To the Editor,
The perioperative risk associated with aortic stenosis during noncardiac surgery (NCS) depends on the presence of symptoms, the severity of aortic stenosis, concomitant cardiovascular diseases, and the risk associated with noncardiac comorbidity. Severe symptomatic aortic stenosis is a major risk factor for postoperative heart failure and a predictor of 30-day and long-term mortality after noncardiac surgery; therefore, an appropriate perioperative strategy is essential in patients undergoing intermediate- or high-risk noncardiac surgery.1,2 Hip and vertebral fractures, which are highly prevalent in the elderly population, are usually due to accidental falls and considered intermediate-risk interventions.3 Nonetheless, these patients are characterized by their advanced age and the presence of concomitant diseases, which increases their surgical risk. In this context, the management of aortic stenosis is associated with reduced morbidity and mortality rates in patients undergoing intermediate or high-risk noncardiac surgery.4,5 The perioperative management of patients with severe symptomatic aortic stenosis requiring uncertain trauma surgery is challenging.
We present a series of 4 consecutive patients with a past medical history of severe symptomatic aortic stenosis with a trauma emergency, 3 of them due to hip fracture and 1 due to vertebral fracture, all after accidental falls, in whom perioperative management of aortic stenosis was optimized by transcatheter aortic valve implantation (TAVI). The study was conducted following the ethical principles for medical research of the Declaration of Helsinki and was approved by the ethics committee of our center. Table 1 lists the patients’ baseline characteristics. Two of the patients were octogenarians and the other 2 were nonagenarians, with a high comorbidity index (Charlson ≥ 7), severe aortic stenosis, and in New York Heart Association functional class II-III. Initially, we evaluated the risk associated with the surgical intervention required, and we considered it to be intermediate-high risk. Regarding waiting time, we considered the procedures to be a priority, which needed to be performed as soon as possible. In this context, we evaluated the patients’ clinical risk, the presence of symptomatic aortic stenosis with echocardiographic repercussions (decreased left ventricular ejection fraction or pulmonary hypertension), and comorbidity, and considered aortic valve replacement as a high-risk procedure. Furthermore, we analyzed the patients’ quality of life and observed that they could all undertake most activities of daily living, with acceptable mobility. The Heart and Outer Teams both made their assessment and decided to optimize the perioperative period of noncardiac surgery by definitively treating aortic stenosis through TAVI, which was accepted by the patients and their families. All patients underwent previous anatomical studies (echocardiography, angiography, computed tomography, or 3-dimensional transesophageal echocardiography). We selected transfemoral access, opting for the unaffected lower limb in the case of hip fracture, with extra radial access support, due to the presence of a certain external rotation and shortening of the affected limb. The implanted valve was self-expandable according to the experience of the center and availability.
Table 1. Patients’ clinical, biochemical, and echocardiographic characteristics, and clinical events
| Variables | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age. years | 89 | 94 | 80 | 91 |
| Sex | Male | Female | Male | Female |
| STS Score (%) | 9.89 | 6.23 | 2.18 | 10.73 |
| Charlson Index | 12 | 7 | 8 | 8 |
| PROFUND Index | 11 | 9 | 3 | 8 |
| Activities of daily living | Independent (lives alone) | Partial (walks alone + caregiver) | Independent (lives alone) | Partial (walks with cane + caregiver) |
| Cognitive status | Normal | Normal | Normal | Normal |
| Coronary heart disease | Yes | No | No | No |
| Clinical event | Right pertrochanteric femur fracture | Left pertrochanteric femur fracture | Vertebral fracture | Left intertrochanteric and distal radius and left styloid apophysis fracture |
