Abstract
Calcified coronary artery disease poses a number of challenges to the interventional cardiologist when performing percutaneous coronary interventions, and patients with calcified coronary artery disease continue to have poorer outcomes at both the short and the long-term follow up. Stent underexpansion is the most feared outcome when performing percutaneous coronary interventions in these patients and is a strong predictor of stent failure. Therefore, intracoronary imaging to guide calcium modification is an important step in the treatment of this disease. The following review outlines a stepwise approach using intracoronary imaging in the assessment of coronary calcification, and in the selection of the appropriate calcium modification tool. Additionally, we describe current calcium modification techniques available, the evidence behind their use, their mechanism of action, and the typical results seen on intracoronary imaging.
Keywords: Coronary calcium. Calcium modification. Atherectomy. Lithotripsy. Optical coherence tomography. Intravascular ultrasound.
RESUMEN
Las intervenciones coronarias percutáneas en enfermedad arterial coronaria calcificada representan un desafío para el cardiólogo intervencionista. Además, los pacientes con enfermedad arterial coronaria calcificada tienden a tener peores resultados en el seguimiento a corto y largo plazo. La infraexpansión del stent es el resultado más temido cuando se realiza una intervención coronaria percutánea en estos pacientes y es un gran predictor de falla del stent. Por lo tanto, la modificación del calcio guiada por imágenes intracoronarias, es un paso importante en el tratamiento de esta enfermedad. La siguiente revisión describe el uso «paso a paso» de imágenes intracoronarias en la evaluación de la calcificación coronaria y en la selección de una técnica de modificación de calcio adecuada. Además, se describen las técnicas actuales de modificación de calcio disponibles, la evidencia para su uso, su mecanismo de acción y los resultados típicos que se observan en las imágenes intracoronarias.
Palabras clave: Calcificación coronaria. Modificación de placa calcificada. Aterectomía. Litoplastia. Tomografía coherencia óptica. Ecografía intravascular.
Abbreviations CAD: coronary artery disease. IVI: intravascular imaging. IVL: intravascular lithotripsy. IVUS: intravascular ultrasound. OA: orbital atherectomy. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. RA: rotational atherectomy.
INTRODUCTION
Calcified coronary stenosis is a relatively common finding present in up to 30% of lesions planned for percutaneous coronary intervention (PCI).1 Calcified atherosclerosis presents a number of difficulties when performing PCI especially stent underexpansion, a strong predictor of stent failure (thrombosis and restenosis).2-4 It comes as no surprise, then, that worse clinical outcomes have been found following PCI in moderate-to-severe calcified disease compared to atherosclerotic plaques without calcium.1 A number of plaque modification techniques are available although there is a paucity of head-to-head comparisons among the techniques making device selection difficult. Understanding calcium morphology can contribute to proper device or technique selection, and is best guided by intravascular imaging (IVI). In this review, we outline the assessment of coronary calcium using IVI, propose a simplified calcium modification algorithm we use at our center, and examine the mechanism of action and evidence behind the use of each of these techniques.
Pathophysiology and prognostic implications of coronary calcium
The pathophysiology of atherosclerosis is well documented and starts with injury to the vessel and accumulation of low density lipoprotein which undergoes oxidative changes that result in the release of proinflammatory cytokines. These attract monocytes that migrate towards the intima layer, mature into macrophages, and eventually form foam cells.5 Further recruitment of smooth muscle cells from the media layer produce extracellular matrix that leads to intimal thickening and plaque progression. In time, and in the presence of risk factors including age, male sex, Caucasian race, hypertension, hyperlipidemia, diabetes, and chronic kidney disease, calcification of atherosclerotic plaques can occur and its pathogenesis has much in common with bone formation.1,5-8 Transformation of vascular smooth muscle cells into an osteoblastic phenotype is thought to be the initiation factor prompted by exposure to bone morphogenetic protein-2 (BMP 2) produced by endothelial cells when exposed to stressors like hypoxia, high pressure, turbulent flow, and inflammation.9 The result is the loss of expression of vascular smooth muscle specific markers, and the expression of genes typically found in bone generating cells.10 Other pathways also play a role including apoptosis of vascular smooth muscle cells, and formation of calcifying matrix vesicles by macrophages.6 The early result is the deposition of microcalcifications that eventually coalesce into larger calcium deposits that can be seen as “spotty calcification” on IVI. Further progression ultimately results in calcium sheets or plates which can extend across multiple quadrants of the vessel causing vessel stiffening and altering compliance.11 Nodular calcification, an important morphological subtype which protrudes into the vessel lumen, forms when there is rupture of the calcium sheets.6 Prognostically, the presence of calcified atherosclerosis is associated with poorer cardiovascular outcomes.12,13 Initial spotty calcification represents an unstable period in the evolution of calcified coronary artery disease (CAD), and these lesions are more commonly associated with plaque rupture and acute coronary syndrome.6,14 Conversely, lesions with a higher percentage calcified plaque volume as seen on computed tomography coronary angiography are more stable and present less frequently with acute cardiovascular events, yet more commonly with chronic coronary syndromes and multivessel disease.6,15
Percutaneous coronary intervention in calcified atherosclerosis
Calcific stenoses are found in up to 30% of all patients presenting for PCI.1 The subsequent reduction of coronary artery compliance presents a number of procedural difficulties. Inadequate lesion dilation can potentially result in stent underexpansion,16 one of the most important predictors of stent failure.2-4 Other difficulties include a higher risk of dissection and perforation, difficulty passing equipment distally, damage to the stent polymer, altered drug elution kinetics from stents, and potentially stent deformation or loss.1,17,18 Furthermore, patients with coronary artery calcification are less likely to undergo complete revascularization and more frequently experience adverse outcomes following PCI. In a pooled analysis of the HORIZONS-AMI and ACUITY studies, the presence of moderate or severe calcification (as assessed angiographically) was associated with poorer outcomes at 1 year for all endpoints including death, cardiac death, myocardial infarction, and overall major adverse cardiovascular events.1 As a matter of fact, at 1 year, the risk of stent thrombosis increased by 62% and that of ischaemic target lesion revascularization (TLR) increased by 44% in calcified compared to non-calcified lesions. These findings have been replicated across numerous other studies at both short and long-term follow-up.1,7,19-21 In a recent analysis of the SYNTAXES trial, heavily calcified lesions were associated with a higher all-cause mortality rate after 10 years regardless of the type of revascularization used (hazard ratio, 1.79; 95% confidence interval, 1.49-2.16; P < .001).21 Optimizing the results of PCI is, therefore, of paramount importance with plaque preparation with calcium modification is an important step in this process.
