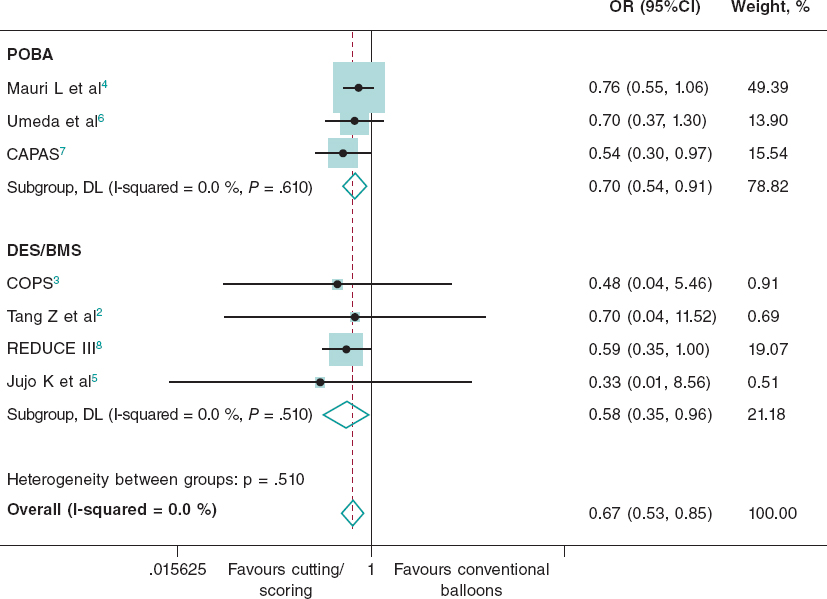
QUESTION: Although we will discuss the aspects of 2 plaque modification techniques, please explain when you resort to intravascular imaging modalities in cases of calcified lesions and how that helps you.
ANSWER: Undoubtedly, intracoronary imaging modalities are an essential tool for interventional cardiologists in dealing with the assessment and treatment of calcified lesions. As we all know, revascularization of these lesions is associated with a higher rate of short- and long-term cardiovascular events, related to a greater risk of stent underexpansion and intraoperative complications.1 In calcified lesions, simple angiographic assessment is insufficient because of its lower sensitivity in the detection of coronary artery calcification, and limitations in the identification of calcium distribution patterns.
In my opinion, since optimizing results is so important, the use of intravascular ultrasound or optical coherence tomography is mandatory in cases of moderate or severe calcification and helps us in several key aspects of the procedure. First, both intravascular ultrasound and optical coherence tomography have high sensitivity and specificity for calcium detection and its morphological characterization: pattern (nodular, parietal), angle, extent, and depth. With this information, we can select the best plaque modification technique for each case and evaluate its effect on the treated lesion. In recent years, several risk scores based on intracoronary imaging modalities have been developed, including decision algorithms for plaque modification systems based on calcium length, depth, and angle.2
Finally, imaging modalities allow us to be precise in selecting the size and length of the stent, as well as to assess its apposition and expansion, and rule out complications and residual disease. This aspect is crucial in the management of calcified lesions, where plaque modification devices can cause deep dissections and fractures, and we encounter more difficulties when trying to achieve adequate stent expansion.
Q.: In your opinion, what are the advantages and disadvantages of intracoronary lithotripsy?
A.: One of the main advantages for the implementation of intracoronary lithotripsy in the daily routine of cath labs is that it is technically simple and reproducible and does not require a long learning curve. The currently available intracoronary lithotripsy (ICL) system—Shockwave Medical, United States—consists of a specific semicompliant rapid-exchange balloon catheter with a 0.042-inch crossing profile, which is advanced inside the coronary arteries through a conventional 0.014-inch guidewire, and is compatible with a 6-Fr guide catheter. Once positioned in the lesion, the balloon is inflated to 4 atm with the sole intention of ensuring good contact between its surface and the vascular wall to facilitate energy transfer. Inside the balloon, there are 2 emitters that receive an electric discharge from the generator, vaporizing the liquid inside and generating sound waves that cause a local effect. The waves run through the soft tissues, causing selective calcium microfractures in the intimal and medial layers of the vascular wall. After pulse emission and the corresponding calcium modification, the balloon is inflated at 6 atm to maximize luminal gain.