| Cardiovascular symptoms | FC II dyspnea | FC II-III dyspnea | FC II dyspnea | FC III dyspnea |
| BNP/NT-proBNP pre-TAVI (pg/mL) | 938 / NA | 320 / 1130 | NA / 2235 | 935 / 4951 |
| BNP/NT-proBNP post-TAVI (pg/mL) | 536 / NA | 96 / 638 | NA / 698 | 406 / 1066 |
| Hb pre-TAVI (g/dL) | 9.8 | 8.8 | 10.8 | 11.2 |
| Hb post-TAVI (g/dL) | 9.1 | 11.7 | 9.9 | 9.8 |
| Creatinine pre-TAVI (mg/dL) | 2.39 | 1.59 | 0.84 | 1.79 |
| Creatinine post-TAVI (mg/dL) | 2.12 | 2.43 | 1.15 | 2.1 |
| High creatinine (mg/dL) | 1.71 | 1.23 | 1.34 | 1.5 |
| Red blood cell transfusion | After surgery | After TAVI | After surgery | After TAVI and surgery |
| Echocardiographic parameters | ||||
| Peak gradient (mmHg) | 69 | 84 | 51 | 66 |
| Mean gradient (mmHg) | 47 | 52 | 34 | 42 |
| Area (cm2) | 0.8 | 0.68 | 0.88 | 0.64 |
| AR | None | Moderate | None | None |
| LVEF (%) | 60 | 61 | 55 | 60 |
| sPAP (mmHg) | 36 | 40 | 32 | 80 |
| Anatomic parameters of the aortic annulus (CT and 3D-TEE) | ||||
| Perimeter (mm) | 70 | 66 | 83.7 | 73 |
| Area (mm2) | 380 | 336 | 540 | 389 |
| Annular diameter (mm) | 21.5 | 20.7 | 26.6 | 22.7 |
| Days event-TAVI | 3 | 3 | 2 | 3 |
| Permanent pacemaker | Yes | No | No | No |
| TAVI access | Transfemoral | Transfemoral | Transfemoral | Transfemoral |
| Transcatheter closure | Double ProGlide | Double ProGlide | Double ProGlide | Double ProGlide |
| Type of valve | Accurate Neo 2 S | Evolut Pro + 26 mm | Evolut Pro 34 mm | Accurate Neo 2 S |
| Echocardiographic parameters post-TAVI | ||||
| Peak gradient (mmHg) | 18 | 12 | 7 | 26 |
| Mean gradient (mmHg) | 11 | 7 | 4 | 13 |
| AR | None | None | None | None |
| LVEF (%) | 63 | 52 | 53 | 65 |
| sPAP (mmHg) | NA | 32 | 28 | 74 |
| Days TAVI-surgery | 2 | 3 | 2 | 3 |
| Events | ||||
| Heart failure | No | No | No | No |
| Myocardial infarction | No | Yes, during TAVI | No | No |
| Vascular complications | No | No | No | No |
| Stroke | No | No | No | No |
| De novo atrial fibrillation | No | No | No | NA |
| Ventricular arrhythmias | No | No | No | No |
| Mortality | No | No | No | No |
| Cardiovascular mortality | No | No | No | No |
| Others | UTI | UTI, membranous colitis | UTI | UTI |
| Antiplatelet/anticoagulant therapy | ||||
| Pre-TAVI | Aspirin + clopidogrel | Aspirin | Acenocumarol* | Apixaban |
| Post-TAVI | Aspirin + LMWH | Aspirin + LMWH | LMWH | LMWH (bemiparin) |
| Discharge | Aspirin + clopidogrel | Aspirin + clopidogrel | Acenocumarol | Apixaban |
| Follow-up | Deceased at the 11-month follow-up due to urothelial cancer | Alive at the 19-month follow-up | Alive at the 20-month follow-up | Deceased at the 3-month follow-up due to pneumonia |
|
3D-TEE, 3-dimensional transesophageal echocardiogram; AR, aortic regurgitation; BNP, B-type natriuretic peptide; CT, computed tomography; FC, New York Heart Association functional class; Hb, hemoglobin; LMWH, low molecular weight heparin; LVEF, left ventricular ejection fraction; NA, not applicable; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sPAP, systolic pulmonary artery pressure; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; UTI, urinary tract infection. * Oral anticoagulation for pulmonary embolism. |
||||
We successfully performed TAVI, with a median of 3 days, and only 1 complication, an acute anterior myocardial infarction due to embolization during implantation, which was resolved by direct stenting of the left anterior descending coronary artery. We performed the orthopedic trauma surgery during admission, between 2 and 3 days after TAVI, without any cardiac complications being reported during or after the intervention, with good tolerance to blood volume and favorable clinical and hemodynamic parameters.
In our series of patients with various degrees of surgical risk and a need for intermediate-high risk noncardiac surgery with priority status, we opted for definitive treatment through TAVI, after establishing inter- and multidisciplinary consensus between an Outer Team and the Heart Team. To date, the approach has been the strict control of blood volume or performing aortic valvuloplasty as a bridge therapy. However, after the consolidation of TAVI, it can be considered an appropriate therapeutic option to facilitate perioperative treatment and reduce mortality.
