Endorsed by the current clinical practice guidelines, the indication to perform percutaneous coronary intervention (PCI) of intermediate coronary stenosis should be guided by either fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) if evidence of ischemia is lacking.1 Despite these clear recommendations, the uptake of physiology in clinical practice remains low supporting the development of new non-invasive tools that no longer mandate the need for dedicated coronary guidewires or microcatheters along with the need to administer hyperemic agents in case of FFR.1
Advances in computational power and three-dimensional quantitative coronary angiography has facilitated the development of angiography-based-FFR indices, thus allowing easy, online physiological lesion assessments. Besides anatomical and angiographic exclusion criteria like severe tortuosity, aorto-ostial lesions or overlapping vessels, pivotal studies demonstrated that with angiography-based-FFR indices the need for invasive coronary artery instrumentation and hyperemic agents can, in most cases, be avoided.2
Currently, 4 angiography-based-FFR indices have emerged and are currently commercially available.1 Despite workflow differences and embedded simplified computational fluid dynamics models, these indices demonstrated to have a good diagnostic performance with pressure guidewire based FFR as a reference.1
Among these, vessel fractional flow reserve (vFFR, CAAS Workstation 8.5 Pie Medical Imaging, Netherlands) uses a computational fluid dynamic approach based on simplified Navier–Stokes equations and 2 angiographic views separated, at least, 30 degrees to generate a 3D reconstruction of the coronary artery. Using aortic pressure as inlet boundary condition, the algorithm applies automated and harmonized optimal end-diastolic frame selection in the 2 views by electrocardiogram triggering, thus allowing physiological lesion assessment without the need for full cardiac tree assessment or manual frame counting.3
This review provides an overview of the currently available clinical evidence on the use of vFFR (table 1 and figure 1).
Vessel fractional flow reserve was first validated in 2 retrospective, single-center studies where the technology demonstrated an excellent diagnostic performance in intermediate coronary artery lesions compared to FFR, which was consistent among different anatomical and patient subsets including tandem lesions, and patients presenting with non-ST-segment elevation acute coronary syndrome.3,4 These findings were later confirmed in the multicenter, prospective FAST II study, in which vFFR computed offline by local site personnel and a blinded core lab showed excellent diagnostic accuracy in identifying lesions with invasive guidewire-based FFR ≤ 0.80 (area under the curve [AUC], 0.93; P < .001). Positive and negative predictive values, sensitivity and specificity of vFFR were 90%, 90%, 81% and 95%, respectively.5 The system allows accurate automated vessel contour detection with manual correction required in merely 9.3% of vessel contours.5 Regarding reproducibility, vFFR showed a low inter-observer variability when computed offline by blinded academic operators (r = 0.95; P < .001) or local personnel vs a blinded core lab (r = 0.87; P < .001). Additionally, a low coefficient of variation (3.92%) was observed when vFFR was analyzed at 2 different timeframes by an independent core lab.6
Following these promising data, we explored the potential value of vFFR in a variety of clinical and procedural settings (table 1 and figure 1).
Table 1. Major studies trials investigating the diagnostic performance of vessel fractional flow reserve (vFFR)
| Study/Author | Year | Study design | Number of vessel (patient) | Primary endpoint |
|---|---|---|---|---|
| Pre-PCI setting | ||||
| FAST study | 2019 | Retrospective | 100 (100) | AUC = 0.93 (95%CI, 0.88-0.97) |
| FAST EXTEND | 2020 | Retrospective | 294 (294) | AUC = 0.94 (95%CI, 0.92-0.97) |
| FAST II | 2021 | Prospective | 334 (334) | AUC = 0.93 (95%CI, 0.90-0.96) |
| FAST Heart Team | 2022 | Retrospective | 1248 (416) | Mismatch between vFFR and revascularization = 29.8% |
| FAST III | Ongoing | Prospective | ||
| Imaging | ||||
| Tomaniak et al. (Left main coronary artery disease) |
2022 | Retrospective | 63 (63) | AUC = 0.95 (95%CI, 0.89-1.0) |
| FAST OCT | Ongoing | Prospective | ||
| Post-PCI setting | ||||
| FAST POST | 2021 | Retrospective | 100 (100) | AUC = 0.98 (95%CI, 0.96-1.0) |