On the other hand, compared with the limitations of noncompliant, very high-pressure, or cutting balloons, which in eccentric calcification can be directed toward noncalcified arterial segments with a risk of dissection at the fibrocalcific interface, ICL allows homogeneous calcium fracture. Another advantage is that ICL avoids the bias of having to follow the direction of the guidewire of rotational and orbital atherectomies, because it fractures calcium on superficial and deep layers circumferentially through acoustic pressure waves.3
Regarding complications, calcium fragmentation caused by the lithotripsy balloon remains in place, without distal embolization, thus reducing the incidence of slow-no reflow.4
In terms of disadvantages, the main limitation of ICL is its crossing profile: it often requires lesion predilatation or combination with atherectomy techniques. Notably, the DISRUPT CAD III trial5 reported ventricular captures during ICL pulses in 41.1% of the patients. Although the drop in systolic pressure is more common in patients in whom ICL induces ventricular capture, it has not been associated with the occurrence of adverse events, or sustained ventricular arrhythmias.
Q.: In which cases do you use intracoronary lithotripsy as a first-line approach?
A.: The available evidence on ICL comes from the DISRUPT CAD trials.5-8 The most relevant of these trials, the DISRUPT CAD III,5 is a prospective registry of 431 patients that assessed the safety and efficacy profile of the ICL balloon to treat calcified lesions. The 30-day rate of adverse cardiovascular events (death, myocardial infarction, or target lesion revascularization) was 7.8%, and the effectiveness rate (procedural success with in-stent stenosis < 50%) was 92.4%. This trial included patients with severely calcified de novo lesions and excluded those with acute myocardial infarction and aorto-ostial or bifurcation lesions.
As I mentioned previously, with the data provided by imaging modalities on calcium distribution and depth, we could consider ICL as the first-line approach to treat concentric calcified lesions with circumferential calcium distribution, especially in cases of deep calcium deposits, where ICL has proven more effective than other plaque modification techniques. Furthermore, ICL is effective in large-caliber vessels since balloons can be up to 4 mm in diameter.
One of the most common scenarios in which ICL is used in routine clinical practice is in calcified lesions that cannot be dilated with conventional or high-pressure balloons. This indication accounts for up to 75% of the cases in real-world registries,9 with very good results, and a procedural success rate of 99%.
Q.: Which calcified lesions benefit most from intracoronary lithotripsy compared with rotational or orbital atherectomy?
A.: While we can’t draw direct comparisons on the safety and efficacy results between ICL and rotational or orbital atherectomy because of the different inclusion criteria, stent types, and study endpoints among trials such as ROTAXUS10 and DISRUPT-CAD, in clinical practice, we choose one technique over the other based on the characteristics of the lesion.
Although, as I will discuss later, both techniques are complementary, atherectomy is an excellent option to treat balloon-uncrossable calcified lesions. However, atherectomy targets superficial calcium shaving, less so the deep calcium deposits. Hence, ICL is a better choice for concentric calcified lesions with circumferential and deep calcium distribution.
Beyond the landmark studies, in recent years, numerous real-world experiences11 have been reported, demonstrating the usefulness of ICL in specific and complex scenarios, such as:
- – Calcified bifurcation lesions: information on the safety and efficacy profile of ICL in complex contexts is limited to case reports and short series of patients describing experiences in substrates such as bifurcation or aorto-ostial lesions with promising results. Unlike rotational or orbital atherectomy techniques, ICL is increasingly used because it allows us to work with 2 different guidewires easily and simplifies the procedure in this context.
- – In-stent stenosis: Although this is an off-label use of ICL, there is growing evidence of the usefulness of ICL in both acute stent underexpansion and restenosis, especially in nondilatable lesions due to calcified neoatherosclerosis.12 In the Spanish multicenter REPLICA registry13 of 426 patients treated with ICL in routine clinical practice, a previously implanted stent was stenosed in 23% of the cases.
- – Chronic occlusions: ICL can be useful to treat chronic occlusions with severe calcification, and its use has increased in recent years, as confirmed by a recently published subanalysis of the PROGRESS-CTO registry14 with data from 82 patients (out of a total of 3301 included in the study [2.5%]) who underwent ICL. Indications were severe vessel calcification, or balloon nondilatable lesions. Technical success was achieved in 94% of the patients and procedural success in 90%.
