ABSTRACT
Invasive coronary angiography is the standard approach in the routine clinical practice. Intracoronary imaging modalities provide real-time images of intracoronary anatomy. On this basis, optical coherence tomography and intravascular ultrasound have a positive impact on diagnosis and percutaneous coronary intervention. This summary provides an insight on these imaging modalities for the interventional and clinical cardiologist with the currently available evidence.
Keywords: Intravascular ultrasound. Optical coherence tomography. Invasive coronary angiography.
RESUMEN
La coronariografía es el método de elección para el estudio de la anatomía coronaria en la práctica clínica diaria. Las diferentes modalidades de imagen intracoronaria permiten valorar en tiempo real la anatomía de la pared arterial coronaria. Sobre esta base, la tomografía de coherencia óptica y la ecografía intravascular tienen un impacto positivo en el diagnóstico y en el intervencionismo percutáneo. La presente revisión proporciona un resumen de las técnicas de imagen intracoronaria basadas en la evidencia actual disponible.
Palabras clave: Ecografía intravascular. Tomografía de coherencia óptica. Coronariografía.
Abbreviations
CA: coronary angiography. CTO: chronic total coronary occlusion. ICI: intracoronary imaging. IVUS: intravascular ultrasound. LMCAD: left main coronary artery disease. MACE: major adverse cardiovascular events. OCT: optical coherence tomography. PCI: percutaneous coronary intervention.
INTRODUCTION
Coronary artery disease is still the leading cause of death across the world and can manifest through a wide range of presentations given its dynamic nature.1 Coronary angiography (CA) is the gold standard approach to assess the presence and severity of coronary artery disease. However, it is limited by qualitative assessment although improvements have been made such as the development of quantitative coronary angiography.2 New imaging modalities for coronary assessment have emerged over the last few decades to improve patient outcomes.3
Intracoronary imaging (ICI) modalities provide an in-depth understanding of the aspects that contribute to the pathogenesis of coronary artery disease, but also help us guide the decision-making process. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) produce real-time cross-sectional images of the coronary artery. Data from clinical studies have suggested improved outcomes during complex ICI-guided percutaneous coronary interventions (PCI).4,5 Both the American and the European clinical guidelines on myocardial revascularization allocate a Class II recommendation—American College of Cardiology/American Heart Association: Class IIa6 and European Society of Cardiology: Class IIa—to IVUS–guided PCI,7 being the OCT-guided PCI an alternative with the exception of ostial left main coronary artery disease.6,7 However, its use is still uneven worldwide.
This review seeks to summarize the evidence available to portray all the potential advantages and downsides of these 2 catheter-based imaging modalities in the routine clinical practice.
IMAGING MODALITIES
IVUS
IVUS catheters are rapid exchange catheters that have a piezoelectric crystal that produces soundwaves through transducers when electrically excited. Soundwaves propagate through the different tissues reflecting on the surfaces according to the acoustic properties of the tissue. These returned soundwaves are formatted into a grayscale image with dynamic contrast resolution. This modality allows the interventional cardiologist to evaluate the integrity of the vessel wall, characterize tissue composition, and tackle PCI challenges (stent malapposition and underexpansion).8
The quality of the images depends on the soundwave, transducer, and tissue properties. The resulting image resolution is greater at shorter distances (near-field), but it appears less clear in the deep fields (far-field) due to beam scattering. Flow properties also make it more difficult to distinguish the lumen from the tissues. Mechanical or rotational catheters work at 40-60 MHz frequencies, as opposed to electronic ones that operate at 20 MHz frequencies and have greater axial and lateral resolution. Overall, the best images are obtained when the catheter is coaxial to the vessel, the beam is perpendicular to the lesion, and with clear lumens.8,9
IVUS image acquisition should be routinely performed with IV anticoagulation and intracoronary nitrates to prevent device-related complications.5 Vessel interrogation can be performed with manual or automatic pullback starting, at least, 10 mm distal to the target lesion until the aorta or the guiding catheter can be seen. In the case of aorto-ostial lesions, the guiding catheter must be disengaged to unmask ostial lesions. Automatic pullbacks have the advantage of providing measurements of lesion length, which is estimated with the average time and pullback speed. Multiple lesions are considered when distance is > 5 mm within the same coronary segment. However, spatial orientation is a major limitation.8,9 The main features of IVUS are shown on table 1 and table 2.
Table 1. General characteristics of intracoronary imaging modalities
| Intracoronary imaging modality | Source of image | Frequency (Mhz) | Wavelength (µm) | Minimal guide catheter (Fr) | Axial resolution (µm) | Lateral resolution (µm) | Tissue penetration (mm) | Pullback length (mm) | Pullback speed (mm/sec) |
|---|---|---|---|---|---|---|---|---|---|
| IVUSa | Ultrasound | 20-60 | 40-50 | 5 | 20-170 | 50-260 | 3-8 | 100 | 0.5-10 |
| OCTb | Infrared light | NA | 1.3 | 5 | 15-20 | 20-40 | 1-3 | 75 | 10-40 |
| Hybrid (OCT/IVUS)c | Ultrasound and Infrared light | 40 | 1.3 | 5 | 200/15 | 200/30 | 3-8 | 100-150 | 0.5-40 |
|
IVUS, intravascular ultrasound; OCT, optical computed tomography; NA, not applicable. a Includes OptiCross (Boston Scientific, United States), Volcano (Philips, United States), Infraredx (Burlington, United States), ACIST CVi (ACIST, United States), and Fastview (Terumo, Japan). b Includes OPTIS (Abbott Vascular, United States), and Lunawave (Terumo, Japan). c Includes Novasight Hybrid (Conavi Medical, Canada), and Dual Sensor (Terumo, Japan). |
|||||||||
Table 2. Main advantages and drawbacks of intracoronary imaging modalities
| Intravascular ultrasound | Optical computed tomography |
|---|---|
| • More penetration capabilities, capable of assessing plaque volume and deeper plaques. • Better suited for CTO, aorto-ostial junction, LMCAD, and stent sizing assessment. • Does not require contrast. |
• Higher resolution, fewer artifacts, and facilitates the identification of subtle details • More user friendly. • Capable of assessing calcium thickness. • Better suited for strut and thrombus evaluation. |
| • Lower resolution. • Requires anticoagulation and additional time. • Image interpretation requires experience and expertise. • Cannot penetrate calcium or adequately evaluate thrombi. • Expensive. |
• Less penetration capabilities, cannot adequately evaluate ostial lesions. • Requires anticoagulation and additional time. • Image acquisition requires blood clearance through contrast or other means. • Image interpretation requires experience and expertise. • Expensive. |
|
CTO, chronic total coronary occlusions; LMCAD, left main coronary artery disease. |
|
Efforts to explore the potential benefits of IVUS over CA have been reported over the years with promising results. In a recent meta-analysis of 27 610 patients that compared IVUS-guided PCI vs CA-guided PCI, IVUS was associated with less cardiac death (risk ratio [RR], 0.63; 95% confidence interval [95%CI], 0.54–0.73), and PCI-related complications. Similarly, the risk of myocardial infarction (RR, 0.71; 95%CI, 0.58–0.86), target lesion revascularization (RR, 0.81; 95%CI, 0.70–0.94), and stent thrombosis (RR, 0.57; 95%CI, 0.41–0.79) was lower with IVUS-guided PCI.10
Optical coherence tomography
OCT image generation is based on infrared light (1.3 µm wavelength). Compared to IVUS, this imaging modality provides greater axial resolution (10-20 µm vs 50-150 µm) with limited soft tissue penetration (1-2 mm vs 5-6 mm except for calcium evaluation).3,4,11
Current devices are 5-Fr compatible through a rapid exchange system that also allows automatic pullbacks with angiography co-registration and automatic lumen measurements and calcium detection. The quality of the images depends on the interaction of light with the surrounding tissues (echo-time delay). As such, light reflection, refraction, and attenuation (absorption) determine the final image resolution. Metal devices and fibrous plaques are considered strong reflectors while low reflectors are calcium and necrotic cores (lipid-rich). Red blood cells cause light scattering that requires contrast washout causing the appearance of a “pseudo-thrombus” image with poor blood clearance.