Imaging for calcium detection
Detecting the presence of coronary calcium prior to PCI is important for procedural planning and a number of imaging techniques may be used as shown on table 1.14,15,22-28
Table 1. Summary of available imaging techniques for the detection of coronary calcium
| Imaging modality | Quantification | Sensitivity | Specificity | Advantages | Disadvantages |
|---|---|---|---|---|---|
|
Computed tomography14,15,22,23  |
• Calcium scoring on non-contrast images • Percentage calcified plaque |
++++ |
++++ |
• Non-invasive • Calcium scoring provides prognostic information • Highlights the presence of calcium prior to undertaking an invasive procedure • Provides some information on the plaque morphology and composition (specific software available) • Percentage calcified plaque is a predictor of future events |
• Blooming artifact can overestimate the degree of calcification • Circumferential arc difficult to assess • Radiation exposure • Contrast use • Does not provide intraprocedural guidance |
 |
• Mild: not visible • Moderate: radiopacities seen only with cardiac motion • Severe: radiopacities seen without cardiac motion, before contrast injection affecting both sides of the arterial wall (tram-track appearance) |
++ +++ in the presence of severe calcification |
+++ |
• Assessment of anatomical complexity, vessel tortuosity, side branch angulation |
• Invasive • No information on calcium morphology (thickness, circumferential arc) |
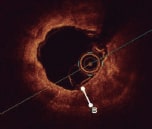 |
• Calcium thickness • Calcium circumferential arc • Calcium length |
++++ |
++++ |
• High resolution, 10 µm to 20 µm • Detailed calcium morphological assessment ○ Distribution/arc ○ Depth ○ Volume ○ Length ○ Presence of calcium nodules • Procedural guidance ○ Landing zones ○ Vessel dimensions ○ Lesion length ○ Stent length ○ Guide stent optimization ○ Assess stent expansion ○ Identify complications (dissection, under-expansion, malapposition, stent distortion) • Co-registration with angiography available |
• Invasive • Requires a blood-free environment for image acquisition • Contrast required for blood clearance • Limited assessment of ostial lesions • Difficult to advance the catheter distally in tortuous vessels |
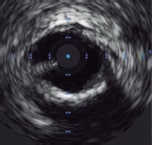 |
• Calcium arc • Calcium length |
++++ |
++++ |
• Moderate-high resolution 100 µm to 150 µm (high-resolution IVUS 20 µm to 30 µm) • High penetration depth into non-calcific vessel wall ~10 mm • No specific imaging requirements • Can assess ostial lesions • Morphological assessment of calcium ○ Distribution/arc ○ Length ○ Presence of calcium nodules • Procedural guidance ○ Landing zones ○ Vessel dimensions ○ Lesion length ○ Stent length ○ Guide stent optimization ○ Assess stent expansion ○ Identify complications (dissection, underexpansion, malapposition, stent distortion) • Co-registration with angiography available |
• Invasive • Acoustic shadowing in severe calcification • Difficult to assess calcium thickness ○ Use of surrogate markers of thickness (reverberations) |
|
IVUS, intravascular ultrasound; OCT, optical computed tomography. |
|||||
Non-invasive imaging for coronary calcification
Coronary computed tomography angiography is highly sensitive and specific for the detection of calcium and is a non-invasive technique. Coronary computed tomography angiography can determine plaque morphology and percentage of calcified plaque volume, which has prognostic significance.15 Its utility in procedural planning is increasingly seen in the planning of chronic total coronary occlusions, but it is less useful in the specifics of guiding intraprocedural strategy.
Invasive imaging for coronary calcification
Invasive coronary angiography has long been known to have low sensitivity but high specificity for the detection of coronary calcium. Compared to intravascular ultrasound (IVUS), its overall sensitivity is ~48%, but it increase up to > 85% in the presence of severe (4 quadrant) calcification.24,25 Nonetheless, an arc > 100° as seen on the IVI is required before calcium can be reliably detected on angiography, thus highlighting the potential for calcium to go undetected when the PCI is guided by angiography alone.25 Calcification on angiography is typically classified as none/mild, moderate or severe (table 1). Although angiography provides valuable information to guide the procedure such as vessel tortuosity, angulation of bifurcations, etc, its limitations are well documented, and studies have consistently shown poorer outcomes when PCI is guided by angiography compared to IVI.29-31
IVI overcomes much of the shortfalls of other imaging modalities. Both optical coherence tomography (OCT) and IVUS are more sensitive for the detection of calcium compared to coronary angiography.25 Furthermore, both imaging modalities provide additional information to guide and optimize the procedure (table 1).27 Co-registration with angiography is available for both modalities, and can reduce the learning curve significantly.32 Although the advantages of IVI over angiography have been shown in a number of studies, no randomized studies have specifically examined its potential benefits regarding calcified CAD. Nonetheless, given the complexity of these lesions, performing IVI-guided PCIs seems reasonable.
Intravascular ultrasound
IVUS has both high sensitivity and specificity (86.7% and 93.3%, respectively compared to histological samples) for the detection of dense calcification, although it is less sensitive for the detection of microcalcifications,33 and in the presence of overlying fibrotic plaque.34 Calcium reflects ultrasound resulting in a bright hyperechoic signal with significant posterior shadowing that often precludes the assessment of calcium thickness (figure 1).35 Surrogate markers for calcium thickness can be used such as the presence of posterior reverberations (correlated with thinner calcium < 0.5 mm) while significant shadowing is suggestive of thicker calcification ( > 1 mm).25 Recently, an IVUS specific scoring system has been found to be useful in predicting stent underexpansion using 4 criteria: calcium arc > 270° for a length of ≥ 5 mm, presence of 360° calcium, presence of calcified nodules, and adjacent vessel diameter of < 3.5 mm. Scores ≥ 2 suggest that calcium modification should be undertaken and therefore operators should aim to measure each of these parameters on IVUS pullbacks.36
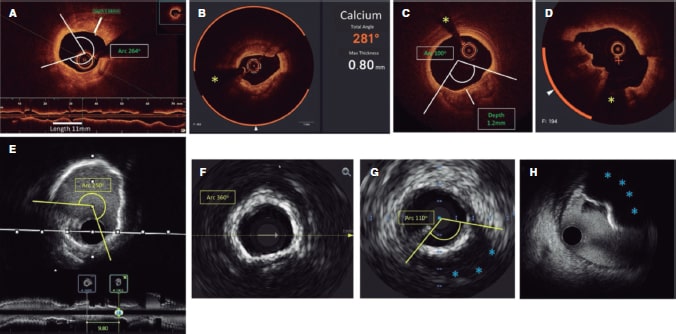
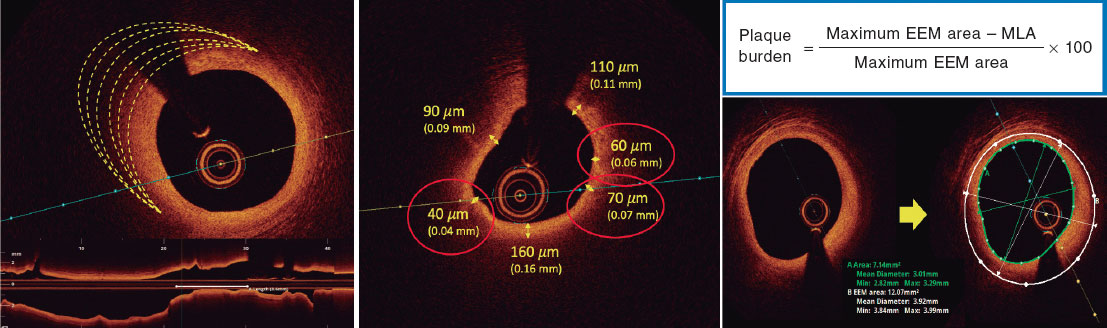
Figure 1. Calcium morphology and measurement using intracoronary imaging. A: concentric calcification on optical coherence tomography (OCT); calcium arc of 264°, depth of 0.68 mm, and length of 11 mm - high risk features by OCT for stent underexpansion and plaque preparation is advised. B: concentric calcification; arc 281°, and depth of 0.8 mm. Automatic calcium detection using Ultreon software; degrees of calcium detected outlined by the orange arc surrounding the OCT image. C: eccentric calcium on the OCT; arc < 180 degrees. Note the sharply demarcated borders of calcium that allow the assessment of calcium depth (1.2 mm) D: calcified nodule on the OCT. Significant posterior shadowing is caused by the nodule precluding the assessment of its posterior border. E: concentric calcification on IVUS with an arc of 250° and a length of 9.8 mm. Posterior shadowing and lack of reverberations suggests thick calcium (~1 mm). These features represent a high risk of stent underexpansion. F: concentric calcification on intravascular ultrasound (IVUS) with an arc of 360°. G: eccentric calcification on IVUS with an arc of < 180°. Significant posterior shadowing (blue asterisk). H: calcified nodule on IVUS protruding into the lumen and casting significant posterior acoustic shadowing (blue asterisk). The yellow asterisk (in all OCT images) denotes wire artefact.
