ABSTRACT
Introduction and objectives: Between 10% and 25% of patients hospitalized due to an acute coronary syndrome develop acute kidney injury, a condition associated with higher morbidity and mortality rates. Scores have been developed to predict the occurrence of post-coronary angiography contrast-induced nephropathy (CIN) in patients with acute coronary syndrome. The objective of this study was to assess the association between microalbuminuria and post-coronary angiography CIN in patients with acute coronary syndrome.
Methods: Patients admitted with acute coronary syndrome in whom a coronary angiography was performed during their hospitalization and with urinary albumin-to-creatinine ratio (ACR) assessment within the first 24 hours were analyzed. The best ACR cutoff value for coronary angiography-induced CIN was determined using the C-statistic measure. The receiver operating characteristic (ROC) curves were built to compare between the predictive ability of the Mehran score alone and also in combination with the ACR.
Results: A total of 148 patients were analyzed. Median age was 64 years (56-73), 35% were women, mean creatinine clearance rate at admission was 86 mL/min (66-107) and the ACR was 5 mg/g (0-14). The analysis showed that 9.6% of the patients developed post-coronary angiography CIN with ACR levels ≥ 20 mg/g compared to 1.6% when these levels were < 20 mg/g. The area under the ROC curve of the Mehran score to predict the development of post-coronary angiography CIN was 0.75 (95%CI, 0.68-0.81) and when the ACR was added it went up to 0.82 (95%CI, 0.76-0.87).
Conclusions: The ACR levels at admission were associated with the development of post-coronary angiography CIN and bring added value to an already validated predictive score. Therefore, the ACR should be used as a simple and accessible tool to detect and prevent this severe complication in patients with acute coronary syndrome.
Keywords: Contrast media. Coronary angiography. Microalbuminuria. Contrast-induced nephropathy. Urine albumin-to-creatinine ratio.
RESUMEN
Introducción y objetivos: Entre el 10 y el 25% de los pacientes hospitalizados por síndrome coronario agudo desarrollan insuficiencia renal aguda, lo que aumenta la morbimortalidad. Existen escalas para predecir la aparición de nefropatía inducida por contraste (NIC) tras la realización de una angiografía coronaria en pacientes con síndrome coronario agudo. El objetivo de este estudio fue evaluar la asociación entre el índice albúmina-creatinina (IAC) urinario y el desarrollo de NIC tras una angiografía coronaria en pacientes con síndrome coronario agudo.
Métodos: Se analizaron pacientes internados por síndrome coronario agudo a quienes se realizó angiografía coronaria durante el ingreso, con el cálculo del IAC en las primeras 24 horas. Se determinó el mejor valor de corte por curva ROC (Receiver Operating Characteristic)del IAC asociado a NIC. Se compararon las curvas ROC de la escala de Mehran sola y con el agregado de la variable de IAC.
Resultados: Se analizaron 148 pacientes. La mediana de la edad fue de 64 años (56-73), el 35% eran mujeres, el aclaramiento de creatinina fue de 86 ml/min (66-107) y el IAC de 5 mg/g (0-14). El 9,6% de los pacientes desarrollaron NIC tras la angiografía coronaria cuando su IAC fue ≥ 20 mg/g y el 1,6% cuando fue < 20 mg/g. El área bajo la curva ROC de la escala de Mehran para predecir el desarrollo de NIC tras la angiografía coronaria fue de 0,75 (intervalo de confianza del 95% [IC95%], 0,68-0,81); cuando se agregó la variable de IAC fue de 0,82 (IC95%, 0,76-0,87).
Conclusiones: El IAC basal se asoció con el desarrollo de NIC tras la angiografía coronaria. Al añadirlo a la escala de Mehran aumentó la capacidad discriminativa. El IAC podría ser una herramienta de simple uso, bajo costo y amplia disponibilidad para detectar pacientes en riesgo de desarrollar NIC y adoptar medidas preventivas apropiadas.