OCT imaging is also routinely performed with IV anticoagulation and intracoronary nitrates to prevent complications. The study of the vessel begins 10 mm distal to the target lesion, the catheter is then purged with contrast, and an automatic pullback with co-registration (if available) is performed. The average pullback speed of 10-40 mm/s usually allows a single bolus injection of contrast to achieve a blood-free environment.12 The general OCT features are shown on table 1 and table 2.
Compared to the CA-guided PCI, observational studies have suggested a potential benefit of OCT-guided PCI with lower rates of major adverse cardiovascular events (MACE) and stent-related complications.13 Furthermore, the OCT provides more reliable and reproducible images with less inter-observer variability compared to the IVUS. In this regard, the OCT may be superior to assess stent and lumen diameters.14,15
SPECIFIC SCENARIOS
Acute coronary syndromes
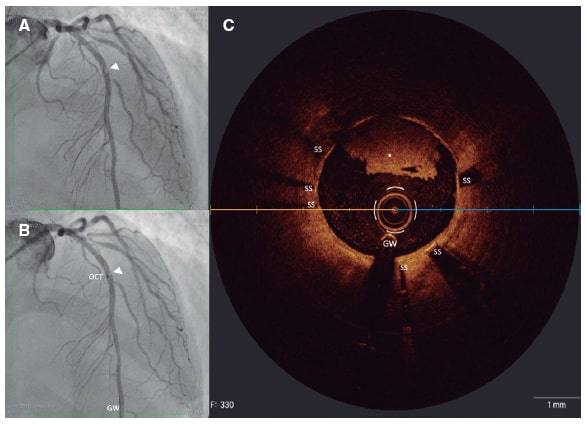
Acute coronary syndromes are mostly caused by coronary thrombosis due to plaque rupture, plaque erosion or an eruptive calcified nodule.16 Accurate diagnosis may have prognostic implications. The rupture of the plaque is associated with a greater rate of no-reflow and distal embolization. Plaque erosion can be conservatively managed in non-critical stenoses. Calcified nodules are associated with a higher rate of stent restenosis and thrombosis17 (figure 1).
Figure 1. Stent thrombosis. A: filling defect (arrowhead) in the mid left anterior descending coronary artery on the right oblique anterior projection. B: co-registration of optical coherence tomography and angiography. C: OCT cross-sectional image showing non-occlusive stent thrombosis (white asterisk). GW, guidewire; OCT, optical coherence tomography; SS, stent struts.
The OCT is often used for the perioperative identification of culprit lesions after careful evaluation of the morphological characteristics of the fibrous cap.18 The plaque classification algorithm through OCT classifies plaques based on the state of fibrous caps, thereby showing an intact fibrous cap in plaque erosion, a disrupted cap as the hallmark of a ruptured plaque or a calcified nodule. The OCT can also determine thrombus burden, but is not necessary to ascertain the culprit lesion. However, a recent publication that compared near infrared spectroscopy combined with IVUS to OCT in 276 patients found that the former can accurately characterize culprit lesions after the characterization of calcium, plaque cavity, and the maximum lipid core burden index with 93% and 100% sensitivity and specificity, respectively.19
Moreover, data supports the preference of ICI-guided PCI to improve outcomes in the management of acute coronary syndrome. A meta-analysis of 26 610 patients reported a net benefit of IVUS regardless of the presence of acute coronary syndrome with a lower rate of MACE (RR, 0.57; 95%CI, 0.41-0.79) compared to the CA-guided PCI.10 Similarly, an observational Korean registry of 11 731 patients treated with primary PCI reported a lower rate of cardiac death, target vessel reinfarction, and target lesion revascularization with either IVUS- or OCT-guidance.20
Bifurcated lesions
Coronary bifurcation lesions are found in 15% to 20% of all patients treated with PCI.21 The main challenge when treating bifurcation lesions is selecting the right PCI strategy to avoid target lesion failure or side-branch occlusion. The importance of careful evaluation is evident with the distal left main coronary artery disease (LMCAD). The European Bifurcation Club recommends intracoronary imaging to treat bifurcated lesions.22
The risk of side-branch compromise can be diminished with both the IVUS and the OCT by selecting the proper stent (type and size), landing zone, and evaluation of post-PCI results (stent expansion and apposition; distal dissection). Intracoronary imaging can identify a “spiky” carina in cases of distal LMCAD, which has been associated with restenosis due to carina shift. Also, some predictors of side-branch compromise with IVUS (minimum lumen area of side-branch and plaque burden)19 and OCT (angle < 50º and branching point to carina tip length < 1.70 mm)23 have been reported.
Also, both imaging modalities can be used for stent sizing in bifurcation lesions; however, areas with high plaque burden or lipid plaques where both imaging modalities are useful should be avoided as landing zones. Overall, ICIs are also useful to treat bifurcations with PCI since they evaluate side-branch wire entry, calcification, lesion length, and post-stent-related complications that may interfere with the clinical outcomes.22 Two randomized control trials are currently evaluating the role of OCT in patients with bifurcated lesions (NCT03171311; NCT03507777).