| FAST OUTCOME | 2022 | Retrospective | 832 (748) | vFFR tertiles = TVF 24.6%, 21.5% vs 17.1% |
| STEMI and multivessel disease | ||||
| FAST STEMI II | Ongoing | Prospective | ||
|
95%CI, 95% confidence interval; AUC, area under the curve; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TVF, target vessel failure. |
||||
Figure 1. Clinical application of vessel fractional flow reserve (vFFR). LM, left main; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
First, the evaluation of left main coronary artery (LMCA) lesions remains challenging and often warrants a multimodality approach, including physiological assessment and intravascular imaging. Since patients with LMCA disease are often under-represented in the studies, a dedicated analysis comparing vFFR to intravascular ultrasound in patients with non-ostial LMCA disease was performed. vFFR was shown to correlate well to the LMCA minimum lumen area (MLA) as assessed by intravascular ultrasound (r = 0.79; P = .001) and to have excellent diagnostic accuracy identifying LMCA lesions with MLA < 6.0 mm2 (AUC = 0.95; P = .001).7
Second, the use of physiology in the ACS setting has been topic of disussion as the benefit of physiology-guided-PCI has been mainly demonstrated in patients with stable disease.1 The latter is an important limitation since most patients present with ACS, which in up to 31% of cases occurs in the context of plaque rupture/erosion or calcium nodules located in intermediate coronary artery lesions. Conversely, a thrombotic component was identified in 602/695 of the culprit lesions (87%), which may affect the validity of both the pressure guidewire and the angiography-based-FFR assessments (TACTIS Registry, TCT 2022). In that perspective, the FAST OCT study (NCT04683133) will assess the agreement between vFFR and optical coherence tomography detected causes of luminal obstruction in intermediate lesions of patients presenting with ACS.
Whether the use of vFFR can be extended to patients with ST-segment elevation acute coronary syndrome and multivessel disease will be explored in the ongoing FAST STEMI program.
Next to the potential for online use, the concept of angiography-based-FFR caries significant potential in an offline setting where this technology could be used for clinical decision-making in patients with multivessel disease or those referred for heart team discussion. In a recent retrospective analysis, 3-vessel vFFR screening demonstrated a discordance between lesion significance and revascularization in 30% of the cases.8
Third, post-PCI physiological assessment has gained attention since several studies demonstrated that low post-PCI FFR values are detectable in up to 58% of vessels.9 Although the relevance of low post-PCI FFR was demonstrated by a significantly increased risk for future adverse cardiovascular events, the uptake of post-PCI FFR in the routine clinical practice is still limited.9 Hypothetically, the concept of having a wire-free method to detect suboptimal stent deployment, residual disease, and additional procedural optimization is promising. In the retrospective, single-center FAST POST study, vFFR demonstrated a good correlation with conventional invasive post-PCI FFR (r = 0.88), and a higher accuracy in the identification of patients with FFR values < 0.90 (AUC = 0.98) compared to three-dimensional quantitative coronary angiography (AUC = 0.62).10 In the light of these results, the hypothesis that post-PCI vFFR may predict future adverse cardiac events was proven in the FAST OUTCOME study.11
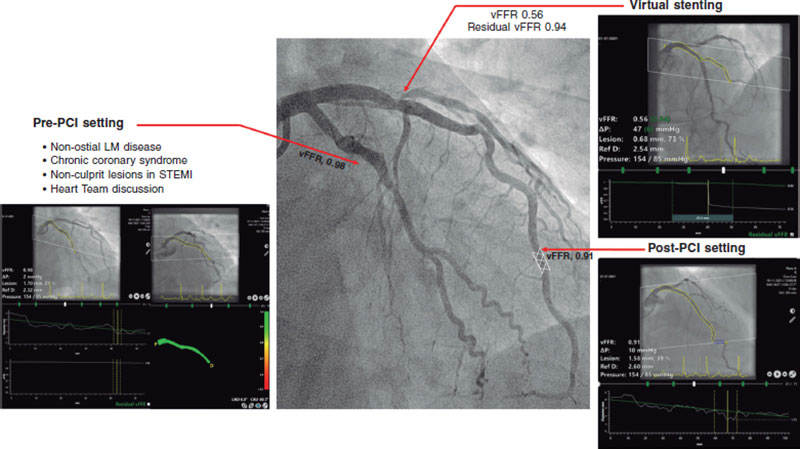
Fourth, the ability to predict functional outcomes of PCI may entail another step forward in the identification of patients who could benefit the most from PCI and thereby avoid the risk of a futile invasive procedure. Recent developments in vFFR software have allowed to simulate the effects of a ‘virtual’ PCI and estimate post-PCI FFR (residual vFFR). Using pre-PCI virtual pullbacks, residual vFFR showed a good correlation with invasive post-PCI FFR and post-PCI vFFR values (r = 0.84, and r = 0.77, respectively), and good discriminative ability to identify post-PCI FFR < 0.90 (AUC = 0.93).12 Of note, the current algorithm assumes an almost perfect PCI result, and thus, cannot account for heavy calcifications or stent underexpansion suggesting a potential need for future hybrid technologies combining multimodality invasive and non-invasive imaging modalities and physiology tools.