Optical coherence tomography
Although significantly more sensitive than angiography OCT is less sensitive compared to the IVUS at detecting coronary calcium. Wang et al. found that ~6% of lesions with IVUS detectable calcium did not show visible calcium on OCT, which was mainly attributed to overlying fibrotic plaque.25 On the OCT, calcium appears as a region of low signal intensity with sharply demarcated borders that facilitate the assessment of calcium depth.26 Fujino et al. demonstrated that calcium arc > 180°, depth > 0.5 mm, and length > 5 mm on the OCT were associated with a higher risk of stent underexpansion and—similar to IVUS—operators should try to analyse each of these parameters.37 Recently, artificial intelligence software has become available (Ultreon OCT system, Abbott, United States), which automatically identifies calcium arc and depth, as well as the external elastic lamina for vessel sizing further simplifying this analysis (figure 1).
In practical terms, therefore, it may be useful to assess the extent of coronary calcification on IVI by considering calcium arc, depth, length, and whether it is superficial or deep as shown on figure 1. Considering the circumferential arc, coronary calcium can be divided into 3 morphological subtypes (figure 1). Eccentric, extending across 2 or less quadrants with an arck < 180°, and concentric, with an arc > 180° and nodular calcification presenting as an eruptive protrusion into the lumen. Calcium can also be divided into superficial (located at < 50% of the depth of the plaque plus media thickness) or deep (located at > 50% of the depth of the plaque plus media thickness).28 Calcium length should be measured on the longitudinal projection on both IVUS and OCT.
Calcium modification
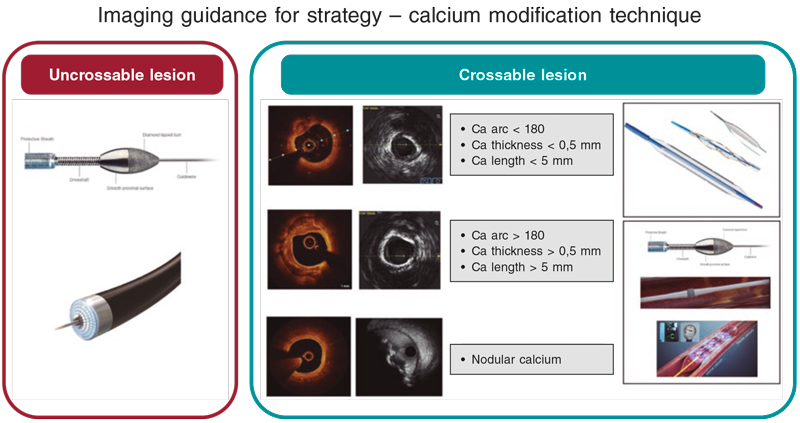
Although there is a lack of clinical trials comparing modification techniques in varying calcium morphologies, consensus in this regard suggests that balloon-based therapies may be effective in eccentric calcification, which is short in length. Ablative and lithotripsy-based therapies may be more useful in concentric calcification or long calcified lesions with lithotripsy being particularly useful in deeper calcium deposits. Nodular calcification presents the greatest challenge; however many advocate for the use of ablative techniques, and some recently presented data suggests lithotripsy may have a role.38 Uncrossable and undilatable lesions may be treated with rotational atherectomy (RA) or excimer laser coronary angioplasty (ELCA). While acknowledging the paucity of data and the lack of head-to-head trials comparing the different techniques available, we have tried to summarize this practice, and the practice at our center, into a simplified calcium modification algorithm that can provide some guidance (figure 2). table 2 summarizes the mechanism of action and specifications for these techniques. The expected results following calcium modification are shown on figure 3.