Coronary artery calcification
Coronary artery calcification increases PCI complexity by impairing stent deployment, expansion, and apposition, which in turn increases the risk of stent thrombosis and restenosis.24 CA can detect—with a low-to-moderate sensitivity—the presence of coronary artery calcification with severe cases being visible without cardiac motion and contrast injection.25
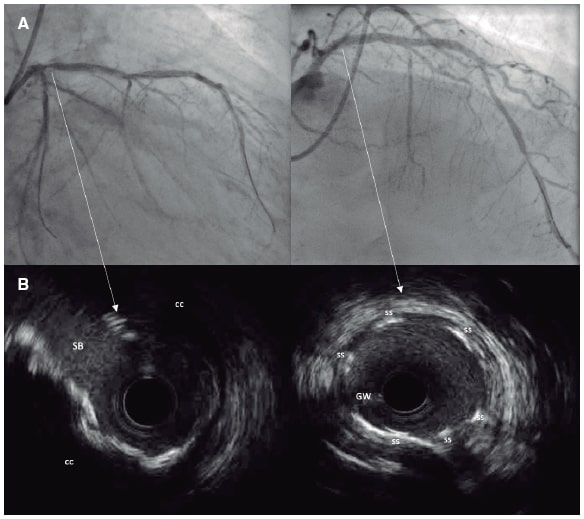
Calcified plaques appear as hyperechoic structures with a characteristic acoustic shadowing on the IVUS (figure 2).8 The IVUS can assess coronary artery calcification quantitatively (angle and length), semiquantitatively (absent or quadrant distribution), and qualitatively (depth of acoustic shadowing based on plaque and medial thickness).25 A study that compared IVUS to CA in 67 chronic total coronary occlusions (CTO) lesions found that IVUS was superior regarding the identification of calcium deposits (96% vs 61%).26 However, IVUS cannot evaluate microcalcifications (> 5 µm), but it can estimate the depth or thickness of calcium deposits.25
Figure 2. Coronary angiography (A) and intravascular ultrasound imaging (B) of ostial left anterior descending coronary artery showing severe concentric coronary calcification. Panels C, and D show the immediate outcomes after definitive stent implantation. CC, concentric calcification; GW, guidewire; SS, stent struts.
On the OCT, calcification appears as a heterogeneous structure with well-defined borders that can be used to offset some of the limitations of IVUS. Although the OCT has less depth penetration capabilities,25 its evaluation of the calcium thickness, area, and volume is more precise and reliable.27
The ICI analysis of calcium features can provide some insights for efficient planning to prevent stent underexpansion or malapposition.28,29 Therefore, calcium circumferences > 180º, thicknesses > 0.5 mm or lengths > 5 mm on the ICIs should involve adjunctive therapies to plaque modification.30 The OCT analysis of 31 patients from the Disrupt CAD study showed that calcium fractures were the leading mechanism of action of coronary lithotripsy and a tendency towards more adequate stent expansion was observed.31 Similar results were reported with rotational atherectomy where a study that evaluated 88 calcified lesions with both ICI modalities confirmed that OCT-guided rotational atherectomy was associated with better stent expansion (83% vs 72%; P = .0004), but with similar survival rates at the 1-year follow-up.32 Nevertheless, the IVUS may help while performing a PCI on the left main artery disease,33 where an early assessment can identify the optimal plaque modification technique to be used.34
Chronic total coronary occlusion lesions
The prevalence of CTO is 20% among patients with coronary artery disease. Performing a PCI on a CTO can improve the patients’ symptoms, workout abilities, and quality of life.35 The reported success rate for CTO procedures is estimated at 70% to 90%. With careful planning, better results can be achieved.36,37
IVUS provides an adequate and more efficient way to evaluate CTO features (cap ambiguity, lesion length, and calcification) allowing optimal re-entries into the true lumen with both antegrade and retrograde approaches.38 Studies that compared IVUS vs CA in PCIs performed on CTOs have yielded conflicting results regarding the rate of MACE (table 3).39-41 Furthermore, the IVUS has proven beneficial to predict restenosis in PCIs performed on CTOs where post-minimal luminal diameters of ≤ 2.4 mm and stent expansion rates of ≤ 70% were independent predictors of post-PCI restenosis at the mid-term follow-up, particularly in complex CTOs.42 A major limitation of IVUS guided PCIs performed on CTOs is the artifact generated by calcium rendering, which complicates the interpretation of images.38
Table 3. Invasive coronary angiography vs intracoronary imaging for percutaneous coronary interventions on chronic total coronary occlusions
| Reference | Type of study | IVUS vs CA (n) | Primary endpoint | Study outcomes |
|---|---|---|---|---|
| Tian et al.39 | Prospective RCT | 130 vs 130 | In-stent late lumen loss | • Rate of stent restenosis (3.9% vs 13.7%; P = .021) • Adverse event rate after 2 years (21.7% vs 25.2%; P = .641) |
| Hong et al.40 | Retrospective | 206 vs 328 | Stent thrombosis | • Similar rate of MACE in the matched cohort • Lower stent thrombosis in IVUS-guided PCI (0% vs 3%; P = .015) |
| Kim et al.41 | RCT | 201 vs 201 | Cardiac death | • Less MACE (HR, 0.35; 95%CI, 0.13–0.97) and stent thrombosis (0% vs 1.5%; P = .11) in IVUS-guided PCI |
|
CA, coronary angiography; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular event; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; OCT. |
||||
The main obstacle of the OCT in PCIs performed on CTOs is the need for contrast washout and the propagation of dissections due to the need for blood clearance, which is why it has been considered inadequate. However, this imaging modality could find its way into optimized PCIs performed on CTOs and follow-up monitorizations. A retrospective study reported a higher rate of stent malapposition and uncovered struts at 6-months after OCT examinations of patients with successful PCIs performed on CTOs.43 The ALSTER-OCT-CTO registry reported similar results after evaluating 111 lesions with OCT and saw a higher rate of malapposed and uncovered stent struts in CTOs vs non-CTO lesions at the 12-month follow-up.44
Coronary artery aneurysms
Coronary artery aneurysms are often clinically silent and can be identified in approximately 5% of all the patients undergoing CA. The most common causes are atherosclerosis in adults and Kawasaki disease in children. Coronary artery aneurysm is defined as a focal dilation of at least > 1.5 times the adjacent normal coronary artery while diffuse dilation is considered as coronary artery ectasia. Morphologically, when looked at from their maximum diameter, saccular and fusiform aneurysms can be seen with the former greater transverse rather than longitudinal diameter.45
The optimal ICI modality remains controversial, but historically IVUS has been the preferred approach for the evaluation and follow-up of coronary artery aneurysm in Kawasaki disease. The deeper penetration capabilities of IVUS allows us to assess the diameter of the vessel. Besides, it can accurately differentiate false from true coronary aneurysms by identifying a single-layered bulging. IVUS-guided preoperative planning is advised as aneurysms often go undersized with CA.45
Dionne et al. conducted an analysis of coronary artery aneurysms using OCT in a pediatric population with a past medical history of Kawasaki disease. The OCT proved to be safe, and similar findings (intimal hyperplasia, fibrosis, and media disruption) were observed in aneurysmal lesions compared to former histopathological studies. Nonetheless, these findings were also seen in non-aneurysmal coronary segments, which could drive the higher risk of ischemia in patients with a past medical history of Kawasaki disease.46
Left main coronary artery disease
The prevalence of LMCAD is 4% and, traditionally, coronary artery bypass graft has been the standard treatment with growing evidence to this date supporting PCI.47 Selecting the right imaging modality is important to determine accurately the clinical significance of LMCAD. CA remains the standard evaluation of choice, but it is subject to a high inter and intra-observer variability in the detection of intermediate lesions (30% to 70%).48 Consequently, intracoronary imaging can improve the assessment of LMCAD, and the long-term outcomes.
