ABSTRACT
Introduction and objectives: Moderate or severe paravalvular leak (PVL) following transcatheter aortic valve implantation (TAVI) has been associated with worse outcomes. Aortic valve (AV) calcification is a strong predictor of PVL. ACURATE neo (Boston Scientific Corporation, United States) is a self-expanding transcatheter heart valve to treat degenerative aortic stenosis. We evaluated PVL after ACURATE neo and neo2 implantation, and the role of AV calcification.
Methods: We analyzed patients referred for TAVI with ACURATE neo and neo2 from a large volume tertiary center. All cardiac computed tomography scans were analyzed using 3Mensio Structural Heart software (Pie Medical Imaging, The Netherlands). The volume of AV calcium was quantified using contrast-enhanced cardiac computed tomography series. The 30-day clinical and echocardiographic data were prospectively recorded.
Results: We included 165 patients referred for TAVI with ACURATE (neo = 87; neo2 = 78). Median age was 82 years-old, 65% were women with a median EuroSCORE II of 4.7 [IQR, 2.4-6.1]. Patients in the neo group showed a larger amount of total AV calcium (320 mm3 vs 200 mm3; P = .0305). We found no significant inter-group differences regarding clinical outcomes both in-hospital or at 30-days. At 30-days, the rate of PVL ≥ mild (61% vs 34%; P < .001) and ≥ moderate (15.9% vs 5.4%; P = .0365) were higher in the neo group. After propensity score matching adjusted by the total amount of AV calcium, neo2 was associated with a lower risk of PVL ≥ mild (OR, 0.35, 95%CI, 0.18-0.69; P = .003), and ≥ moderate (OR, 0.16; 95%CI, 0.03-0.74; P = .019).
Conclusions: TAVI with ACURATE neo2 vs neo is associated with a lower risk of any degree of PVL and a reduced risk of PVL ≥ moderate. After adjusting for AV calcium volume, ACURATE neo2 was still associated with a lower risk of PVL.
Keywords: Transcatheter aortic valve implantation Transcatheter heart valve Paravalvular leak
RESUMEN
Introducción y objetivos: La fuga paravalvular (FPV) moderada o grave tras el implante percutáneo de válvula aórtica (TAVI) se ha asociado a peores resultados. La calcificación de la válvula aórtica constituye un importante factor predictivo de FPV. ACURATE neo (Boston Scientific Corporation, Estados Unidos) es una válvula cardiaca transcatéter autoexpandible para el tratamiento de la estenosis aórtica degenerativa. Se evaluó la presencia de FPV tras el implante de ACURATE neo y neo2, así como el papel de la calcificación de la válvula aórtica.
Métodos: Se analizaron pacientes intervenidos de TAVI con ACURATE neo y neo2 de un hospital terciario de alto volumen. Todas las tomografías computarizadas cardiacas se analizaron con el software 3Mensio Structural Heart (Pie Medical Imaging, Países Bajos). El volumen de calcio aórtico se cuantificó mediante tomografía computarizada cardiaca con contraste. Se registró prospectivamente la evolución clínica y ecocardiográfica a 30 días.
Resultados: Se incluyeron 165 pacientes intervenidos de TAVI con ACURATE (neo = 87; neo2 = 78). La mediana de edad fue de 82 años, el 65% eran mujeres y la mediana de EuroSCORE II fue de 4,7 [rango intercuartílico, 2,4-6,1]. Los pacientes del grupo con neo presentaban una mayor cantidad de calcio total aórtico (320 frente a 200 mm3; p = 0,0305). No se hallaron diferencias significativas entre los grupos en cuanto a los resultados clínicos tanto durante el ingreso como a los 30 días. A los 30 días, la tasa de FPV ≥ leve (61 frente a 34%; p < 0,001) y de FPV ≥ moderada (15,9 frente a 5,4%, p = 0,0365) fue más alta en el grupo con neo. Tras el emparejamiento por puntuación de propensión ajustado por la cantidad total de calcio aórtico, neo2 se relacionó con un menor riesgo de FPV ≥ leve (odds ratio [OR] = 0,35; intervalo de confianza del 95% [IC95%], 0,18-0,69; p = 0,003) y de FPV ≥ moderada (OR = 0,16; IC95%, 0,03-0,74; p = 0,019).
Conclusiones: El TAVI con ACURATE neo2, en comparación con neo, se asocia a un menor riesgo de cualquier grado de FPV y a un menor riesgo de FPV ≥ moderada. Tras el ajuste por volumen de calcio aórtico, ACURATE neo2 se asocia a un menor riesgo de FPV.