Table 2. Calcium modification tools: description, mechanism of action, and specifications
| Cutting balloons | Scoring balloons | High-pressure noncompliant NC balloons | Rotational atherectomy | Orbital atherectomy | Excimer LASER | Lithotripsy | |
|---|---|---|---|---|---|---|---|
|
Technology description |
Balloon platform with a number of microblades |
Several nitinol wires wrapped around a semi- or noncompliant balloon |
Double layered noncompliant balloon |
Diamond coated burr capable of atherectomy in a forward motion |
Eccentrically mounted diamond coated crown capable of atherectomy in a forward and a backward motion |
Concentric or eccentric array of laser fibers. Uses a mixture of rare gas and halogen to generate brief pulses of high-frequency, short wavelength UV light |
Series of emitters encased within a balloon delivery system |
|
Mechanism of action |
Controlled incisions into calcium |
Controlled incisions into calcium |
Super high-pressure dilation with a rated burst pressure of 35 atm (often dilated at ~50 atm) |
High speed burr rotation (140-160 000 rpm) results in differential atherectomy of fibrocalcific tissue Additional effect due to burr vibration (+) |
Centrifugal force causes the crown to orbit at high speeds (80 or 120 000 rpm) resulting in calcium sanding Additional effect due to crown vibration (+++) |
Disrupts plaque through 3 mechanisms Photochemical: by breaking carbon bonds between molecules Photothermal: by the production of thermal energy and vapour bubbles Photomechanical: by the expansion of vapour bubbles causing plaque disruption The light energy (fluence) used ranges between 30 mL/mm2 and 80 mL/mm2 Pulse repetition rate is between 25 Hz and 80 Hz |
Emitters generate sparks creating a vapour bubble that expands and propagates an acoustic wave through the vessel wall. Causes compressive and decompressive forces when calcium is found resulting in fracture |
|
Sizes available |
A number of brands available in sizes ranging from 2.0 mm to 4.0 mm |
A number of brands available in sizes that range from 1.75 mm to 4.0 mm |
1.5 mm to 4.5 mm balloons |
1.25, 1.5, 1.75, 2.0, 2.15, 2.25, 2.38, 2.5 mm burr |
1.25 mm crown |
0.9 mm, 1.4 mm, 1.7 mm, and 2.0 mm |
2.5 mm, 3.0 mm, 3.5 mm, and 4.0 mm diameters All sizes are 12 mm in length |
|
Guide catheter compatibility |
6-Fr |
Some balloon sizes are compatible with 5-Fr and 6-Fr systems |
6-Fr |
6-Fr; 1.25 & 1.5 mm 7-Fr; 1.75 mm 8-Fr; 2.0, 2.15 mm 9-Fr; 2.25, 2.38 mm 10-Fr; 2.50 mm |
6-Fr |
6-Fr: 0.9 & 1.4 mm 7-Fr: 1.7 mm 8-Fr: 2.0 mm |
6-Fr |
|
Wire compatibility |
Conventional 0.014 in guidewires |
Conventional 0.014 in guidewires |
Conventional 0.014 in guidewires |
Specialized 0.009 or 0.014 in wire required |
Specialized 0.012 or 0.014 in wire required Viper wire |
Conventional 0.014 in guidewires |
Conventional 0.014 in guidewires |
|
Other caveats |
1:1 balloon: vessel sizing Rotating the balloon followed by repeat inflation can increase the number of incisions |
1:1 balloon: vessel sizing |
1:1 balloon: vessel sizing |
Burr-to-artery ratio of 0.5 to 0.6 Lubricant available but not mandatory and contraindicated in egg and olive oil allergies |
Specific lubricant required which is contraindicated in egg and soy allergies |
Catheter-to-artery ratio of 0.5 to 0.6 Requires continuous infusion of saline through the guide catheter Contrast infusion increases effectiveness but can also increase the risk of thermal damage |
1:1 balloon: vessel sizing Rigorous balloon preparation to remove all air May require de-airing while being used |
|
Advantages |
Easy to use Compatible with conventional guidewires |
Easy to use Compatible with conventional guidewires |
Easy to use Compatible with conventional guidewires |
Useful in undilatable lesions May be more useful for nodular calcium than other technologies |
Useful in undilatable lesions May be more useful for nodular calcium than other technologies Can ablate in both a forward and a backward motion Produces smaller particles than rotational atherectomy |
Easy to use Compatible with conventional guidewires |
Easy to use Compatible with conventional guidewires Modifies superficial and deep calcification No particulate matter created so lower risk of slow flow or no-reflow |
|
Disadvantages |
May not be sufficient as monotherapy Bulky profile |
May not be sufficient as monotherapy Bulky profile |
Bulky profile |
Specialized wire required Wire bias may result in differential atherectomy. Ablation in a forward motion only Cannot maintain a wire in a side branch during atherectomy. Produces larger particles compared to orbital atherectomy Distal embolization can result in slow flow or no-reflow |
Specialized wire required Specialized lubrication infusion required Cannot maintain a wire in a side branch during atherectomy. Distal embolization can result in slow flow or no-reflow |
Set up time Additional UV protection required |
Bulky profile for lesion crossing 80 pulses per catheter may require the use of > 1 catheter to treat long lesions |
|
Potential complications |
Perforation Dissection Slow flow/no reflow |
Perforation Dissection Slow flow/no reflow |
Perforation Dissection Slow flow/no reflow |
Perforation Dissection Burr entrapment Wire fracture Slow flow/no reflow Transient heart block |
Perforation Dissection Crown entrapment Slow flow/no reflow |
Perforation Dissection Thermal injury |
Perforation Dissection |
|
Fr, French; Hz, Hertz; in, inches; NC, noncompliant; rpm, revolutions per minute; UV, ultraviolet. |
|||||||
Figure 2. Calcium modification algorithm. Intravascular imaging (IVI) for lesion assessment is advised prior to undertaking plaque modification. Uncrossable lesions usually require rotational atherectomy or excimer laser coronary angioplasty (ELCA). Crossable lesions with eccentric calcification without high-risk features for stent underexpansion can be treated using noncompliant, cutting or scoring balloons. Concentric calcification or calcium with high-risk features for stent underexpansion can be treated with atherectomy techniques or intravascular lithotripsy (IVL). Nodular calcium can be modified using atherectomy techniques with emerging evidence that IVL may also be effective. Post plaque modification IVI is key for proper plaque modification assessment. Ca, calcium.
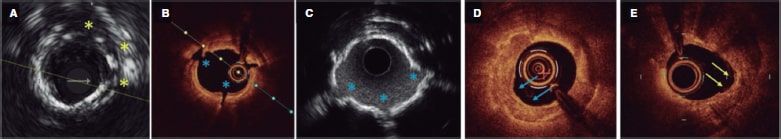
Figure 3. Calcium morphologies and results of different modification techniques on intravascular imaging. A: discrete calcium incisions and fracture following cutting balloon (yellow asterisk). B, C: calcium fractures following intravascular lithotripsy on optical coherence tomography and intravascular ultrasound (IVUS) (blue asterisk). Note how reverberations can be seen at fracture points (blue asterisk) on the IVUS due to acoustic waves now being able to pass through the fracture sites. D: results of calcium modification using rotational atherectomy in an uncrossable lesion. A “cored out” appearance can be seen with widening of the lumen and a semilunar appearance in some regions (blue arrow). E: results of calcium modification following orbital atherectomy. The semilunar shape of the orbital atherectomy crown can be seen at the yellow arrows.