The importance of IVUS assessing the anatomy of LMCAD is evident given its more consistent tissue penetration capabilities that allows proper plaque evaluations. Former studies (table 4) described significant LMCAD with minimal lumen areas between 6 mm2 and 9 mm2 estimated using IVUS33,49 with values < 6 mm2 showing a good correlation with fractional flow reserve < 0.75.50 However, smaller areas have been reported in the Asian population.51 A multicenter prospective study that evaluated LMCAD with IVUS reported a similar rate of cardiac events after 2-years in patients undergoing revascularization with minimum lumen areas (MLA) < 6 mm2 (5.5%), as well as in those with MLAs ≥ 6 mm2 (2.3%) with revascularization deferral.33 Therefore, an angiographically ambiguous LMCAD with an IVUS-derived MLA > 6 mm2 can be considered non-ischemic whereas those with a MLA ≤ 4.5 mm2 could be deemed as ischemia-generating LMCAD. However, for those with a MLAs from 4.5 mm2 to 6 mm2, additional invasive or non-invasive assessment tools are required to rule out the presence of ongoing ischemia.52
Table 4. Summary of the studies that evaluated invasive coronary imaging for the assessment of left main coronary artery disease
| Reference | Type of study | ICI use | Follow-up time | Outcomes |
|---|---|---|---|---|
| De la Torre Hernandez et al.33 | Prospective multileft | IVUS | 2 years | Defer PCI with MLA > 6 mm2 is safe |
| Fassa et al.49 | Prospective | IVUS | 3 years | Defer PCI with MLA ≥ 7.5 mm2 is safe |
| Jasti et al.50 | Prospective | IVUS | 3 years | MLA < 5.9 mm2 is well correlated with a FFR < 0.75 |
| Park et al.51 | Prospective | IVUS | NA | FFR < 0.8 had a good correlation with MLA ≤ 4.5 mm2 among Asians |
|
ICI, intracoronary imaging; IVUS, intravascular ultrasound; MLA, minimum lumen area; PCI, percutaneous coronary intervention. |
||||
Former studies have demonstrated that plaque burdens > 60% in non-LMCAD is a predictor of MACE and can be recognised when assessing the risk of future events after PCI.4 Through IVUS analysis, it was shown that the larger the plaque burden in the LMCAD, the greater the overall plaque burden in the coronary tree.53 However, in the PROSPECT study a greater plaque burden was not associated with a higher rate of MACE as opposed to the overall plaque burden (hazard ratio, 1.06; 95%CI, 1.01–1.11; P = .02).54 Therefore, the IVUS assessment of the LMCAD plaque burden can identify high-risk patients with coexisting non-LMCAD atherosclerotic disease.
The role of IVUS in LMCAD is not limited to diagnosis only (table 5).55-59 A meta-analysis that compared IVUS-guided vs CA-guided PCI in LMCAD found that the former was associated with less cardiovascular mortality (RR, 0.47; 95%CI, 0.33–0.66; P < .001), new target lesion revascularization (RR, 0.43; 95%CI, 0.25–0.73; P = .002), and stent thrombosis (RR, 0.28; 95%CI, 0.12–0.67; P = .004).60 Also, de la Torre Hernández et al. reported that IVUS-guided PCI was particularly useful in distal lesions with a lower event rate compared to non-IVUS guided PCI (hazard ratio, 0.54; 95%CI, 0.34–0.90).56 Other studies have proposed a role for IVUS in the optimization of LMCAD after stent deployment where minimum lumen areas were associated with stent underexpansion and could predict in-stent restenosis with different thresholds regarding the assessed segment (8 mm2 for the proximal left main coronary artery, 6 mm2 for the ostium of the left anterior descending coronary artery, and 5 mm2 for the ostium of the left circumflex artery).61
Table 5. Summary of the studies that compared IVUS-guided vs coronary angiography-guided percutaneous coronary interventions on left main coronary artery disease
| Reference | Endpoints | Outcomes |
|---|---|---|
| Park et al.55 | • Primary endpoint was all-cause mortality • Secondary endpoints were MI, TVR, and the composite endpoint |
• IVUS-guided PCI was associated with a lower rate of overall mortality (HR, 0.31; 95%CI, 0.19–0.51), and MI (HR, 0.470; 95%CI, 0.33-0.67). • The risk of TVR (HR, 0.47; 95%CI, 0.33-0.67) did not decrease with IVUS guidance |
| De la Torre Hernandez et al.56 | • Primary endpoint was MACE (cardiac death, MI, TLR) • Secondary endpoints were all-cause mortality, cardiac death, infarction-free survival, TLR-free survival, and the rate of ST |
• The 3-year rate of all-cause mortality was lower with IVUS-guided PCI (4.7% vs 16%; P = .048) • Lower rate of ST with IVUS-guided PCI (0.6% vs 2.2%; P = .04) • IVUS-guided PCI of LMCAD was associated with minor adverse events in distal lesions (HR, 0.34; 95%CI, 0.34-0.90), and in the overall population (HR, 0.70; 95%CI, 0.52-0.99) |
| Gao et al.57 | • Primary endpoint was the 1-year rate of MACE (cardiac death, MI, TVR) • Safety outcome was ST |
• The 1-year rate of MACE in the IVUS-guided group was lower (14.8% vs 27.7%) • Coronary angiography-guided PCI was associated with a higher rate of ST (2.7% vs 0.6%; P = .026) |
| Tan et al.58 | • 2-year rate of MACE (death, MI or TLR) | • Similar event rate regarding SR (3.28% vs 8.15%; P = .11), and ST (1.6% vs 3.2%; P = .568) • The IVUS-guided PCI was associated with a lower rate of MACE (OR, 0.414; 95%CI, 0.129-0.867), and TLR (8.2% vs 19%; P = .045) |
| Andell et al.59 | • Primary endpoint was a composite endpoint of all-cause mortality, SR, and ST) • Secondary endpoints were all-cause mortality, SR, ST, and unexplained death within 30-days |
• The IVUS group was associated with fewer composite endpoints (HR, 0.65; 95%CI, 0.50–0.84) and a lower all-cause mortality rate (HR, 0.62; 95%CI, 0.47–0.82) • Not differences were seen in the rate of ST and SR |
|
95%CI, 95% confidence interval; HR, hazard ratio; IVUS, intravascular ultrasound; LMCAD, left main coronary artery disease; MACE, major adverse cardiovascular event; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; SR, stent restenosis; ST, stent thrombosis; TLR, target lesion revascularization; TVR, target vessel revascularization. |
||
On the contrary, the OCT has limited utility in the assessment of LMCAD given its average diameter (3 mm to 5 mm) and inability to evaluate aorto-ostial lesions where blood-free fields are difficult to achieve.48 A multicenter retrospective study (ROCK cohort II) recently reported a lower 1-year rate of target lesion failure in intravascular imaging guided vs angiographically guided distal PCIs on the LMCA (12.7% vs 21.2%; P = .039) with similar outcomes between the OCT and the IVUS (P = .26).62 However, future prospective data supporting OCT-guided PCI is expected to better define the optimal clinical management of patients with LMCAD (NCT04248777, NCT04391413, NCT03474432, NCT03820492, and NCT04531007).