Palabras clave: Implante percutáneo de válvula aórtica Válvula cardiaca transcatéter Fuga paravalvular
Abbreviations
AV: aortic valve. CCT: cardiac computed tomography scan. LVOT: left ventricular outflow tract. PVL: paravalvular leak. TAVI: transcatheter aortic valve implantation. THV: transcatheter heart valve.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has become an increasingly popular therapeutic option in patients with degenerative severe aortic stenosis in the entire spectrum of estimated procedure-related risks.1,2 Compared to surgical aortic valve replacement, transcatheter heart valves (THV) have to deal with a higher risk of paravalvular leak (PVL), a matter of especial concern in younger and low-risk patients referred for aortic replacement.3 In addition, the presence of residual moderate or severe PVL following TAVI has been associated with an increased risk of short- and long-term mortality.4,5 The prognostic role of mild PVL is still controversial, still some studies have also shown worse outcomes in patients with mild compared with none or trace PVL.5 Former studies have identified the presence and distribution of calcium in the aortic valve (AV) as a predictor of residual PVL after TAVI.6,7 In addition, left ventricular outflow tract (LVOT) calcification has also been associated with a higher risk of residual PVL.8 ACURATE neo (Boston Scientific Corporation, United States) is a self-expanding THV indicated for the treatment of AS. However, early data with ACURATE neo showed a higher rate of moderate or severe PVL compared to other self-expanding THV.9 Its last iteration, the ACURATE neo2, added an inner and outer pericardial skirt, potentially capable of reducing the rate of PVL.
The aim of this study was to compare the rate of PVL at 30-days after ACURATE neo and neo2 THV implantation, and analyze the role of aortic calcium volume in the development of significant PVL after ACURATE neo and neo2 THV implantation.
METHODS
We selected patients from our prospective TAVI registry referred for TAVI with ACURATE neo and neo2 in our center, a large volume tertiary hospital and reference center for structural heart procedures. In all cases, the decision to perform TAVI over surgical replacement was agreed by the heart team based on the patients’ characteristics, comorbidities, and estimated risk scores. Per site protocol, 30-day follow-up clinical visits, and follow-up transthoracic echocardiograms were scheduled in all the patients. Due to the COVID-19 outbreak in the Netherlands that started back in March 2020 some of the 30-day follow-up visits were performed via telephone call. All transthoracic echocardiograms were performed by an experienced imaging cardiologist in THV evaluation following current clinical guidelines on the evaluation of PVL after TAVI.10 Consequently, PVL was classified as none/trace, mild, moderate, and severe following the Valve Academic Research Consortium definitions regarding AV clinical research (VARC-3) recommendations.10 Data on baseline admission, demographics, clinical, and procedural characteristics, in-hospital events, and 30-day follow-up were prospectively collected and managed using REDCap (Research Electronic Data Capture), a secure, web-based software platform designed to support data capture for research studies. The study received the approval of the local research ethics committee (St. Antonius Hospital, Nieuwegein, The Netherlands). Informed consent for this study was waived due to its retrospective and observational design.
Event definitions
For both in-hospital and 30-day adverse events, the last published VARC-3 definitions regarding AV clinical research were followed.10
Cardiac computed tomography calcium analysis
The 3Mensio Structural Heart software version 10.3 (Pie Medical Imaging, Bilthoven, The Netherlands) was used for all cardiac computed tomography (CCT) analyses. All CCT scans were independently analysed by 1 operator experienced in THV sizing and specific training in 3Mensio software. In the early cases, our CCT protocol for TAVI evaluation did not include non-contrast-enhanced series. Therefore, non-contrast-enhanced series were unavailable for a significant number of patients. Thus, we decided to evaluate calcium volume using contrast-enhanced series. We followed the method recently described by Angelillis et al. that showed a good correlation with Agatston score as measured in non-contrast-enhanced scans.11 In short, this method for calcium volume estimation modifies the Hounsfield Units threshold depending on the average found in the LVOT. In highly contrasted LVOT (> 300 HU) the threshold used was 850. Regarding low contrasted LVOT (< 300 HU), the threshold was set at 450. After the aortic annulus identification (baseline plane), the ´calcium scoring tool´ included in the software was used to estimate the aortic volume. The aortic box displayed a 15 mm length from the baseline plane to the aortic root. All calcium on the aortic wall and/or the coronary arteries was carefully identified and excluded from the volume estimate. The estimate of LVOT calcium volume was performed too. For this, a 10 mm-long box was included from the baseline plane to the LVOT. All calcium associated with the anterior mitral leaflet was identified and excluded from the estimated volume. To analyze the impact of the valve size, and the prosthesis to annular size, the cover index was calculated (100 × [valve diameter - CCT perimeter-derived annulus diameter in systole]/valve diameter) following the previous description established by Kim et al.12
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median (interquartile range). The Kolmogorov-Smirnov test was used to evaluate the adjustment to normality. Categorical variables are expressed as numbers (percentage). The Student t test or the Mann–Whitney U test were used to compare continuous variables. Pearson’s chi-square test was used for categorical variables. Predictors of binary PVL were analyzed using logistic regression. Test for a trend across a categorial variable was performed using the nptrend command (STATA). Two propensity score matching models (1:1 matching) were estimated, the first model adjusted for the total amount of AV calcium according to the CCT, and the second one for the total amount of LVOT calcium according to the CCT too. A 2-tailed P value of < .05 was considered statistically significant. All tests were performed with STATA 12 (StataCorp LLC, United States).