Palabras clave: Contraste intravenoso. Angiografía coronaria. Microalbuminuria. Nefropatía inducida por contraste. Índice albúmina-creatinina urinario.
Abbreviations: ACR: Albumin-to-creatinine ratio. ACS: Acute coronary syndrome. AKI: Acute kidney injury. CIN: Contrast-induced nephropathy.
INTRODUCTION
Renal function impairment is associated with poor prognosis in patients with stable or acute coronary syndrome (ACS). One of the most common causes of acute kidney injury (AKI) in hospitalized patients is the nephropathy induced by the IV administration of contrast agents.1Its incidence varies between 1% and 6%, and increases considerably in high-risk conditions like in the ACS setting. The reported frequency of post-coronary angiography contrast-induced nephropathy (CIN) goes from 12% to 46% in patients with ACS.2,3
There are several potential causes that trigger CIN in patients without a past medical history of kidney failure such as hemodynamic instability, the IV administration of contrast agents, thromboembolic events, and adverse drug reactions, among others. Also, it is important to consider the type of contrast used, its osmolarity, the volume administered, and the lack of preventive measures.4-6
Because CIN is associated with poor prognosis in hospitalized patients, predictive scores have been designed to identify the most vulnerable patients who can develop this complication. The Mehran score is one of the most popular indices to estimate the chances of post-coronary angiography CIN.7
It is well-established that microalbuminuria is a predictor of kidney dysfunction mainly in diabetic and hypertensive patients.8-14Also, there is a correlation between high levels of microalbuminuria and the poor outcomes seen in patients with ACS.15-16 Currently, microalbuminuria is estimated through the dosage of the albumin-to-creatinine ratio (ACR) through a simple urine sample.17
The objective of this study is to calculate microalbuminuria using the ACR as a predictive variable of post-coronary angiography CIN in patients with ACS.
METHODS
Population
Patients with ACS consecutively admitted to the coronary care unit of a community hospital were analyzed. Those undergoing an in-hospital coronary angiography with non-ionic, hyperosmolar IV contrast agents such as iopamidol, optiray or xenetix, were included in the study. The volume of IV contrast for each angiographic study was calculated retrospectively. It was estimated that each injection of contrast material into the left coronary artery required an average 10 cc to 8 cc for the right coronary artery.
Patients with a past medical history of renal failure, macroalbuminuria, treatment with diuretics and patients with secondary angina were excluded from the study.
The urinary ACR was assessed in all patients included in the study using an immunoturbidimetric assay in simple urine samples within the first 24 hours after hospitalization.
Definitions
IV contrast-induced nephropathy(CIN) was defined as an increase in serum creatinine levels ≥ 25% 48 hours after performing the coronary angiography or an absolute increase of ≥ 0.5 mg/dL compared to levels at admission.
Microalbuminuria was defined as an abnormal urinary albumin excretion rate between 30 to 200 mg/min or 30 to 229 mg/day.
The study protocol was approved by the center review board and conducted in compliance with the Declaration of Helsinki, good clinical practice guidelines, and local regulatory requirements. Informed consents were obtained from all patients.
Biochemical considerations
A urine sample collected within the first 24 hours after admission (preferably during morning hours) was centrifuged at 3000 rpm and stored at -20° Celsius until biochemical analysis was conducted. The principle of the ACR test is immunoturbidimetry. This method is based on the reaction of human albumin antibodies to the antigen. Complexes are then measured after agglutination. The COBAS 6000 analyzer (ROCHE, Switzerland) was used to process the sample. The analytical detection limits of the assay were between 3 mg/g and 400 mg/g. The test variation coefficient was 3.8%.
Statistical analysis
The Kolmogorov-Smirnov test was used to analyze the distribution of continuous variables and their kurtosis-skewness measures. Data were expressed as mean and standard deviation or as median with interquartile range (25%-75%) and compared using Student’s t test or Mann-Whitney-Wilcoxon test for independent groups according to their parametric or non-parametric distribution, respectively.