Spontaneous coronary artery dissections
Spontaneous coronary artery dissection is a classically misdiagnosed life-threatening condition that can occur in otherwise healthy individuals. Coronary flow is compromised after the development of a false lumen through an “inside-out” or “outside-in” mechanism. The Yip-Saw coronary classification has revealed the limitations of coronary angiography. Diagnosis is particularly challenging with type 2 (diffuse smooth stenosis) and type 3 (mimic atherosclerotic stenosis) spontaneous coronary artery dissections.63,64
The benefits of implementing ICIs (table 6) for diagnostic purposes or even to guide coronary intervention in spontaneous coronary artery dissections are their higher resolution.65,66 IVUS has a deeper power of penetration to visualize the vessel wall and intramural hematoma, consequently, it is also recommended for proximal dissections8. It can also differentiate between true and false lumens once fused with color interpolation. However, the OCT is more sensitive regarding the identification of subtle signs such as an intimal tear (entry site to the false lumen), and Ribero et al. used it for establishing the mechanism behind coronary dissection.67
Table 6. Benefits of intravascular imaging modalities in spontaneous coronary artery dissection
| IVUS | OCT |
|---|---|
| Intramural hematoma (complete visualization of the vessel wall) | Detail characterization of the intimal flap (intimal-medial disruption) |
| True and false lumen (with IVUS and ChromaFlo*) | Connection between true-false lumen (entry tear) |
| Thrombosis of the false lumen | Involvement of side branches and/or thrombus |
| Guidewire position | Guidewire position |
|
IVUS, intravascular ultrasound; OCT, optical computed tomography. * Chromaflo Volcano (Philips, United States). |
|
If the ICI is deemed necessary during the management of spontaneous coronary dissection, it is important to acknowledge that there is a risk of procedural complications (eg, dissections with contrast injection during the OCT, especially in type I spontaneous dissections or lead to vessel occlusion). A study of 28 patients with spontaneous coronary artery dissections found that intracoronary imaging assessment was associated with iatrogenic wire (3.5%) and guide catheter dissections (3.5%), but also with propagation with wiring (10.7%) or advancement of the OCT catheter (3.5%).68 Therefore, the benefit may be greater in cases of diagnostic uncertainty or with complex dissections requiring PCI.
Heart transplant vasculopathy
The clinical presentation of cardiac allograft vasculopathy is often silent. Still, it is characterized by aggressive and concentric diffuse fibromuscular dysplasia. The International Society for Heart and Lung Transplantation classifies allograft vasculopathy into 4 categories based on graft function and angiographic findings having CAV2 and CAV3 the worst prognosis of all.69 CAV is considered the gold standard technique for routine screening and definitive diagnosis.
Heart transplant patients may present with an intimal thickening identifiable through IVUS only. Former studies have reported that an intimal thickening > 0.5 mm from baseline is associated with a higher rate of adverse events within the first year after the heart transplant.70,71 Consistent with these findings, volumetric studies with IVUS have shown that the combination of intimal thickening plus negative remodelling of the proximal left anterior descending coronary artery were associated with acute rejection and major adverse events within the first year.72 However, the OCT can identify early stages of intimal thickening in the form of intimal hyperplasia (thickness > 100 µm), and improve the clinical outcomes.73
Post-stent findings
Both imaging modalities have been used to identify stent underexpansion, incomplete apposition, and edge dissection as potential causal mechanisms of stent failure.
In this regard, minimal stent area (MSA) is associated with both restenosis and stent thrombosis. IVUS studies reported MSAs between 5.3 mm2 and 5.7 mm2 with smaller areas identified in patients with definitive stent restenosis at the short-term follow-up after stent implantation.74,75 Similarly, 2 studies reported that MSAs < 5 mm2 as seen on the OCT were associated with a higher rate of target lesion revascularization and stent thrombosis with drug-eluting stents.76,77 On the contrary, stent patency assessed through the OCT suggested that values > 4.5 mm2 had a lower rate of MACE,76 but higher cut-off values for proximal (> 8 mm2) and distal (> 7 mm2) LMCA with IVUS assessment. Therefore, clinical guidelines recommend a post-PCI MSA/mean reference lumen of > 80%.
A series of OCT registries observed that a common leading mechanism responsible for early (1 to 30 days), late (1 to < 12 months), and very late (> 1 year) thrombus formation is the malapposition (axial distance > 0.4 mm with a longitudinal extension > 1 mm) of stented segments.78-80 Consistent with this, stent edge dissection is also associated with adverse events as seen on the CLI-OPCI II study, where distal stent edge dissections > 200 µm had a higher rate of MACE.76
SAFETY
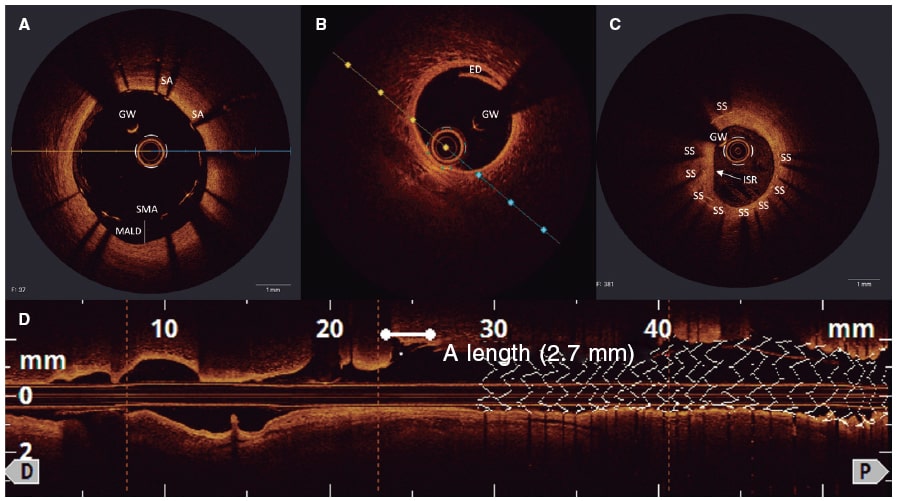
The development of ICI techniques has resulted in significant clinical improvements, but they are not free from procedural complications (figure 3).