- – Acute coronary syndrome: available data on the use of ICL in calcified lesions in patients with acute coronary syndrome are scarce. These cases were excluded from the DISRUPT-CAD trials, and again, the experience reported in the medical literature is limited to short case series. However, as the REPLICA registry results show, where a high percentage of patients with calcified lesions treated with ICL presented with acute coronary syndrome (62.8%), this technique is commonly used in the routine clinical practice in this group of patients who require a quick and safe technique.
Q.: How do you integrate the 2 techniques into your protocol to treat calcified lesions?
A.: The combined use of the ICL balloon and other plaque modification techniques, such as rotational15 or orbital atherectomy,16 has shown promising results in short patient series, and seems to be a highly attractive strategy when the target lesion cannot be reached with the ICL balloon.
In my opinion, the combination of atherectomy and ICL techniques is a suitable option to treat diffuse, superficial, and deep calcium deposits. By combining the 2 techniques, we can leverage the advantages of each. On the one hand, atherectomy allows the advancement of the ICL balloon in long lesions with severe stenosis that prevent its passage. On the other, ICL is very useful in balloon nondilatable lesions after atherectomy. This combination of techniques can be particularly useful in one of the most complex scenarios: the management of calcium nodules.
FUNDING
None declared.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used in the preparation of this article.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Généreux P, Redfors B, Witzembichler B, et al. Two year outcomes after percutaneous coronary intervention of calcified lesions with drug eluting stents. Int J Cardiol. 2017;231:61-67.
2. Fujino A, Mintz G, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
3. Jurado-Román A, Gómez-Menchero A, Gonzalo N, et al. Documento de posicionamiento de la ACI-SEC sobre la modificación de la placa en el tratamiento de las lesiones calcificadas. REC Interv Cardiol. 2023;5:43-61.
4. Rodriguez Costoya I, Tizón Marcos H, Vaquerizo Montilla B, et al. Coronary Lithoplasty:Initial Experience in Coronary Calcified Lesions. Rev Esp Cardiol. 2019;72:788-790.
5. Hill J, Kereiakes DJ, Shlofmitz RA, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol. 2020;76:2635-2646.
6. Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139:834-836.
7. Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses:the Disrupt CAD II study. Circ Cardiovasc Interv. 2019;12:e008434.
8. Saito S, Yamazaki S, Takahashi A, et al. Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement —primary outcomes from de Japanese Disrupt CAD IV Study. Circ J. 2022;85:826-833.
9. Azir A, Bhatia G, Pitt M, et al. Intravascular lithotripsy in calcified-coronary lesions:A real-world observational, European multicenter study. Catheter Cardiovasc Interv. 2021;98:225-235.
10. Abdel-Wahab M, Richardt G, Joachim Buttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions:the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19.
11. Vilalta V, Rodriguez-Leor O, Redondo A, et al. Litotricia coronaria en pacientes de la vida real:primera experiencia en lesiones complejas y gravemente calcificadas. REC Interv Cardiol. 2020;2:76-81.
12. Tovar N, Sardella G, Salvi N, et al. Coronary litotripsy for the treatment of underexpanded stents:CRUNCH registry. Eurointervention. 2022;18:574.8112.
13. Rodriguez-Leor O, Cid-Alvarez AB, Lopez-Benito M, et al. A Prospective, Multicenter, Real-World Registry of Coronary Lithotripsy in Calcified Coronary Arteries: The REPLICA-EPIC18 Study. JACC Cardiovasc Interv.. 2024:76(12):1021-1031.
14. Kostantinis S, Simsek B, Karaksonyi J, et al. Intravascular lithotripsy in chronic total occlusion percutaneous coronary intervention:Insights from the PROGRESS?CTO registry. Catheter Cardiovasc Interv. 2022;100:512-519.
15. Gonzalvez-Garcia A, Jimenez-Valero S, Galeote G, et al. “RotaTripsy”:combination of rotational atherectomy and intravascular lithotripsy in heavily calcified coronary lesions:a case series. Cardiovasc Revasc Med. 2022;35:179-184.
16. Yarusi BB, Jagadeesan VS, Hussain S, et al. Combined coronary orbital atherectomy and intravascular lithotripsy for the treatment of severely calcified coronary stenoses:the first case series. J Invasive Cardiol. 2022;34:E210-E217.