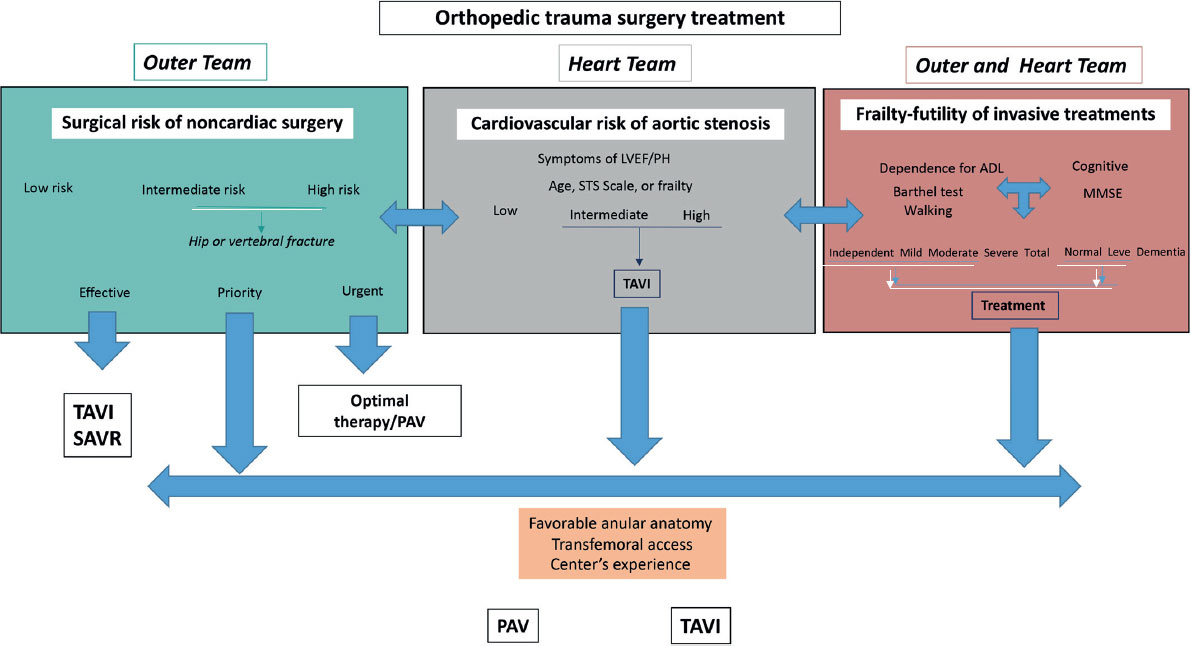
Based on our results and the clinical outcomes of the patients in our series, as well as the European clinical guidelines,3 we developed an algorithm to evaluate perioperative management (figure 1). The risk of noncardiac surgery must be assessed and stratified as low, intermediate, or high. Furthermore, the Outer Team must establish the waiting time for surgery, categorizing it as emergent, urgent, elective prioritized, or elective. Subsequently, the heart team must evaluate the risk of aortic stenosis based on severity, symptoms, and hemodynamic repercussions. Then, the patient’s frailty indices6 need to be evaluated, supported by their quality of life; in our series, one of the markers that allowed us to make decisions was the extent of dependency and the ability to walk, with or without assistance, and the absence of cognitive impairment. Finally, perioperative TAVI should be considered as the treatment of aortic stenosis if the risk of the transcatheter procedure, including anatomical criteria and the experience of the center, does not entail excessive morbidity and mortality (transfemoral access). We prioritized a bridge therapy such as aortic valvuloplasty in patients requiring emergency surgery because of time constraints, mainly for organizational reasons; nevertheless, TAVI has been reported in patients in cardiogenic shock with encouraging results. In fact, for interventions such as that presented in our series, perhaps definitive treatment (TAVI)—compared with a bridge therapy that also carries risks—can minimize cardiac complications and better address blood volume in surgical interventions requiring transfusions.
Figure 1. Therapeutic decision-making algorithm in patients with aortic stenosis requiring noncardiac surgery. ADL, activities of daily living; LVEF, left ventricular ejection fraction; MMSE, Mini-Mental State Examination; PAV, percutaneous aortic valvuloplasty; PH, pulmonary hypertension; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Therefore, in our small series, the strategy for treating severe symptomatic aortic stenosis with transfemoral TAVI as a minimally invasive procedure in the perioperative approach of patients requiring intermediate-high risk trauma surgery with priority status, is a safe and feasible approach, without postoperative cardiac complications.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
The present study complies with the criteria of the Declaration of Helsinki and was approved by the ethics committee of our center. Patients signed their informed consent forms for procedures and the use of their data for registration. SAGER guidelines have been followed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
No artificial intelligence has been used.
AUTHORS’ CONTRIBUTIONS
M. Muñoz-García: project management, data collection, and manuscript drafting. R. Rivera López, J. Sánchez Gila, and E. Molina Navarro: project management, data interpretation, and supervision. R. Parrilla Linares and J.M. Romero León: data interpretation and manuscript review. All authors have substantially contributed to the design, formal analysis, research, and critical review of the study, and given their final approval.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Luis SA, Dohaei A, Chandrashekar P, et al. Impact of aortic valve replacement for severe aortic stenosis on perioperative outcomes following major noncardiac surgery. Mayo Clin Proc. 2020;95:727-737.
2. Calleja AM, Dommaraju S, Gaddam R, Cha S, Khandheria BK, Chaliki HP. Cardiac risk in patients aged 75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 2010;105:1159-1163.
3. Halvorsen S, Mehilli J, Cassese S, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2023;44:4421.
4. Taniguchi T, Morimoto T, Shiomi H, et al. Elective non-cardiac surgery in patients with severe aortic stenosis –observations from the CURRENT AS Registry. Circ J. 2020;84:1173-1182.
5. Okuno T, Yahagi K, Horiuchi Y, et al. The role of transcatheter aortic valve replacement in the patients with severe aortic stenosis requiring major noncardiac surgery. Cardiovasc Interv Ther. 2019;34:345-351.
6. Gutiérrez J, Avanzas P, Solla P, Díaz R, Solano JJ, Morís C. Comprehensive geriatric assessment in older patients with severe aortic stenosis:usefulness in detecting problems and planning interventions. Rev Esp Cardiol. 2020;73:336-338.