzed. The baseline characteristics are presented in table 1, which shows increased levels of serum lactate in 30% of patients, troponin in 97%, and N-terminal pro-B-type natriuretic peptide in 92%. At admission, 18% of patients were stratified as high-risk. The admission echocardiogram revealed right ventricle (RV) dilation in 95% of patients, with RV systolic dysfunction in 69% of them.
Table 1. Baseline characteristics and procedure data
| Baseline characteristics (n = 39) | |
| Age, years | 60.0 ± 17.6 |
| Gender, male | 46.2% (18) |
| Previous VTE | 12.8% (5) |
| Oncologic disease | 10.3% (4) |
| Clinical and laboratorial findings (n = 39) | |
| Syncope at presentation | 28.2% (11) |
| Dyspnea at presentation | 76.9% (30) |
| Days from symptoms onset | 1.0 [1.8] |
| High-risk pulmonary embolism | 17.9% (7) |
| Failed systemic fibrinolysis | 0% |
| Contraindication to fibrinolysis | 10.3% (4) |
| Systolic blood pressure, mmHg | 116 ± 26 |
| Heart rate, bpm | 102 ± 21 |
| PaO2/FiO2 ratio | 262 ± 96 |
| Serum lactate, mmol/L (N < 1.8) | 1.7 ± 1.6 |
| hs-troponin I, pg/mL (N < 14) | 262 [520] |
| NT-proBNP, pg/mL (N < 150) | 2775 [3910] |
| Peak D-dimer, ng/mL (N < 500) | 8835 [12 254] |
| Positive lactate | 30.8% (12) |
| Positive troponin | 97.4% (38) |
| Positive NT-proBNP | 92.3% (36) |
| Imaging findings – initial work-up (n = 39) | |
| Central PE in angio-CT scan | 34.2% (13) |
| RV/LV ratio angio-CT scan | 1.4 ± 0.2 |
| Dilated RV in TTE | 94.6% (35) |
| RV dysfunction in TTE | 69.4% (25) |
| Procedural data and complications (n = 39) | |
| Thrombectomy + local fibrinolysis | 17.9% (13) |
| Isolated thrombectomy | 10.3% (4) |
| Isolated local fibrinolysis | 71.2% (28) |
| Any procedure complication | 5.1% (2) |
| Cardiovascular death | 2.6% (1) |
| Cardiogenic shock | 2.6% (1) |
| Major bleeding | 0% |
| Cardiac tamponade | 0% |
| Pulmonary artery perforation | 0% |
| Pulmonary artery dissection | 2.6% (1) |
| Penumbra burr avulsion | 2.6% (1) |
| Moderate-to-severe PR | 0% |
| Moderate-to-severe TR (previous) | 25.6% (10) |
| Moderate-to-severe TR (post) | 7.7% (3) |
|
CT, computed tomography; LV, left ventricle; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PE, pulmonary embolism; PR, pulmonary regurgitation; RV, right ventricle; TR, tricuspid regurgitation; TTE, transthoracic echocardiogram; VTE, venous thromboembolism. The data are expressed as No. (%), mean ± standard deviation, or median [interquartile range]. |
|
Local fibrinolysis was performed in 71% of the patients, isolated penumbra aspiration in 10% and combined therapy in 18%. No major bleeding leading to death or requiring medical intervention or transfusion was observed during or after the procedure. There was 1 pulmonary artery dissection and 1 partial avulsion of the penumbra burr, both of which were managed conservatively with good outcomes. One patient developed persistent and refractory cardiogenic shock, leading to death. The procedural data are shown in table 1.