RESULTS
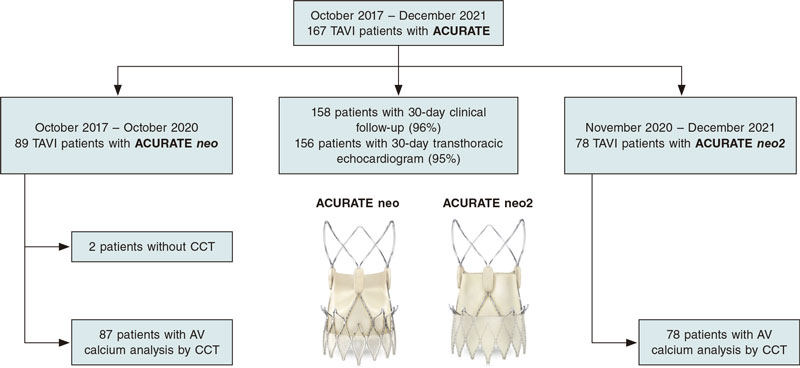
A total of 167 patients were referred for TAVI with ACURATE at our center from October 2017 through December 2021. Of those, a total of 89 patients were referred for TAVI with an ACURATE neo from October 2017 through October 2020. A total of 78 patients were referred for TAVI with ACURATE neo2 from November 2020 through December 2021. In 2 patients from the ACURATE neo group, preoperative CCT information was not available and both patients were eventually excluded from the analysis. A 30-day clinical follow-up was available in 158 patients (96%), information regarding the presence of PVL at 30-days by transthoracic echocardiogram was available in 156 patients (95%). The study flowchart is shown on figure 1.
Figure 1. Study flow-chart. AV, aortic valve; CCT, cardiac computed tomography; TAVI, transcatheter aortic valve implantation. Images provided courtesy of Boston Scientific. Reproduced with permission from Boston Scientific Corporation or its affiliates.
The cohort baseline characteristics are shown on table 1. We found no differences in the distribution of age, sex, and cardiovascular risk factors between the groups (median age, 82 years-old; 65%, female). There were not significant inter-group differences regarding risk estimate with EuroSCORE II (4.2 vs 4.8; P = .5371). A third of the patients had atrial fibrillation, and a fourth had been treated with previous percutaneous coronary intervention, and 9% with previous coronary artery bypass graft with no inter-group differences. Almost 2 thirds of the patients had functional New York Heart Association class III-IV before intervention while 17% showed systolic dysfunction (left ventricular ejection fraction < 50%) by transthoracic echocardiogram.
Table 1. Baseline characteristics
| Global cohort N = 165 | ACURATE neo N = 87 | ACURATE neo2 N = 78 | P | |
|---|---|---|---|---|
| Age, years | 82 [79-85] | 82 [79-85] | 82 [79-85] | .7536 |
| Sex (female) | 107 (65) | 57 (66) | 50 (64) | .8493 |
| Hypertension | 107 (65) | 61 (70) | 46 (59) | .1345 |
| Diabetes mellitus | 43 (26) | 20 (23) | 23 (30) | .3173 |
| COPD | 43 (26) | 13 (15) | 11 (14) | .8786 |
| CKD | 47 (28) | 26 (30) | 21 (27) | .6739 |
| Peripheral artery disease | 20 (12) | 9 (10) | 11 (14) | .4603 |
| Porcelain aorta | 5 (3) | 1 (1) | 4 (5) | .1402 |
| EuroSCORE II | 4.7 [2.4-6.1] | 4.2 [2.2-6.1] | 4.8 [2.7-6.5] | .5371 |
| Previous AF | 60 (36) | 33 (38) | 27 (35) | .6585 |
| Previous TIA/stroke | 10 (6) | 4 (5) | 6 (8) | .4056 |
| Previous PCI | 45 (27) | 25 (29) | 20 (26) | .6559 |
| Previous CABG | 15 (9) | 7 (8) | 8 (10) | .6219 |
| Height, cm | 167 ± 9 | 167 ± 9 | 167 ± 9 | .6656 |
| Weight, kg | 76 ± 13 | 75 ± 14 | 77 ± 13 | .3275 |
| BMI | 27 ± 5 | 27 ± 5 | 27 ± 5 | .4786 |
| Angina | 19 (12) | 9 (10) | 10 (13) | .6189 |
| NYHA class III-IV | 105 (64) | 56 (64) | 49 (63) | .8366 |
| Systolic disfunction (LVEF < 50%) | 28 (17) | 15 (17) | 13 (17) | .9218 |
|
AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischemic attack. |
||||
Main data of the CCT analysis is shown on table 2. We found no differences in the aortic annulus mean diameter, area or perimeter between the groups. Regarding calcium analysis, patients from the neo group showed a larger amount of estimated total calcium in the AV (320 mm3 vs 200 mm3; P = .0305) driven by differences in the amount of calcium in the non-coronary and left coronary cups (154 mm3 vs 98 mm3; P = .0149; 86 mm3 vs 37 mm3; P = .0335, respectively). We found no inter-group differences in the quantification of calcium in the LVOT. However, the presence of any amount of calcium in the LVOT was more frequently seen in the neo group (61% vs 41%; P = .0107). No differences were reported in the cover index between groups with a median of 6.8% [IQR, 4.4-8.5] oversize in the overall cohort.