Discrete variables were expressed as percentages and compared using the chi-square test. The cross-product ratio was expressed as odds ratio (OR) with its 95% confidence interval (95%CI). The C-statistic measure was used to detect the best ACR cutoff value associated with the primary endpoint and compare the discrimination capacity of the Mehran score alone and with the ACR combined.
A multivariable regression analysis will be built to predict CIN including ACR and adjusted using the Mehran score.
Both the IBM SPSS Statistics version 19 software and the MedCalc version 11.6.1 software (Mariakerke, Belgium) were used for statistical analysis and to calculate and compare the C-statistic measure. To test the additional predictive value of ACR, the C-statistic measurewas compared using the Mehran score alone and after adding the ACR information obtained.
RESULTS
Out of a total of 397 patients diagnosed with ACS, 148 (59.4%) underwent a coronary angiography during hospitalization and this was the study population. The mean age was 64 ± 12 years; 35% were women, 20% had diabetes, 54% dyslipidemia, 65% hypertension, and 42% were active smokers. The mean blood sugar levels on admission were 110 mg/dL (98-133 mg/dL), the median creatinine clearance rate (estimated using the MDRD) was 86 mL/min (66-107), and the ACR was 5 mg/g (0-14) (table 1). The patient comparison between these groups with or without CIN showed a higher rate of overweight and obesity, left bundle branch block, atrial fibrillation, and AMI Killip and Kimball class III-IV (table 2).
Table 1. Baseline characteristics of the patients
| Total number of patients | N = 148 |
|---|---|
| Age (years), median [25-75] | 64 [56-73] |
| Women | 35 |
| Hypertension | 65 |
| Diabetes mellitus | 20 |
| Dyslipidemia | 54 |
| Smoking | 42 |
| Previous AMI | 24.5 |
| STEAMI | 20.9 |
| NSTEACS | 79.1 |
| Fasting blood glucose levels, mg/dL | 110 [98-133] |
| Serum creatinine levels, mg/dL | 0.9 [0.8-1.0] |
| Creatinine clearance rate, mL/min | 86 [66-107] |
| Urinary albumin-to-creatinine ratio, mg/gr | 5 [0-14] |
| CPK, IU/L | 121 [73-264] |
| CK-MB, IU/L | 16 [12-34] |
| Troponin T levels, ng/mL | 0.01 [0.01-0.27] |
| Moderate to severe LVSF impairment (EF < 40%) | 5.79 |
|
Unless specified otherwise, data are expressed as % or mean and standard deviation. AMI, acute myocardial infarction; CK-MB, creatine kinase myocardial band; CPK, creatine phosphokinase; EF, ejection fraction; IQR, interquartile range; IU, international units; LVSF, left ventricular shortening fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction. |
|
Table 2. Comparison of patients with and without contrast-induced nephropathy
| CIN - (136) | CIN + (12) | P | |
|---|---|---|---|
| Age (years) | 63.7 [55-74] | 68 [61-76] | NS |
| Women | 25.3 | 16.7 | NS |
| Hypertensive | 67.7 | 58.3 | NS |
| Diabetic | 20 | 33.3 | NS |
| Body mass index | 26 [24-29] | 29 [25-31] | .05 |
| Creatinineclearence rate mg/dL | 85 [65-108] | 74 [50-98] | NS |
| Blood glucose levels at admission, mg/dL | 112 [100-142] | 143 [108-209] | NS |
| Previous AMI | 26 | 16 | NS |
| Previous PCI | 17 | 8.3 | NS |
| Previous stroke or TIA | 3.6 | 8.3 | NS |
| NSTEACS | 17.7 | 25 | NS |
| STEMI | 30.8 | 33.3 | NS |
| Left bundle branch block | 3.6 | 16.7 | .02 |
| Atrial fibrillation | 0.9 | 8.3 | .02 |
| Killip and Kimball III-IV | 4.1 | 22 | .001 |
|