Figure 3. Post-stent findings with optical coherence tomography: A: stent malapposition from the 16 o’clock to the 19 o’clock position; B and D: edge dissection with flap (white asterisk) distal to an implanted drug-eluting stent; C: stent restenosis (white arrow) due to concentric neointimal proliferation; the stent struts are visible under the homogenous bright layer. ED, edge dissection; GW, guidewire; ISR, in-stent restenosis; MALD, malapposed distance; SA, strut apposition; SMA, strut malapposition; SS, stent struts.
Safety trials on IVUS have reported an estimated rate of complications between 1% to 3%, mostly associated with the size of the catheter. The setback of CA is the use of contrast materials to enhance image quality with the inherent risk of contrast-induced nephropathy.81 On this regard, a small retrospective study of 37 patients with advanced kidney disease evaluated the safety of IVUS-guided zero contrast PCI without a higher rate of renal replacement therapy or MACE being reported.82 Similar findings were described in a prospective and multicenter study,83 and a randomized control trial.84 Safety and feasibility have also been assessed on the OCT without a higher rate of MACE,85 procedural complications or acute kidney injury being reported.86 Additionally, data from 2 prospective studies suggests that contrast-less OCT would be a feasible imaging modality.87,88
In former studies that compared ICI modalities (table 7), similar complication rates were reported.89-91 Van der Sijde et al. used a prospective study to compare the procedural complications of both ICIs and did not observe a higher event rate during image acquisition. Also, they did not identify any potential risk factors regarding major adverse events suggesting that both the safety and feasibility of ICIs are greater than expected and unrelated to the operator’s experience.92
Table 7. Summary of studies comparing IVUS vs OCT and/or CA for PCI guidance
| Reference | Type of study | ICI modality | Outcomes |
|---|---|---|---|
| Ali et al.89 | Multileft RCT | OCT vs IVUS vs CA | No differences in procedural MACEa were reported between OCT (3%) and IVUS (1%; P = .37) and CA (1%; P = .37) Similar rate of procedural complication |
| Habara et al.90 | Prospective RCT | OCT vs IVUS | Similar rate of procedural time (40 ± 16.4 min vs 47 ± 17.6 min; P = .09), and fluoroscopy time (20.4 ± 8.4 min vs 24.8 ± 10.4 min; P = .05) Similar rate of complications, no deaths reported (P > .99) |
| Kubo et al.91 | Prospective multileft RCT | OCT vs IVUS | Similar rates of cardiac death (0% vs 0.2%; P = .99) and MACEb (2.9% vs 3.5%; P = .81) No contrast-induced nephropathy reported with a similar rate of complications between the groups |
| Van der Sijde et al.92 | Single-left Prospective |
OCT vs IVUS | Similar rate of procedural cardiac events (< 1%) No predictors of adverse events were identified |
|
CA, coronary angiography; ICI, intracoronary imaging; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular event; OCT, optical computed tomography; RCT, randomized controlled trial. a Defined as procedural complications (angiographic dissection, perforation, thrombus, or acute closure), and active procedures (balloon inflations, additional stent implantations or pericardiocentesis). b Defined as a composite of cardiac death, myocardial infarction or ischemia-driven target lesion revascularization. |
|||
CONCLUSIONS
Beyond the limitations of coronary angiography, coronary assessment remains complex given the different forms of presentation. Therefore, the ideal imaging modality would be one that is easy to use, interpret, and safe. Intracoronary imaging guidance is widely recognized for diagnosis, PCI planning, and to guide post-PCI treatment. However, there is still room for improvement, and future randomized studies will contribute to the wider adoption of these imaging modalities in all cath labs.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Á. Aparisi drafted the original manuscript. Á. Aparisi, H. Cubero-Gallego, and H. Tizón-Marcos were involved in the process of critical revision of the manuscript regarding significant intellectual content, and eventually wrote the final version. All authors read and gave their publication consent for this version of the manuscript.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812-16823.
2. Collet C, Grundeken MJ, Asano T, Onuma Y, Wijns W, Serruys PW. State of the art:coronary angiography. EuroIntervention. 2017;13:634-643.
3. Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease [published correction in Lancet. 2017;390:1026]. Lancet. 2017;390:793-809.
4. Räber L, Mintz GS, Koskinas KC, et al. Clinical use of intracoronary imaging. Part 1:guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions [published correction in Eur Heart J. 2019;40:308]. Eur Heart J. 2018;39:3281-3300.
5. Garcia-Garcia HM, Fernández-Peregrina E, and KOK, Diletti R. Ongoing large randomized clinical trials on complex percutaneous coronary interventions:intravascular imaging-guided trials. REC Interv Cardiol. 2021;3:297-303.
6. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization:A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;79:e21-e129.
7. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization [published correction in Eur Heart J. 2019;40:3096]. Eur Heart J. 2019;40:87-165.
8. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
9. Xu J, Lo S. Fundamentals and role of intravascular ultrasound in percutaneous coronary intervention. Cardiovasc Diagn Ther. 2020;10:1358-1370.
10. Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular Ultrasound Imaging-Guided Vs Coronary Angiography-Guided Percutaneous Coronary Intervention:A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e013678.
11. Ali ZA, Karimi Galougahi K, Maehara A, et al. Intracoronary Optical Coherence Tomography 2018:Current Status and Future Directions. JACC Cardiovasc Interv. 2017;10:2473-2487.
12. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies:a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072.
13. Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone vs angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention:the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823-829.
14. Magnus PC, Jayne JE, Garcia-Garcia HM, et al. Optical coherence tomography vs intravascular ultrasound in the evaluation of observer variability and reliability in the assessment of stent deployment:the OCTIVUS study. Catheter Cardiovasc Interv. 2015;86:229-235.