Table 2. Computed tomography analysis
| Global cohort N = 165 | ACURATE neo N = 87 | ACURATE neo2 N = 78 | P | |
|---|---|---|---|---|
| CCT annular sizing | ||||
| Minimum aortic annulus diameter | 21 ± 2 | 20 ± 2 | 21 ± 2 | .0757 |
| Maximum aortic annulus diameter | 26 ± 2 | 26 ± 2 | 27 ± 2 | .7989 |
| Mean aortic annulus diameter | 24 ± 1 | 23 ± 1 | 24 ± 1 | .2337 |
| Aortic annulus area | 427 ± 51 | 421 ± 50 | 434 ± 51 | .1134 |
| Aortic annulus perimeter | 74 ± 4 | 74 ± 4 | 75 ± 4 | .1159 |
| Calcium quantification | ||||
| Total calcium volume at the AV, mm3 | 266 [100-566] | 320 [123-696] | 200 [90-377] | .0305 |
| Calcium volume at the NCC-AV, mm3 | 125 [50-275] | 154 [68-305] | 98 [32-203] | .0149 |
| Calcium volume at the RCC-AV, mm3 | 62 [19-150] | 81 [25-193] | 51 [12-126] | .2122 |
| Calcium volume at the LCC-AV, mm3 | 56 [20-148] | 86 [24-194] | 37 [14-96] | .0335 |
| Total calcium volume at the LVOT, mm3 | 1 [0-29] | 4 [0-34] | 0 [0-22] | .2897 |
| Calcium volume at the NCC-LVOT, mm3 | 0 [0-5] | 0 [0-6] | 0 [0-0] | .5474 |
| Calcium volume at the RCC-AV, mm3 | 0 [0-0] | 0 [0-0] | 0 [0-0] | .8615 |
| Calcium volume at the LCC-AV, mm3 | 0 [0-3] | 0 [0-7] | 0 [0-0] | .3521 |
| Any calcium at the LVOT | 85 (52) | 53 (61) | 32 (41) | .0107 |
| Cover index (oversizing) % | 6.8 [4.4-8.5] | 6.8 [3.7-8.4] | 6.9 [5.2-9] | .1769 |
|
AV, aortic valve; LCC, left coronary cusp; LVOT, left ventricle outflow tract; NCC, non-coronary cusp; RCC, right coronary cusp. |
||||
Data on procedural characteristics and in-hospital evolution are shown on table 3. All cases were performed via transfemoral access. The first 8 cases from the neo group were performed under general anaesthesia while the remaining cases from both groups were performed under local anaesthesia. There was a non-significant trend towards a lower use of the small 23 mm ACURATE in the neo2 cohort (P = .0523). Predilatation was more common in the neo2 group (99% vs 92%; P = .0434), and postdilatation in the neo group (43% vs 22%; P = .0046). We found no differences in the presence of residual PVL ≥ moderate on angiography immediately after implantation (7% vs 5%; P = .6346). Regarding in-hospital outcomes, only 1 patient died at the index admission due to sepsis 59 days after TAVI. A total of 3 patients suffered a stroke without inter-group differences being reported. There was a non-significant trend towards a higher bleeding risk in the neo group (18% vs 12%; P = .2203) without any differences being reported in the rates of major or life-threatening bleeding. The rate of permanent pacemaker implantation was 7.3% without any inter-group differences. Median length of stay was 2 days [IQR, 2-4]. Half of the patients were discharged on aspirin with patients in the neo group being more frequently treated with dual antiplatelet therapy at discharge (41% vs 10%; P < .001). A total of 40% of the cohort were discharged with oral anticoagulation.