Unless specified otherwise, data are expressed as % or mean and standard deviation 25%-75%. ACEI, angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARA II, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; CK-MB, creatine kinase myocardial band; CPK, creatine phosphokinase; EF, ejection fraction; HR, heart rate; IQR, interquartile range; IU, international units; LVSF, left ventricular shortening fraction; NS, not significant; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack. |
|||
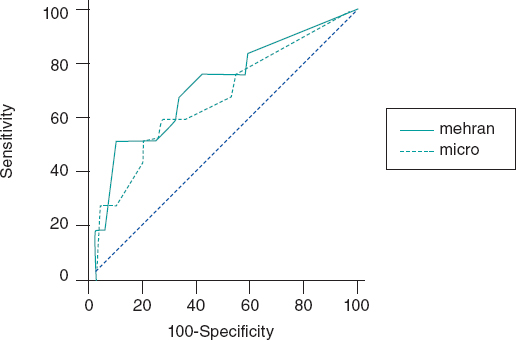
The C-statistic measure showed that the best CIN related ACR cutoff value was 20 mg/g. Twelve patients developed CIN (8.1%) and the ACR of 22% of the patients was >20 mg/g. The rate of ACR > 20 mg/g among patients without CIN was 2.9% and 11.3% (P = .01) among patients with CIN. Contrast-induced nephropathy was significantly higher when the ACR was ≥ 20 mg/g compared to when it was < 20 mg/g (≥ 9.6% vs 1.6%, res- pectively, P < .001). When the ACR was added to the Mehran score, its predictive power went up to 0.82 (95%CI, 0.76-0.87).(Figure 1).
Figure 1. Effect of albumin-to-creatinine ratio when added to the Mehran score. When the albumin-to-creatinine ratio was added to the Mehran score, its predictive power went up from 0.75 to 0.82 (95%CI, 0.76-0.87).
Using a multivariable regression analysis model the ACR > 20 mg/g turned out to be an independent predictor for CIN: OR, 3.2 (0.7-6.2); P = .01, adjusted by the Mehran score variables (age, women, body mass index, atrial fibrillation, Killip Class III-IV, and creatinine clearance rate).
DISCUSSION
Our study proved the association between the ACR and the development of CIN in patients admitted with ACS.
Acute kidney injury in the ACS setting predisposes to more complications such as in-hospital and long-term mortality; therefore, predicting it is of critical clinical importance. A recent study reported that the rate of AKI was close to 17% in the ACS setting with significant peaks of cardiovascular complications. In this study, the ACR was not used as an early marker of AKI. The development of CIN was not specifically analyzed either as a post-coronary angiography complication.18-22
Microalbuminuria calculated through the ACR obtained from a simple urine sample is also an established marker of endothelial dysfunction that has been validated to predict cardiovascular events and mortality in different clinical settings. A previous analysis of our group revealed that higher ACR levels are associated with significantly worse outcomes in patients with non-ST-segment elevation ACS, and with a higher rate of hard endpoints like mortality and/or non-fatal acute myocardial infarction at the long-term follow-up (12% vs 2.2%, P =/< .0001).23 Also, other authors proved its utility to assess the risk of developing AKI, mainly in the ACS setting or while being exposed to cardiac surgery.24Tziakas et al confirmed the significant correlation between AMI related higher ACR levels and the development of AKI after this event (area under the ROC curve 0.72; 95%CI, 0.67-0.77). However, the authors did not report on the clinical impact of this complication on the patient’s clinical course or its association with the use of contrast during coronary angiography.25
Special attention should be paid to patients with post-angiographic AKI in the ACS setting. Several studies have shown that CIN negatively impacts the prognosis of hospitalized and long-term patients. In our population, mortality in patients with CIN was significantly higher compared to those without this disease (33% vs 1.8%).