Finally, following the positive data from the FAVOR III outcome trial that proved the superiority of quantitative flow ratio (QFR, Pulse Medical Imaging Technology, China) vs angiography-guided-PCI in a Chinese population, the results of, at least, 5 currently ongoing angiography based FFR outcome trials (FAVOR III Europe Japan trial [NCT03729739], PIONEER IV [NCT04923191], FAST III [NCT04931771], LIPSIA STRATEGY [NCT03497637], FLASH FFR II [NCT04575207]) are eagerly awaited and may enhance guideline adoption.2 Specific to vFFR, the ongoing multicenter, randomized FAST III trial will assess whether a vFFR-based diagnostic strategy yields non-inferior clinical outcomes compared to an FFR-based strategy.
Up until the results of these studies will be released, angiography-based-FFR indices, including vFFR, remains an appealing alternative to conventional physiological indices in a broad selection of anatomical and clinical scenarios with the potential to increase the use of physiology and improve patient outcome.
FUNDING
None whatsoever.
AUTHOR’S CONTRIBUTIONS
A. Scoccia contributed to the drafting of this manuscript, and made a critical review of its intellectual content. J. Daemen also contributed to the drafting of this manuscript, made a critical review of its intellectual content, and gave his final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
J. Daemen received institutional grant/research support from Astra Zeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, Microport, Pie Medical, and ReCor medical; and consultancy and speaker fees from Abiomed, ACIST medical, Boston Scientific, ReCor Medical, PulseCath, Pie Medical, Siemens Health Care and Medtronic. A. Scoccia declared no conflicts of interest.
REFERENCES
1. Kogame N, Ono M, Kawashima H, et al. The Impact of Coronary Physiology on Contemporary Clinical Decision Making. JACC Cardiovasc Interv. 2020;13:1617-1638.
2. Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398:2149-2159.
3. Masdjedi K, van Zandvoort LJC, Balbi MM, et al. Validation of a three-dimensional quantitative coronary angiography-based software to calculate fractional flow reserve: the FAST study. EuroIntervention. 2020;16:591-599.
4. Neleman T, Masdjedi K, Van Zandvoort LJC, et al. Extended Validation of Novel 3D Quantitative Coronary Angiography-Based Software to Calculate vFFR: The FAST EXTEND Study. JACC Cardiovasc Imaging. 2021;14:504-506.
5. Masdjedi K, Tanaka N, Van Belle E, et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity: the FAST II study. EuroIntervention. 2022;17:1498-1505.
6. Scoccia A, Neleman T, Kardys I, et al. Reproducibility of 3D vessel Fractional Flow Reserve (vFFR): A core laboratory variability analysis of FAST II study. Cardiovasc Revasc Med. 2022;44:101-102.
7. Tomaniak M, Masdjedi K, van Zandvoort LJ, et al. Correlation between 3D-QCA based FFR and quantitative lumen assessment by IVUS for left main coronary artery stenoses. Catheter Cardiovasc Interv. 2021;97:E495-E501.
8. Tomaniak M, Masdjedi K, Neleman T, et al. Three-dimensional QCA-based vessel fractional flow reserve (vFFR) in Heart Team decision-making: a multicentre, retrospective, cohort study. BMJ Open. 2022;12:e054202.
9. Hwang D, Koo BK, Zhang J, et al. Prognostic Implications of Fractional Flow Reserve After Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5(9):e2232842.
10. Masdjedi K, van Zandvoort LJ, Balbi MM, et al. Validation of novel 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve post stenting. Catheter Cardiovasc Interv. 2021;98:671-677.
11. Neleman T, Scoccia A, Masdjedi K, et al. The prognostic value of angiography-based vessel fractional flow reserve after percutaneous coronary intervention: The FAST Outcome study. Int J Cardiol. 2022;359:14-19.
12. Tomaniak M, Neleman T, Ziedses des Plantes A, et al. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. J Clin Med. 2022;11:1397.