Table 3. Procedural characteristics, in-hospital evolution, and 30-day follow-up
| Global cohort N = 165 | ACURATE neo N = 87 | ACURATE neo2 N = 78 | P | |
|---|---|---|---|---|
| Type of anesthesia | .0060 | |||
| General anesthesia | 8 (5) | 8 (9) | 0 | |
| Local anesthesia | 157 (95) | 79 (91) | 78 (100) | |
| ACURATE valve size | .0523 | |||
| 23 mm (small) | 35 (21) | 24 (28) | 11 (14) | |
| 25 mm (medium) | 78 (47) | 39 (44) | 39 (50) | |
| 27 mm (large) | 52 (32) | 24 (28) | 28 (36) | |
| Predilatation | 157 (95) | 80 (92) | 77 (99) | .0434 |
| Postdilatation | 54 (33) | 37 (43) | 17 (22) | .0046 |
| Residual PVL (angio) ≥ moderate | 10 (6) | 6 (7) | 4 (5) | .6346 |
| In-hospital outcomes | ||||
| Death | 1 (0.6) | 0 | 1 (1.3) | .2894 |
| Myocardial infarction | 0 | 0 | 0 | |
| Stroke | 3 (1.8) | 1 (1.2) | 2 (2.6) | .4971 |
| Any bleeding | 25 (15) | 16 (18) | 9 (12) | .2203 |
| Major or life-threatening bleeding | 4 (2.4) | 2 (2.3) | 2 (2.6) | .8634 |
| New LBBB | 23 (14) | 13 (15) | 10 (13) | .2172 |
| New PPM | 12 (7.3) | 6 (6.9) | 6 (7.7) | .8442 |
| Length of stay, days | 2 [2-4] | 2 [2-4] | 2 [2-3] | .5119 |
| Treatment at discharge | ||||
| Aspirin | 87 (53) | 46 (53) | 41 (53) | .9683 |
| P2Y12 inhibitors | 73 (44) | 55 (63) | 18 (23) | < .001 |
| DAPT | 44 (27) | 36 (41) | 8 (10) | < .001 |
| Oral anticoagulation | 66 (40) | 36 (41) | 30 (38) | .7025 |
| 30-day follow-up (N = 158) | ||||
| Death | 4 (2.5) | 1 (1.2) | 3 (4) | .2700 |
| Myocardial infarction | 0 | 0 | 0 | |
| Stroke | 7 (4.4) | 2 (2.4) | 5 (6.7) | .1941 |
| Any bleeding | 27 (17) | 18 (22) | 9 (12) | .0986 |
| Mean AV gradient | 8 ± 3 | 7 ± 3 | 8 ± 4 | .3160 |
| Mean AV gradient ≥ 20 mmHg | 1 (0.6) | 0 | 1 (1.4) | .2909 |
| Peak AV gradient | 15 ± 7 | 14 ± 6 | 15 ± 7 | .3365 |
| Peak AV velocity ≥ 3 m/s | 3 (2) | 1 (1.2) | 2 (2.7) | .5006 |
| PVL ≥ mild | 75/156 (48) | 50 (61) | 25 (34) | .0007 |
| PVL ≥ moderate | 17/156 (10.9) | 13 (15.9) | 4 (5.4) | .0365 |
|
AV, aortic valve; DAPT, dual antiplatelet therapy; LBBB, left bundle branch block; PPM, permanent pacemaker implantation; PVL, paravalvular leak. |
||||
Information on 30-day follow-up is also shown on table 3. One-month clinical follow-up information was available in 158 patients. We found no differences in the rates of death, myocardial infarction, and stroke between the groups. However, there was a trend towards a higher bleeding risk in patients from the neo group (22% vs 12%; P = .0986). Regarding the follow-up echocardiogram, we found no differences in mean and peak AV gradients between the groups (mean, 7 ± 3 mmHg vs 8 ± 4 mmHg; P = .3160; peak 14 ± 6 mmHg vs 15 ± 7 mmHg; P = .3365) with good performance in both groups regarding aortic gradients. The rate of PVL ≥ mild at 30 days was significantly higher in the neo vs neo2 group (61% vs 34%; P < .001). Also, the rate of PVL ≥ moderate was also significantly higher in the neo group (15.9% vs 5.4%; P = .0365) compared to the last THV generation.
A crude analysis showed a significant univariate relationship between neo2 vs neo with a lower rate of both PVL ≥ mild (OR, 0.33; 95%CI, 0.17-0.63; P = .001) and ≥ moderate (OR, 0.30; 95%CI, 0.10-0.98; P = .045) at 30-day follow-up. A trend analysis showed a significant correlation between the AV calcium quartile and the presence of PVL ≥ mild (Q1 37%, Q2 38%, Q3 54%, Q4 63%; P-value for trend = .012). This analysis failed to show any significant correlations between the AV calcium quartile and PVL ≥ moderate (P = .125). However, Q4 showed a significantly higher rate of PVL ≥ moderate on binary comparison (Q4 vs Q1-3, 20% vs 7.8%; P = .0322). A trend analysis showed a no significant correlation between the cover index and the presence of PVL ≥ mild (P = .961) or ≥ moderate (P = .596). Figure 2 illustrates a case of moderate PVL in a severely calcified AV after ACURATE neo implantation.