15. Abnousi F, Waseda K, Kume T, et al. Variability in quantitative and qualitative analysis of intravascular ultrasound and frequency domain optical coherence tomography. Catheter Cardiovasc Interv. 2013;82:E192-E199.
16. Collet C, Conte E, Mushtaq S, et al. Reviewing imaging modalities for the assessment of plaque erosion. Atherosclerosis. 2021;318:52-59.
17. Khalifa AKM, Kubo T, Ino Y, et al. Optical Coherence Tomography Comparison of Percutaneous Coronary Intervention Among Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. Circ J. 2020;84:911-916.
18. Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748-1758.
19. Terada K, Kubo T, Kameyama T, et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc Imaging. 2021;14:1440-1450.
20. Kim N, Lee JH, Jang SY, et al. Intravascular modality-guided vs angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter Cardiovasc Interv. 2020;95:696-703.
21. Sawaya FJ, Lefèvre T, Chevalier B, et al. Contemporary Approach to Coronary Bifurcation Lesion Treatment. JACC Cardiovasc Interv. 2016;9:1861-1878.
22. Burzotta F, Lassen JF, Lefèvre T, et al. Percutaneous coronary intervention for bifurcation coronary lesions:the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307-1317.
23. Watanabe M, Uemura S, Sugawara Y, et al. Side branch complication after a single-stent crossover technique:prediction with frequency domain optical coherence tomography. Coron Artery Dis. 2014;25:321-329.
24. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression:What Does it Really Mean?. JACC Cardiovasc Imaging. 2018;11:127-142.
25. Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461-471.
26. Fujii K, Ochiai M, Mintz GS, et al. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006;97:1455-1462.
27. Mehanna E, Bezerra HG, Prabhu D, et al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J. 2013;77:2334-2340.
28. Wang X, Matsumura M, Mintz GS, et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc Imaging. 2017;10:869-879.
29. Kobayashi Y, Okura H, Kume T, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209-2214.
30. Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189.
31. Ali ZA, Brinton TJ, Hill JM, et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions:First Description. JACC Cardiovasc Imaging. 2017;10:897-906.
32. Teng W, Li Q, Ma Y, et al. Comparison of optical coherence tomography-guided and intravascular ultrasound-guided rotational atherectomy for calcified coronary lesions. BMC Cardiovasc Disord. 2021;21:290.
33. de la Torre Hernandez JM, Hernández Hernandez F, Alfonso F, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351-358.
34. Sakakura K, Yamamoto K, Taniguchi Y, Tsurumaki Y, Momomura SI, Fujita H. Intravascular ultrasound enhances the safety of rotational atherectomy. Cardiovasc Revasc Med. 2018;19(3 Pt A):286-291.
35. Khan AA, Khalid MF, Ayub MT, et al. Outcomes of Percutaneous Coronary Intervention Vs Optimal Medical Treatment for Chronic Total Occlusion:A Comprehensive Meta-analysis. Curr Probl Cardiol. 2021;46:100695.
36. Wang N, Fulcher J, Abeysuriya N, Adams M, Lal S. Predictors of successful chronic total occlusion percutaneous coronary interventions:a systematic review and meta-analysis. Heart. 2018;104:517-524.
37. Ellis SG, Burke MN, Murad MB, et al. Predictors of Successful Hybrid-Approach Chronic Total Coronary Artery Occlusion Stenting:An Improved Model With Novel Correlates. JACC Cardiovasc Interv. 2017;10:1089-1098.
38. Galassi AR, Sumitsuji S, Boukhris M, et al. Utility of Intravascular Ultrasound in Percutaneous Revascularization of Chronic Total Occlusions:An Overview. JACC Cardiovasc Interv. 2016;9:1979-1991.
39. Tian NL, Gami SK, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound- vs angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions:two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409-1417.
40. Hong SJ, Kim BK, Shin DH, et al. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second-generation drug-eluting stents for chronic total occlusions (from the Multicenter Korean-Chronic Total Occlusion Registry) [published correction in Am J Cardiol. 2014;114:1937]. Am J Cardiol. 2014;114:534-540.
41. Kim BK, Shin DH, Hong MK, et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Vs Biolimus-Eluting Stent Implantation:Randomized Study. Circ Cardiovasc Interv. 2015;8:e002592.
42. Kang J, Cho YS, Kim SW, et al. Intravascular Ultrasound and Angiographic Predictors of In-Stent Restenosis of Chronic Total Occlusion Lesions. PLoS One. 2015;10:e0140421.
43. Jia H, Hu S, Liu H, et al. Chronic total occlusion is associated with a higher incidence of malapposition and uncovered stent struts:OCT findings at 6 months following DES implantation. Catheter Cardiovasc Interv. 2017;89:582-591.
44. Heeger CH, Busjahn A, Hildebrand L, et al. Delayed coverage of drug-eluting stents after interventional revascularisation of chronic total occlusions assessed by optical coherence tomography:the ALSTER-OCT-CTO registry. EuroIntervention. 2016;11:1004-1012.
45. Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M. Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018;11:1211-1223.
46. Dionne A, Ibrahim R, Gebhard C, et al. Coronary Wall Structural Changes in Patients With Kawasaki Disease:New Insights From Optical Coherence Tomography (OCT). J Am Heart Assoc. 2015;4:e001939.
47. Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents vs coronary artery bypass grafting in left main coronary artery disease:an individual patient data meta-analysis. Lancet. 2021;398:2247-2257.
48. Collet C, Capodanno D, Onuma Y, et al. Left main coronary artery disease:pathophysiology, diagnosis, and treatment. Nat Rev Cardiol. 2018;15:321-331.
49. Park SJ, Ahn JM, Kang SJ, Yoon SH, Koo BK, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Park SW. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7:868-874.
50. Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831-2836.
51. Park SJ, Ahn JM, Kang SJ, Yoon SH, Koo BK, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Park SW. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7:868-874.
52. Johnson TW, Räber L, di Mario C, et al. Clinical use of intracoronary imaging. Part 2:acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making:an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40:2566-2584.
53. Uchida Y, Ichimiya S, Ishii H, et al. Impact of plaque burden in the left main coronary artery determined by intravascular ultrasound on cardiovascular events in a Japanese population undergoing percutaneous coronary intervention. Am J Cardiol. 2012;109:352-358.
54. Shimizu T, Mintz GS, De Bruyne B, et al. Relationship between left main coronary artery plaque burden and nonleft main coronary atherosclerosis:results from the PROSPECT study. Coron Artery Dis. 2018;29:397-402.
55. Park SJ, Kim YH, Park DW, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167-177.
56. de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease:pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014;7:244-254.