The use of urinary ACR has been less studied in this context. Meng et al. reported that high microalbuminuria levels (ACR in between 30 mg/g and 300 mg/g) were associated significantly with the development of post-contrast acute kidney injury in patients undergoing coronary catheterization (12.1% vs. 5.0%; P = .005). A key point here that distinguishes this study from ours is that they included patients with scheduled coronary angiographies only and out of the ACS setting.26Another relevant point is that the ACR cutoff value to develop CIN was determined from the analysis of the area under the ROC curve, and its value of 20 mg/g was even lower compared to the conventional standard threshold of 30 mg/g, a finding that was consistent with what other clinical studies reported.27
The rate of CIN and its impact on the clinical outcome of coronary patients triggered the development of predictive scores for this disease. One of the best known indices is the Mehran score that includes variables like age > 75 years, hypertension, functional class III/IV heart failure, diabetes mellitus, anemia, use of intra-aortic balloon pump, volume of contrast administered, and past medical history of renal dysfunction and is capable of identifying who the most vulnerable patients are to develop post-coronary angiography CIN (the area under the ROC curve was 0.75). Adding the ACR to this score showed an even greater discriminatory power to predict post-coronary angiography CIN in patients with ACS. This would prove the practical utility of adding this index as a variable to the Mehran score.
CIN, one of the most common causes for acute nephrotoxicity, is a multi-factor event. Among its causes we should mention the direct nephrotoxic effect of the contrast substances used during endovascular procedures on the renal endothelium and the development of acute tubular necrosis. It is estimated that the nephrotoxicity of hyperosmolar contrast enhanced by the hemodynamic alterations produced by the ongoing ACS could alter vascular resistance with changes in the regulation of the release and balance of vasoactive substances like adenosine, endothelin, and nitric oxide. The damage perpetuates the slowing down of renal perfusion, spinal hypoxemia, ischemic injury, and ultimately cell death. In addition to reducing the clearance of oxidative stress products, the lower glomerular filtration rate levels increase the concentration of inflammatory mediators triggering structural alterations at renal tubular epithelium level like edema, vacuolization, and death.28,29
We believe that these findings could help identify patients at high-risk of developing post-coronary angiography CIN in the ACS setting to promote preventive measures, behaviors, and strategies to avoid this complication.
Limitations
First, one of the main limitations of our work is its single center nature. However, we should mention that the population included was representative and covered the entire spectrum of patients with ACS admitted to our coronary care unit, which secures the internal validity and representativeness of our study. Secondly, the underpowered sample may have conditioned the appearance of false negative results due to its alpha error or lower power and stopped us from performing a proper multivariable analysis. Finally, certain data such as the volume of contrast used in each study was calculated retrospectively with the usual biases of this type of analysis.
CONCLUSIONS
The albumin-to-creatinine ratio, a recognized predictor of renal and endothelial dysfunction, was also a marker of CIN in patients with ACS with an added value when it was included in a widely validated clinical score. These results may be the beginning of a hypothesis-generating study to be confirmed prospectively at a multi-center level.
FUNDING
No funding or grants were received for this work.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
We wish to thank the entire staff of the Hospital Alemán Coronary Care Unit, particularly the nursing staff who helped collect the urine samples that were crucial to conduct this study.
WHAT’S KNOWN ABOUT THE TOPIC?
- CIN is one of the most common causes for AKI in hospitalized patients. Microalbuminuria is an established marker of endothelial dysfunction and has been validated to predict cardiovascular events and mortality in different clinical settings. The ACR is useful to assess the risk of developing CIN basically in the ACS setting or while exposed to cardiac surgery.
WHAT DOES THIS STUDY ADD?
- Our study proved the association that exists between the ACR and the development of post-coronary angiography CIN in patients admitted with ACS. The C-statistic measure showed that the best CIN related ACR cutoff value was 20 mg/g. The ACR brings an added value when included in the Mehran score to assess the risk of developing post-coronary angiography CIN in the ACS setting.
REFERENCES
1. Hsiao PG, Hsieh CA, Yeh CF, et al. Early prediction of acute kidney injury in patients with acute myocardial injury. J Crit Care. 2012;27:525.e1-e7.
2. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419-1428.
3. Mehran R, Nikolski E. Contrast-induced nephropathy:Definition, epidemiology, and patients at risk. Kidney International. 2006;69:S11-S15.