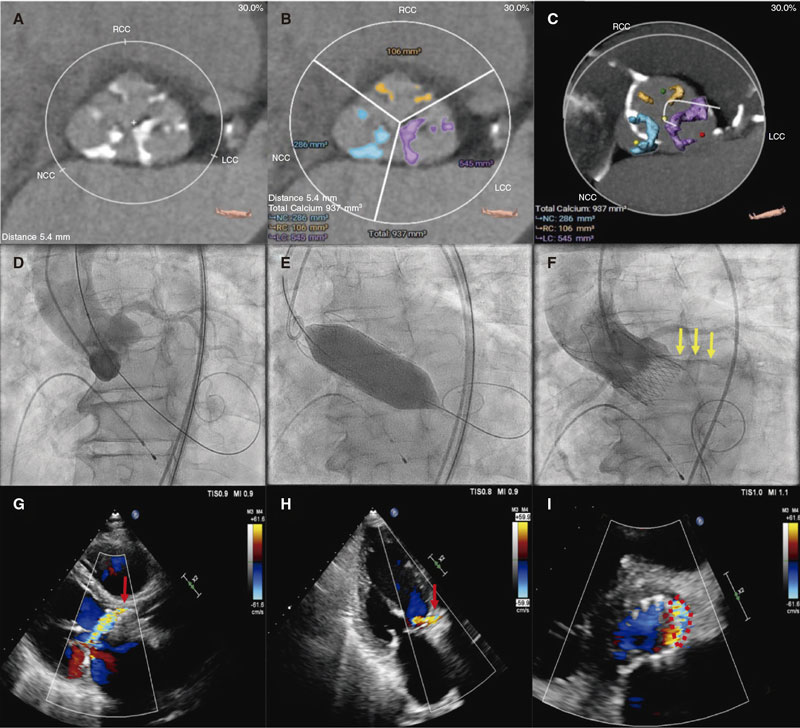
Figure 2. A: 79-year-old man with severe aortic stenosis and New York Heart Association functional class III. Cardiac computed tomography (CCT) shows calcification of aortic valve (AV) leaflets (mostly affecting the left and non-coronary cusps). B-C: the CCT AV calcium estimate using the 3Mensio software showed a total calcium volume of 937 mm3 (quartile Q4). D: conventional 3-cusp coplanar implantation view. After predilatation with a 24 mm non-compliant balloon (not recorded), a large 27 mm ACURATE neo device was successfully implanted. E: transcatheter heart valve was postdilated with a 25 mm non-compliant balloon. F: nonetheless, the final angiogram revealed the presence of mild-to-moderate paravalvular leak (PVL) associated with the left coronary cusp (yellow arrows). G-I: 30-day transthoracic echocardiogram confirmed the presence of a moderate PVL with a jet coming from the area of the native left coronary cusp (red arrows and red dotted line). Videos of both aortography and follow-up echocardiogram have been included as supplementary data.
After propensity score matching adjusted for the total amount of AV calcium (71 pairs), the use of neo2 vs neo was associated with a lower risk of PVL ≥ mild (OR, 0.35; 95%CI, 0.18-0.69; P = .003) and ≥ moderate (OR, 0.16; 95%CI, 0.03-0.74; P = .019) at follow-up. An alternative propensity score matching model adjusted for the total amount of LVOT calcium was also used (71 pairs). In this model, the use of neo2 vs neo was also associated with a lower risk of PVL ≥ mild (OR, 0.39; 95%CI, 0.20-0.78; P = .008) and showed a non-significant trend towards a lower risk of PVL ≥ moderate at follow-up (OR, 0.33; 95%CI, 0.10-1.08; P = .066).
DISCUSSION
Our study analyzed consecutive cases referred for TAVI with ACURATE THV technology in a large volume tertiary center. The main findings of our study are a) the rate of PVL ≥ moderate was dramatically reduced with the use of the ACURATE neo2 compared to its former version; and b) this reduced residual PVL seen with the ACURATE neo2 was not determined by differences in the degree of calcification in the AV.
The ACURATE neo THV received the CE marking back in 2014 for the management of degenerative severe aortic stenosis. Despite early registries showed a good performance of this technology, clinical trials that compared it to other balloon-expandable and self-expandable THVs showed worse results. In the SCOPE-I trial, TAVI with ACURATE neo did not meet non-inferiority criteria compared to the SAPIEN 3 (Edwards Lifesciences, Irvine, CA, United States) with differences mainly driven by a higher rate of moderate or severe PVL with ACURATE (9% vs 3%).13 In the SCOPE-2 trial, TAVI with ACURATE neo failed to reach the non-inferiority margin compared to the CoreValve Evolut THV (Medtronic, United States) regarding mortality or stroke at 12 months. In this study, once again, the use of ACURATE was associated with a higher rate of moderate or severe PVL compared to the Evolut THV (10% vs 3%).9 This data lead to the development of a new iteration of the device, the ACURATE neo2 including a 60% larger outer sealing skirt to minimize PVL.