57. Gao XF, Kan J, Zhang YJ, et al. Comparison of one-year clinical outcomes between intravascular ultrasound-guided vs angiography-guided implantation of drug-eluting stents for left main lesions:a single-center analysis of a 1,016-patient cohort. Patient Prefer Adherence. 2014;8:1299-1309.
58. Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36:549-553.
59. Andell P, Karlsson S, Mohammad MA, et al. Intravascular Ultrasound Guidance Is Associated With Better Outcome in Patients Undergoing Unprotected Left Main Coronary Artery Stenting Compared With Angiography Guidance Alone. Circ Cardiovasc Interv. 2017;10:e004813.
60. Ye Y, Yang M, Zhang S, Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound:A meta-analysis. PLoS One. 2017;12:e0179756.
61. Kang SJ, Ahn JM, Song H, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562-569.
62. Cortese B, de la Torre Hernandez JM, Lanocha M, et al. Optical coherence tomography, intravascular ultrasound or angiography guidance for distal left main coronary stenting. The ROCK cohort II study. Catheter Cardiovasc Interv. 2021;99:664– 673.
63. Adlam D, Alfonso F, Maas A, Vrints C;Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group:a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368.
64. Hayes SN, Tweet MS, Adlam D, et al. Spontaneous Coronary Artery Dissection:JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:961-984.
65. Cerrato E, Giacobbe F, Rolfo C, et al. Role of Invasive and Non-invasive Imaging Tools in the Diagnosis and Optimal Treatment of Patients with Spontaneous Coronary Artery Dissection. Curr Cardiol Rep. 2019;21:122.
66. Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection:novel insights on diagnosis and management. Cardiovasc Diagn Ther. 2015;5:133-140.
67. Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073-1079.
68. Macaya F, Salazar CH, Pérez-Vizcayno MJ, et al. Feasibility and Safety of Intracoronary Imaging for Diagnosing Spontaneous Coronary Artery Dissection. JACC Cardiovasc Imaging. 2019;12:763-764.
69. Lee MS, Tadwalkar RV, Fearon WF, et al. Cardiac allograft vasculopathy:A review. Catheter Cardiovasc Interv. 2018;92:E527-E536.
70. Olymbios M, Kwiecinski J, Berman DS, Kobashigawa JA. Imaging in Heart Transplant Patients. JACC Cardiovasc Imaging. 2018;11:1514-1530.
71. Javaheri A, Saha N, Lilly SM. How to Approach the Assessment of Cardiac Allograft Vasculopathy in the Modern Era:Review of Invasive Imaging Modalities. Curr Heart Fail Rep. 2016;13:86-91.
72. Okada K, Kitahara H, Yang HM, et al. Paradoxical Vessel Remodeling of the Proximal Segment of the Left Anterior Descending Artery Predicts Long-Term Mortality After Heart Transplantation. JACC Heart Fail. 2015;3:942-952.
73. Ichibori Y, Ohtani T, Nakatani D, et al. Optical coherence tomography and intravascular ultrasound evaluation of cardiac allograft vasculopathy with and without intimal neovascularization. Eur Heart J Cardiovasc Imaging. 2016;17:51-58.
74. Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305-1310.
75. Doi H, Maehara A, Mintz GS, et al. Impact of Post-Intervention Minimal Stent Area on 9-Month Follow-Up Patency of Paclitaxel-Eluting Stents An Integrated Intravascular Ultrasound Analysis From the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interventions. 2009;2:1269-1275.
76. Prati F, Romagnoli E, Burzotta F, et al. Clinical Impact of OCT Findings During PCI The CLI-OPCI II Study. JACC Cardiovasc Imaging. 2015;8:1297-1305.
77. Meneveau N, Souteyrand G, Motreff P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non–ST-Elevation Acute Coronary Syndrome. Circulation. 2016;134:906-917
78. Adriaenssens T, Joner M, Godschalk TC, et al. Optical Coherence Tomography Findings in Patients With Coronary Stent Thrombosis. Circulation. 2017;136:1007-1021.
79. Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography:insights from the national PESTO French registry. Eur Heart J. 2016;37:1208-1216.
80. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133:650-660.
81. Batkoff BW, Linker DT. Safety of intracoronary ultrasound:Data from a multicenter European registry. Catheter Cardio Diag. 1996;38:238-241.
82. Ali ZA, Galougahi KK, Nazif T, et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure:a feasibility, safety, and outcome study. Eur Heart J. 2016;37:3090-3095.
83. Sakai K, Ikari Y, Nanasato M, et al. Impact of intravascular ultrasound-guided minimum-contrast coronary intervention on 1-year clinical outcomes in patients with stage 4 or 5 advanced chronic kidney disease. Cardiovasc Intervention Ther. 2019;34:234-241.
84. Mariani J, Guedes C, Soares P, et al. Intravascular Ultrasound Guidance to Minimize the Use of Iodine Contrast in Percutaneous Coronary Intervention The MOZART (Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasTy) Randomized Controlled Trial. JACC Cardiovasc Interventions. 2014;7:1287-1293.
85. Lehtinen T, Nammas W, Airaksinen JKE, Karjalainen PP. Feasibility and safety of frequency-domain optical coherence tomography for coronary artery evaluation:a single-center study. Int J Cardiovasc Imaging. 2013;29:997-1005.
86. Khurwolah MR, Meng H-Y, Wang Y-S, Wang L-S, Kong X-Q. Safety and efficacy of frequency-domain optical coherence tomography in evaluating and treating intermediate coronary lesions. World J Cardiol. 2018;10:222-233.
87. Gupta A, Chhikara S, Vijayvergiya R, et al. Saline as an alternative to radio-contrast for optical coherence tomography guided percutaneous coronary intervention:A prospective comparison. Cardiovasc Revasc Med. 2022;34:86-91.
88. Gore AK, Shlofmitz E, Galougahi KK, et al. Prospective Comparison Between Saline and Radiocontrast for Intracoronary Imaging With Optical Coherence Tomography. JACC Cardiovasc Imaging. 2020;13:2060-2062.
89. Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III:OPTIMIZE PCI):a randomised controlled trial. Lancet. 2016;388:2618-2628.
90. Habara M, Nasu K, Terashima M, et al. Impact of Frequency-Domain Optical Coherence Tomography Guidance for Optimal Coronary Stent Implantation in Comparison With Intravascular Ultrasound Guidance. Circ Cardiovasc Interv. 2012;5:193-201.
91. Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs intravascular ultrasound in percutaneous coronary intervention (OPINION trial):one-year angiographic and clinical results. Eur Heart J. 2017;38:3139-3147.
92. Sijde JN van der, Karanasos A, Ditzhuijzen NS van, et al. Safety of optical coherence tomography in daily practice:a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imaging. 2017;18:467-474.