Eccentric calcification therapies
Specialized balloon-based technologies
Specialized balloon-based technologies are most commonly used for eccentric calcification although they have some utility in concentric calcification in combination with other techniques. Cutting balloons consist of a number of microblades mounted on a balloon, while scoring balloons consist of a semi-compliant balloon around which several nitinol wires are wrapped. Both aim to make incisions into the calcium to facilitate vessel dilation. The advantage of these technologies is that they anchor to the calcium and are less likely to slip (watermelon seeding phenomenon) thus avoiding dissection of adjacent areas. Although sometimes used interchangeably, a study conducted by Matsukawa et al. using IVI demonstrated better calcium modification and increased luminal gain with cutting balloons vs scoring balloons.39 However, regarding severe calcification, cutting balloons have lower rates of procedural success compared to RA.40 Combining cutting balloons with other technologies may be useful. Observational studies have demonstrated increased luminal gain with cutting balloons following RA compared to conventional balloons or RA alone.41,42
Very high-pressure balloons may be effective to cause calcium fracture in both eccentric and concentric calcification. They are generally not first-line therapies and are most often used in undilatable lesions. They consist of a noncompliant twin-layered balloon with rated burst pressure of ~35 atm. However, in practice they are often dilated at ~50 atm. In a retrospective series of 326 consecutive undilatable lesions, Secco et al. reported angiographic success in > 90% using the OPN high-pressure balloon (OPN NC; SIS Medical AG, Switzerland).43 Calcific lesions with calcium arcs > 270° were more likely to require pressures > 40 atm. More recently, the ISAR-CALC trial randomized lesions with residual stenosis > 30% following standard balloons to receive a scoring balloon or a super high-pressure balloon.44 No differences on OCT defined stent expansion index between groups were found (0.72 vs 0.68; P = .22) nor were there differences in angiographic, procedural, or strategy success. Patients in the super high-pressure balloon group, however, less frequently required further dilation with NC balloons prior to stenting, had larger angiographically assessed minimal lumen diameters, and less residual stenosis compared to those in the scoring balloon cohort. Therefore, super high-pressure balloons play a role in the management of undilatable, but crossable lesions.
Concentric and nodular calcification
Lithotripsy
Intravascular lithotripsy (IVL) (SHOCKWAVE Medical inc, United States) is a recently introduced technique based on the use of acoustic energy. It consists of a balloon-based delivery system containing a number of emitters that generate short electric sparks. The sparks produce a vapour bubble in the fluid inside the balloon that is dilated to 4 atm. The vapour bubble expands creating an acoustic pressure wave that propagates through the vessel wall causing compression and decompression stress when calcium is encountered resulting in fracture.45 Each short-lived pulse delivers an equivalent of ~50 atm of pressure. Nonrandomized studies to date have demonstrated significant fissuring of both superficial and deep calcium on IVI (figure 3). A pooled analysis of the DISRUPT CAD series of studies has demonstrated procedural success (residual angiographic stenosis ≤ 30%) in > 90% of the lesions.46 Although to date, IVL has been predominantly used in concentric calcium, analysis of angiographically defined eccentric vs concentric calcification suggests similar success in these 2 calcium morphologies.47 Also, recently presented data suggests no differences in minimal stent area on OCT when IVL was used to treat eccentric, concentric, and nodular calcium.38 Although still an off-label indication, a number of cases and series have reported on the use of IVL to treat stent underexpansion due to severe calcification, and calcified neoatherosclerosis.48-51 The use of IVL in a newly deployed but underexpanded stent has not been widely reported and there are theoretical concerns regarding damage to the polymer. Our practice to date has been to use IVL predominantly in concentric calcification while further data is awaited. figure 4 shows a case of plaque modification using OCT-guided IVL.
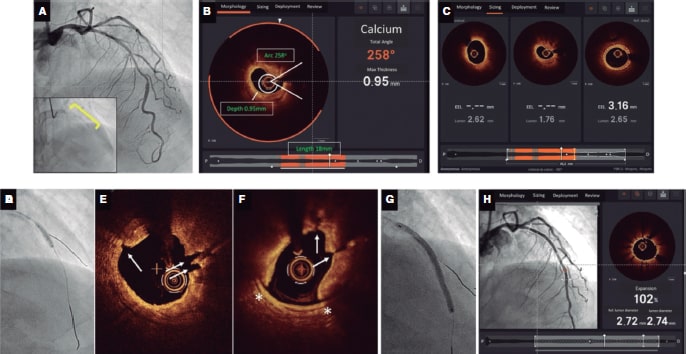
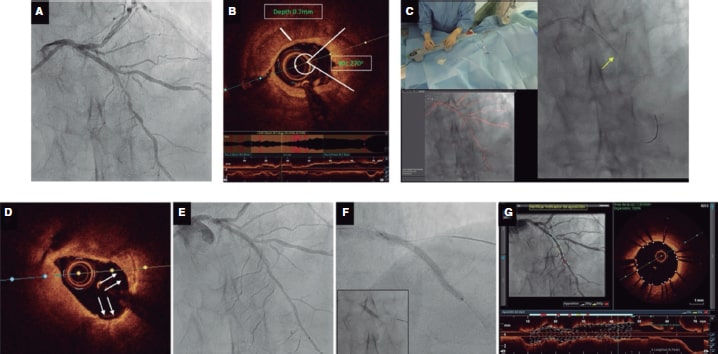
Figure 4. Case example demonstrating calcium modification using intravascular lithotripsy (IVL) guidance with co-registered optical coherence tomography (OCT) imaging using Ultreon software. A: severely calcified left anterior descending coronary artery stenosis with calcium visible on fluoroscopy (inset), B: OCT revealing concentric calcium; arc 258o and depth of 0.95 mm as identified automatically using the Ultreon software with a length of 18 mm. These parameters suggest a high risk of stent underexpansion. C: proximal and distal landing zones and length of stent required. The distal landing zone external elastic lamina to external elastic lamina (dashed white lines automatically detected by Ultreon system) measures 3.16 mm while, proximally, the external elastic lamina cannot be visualized, and lumen diameter is 2.62 mm. The predicted length of the stent required is 45 mm. D: 3.0 mm x 12 mm IVL balloon (1:1 sizing). Sixty pulses delivered along the calcified lesion. E, F: extensive calcium fracture seen on the OCT after IVL (white arrows). A dissection is also noted (white asterisk). G: stent implantation with a 3.0 mm x 48 mm drug-eluting stent according to the sizing by OCT. Optimized with a 3.0 mm x 12 mm noncompliant balloon. F: final OCT; optimal stent expansion (> 90%), no malapposition or complications (eg, dissection) at the proximal and distal landing sites.
Rotational atherectomy
RA (Rotablator, Boston Scientific, United States) uses a diamond-tipped burr that rotates at 140-180 000 rpm Resulting in the differential ablation of calcified tissue while avoiding disruption of healthy elastic tissue. Ablation occurs only in a forward motion. A specialized wire (RotaWire Floppy or RotaWire Extra Support, Boston Scientific, United States) is required and the burr size should not exceed 0.5-0.6 times the size of the vessel. Previously, the infusion of nitroglycerin, verapamil or heparin were advocated to mitigate the effects of debris embolization while temporary pacing wire insertion or aminophylline infusion were used to combat bradycardia particularly when performing RA in the right coronary artery. However, changes to RA techniques have reduced these complications. Aggressive debulking with RA has been replaced by the use of shorter runs (10-15 seconds), a pecking motion of the burr, smaller burr sizes, and resting periods to allow clearance of embolized particles. On IVI, a smoothing out of the calcium can be seen sometimes with a semilunar shape from where the burr has ablated (figure 3).