Data comparing both ACURATE technologies are still scarce. Rück et al. reported comparative data on PVL with neo and neo2 THV.14 The authors retrospectively analyzed the presence of PVL by angiography in 228 patients treated with ACURATE (neo = 108, neo2 = 120) from 2 European centers. In this study, ACURATE neo2 showed a lower rate of moderate or severe PVL (1.7% vs 13.9%). However, in this study, the authors do not provide any information regarding AV calcification. In addition, the 30-day echocardiographic data is not available. Recently, Scotti et al. reported data from the NEOPRO and NEOPRO-2 multicenter retrospective registries (n = 2026) on performance and outcomes of both ACURATE technologies.15 The authors found that the presence of moderate or severe pre-discharge PVL was lower in the neo2 group (2% vs 5%). Furthermore, this reduced PVL was mainly seen in patients with heavy AV calcification. However, this study has some inherent limitations as only pre-discharge echocardiographic data were reported (not at 30-day), and AV calcification was assessed using a scoring system that relies on visual estimation. Therefore, our study adds additional valuable information on the role of AV calcification and risk of PVL with ACURATE THV technology. In our study, we found that, compared to neo, the neo2 TVH was associated with a lower risk of any degree of PVL and a decreased risk of PVL ≥ moderate. Furthermore, we found a significant linear trend between AV calcium volume quartiles and the risk of any degree of PVL. Moreover, propensity score matching analysis allowed us to ascertain that the lower risk of PVL seen with neo2 was independent of the AV calcium estimate.
However, we also have some additional findings that are worth discussing. We found a trend towards a higher rate of bleeding with neo compared to neo2, both in-hospital and at 30-day follow-up. This can be potentially explained by baseline differences in the antithrombotic treatment between both groups. Patients from the neo cohort were more commonly treated with P2Y12 inhibitors and dual antiplatelet therapy. Nevertheless, current evidence, adopted for most of our neo2 cases, has demonstrated a higher bleeding risk associated with dual antiplatelet therapy compared to aspirin alone in patients referred for TAVI.16-18 Our data also seem to support these findings. Alternatively, acquired von Willebrand syndrome associated with aortic stenosis can persist after TAVI in patients with significant residual PVL.19 The higher rate of PVL associated with neo compared to neo2 may explain, by itself, our finding of a trend towards a higher risk of bleeding in the neo cohort.
We are aware that the learning curve seen with the ACURATE device may have altered our results. We found a higher AV calcium volume estimate in neo vs neo2 cases. Our early experience with the neo device may lead interventional cardiologists involved in the heart team to be more prone to selecting a different THV (with a lower estimated risk of PVL) in patients with a high degree of AV calcification. Also, despite being high in both groups (92% and 99%, respectively), the rate of predilatation was significantly higher in the neo2 group, even when this group showed a lower amount of estimated AV calcium, which confirms the impact the learning curve has on device implantation. We should mention that this procedural step can be of special relevance to avoid residual PVL, especially in a THV as the ACURATE one that has a lower radial force. However, the benefits seen with neo2 reducing the risk of any degree of PVL, even after adjusting for AV calcification, seem to exceed what could be justified by these 2 potential biases. The different postdilatation rates reported may be explained by the fact that operators more commonly identified and attempted to correct an angiographically significant PVL immediately after THV implantation in the neo vs neo2 group.
Limitations
Among the limitations of this study are those regarding its non-randomized observational design. The current study is limited by its small sample size and for being a single-center experience. A strict methodology for CCT calcium volume estimate was followed, and clinical and echocardiographic information was prospectively recorded. However, the degree of PVL at 30-day follow-up and adverse outcomes were not independently assigned but based on clinical reports. Current standards to estimate AV calcium volume are based on the analysis of non-contrast-enhanced CCT. However, we applied a method for calcium volume estimate based on contrast enhanced CTT as this was the only method that allowed us to analyze our oldest cases since, at that time, non-contrast-enhanced series were not part of our pre-TAVI CCT protocol. Finally, our report is limited by a short-term follow-up. Longer clinical follow-up is needed to assess clinical outcomes, and the prognostic implication of residual PVL.
Impact on the routine daily practice
With data from our real-world registry, compared to its former iteration, TAVI with ACURATE neo2 is associated with a lower risk of residual PVL. These results were not affected by differences in the total amount of calcium in the AV detected by CCT.