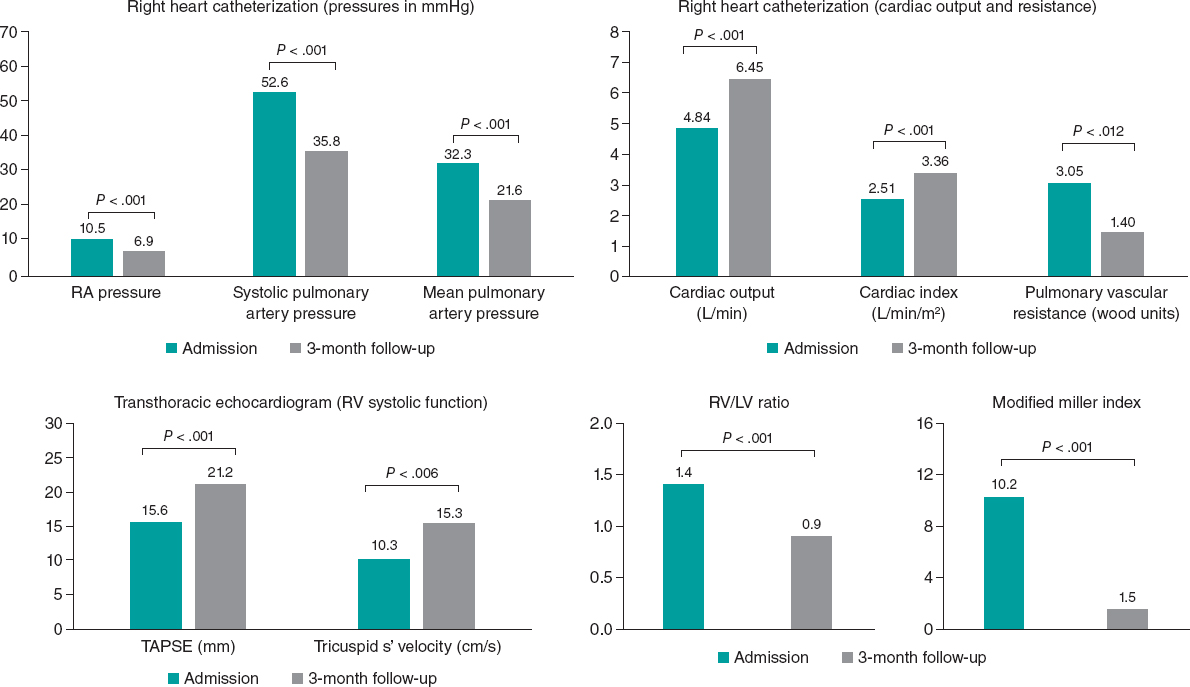
A total of 23 patients completed the 3-month follow-up, while 4 patients died, mainly from noncardiovascular causes. There was a significant rate of incomplete follow-up for various reasons, including 4 foreign patients who were unable to complete the follow-up, 5 patients who withdrew their consent, and 3 who were lost to follow-up. Among the 23 patients who completed the follow-up, the hemodynamics showed significant improvement. The data from RHC revealed a mean drop of 3.6 mmHg, 16.8 mmHg, and 10.7 mmHg in right atrial, systolic pulmonary artery, and mean pulmonary artery pressures, respectively (P < .001). In addition, there was a mean increase of 1.61 L/min and 0.85 L/min/m2 in cardiac output and index, respectively (P < .001), and a 1.65 Wood units decrease in pulmonary vascular resistance (P = .012). There was also an improvement in perfusion defects, with a mean drop of 8.7 points in the modified Miller index (P <.001). Improvement was also observed in RV function, with a mean decrease of 0.5 in the RV/left ventricle (LV) ratio on computed tomography (CT) scan (P < .001), a mean increase of 5.4 mm in tricuspid annular plane systolic excursion (TAPSE) (P < .001), and a mean increase of 5.0 cm/s in tricuspid annular s’ velocity (P = .006). These results are illustrated in figure 1. At 3 months, 9 out of the 23 patients (39%) had a mean pulmonary artery pressure above 20 mmHg.
Figure 1. Invasive hemodynamic, echocardiographic, morphological, and thrombotic burden data on admission and at 3 months. LV, left ventricle; RA, right atrial; RV; right ventricle; TAPSE, tricuspid annular plane systolic excursion.
During the follow-up period, 4 patients died, resulting in an overall mortality rate of 10.3%. However, only 1 patient died from a cardiac cause, which was secondary to worsening refractory cardiogenic shock. One patient died due to oncologic disease progression, and 2 patients died from noncardiovascular causes.