The ROTAXUS trial randomized 240 patients with calcified CAD to RA or conventional therapy prior to drug-eluting stenting.52 Both procedural success and luminal gain (1.56 mm vs 1.44 mm, P < .01) were higher in the RA group at the index procedure. However, higher late luminal loss in the RA group was seen at 9 months (0.44 mm vs 0.31 mm, P = .04). Furthermore at 2-year follow-up no differences were seen between groups regarding major adverse cardiovascular events, myocardial infarction, target lesion revascularization or target vessel revascularization (P > .05 for all comparisons).53 The PREPARE-CALC study examined RA vs modified balloons (cutting or scoring) in the treatment of severely calcified disease. Similar to the ROTAXUS trial, increased strategy success was seen in the RA arm vs the modified balloon arm (98% vs 81%, P = .0001) mainly attributed to a higher crossover rate in the modified balloon group (10% of modified balloon group).40 However, improved strategy success in the RA arm did not translate into differences in clinical or angiographic outcomes at 9 months.40 This may be partially explained by the fact that final stent expansion as seen on OCT was not different between groups (73.5% vs 73.1% for modified balloons vs RA respectively, P = .85).54
Combinations of complementary calcium modification therapies are increasingly being used. A study of 92 patients conducted by Tang et al. found greater decrease in percent stenosis (54.5% to 36.1% vs 55.7% to 46.9%, P < .001), and greater stent expansion (71.7% vs 54.5%) with RA followed by cutting balloon compared to RA alone.41 Similarly, Amemiya et al. found greater calcium fracture and stent expansion (78.9% vs 66.7%, P < .01) on OCT with cutting balloon vs standard balloon angioplasty after RA.42 Additionally, there have been numerous case reports regarding the use of IVL following RA with good effect.55,56 Larger scale observational and randomized studies are required to determine if improved longer term outcomes can be achieved by these (and other) combinations. In practical terms and in our own clinical practice, RA plays a role in uncrossable and undilatable lesions, and severe concentric calcification (figure 2) often in combination with other techniques.
Orbital atherectomy
OA (DIAMONDBACK 360 orbital atherectomy system, Cardiovascular systems Inc., United States) consists of a diamond coated crown that uses centrifugal force to orbit resulting in preferential calcium sanding while flexing away from healthy elastic tissue. It requires a dedicated wire (ViperWire advance), and lubricant infusion (ViperSlide both Cardiovascular systems Inc., United States) during ablation. The 1.25 mm crown orbits at 1 of 2 speed settings (80 or 120 000 rpm), which results in widening or narrowing of the orbital arc. Unlike RA, the OA can ablate both in forward and backward motion, and requires slow smooth movements (~1mm/second). Atherectomy runs should be ≤ 30 seconds with resting periods to allow clearance of debris. IVI following OA demonstrates smoothed out calcium often with a visible arc or semilunar shape where sanding occurred (figure 3). The nonrandomized ORBIT I and II studies examined the safety and effectiveness of OA finding a reduction in percentage diameter stenosis to ≤ 50% in > 98% of the lesions.57,58 Significant dissection occurred in 2.3% of the cases. However, the rate of other complications such as perforation, slow, and no-reflow was low and < 1%.58 The 3-year follow-up of the ORBIT II study demonstrated cumulative rates of major adverse cardiovascular events and target lesion revascularization of 23.5%, and 7.8%, respectively.59 The single arm prospective COAST study examined a modified OA system with a distal microcrown to improve penetration with a reduction in percentage diameter stenosis to ≤ 50% in > 99% of the lesions.60 There are currently no randomized trials comparing OA to other forms of calcium modification. However, a small OCT study suggested deeper calcium modification with OA vs RA,61 and a meta-analysis of observational studies found no difference in procedural complications or 30-day events including death, myocardial infarction, and target vessel revascularization between OA and RA.62 However, although more data is required, our practice is to use OA over RA in larger vessels with concentric or nodular calcium. figure 5 demonstrates an example of OA plaque modification and table 3 summarizes the current data for both OA and RA.
Table 3. Summary of the main prospective studies examining outcomes in RA and OA techniques
| Technique | Study name | Design | Number of participants | Procedural outcomes | Short-to-medium term outcomes | Long-term outcomes |
|---|---|---|---|---|---|---|
|
Rotational atherectomy |
Randomized controlled trial |
240 • 120 RA • 120 Standard therapy (Std Tx) |
Strategy success • RA, 92.5% vs Std Tx, 83.3%, P = .03 Acute luminal gain • RA, 1.56mm vs Std Tx, 1.44, P < .01 Dissection • RA, 3.3% vs Std Tx, 3.3%, P = .99 Perforation • RA, 1.7% vs Std Tx, 0.8%, P = .56 Slow/no flow • RA, 0% vs Std Tx, 0.8%, P = .32 |
9-month out comes In-stent LLL • RA, 0.44mm vs Std Tx, 0.31, P = .04 Mortality • RA, 5.0% vs Std Tx, 5.8%, P = .78. MI • RA, 6.7% vs Std Tx, 5.8%, P = .79 TVR • RA, 16.7% vs Std Tx, 18.3%, P = .73 MACE • RA, 24.2% vs Std Tx, 28.3%, P = .46. TLR • RA, 11.7% vs Std Tx, 12.5%, P = .84 |
2-year outcomes MACE • RA, 29.4% vs Std Tx, 34.3%, P = .47 Death • RA, 8.3% vs Std Tx, 7.4%, P = 1.00) Myocardial infarction • RA, 8.3% vs Std Tx, 6.5%, P = .80), TLR • RA, 13.8% vs Std Tx, 16.7%, P = .58 TVR • RA, 19.3% vs Std Tx, 22.2%, P = .62) |
|
|
PREPARE-CALC40 |
Randomized controlled trial |
200 • 100 RA • 100 MB |
Strategy success • RA, 98% vs MB, 81%, P = .0001 Dissection • RA, 3% vs MB, 7%, P = .33 Perforation • RA, 4% vs MB, 2%, P = .68 Slow/no flow • RA, 2% vs MB, 0%, P = .49 |
9 months In-stent LLL • RA, 0.22 vs MB, 0.16mm, P = .21 Mortality • RA, 2% vs MB, 2%, P = 1.00 TVR • RA, 3% vs MB, 6%, P = .50 TLR • RA, 2% vs MB, 7%, P = .17 Definite/probable stent thrombosis • RA, 0% vs MB, 0%, P = 1.00 TVF • RA, 6% vs MB, 8%, P = .78 |
||
|
Orbital atherectomy |
ORBIT I57 |
Prospective non-randomized |
50 |
• Device success, 98%, • Procedural success, 94% • Dissection, 12% • Perforation, 2% • In-hospital MACE, 4% |
MACE • 30-days, 6% • 6 months, 8% |
|
|
Prospective multicentre non-randomized |
443 |
• Procedural success, 88.9% • Angiographic success, 91.4% • Severe dissection, 2.3% • Perforation, 0.9% • Slow/no flow, 0.2% • In-hospital MACE, 9.8% |
MACE • 30-day, 10.4% |
3-years • MACE, 23.5% • Cardiac death, 6.7% • MI, 11.2% • TVR, 10.2% • TLR, 7.8% |
||
|
COAST60 |
Prospective multicentre single-arm |
100 |
• Procedural success, 85% • In-hospital MACE, 14% • Dissection, 2% • Perforation, 2% • Slow/no flow, 2% |
MACE • 30-day, 15% |
1 year • MACE, 22.2% |
|
|