CONCLUSIONS
Compared to ACURATE neo, TAVI with ACURATE neo2 THV was associated with a lower risk of any degree of PVL, as well as a lower risk of PVL ≥ moderate. After adjusting for AV calcium volume, ACURATE neo2 was still associated with a lower risk of PVL compared to ACURATE neo THV.
FUNDING
This study was partially funded by an Education and Training Grant from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology (ESC) granted to the lead author (M. García-Guimarães).
AUTHORS’ CONTRIBUTIONS
M. García-Guimarães, M.J. Swaans, and L. Timmers contributed to the study idea and design. M. García-Guimaraes acquired data, performed the analysis, and drafted the manuscript. D.-J. Van Ginkel contributed to data acquisition. B.J. Rensing, J.M. Ten Berg, U. Sonker, T.L. de Kroon, and R.H. Heijmen made a critical revision of its intellectual content. All authors give their final approval to this version for publication.
CONFLICTS OF INTEREST
L. Timmers is a proctor and a member of Boston Scientific advisory board. M.J. Swaans received lecturer fees from Boston Scientific. The remaining authors did not declare any conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Presence of significant PVL following TAVI is associated with worse clinical outcomes. Aortic valve calcification is a strong predictor of PVL after TAVI. ACURATE neo is a self-expanding transcatheter heart valve for the management of degenerative aortic stenosis. Its last iteration, the ACURATE neo2, aims to reduce the incidence of residual PVL.
WHAT DOES THIS STUDY ADD?
- In our study with data from a real-world TAVI registry, compared to its former ACURATE neo, ACURATE neo2 was associated with a lower risk of residual PVL. Also, these results were not affected by differences in the total amount of calcium detected in the aortic valve as evaluated by cardiac computed tomography.
SUPPLEMENTARY DATA
Vídeo 1. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 2. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 3. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 4. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 5. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 6. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 7. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
Vídeo 8. García-Guimarães M. DOI: 10.24875/RECICE.M23000369
REFERENCES
1. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632.
2. Writing Committee Members, Otto CM, Nishimura RA, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450-500.
3. Koshy AN, Murphy A, Farouque O, Horrigan M, Yudi MB. Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. Hear Lung Circ. 2020;29:1527-1533.
4. Sinning JM, Vasa-Nicotera M, Chin D, et al. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:11-20.
5. Arnold S V., Zhang Y, Baron SJ, et al. Impact of Short-Term Complications on Mortality and Quality of Life After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:362-369.
6. Wiktorowicz A, Wit A, Malinowski KP, et al. Paravalvular leak prediction after transcatheter aortic valve replacement with self-expandable prosthesis based on quantitative aortic calcification analysis. Quant Imaging Med Surg. 2021;11:652-664.
7. Ludwig S, Goßling A, Waldschmidt L, et al. TAVR for low-flow, low-gradient aortic stenosis: Prognostic impact of aortic valve calcification. Am Heart J. 2020;225:138-148.
8. Okuno T, Asami M, Heg D, et al. Impact of Left Ventricular Outflow Tract Calcification on Procedural Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:1789-1799.
9. Tamburino C, Bleiziffer S, Thiele H, et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients with Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial. Circulation. 2020;142:2431-2442.
10. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77:2717-2746.
11. Angelillis M, Costa G, De Backer O, et al. Threshold for calcium volume evaluation in patients with aortic valve stenosis: correlation with Agatston score. J Cardiovasc Med (Hagerstown). 2021;22:496-502.
12. Kim WK, Möllmann H, Liebetrau C, et al. The ACURATE neo Transcatheter Heart Valve: A Comprehensive Analysis of Predictors of Procedural Outcome. JACC Cardiovasc Interv. 2018;11:1721-1729.
13. Lanz J, Kim WK, Walther T, et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet. 2019;394:1619-1628.
14. Rück A, Kim WK, Kawashima H, et al. Paravalvular aortic regurgitation severity assessed by quantitative aortography: Acurate neo2 versus acurate neo transcatheter aortic valve implantation. J Clin Med. 2021;10:4627.
15. Scotti A, Pagnesi M, Kim W-K, et al. Haemodynamic performance and clinical outcomes of transcatheter aortic valve replacement with the self-expanding ACURATE neo2. EuroIntervention. 2022;18:804-811.
16. Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;383):1447-1457.
17. Capodanno D, Collet JP, Dangas G, et al. Antithrombotic Therapy After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2021;14:1688-1703.
18. Nijenhuis VJ, Brouwer J, Delewi R, et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N Engl J Med. 2020;382:1696-1707.
19. Kibler M, Marchandot B, Messas N, et al. CT-ADP Point-of-Care Assay Predicts 30-Day Paravalvular Aortic Regurgitation and Bleeding Events following Transcatheter Aortic Valve Replacement. Thromb Haemost. 2018;118:893-905.