4. Parikh CR, Coca SG, Wang Y et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987-995
5. Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy, Kidney Int. 2005;68:14-22.
6. Tsai TT, Patel UD, Chag TI, et al. Contemporary incidence, predictors and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions:insights from the NCDR Cath-PCI Registry. JACC Cardiovasc Interv. 2014;7:1-9.
7. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:1393-1399.
8. Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;31:89-93.
9. Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease morbidity associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160:1093-1100.
10. Dogra G, Rich L, Stanton K, Watts Parving H. Microalbuminuria in essential hypertension and diabetes. J Hypertens. 1996;14:S89-S94.
11. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects under-spread vascular damage:the steno hypothesis. Diabetologia. 1989;32:219-226.
12. Estacio RO, Dale RA, Schrier R, Krantz MJ. Relation of reduction in urinary albumin excretion to ten-year cardiovascular mortality in patients with type 2 diabetes and systemic hypertension. Am J Cardiol. 2012;109:1743-1748.
13. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease:Analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106-2111.
14. Bennett PH, Haffner S, Kasiske BL, et al. Screening and management of microalbuminuria in patients with diabetes mellitus:recommendations to the Scientific Advisory Board of the National Kidney Foundations from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107-12.
15. Berton G, Cordiano R, Palmieri F, Cucchini R, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction. A strong predictor for 1-year morbidity. Eur Heart J. 2001;22:1466-1475.
16. Nazer B, Ray KK, Murphy SA, Gibson M, Cannon CP. Urinary albumin concentration and long-term cardiovascular risk in acute coronary syndrome patients:A PROVE IT-TIMI 22 sub-study. J Thromb Thrombolysis. 2013;36:233-239.
17. Jensen JS, Clausen P, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Detecting microalbuminuria by urinary albumin/keratinize concentration ratio. Nephrol Dial Transplant. 1997;12S2:6-9.
18. Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150:170-177.
19. Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of Acute Renal Failure after percutaneous coronary intervention. Circulation. 2002;105:2259-2264.
20. Garcia S, Ko B, Adabag S. Contrast-induced nephropathy and risk of acute kidney injury and mortality after cardiac operations. Ann Thorac Surg. 2012;94:772-777.
21. Bouzas-Mosquera A, Vázquez-Rodríguez JM, Calviño-Santos R, et al. Contrast-Induced Nephropathy and Acute Renal Failure Following Emergent Cardiac Catheterization:Incidence, Risk Factors and Prognosis. Rev Esp Cardiol. 2007;60:1026-1034.
22. Ueda J, Nygren A, Hansell P, Ulfendahl HR. Effect of intravenous contrast media on proximal and distal tubular hydrostatic pressure in the rat kidney. Acta Radiologica. 1993;34:83-87.
23. Higa CC, Novo FA, Nogues I, Ciambrone MG, Donato MS, Gambarte MJ, Rizzo N, Catalano MP, Korolov E, Comignani PD. Single spot albumin to creatinine ratio:A simple marker of long-term prognosis in non-ST segment elevation acute coronary syndromes. Cardiology J. 2016;23:236-241
24. Coca SG, Jammalamadaka D, Sint K, et al. Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143:495-502.
25. Tziakas D, Chalikias G, Kareli D, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol. 2015;197:48-55.
26. Meng H, Wu P, Zhao Y, et al. Microalbuminuria in patients with preserved renal function as a risk factor for contrast-Induced acute kidney injury following invasive coronary angiography. Eur J Radiol. 2016;85:1063-1067.
27. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus:Results of the HOPE study and MICRO HOPE sub-study. Lancet. 2000;355:253-259.
28. Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444-449.
29. Goldenberg I, Matetzky S. Nephropathy induced by contrast media:pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461-1471.
Corresponding author: Servicio de Cardiología, Hospital Alemán, Avenida Pueyrredón 1640, Ciudad Autónoma de Buenos Aires C1118, Argentine Republic.
E-mail address: yeni_vin@yahoo.com.ar (Y. Korolov).