MACE, major adverse cardiovascular events; MB, modified balloons; MI, myocardial infarction; OA, orbital atherectomy; RA, rotational atherectomy; Std Tx, standard therapy, RS, residual stenosis; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; TVR, target vessel revascularization. Definitions • Strategy success: Successful stent delivery, < 20% in-stent RS, TIMI grade-3 flow without crossover or stent failure • Device success: < 50% RS following OA without device malfunction • Angiographic success: stent delivery with RS < 50% ROTAXUS • MACE: MI, TVR, and cardiac death ORBIT I • Procedural success: < 20% in-stent RS • MACE: cardiac death, MI or TLR ORBIT II • Procedural success: stent delivery with a < 50% RS without in-hospital MACE. • MACE: MI, TVR, and cardiac death COAST • MACE: cardiac death, MI or TVR |
||||||
Figure 5. Calcium modification using orbital atherectomy guided by co-registered optical coherence tomography (OCT) imaging. A: severely calcified mid-left anterior descending coronary artery stenosis B: OCT showing severe circumferential calcification; arc of ~270°, depth of 0.7 mm, and length > 5 mm suggesting a high risk of stent underexpansion according to OCT criteria. Distal and proximal reference luminal diameters of 2.5 mm, and 3.25 mm, respectively, with a predicted stent length of 33 mm. C: orbital atherectomy (yellow arrow) using the DIAMONDBACK 360 orbital atherectomy system, and a 1.25 mm crown advanced at 1 mm/s. Sanding/atherectomy was performed in a forward and a backward motion. Dynamic road mapping was also used to guide the procedure (bottom left). D: smoothed out appearance after orbital atherectomy. Image shows that calcium ‘cap’ has been greatly reduced by the sanding effect of orbital atherectomy (OA). A semilunar shape can be seen as an effect of the orbiting crown (white arrows). E: post-OA angiography demonstrating significantly reduced percent stenosis. F: implantation of a 2.5 mm x 36 mm drug-eluting stent with proximal optimization using a 3.5 mm x 10 mm noncompliant balloon (inset). G: final co-registered OCT post-OA, and stenting demonstrating adequate stent expansion and apposition without complications.
Excimer laser coronary angioplasty
Excimer laser coronary angioplasty (ELCA) uses a mixture of rare gas and halogen to generate brief pulses of high-frequency ultraviolet light which disrupts atherosclerotic plaque through 3 mechanisms: photochemical by breaking down the carbon bonds between the molecules, photothermal due to the production of heat and vapour bubbles causing cell rupture, and photomechanical by the expansion of vapour bubbles causing the disruption of the plaque. Fluence (energy measured in mJ/mm2), and pulse frequency can be altered to increase its effectiveness. Constant saline infusion is advised to avoid thermal injury. Also, the short wavelength (~308 nm) of ultraviolet light used reduces the depth of penetration, thus avoiding damage to healthy tissues. Evidence on the use of ELCA in calcified CAD is limited. A prospective multicentre study of 100 uncrossable/undilatable lesions demonstrated technical success in 92% of lesions63 while a more recent prospective multicentre study of 126 uncrossable lesions demonstrated success in ~82% of cases.64 However, severe calcification was significantly associated with ELCA failure. In the setting of in-stent restenosis, more calcium fracture on OCT was seen in the ELCA vs conventional treatment group.65 Given the paucity of large-scale studies and considering the data available to date, ELCA has a relatively niche role predominantly for the management of uncrossable lesions although we prefer to use RA as the first-line ablative therapy in this circumstance.
CONCLUSIONS
Calcified CAD continues to present a barrier for successful PCI. Furthermore, our ageing population suggests that the proportion of patients with calcified CAD who will present for PCI is likely to increase. Its presence is associated not just with poorer acute outcomes, but also with more adverse events at long-term follow-up. Stent underexpansion is one of the most powerful predictors of stent failure, and often occurs in the presence of significant coronary calcification. Identifying the presence of coronary calcium is key in planning a PCI, and is more accurately done using IVI. A number of technologies with different mechanisms of action are now available to modify coronary calcium although head-to-head comparisons between these techniques are lacking. Nonetheless, we propose a simplified calcium modification algorithm based on IVI findings that is currently used at our center. Future studies should aim to compare techniques and elucidate the best technique combinations to ensure improved outcomes in these complex patients.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. McInerney: concept, design, and drafting of the manuscript. J. Escaned: contributed clinical images, and was involved in the critical review of the manuscript. N. Gonzalo: concept, design, drafting, and critical review of the manuscript. Contributed clinical images.
CONFLICTS OF INTEREST
N. Gonzalo reports consultancy and speaker fees from Abbott and Boston Scientific. The remaining authors reported no conflicts of interest pertaining to the current publication.
REFERENCES
1. Genereux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63:1845-1854.
2. Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995-998.
3. Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305-1310.
4. Stefanini GG, Alfonso F, Barbato E, et al. Management of myocardial revascularisation failure: an expert consensus document of the EAPCI. EuroIntervention. 2020;16:e875-e890.
5. Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56.
6. Mori H, Torii S, Kutyna M, et al. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc Imaging. 2018;11:127-142.
7. Bourantas CV, Zhang YJ, Garg S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100:1158-1164.
8. Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703-1714.
9. Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044-1059.
10. Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends Cardiovasc Med. 2012;22:133-137.
11. Alfonso F, Macaya C, Goicolea J, et al. Determinants of coronary compliance in patients with coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 1994;23:879-884.
12. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
13. Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572-577.
14. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319-326.
15. Jin HY, Weir-McCall JR, Leipsic JA, et al. The Relationship Between Coronary Calcification and the Natural History of Coronary Artery Disease. JACC Cardiovasc Imaging. 2021;14:233-242.
16. Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209-2214.
17. Genereux P, Madhavan MV, Mintz GS, et al. Relation between coronary calcium and major bleeding after percutaneous coronary intervention in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy and Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trials). Am J Cardiol. 2014;113:930-935.