This study reports a minor procedural complication rate of 5.1%, which enhances the feasibility and safety of CDT. In the EXTRACT-PE trial (Indigo Aspiration System for Treatment of Pulmonary Embolism), a procedural complication rate of 2.5% was reported, with 1.7% being major bleeding and 0.8% being device-related pulmonary vascular injury.5 Furthermore, both complications were nonfatal and managed conservatively, with good angiographic outcomes upon reevaluation. These complications were associated with the use of mechanical thrombectomy devices, and both occurred early in the learning curve of this device, leading the authors to believe that such complications may be minimized as operator experience increases. Despite the administration of catheter-directed fibrinolysis in nearly 90% of the patients, there were no major or life-threatening bleeding events in the first 48 hours after the procedure, possibly related to the low dose of alteplase.
Most previous trials have used imaging parameters as surrogate markers to evaluate the immediate effect of CDT. The most commonly used parameter is the RV/LV ratio, as seen in the SEATTLE II trial (A prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism), EXTRACT-PE and FLARE (A prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism) trials.4-6 Our study provides more extensive and mid-term data on the benefits of CDT, including invasive direct assessment of hemodynamics instead of imaging surrogate parameters. At 3 months, we obtained a mean reduction in RV/LV ratio of 0.5, which is similar to the rates described in previous studies. Moreover, our study also reports a significant improvement in RV systolic function, as measured by transthoracic echocardiography, a significant reduction in pulmonary vascular pressures and resistance, and an increase in cardiac output, both measured invasively at 3 months after the procedure.
The optimal treatment for intermediate-risk PE is still not well established, and current guidelines recommend anticoagulation alone, with catheter intervention reserved for patients not responding to conservative therapy.3 The PEITHO trial showed that systemic fibrinolysis significantly reduced the combined primary endpoint of death or clinical deterioration, at the expense of a significant increase in major bleeding and intracranial hemorrhage.2 Although CDT has not been directly compared with anticoagulation alone in these patients, the authors believe that CDT has several advantages. First, catheter-directed fibrinolysis may provide the same intrapulmonary benefits as systemic fibrinolysis without the risk of major bleeding. Second, aspiration systems allow for faster and more immediate reperfusion in main branches, preventing further irreversible deterioration in unstable patients. Third, catheter-directed mechanical thrombectomy is a safe and efficient alternative for patients who cannot receive fibrinolytic agents. Fourth, both techniques seem to have an additive benefit in long-term anticoagulation by reducing perfusion and pulmonary vascular pressures, thus reducing progression to chronic thromboembolic pulmonary hypertension.
The evaluation of hemodynamics at 3 months offers new insights into the high rates of patients who develop pulmonary hypertension. This is especially relevant when considering the new cutoff of 20 mmHg for the mean pulmonary artery pressure, as established by the 2022 ESC guidelines for pulmonary hypertension.
The main limitation of this study is the absence of a comparator arm. Other limitations are the incomplete follow-up in almost 33% of patients, the small sample size, and the use of 2 different catheter-directed strategies.
In conclusion, for patients with intermediate-high and high-risk PE, CDT is a feasible and safe treatment option that improves hemodynamics, RV function, and perfusion defects at 3 months after the procedure.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The study was carried out according to the principles of the Declaration of Helsinki and was approved by the local ethics committee. Informed consent was obtained from all participants involved in the study. The possible variables of sex and gender have been taken into account in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used during the performance of this study or during the drafting of this manuscript.
AUTHORS’ CONTRIBUTIONS
A. Grazina and L. Almeida Morais designed the study protocol, with help from A. Fiarresga and D. Cacela. A. Grazina and B. Lacerda Teixeira collected and analyzed the data. A. Grazina and B. Lacerda Teixeira wrote the manuscript with support from L. Almeida Morais. A. Fiarresga and D. Cacela coordinated the project. All authors read and approved this manuscript.
CONFLICTS OF INTEREST
The authors declare that this is an original article and has not been previously published or submitted to another journal. The authors have no conflicts of interest.
REFERENCES
1. Giri J, Sista AK, Weinberg I et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140:774-801.
2. Meyer G, Vicaut E, Danays T et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014; 370:1402-1411.
3. Konstantinides S, Meyer G, Becattini C et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;41:543-603.
4. Piazza G, Hohlfelder B, Jaff MR et al. A prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8:1382-1392.
5. Sista AK, Horowitz JM, Tapson VF et al. Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc Interv. 2021;14:319-329.
6. Tu T, Toma C, Tapson VF et al. A prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc Interv. 2019;12:859-869.