18. Hendry C, Fraser D, Eichhofer J, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;8:79-86.
19. Konigstein M, Madhavan MV, Ben-Yehuda O, et al. Incidence and predictors of target lesion failure in patients undergoing contemporary DES implantation-Individual patient data pooled analysis from 6 randomized controlled trials. Am Heart J. 2019;213:105-111.
20. Guedeney P, Claessen BE, Mehran R, et al. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc Interv. 2020;13:1417-1428.
21. Kawashima H, Serruys PW, Hara H, et al. 10-Year All-Cause Mortality Following Percutaneous or Surgical Revascularization in Patients With Heavy Calcification. JACC Cardiovasc Interv. 2022;15:193-204.
22. Knez A, Becker A, Leber A, et al. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol. 2004;93:1150-1152.
23. Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105:1791-1796.
24. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959-1965.
25. Wang X, Matsumura M, Mintz GS, et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc Imaging. 2017;10:869-879.
26. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072.
27. Raber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281-3300.
28. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
29. Gao XF, Ge Z, Kong XQ, et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:247-257.
30. Hong SJ, Mintz GS, Ahn CM, et al. Effect of Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation: 5-Year Follow-Up of the IVUS-XPL Randomized Trial. JACC Cardiovasc Interv. 2020;13:62-71.
31. Kinnaird T, Johnson T, Anderson R, et al. Intravascular Imaging and 12-Month Mortality After Unprotected Left Main Stem PCI: An Analysis From the British Cardiovascular Intervention Society Database. JACC Cardiovasc Interv. 2020;13:346-357.
32. Mc Inerney A, Escaned J, Gonzalo N. Online Co-Registration Of IVUS and OCT. Minerva Cardiol Angiol. 2021;69:641-654.
33. Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 2014;63:2220-2233.
34. Kim SW, Mintz GS, Lee WS, et al. DICOM-based intravascular ultrasound signal intensity analysis: an Echoplaque Medical Imaging Bench study. Coron Artery Dis. 2014;25:236-241.
35. Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461-471.
36. Zhang M, Matsumura M, Usui E, et al. Intravascular Ultrasound-Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ Cardiovasc Interv. 2021;14:e010296.
37. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
38. Ali Z, Price M, Maehara A, Lansky A. New Insights on the Consistency of Coronary IVL Data. Proceedings of Transcatheter Cardiovascular Therapeutics; 2021 05/11/2021. Available online: https://tct2021.crfconnect.com/ondemand/world-connect/86455. Accessed 12 Dec 2021.
39. Matsukawa R, Kozai T, Tokutome M, et al. Plaque modification using a cutting balloon is more effective for stenting of heavily calcified lesion than other scoring balloons. Cardiovasc Interv Ther. 2019;34:325-334.
40. Abdel-Wahab M, Toelg R, Byrne RA, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415.
41. Tang Z, Bai J, Su SP, et al. Aggressive plaque modification with rotational atherectomy and cutting balloon for optimal stent expansion in calcified lesions. J Geriatr Cardiol. 2016;13:984-991.
42. Amemiya K, Yamamoto MH, Maehara A, et al. Effect of cutting balloon after rotational atherectomy in severely calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;94:936-944.
43. Secco GG, Buettner A, Parisi R, et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc Revasc Med. 2019;20:1083-1087.
44. Rheude T, Rai H, Richardt G, et al. Super high-pressure balloon versus scoring balloon to prepare severely calcified coronary lesions: the ISAR-CALC randomised trial. EuroIntervention. 2021;17:481-488.
45. Kereiakes DJ, Virmani R, Hokama JY, et al. Principles of Intravascular Lithotripsy for Calcific Plaque Modification. JACC Cardiovasc Interv. 2021;14:1275-1292.
46. Kereiakes DJ, Di Mario C, Riley RF, et al. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions: Patient-Level Pooled Analysis of the Disrupt CAD Studies. JACC Cardiovasc Interv. 2021;14:1337-1348.
47. Blachutzik F, Honton B, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy in eccentric calcified coronary lesions: a patient-level pooled analysis from the Disrupt CAD I and CAD II Studies. Clin Res Cardiol. 2021;110:228-236.
48. Salazar C, Escaned J, Tirado G, Gonzalo N. Intravascular lithotripsy for recurrent restenosis caused by severe calcific neoatherosclerosis. EuroIntervention. 2020;16:e351-e352.
49. Salazar C, Escaned J, Tirado G, Gonzalo N. Undilatable Calcific Coronary Stenosis Causing Stent Underexpansion and Late Stent Thrombosis: A Complex Scenario Successfully Managed With Intravascular Lithotripsy. JACC Cardiovasc Interv. 2019;12:1510-1512.
50. Yeoh J, Cottens D, Cosgrove C, et al. Management of stent underexpansion using intravascular lithotripsy-Defining the utility of a novel device. Catheter Cardiovasc Interv. 2021;97:22-29.
51. Ali ZA, McEntegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2020;41:485-486.
52. Abdel-Wahab M, Richardt G, Joachim Buttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10-19.
53. de Waha S, Allali A, Buttner HJ, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. 2016;87:691-700.
54. Hemetsberger R, Gori T, Toelg R, et al. Optical Coherence Tomography Assessment in Patients Treated With Rotational Atherectomy Versus Modified Balloons: PREPARE-CALC OCT. Circ Cardiovasc Interv. 2021;14:e009819.
55. Kaur N, Pruthvi CR, Sharma Y, Gupta H. Rotatripsy: synergistic effects of complementary technologies: a case report. Eur Heart J Case Rep. 2021;5:ytab083.
56. Gonzálvez-García A, Jiménez-Valero S, Galeote G, et al. “RotaTripsy: Combination of rotational atherectomy and intravascular lithotripsy in heavily calcified coronary lesions: A case series”. Cardiovasc Revasc Med. 2022;35:179-184.
57. Parikh K, Chandra P, Choksi N, Khanna P, Chambers J. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81:1134-1139.
58. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-518.
59. Lee M, Genereux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261-264.
60. Redfors B, Sharma SK, Saito S, et al. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification: Coronary Orbital Atherectomy System Study (COAST). Circ Cardiovasc Interv. 2020;13:e008993.
61. Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86:1024-1032.
62. Goel S, Pasam RT, Chava S, et al. Orbital atherectomy versus rotational atherectomy: A systematic review and meta-analysis. Int J Cardiol. 2020;303:16-21.
63. Bilodeau L, Fretz EB, Taeymans Y, et al. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-161.
64. Ojeda S, Azzalini L, Suarez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
65. Lee T, Shlofmitz RA, Song L, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention. 2019;15:e279-e288.
* Corresponding author:
E-mail address: nieves_gonzalo@yahoo.es (N. Gonzalo).