ABSTRACT
Introduction and objectives: The Lotus Valve device (Boston Scientific) is a second-generation fully-retrievable and repositionable transcatheter aortic valve. We report the initial multicenter experience with the Lotus valve in the management of patients with severe aortic stenosis.
Methods: Observational study that described the short and long-term results of implanting the Lotus valve in 8 Spanish and Portuguese centers from March 2014 through April 2016.
Results: The study included 102 patients (mean age 80.4 ± 6.1 years; STS score 5.2% ± 3.3%) with severe symptomatic aortic stenosis (mean aortic valve area 0.66 ± 0.17 cm2, aortic gradients 74.3 / 45.6 mmHg). The valve was successfully implanted in 100 patients (98%), with significant improvement in both the peak and mean aortic valve gradients and with only one patient showing moderate paravalvular regurgitation. Upon hospital discharge, mortality rate was 3.9% while the stroke rate was 2.9%. No cases of valve embolization, ectopic valve deployment or additional valve implantation (valve-in-valve) were seen. Thirty-three patients (32.3%) received a permanent pacemaker.
Conclusions: The Lotus Valve System is effective and safe for the management of patients with severe symptomatic aortic stenosis. In particular, considering the low rate of periprosthetic regurgitation and lack of complications like embolization or ectopic valve deployment; however at the expense of a high pacemaker implantation rate.
Keywords: Transcatheter Aortic Valve. Aortic Stenosis.
RESUMEN
Introducción y objetivos: El dispositivo Lotus (Boston Scientific, Estados Unidos) es una prótesis valvular aórtica transcatéter de segunda generación, completamente recuperable y reposicionable. Se presenta la experiencia inicial con la prótesis Lotus en un registro multicéntrico.
Métodos: Estudio observacional que reporta los resultados a corto y largo plazo del implante transfemoral de prótesis Lotus entre marzo de 2014 y abril de 2016 en 8 centros de España y Portugal.
Resultados: Se incluyeron 102 pacientes (edad media 80,4 ± 6,1 años, índice STS medio 5,2% ± 3,3%) con estenosis aórtica grave sintomática (área valvular media 0,66 ± 0,17 cm2, gradientes 74,3/45,6 mmHg). Se implantó con éxito el dispositivo en 100 pacientes (98%), con mejoría significativa de los gradientes máximo y medio valvular, y un solo caso de regurgitación periprotésica moderada. No hubo ninguna embolización ni necesidad de implante de una nueva prótesis intravalvular. Hasta el alta hospitalaria, la mortalidad fue del 3,9% y la tasa de ictus fue del 2,9%. En 33 pacientes (32,3%) fue necesario el implante de marcapasos definitivo.
Conclusiones: La válvula Lotus es eficaz y segura para el tratamiento de pacientes con estenosis aórtica grave sintomática. Destacan la escasa tasa de insuficiencia periprotésica y la ausencia de complicaciones derivadas del mal posicionamiento o la embolización de la prótesis, a costa de un alta incidencia de implante de marcapasos.
Palabras clave: Prótesis aórtica transcatéter. Estenosis aórtica.
Abbreviations AR: aortic regurgitation. AS: aortic stenosis. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is a therapeutic option in patients with severe symptomatic aortic stenosis (AS) that has proven to be non-inferior to surgical aortic valve replacement even in low-risk patients.1-8
However, TAVI-related persistent complications can have a negative impact on the short and medium-long-term results including periprosthetic aortic regurgitation (AR)—associated with more in-hospital and medium and long-term mortality after TAVI.9-12 Several factors have been associated with the development of periprosthetic regurgitation like valve underexpansion following the severe calcification of the aortic annulus or valve malapposition. The latter is a factor associated with other complications like valve embolization. In order to minimize these setbacks, innovative, second-generation, fully or partially repositionable devices have been developed.
The Lotus device (Boston Scientific, United States) is a fully retrievable and repositionable second-generation transcatheter aortic valve. It has been designed to minimize the risk of complications related to valve malapposition, in particular periprosthetic AR and valve embolization.13
The objective of this study is to present the initial experience in Spain and Portugal in the management of AS with the Lotus valve.
METHODS
Patient selection
This observational study included all consecutive patients with severe AS treated with transfemoral Lotus valve implantation between March 2014 and April 2016 in Spanish and Portuguese centers that disclosed their databases voluntarily. All patients had symptomatic, severe AS (aortic valve area < 1 cm2) or with left ventricular dysfunction according to the recommendations from the European Society of Cardiology guidelines on the management of valvular heart disease;14 in any case, the indication was established according to the local protocols of each center after each particular case was individually assessed by the heart team. Surgical risk was assessed using the STS risk score.15 However, its value was not considered an inclusion or exclusion criterion in the registry because in the selection of patients, clinical and anatomical aspects not found in the surgical risk scores were also considered (porcelain aorta, patency of mammary artery bypass graft, hostile chest, etc.).
Study variables
The patients’ main baseline clinical and echocardiographic variables, procedural details, and clinical and echocardiographic results until hospital discharge were gathered. Special attention was paid to peri- and postoperative complications. Data mining was prospective in every center, although there was no common protocol for it or for the allocation of clinical and echocardiographic results. Each center disclosed its own database and they were all compiled in a single database.
The clinical assessment and diagnostic tests prior to the implant were similar to those of common recommendations.14 A few variables were not systematically collected in all the centers and, therefore, not included in the study final analysis.
Regarding procedural data, the main variables studied were the performance or not of a prior valvuloplasty, the device total or partial recapture, the need for post-dilation, the degree of valvular regurgitation, and postoperative transvalvular gradients. Finally, a comparison was drawn between mean and peak gradients and the prevalence of moderate AR before and after device implantation.
Procedural complications were gathered according to the recommendations established in the Valve Academic Research Consortium 2 consensus document.16 The following complications were analyzed: mortality, strokes, hemorrhagic complications, major and minor vascular complications, definitive pacemaker implantation, renal failure, echocardiographic data suggestive of prosthetic valve dysfunction (mean valve gradient > 20 mmHg, effective valvular area < 0.9-1.1 cm2, Doppler velocity index < 0.35, and moderate or severe AR). The combined efficacy parameter used this definition established according to the criteria of the Valve Academic Research Consortium 2: proper single valve implantation + lack of in-hospital mortality + lack of mean gradient > 20 mmHg, aortic valve area ≥ 1.2 cm2, Doppler velocity index < 0.35 or moderate or severe AR. The combined initial safety parameter (until hospital discharge) was defined as: lack of all-cause mortality, stroke, life-threatening bleeding, stage 2-3 renal failure, coronary obstruction requiring intervention, major vascular complication or valve dysfunction requiring reintervention.
Finally, patients were followed retrospectively 3 years after finishing the registry recruitment phase, and clinical (mortality and cardiovascular events) and echocardiographic parameters were collected.
Boston Scientific has not been involved in the design or development of this study whatsoever.
Description of the device
The Lotus device used in the registry is a bovine pericardial heart valve (3 cusps) mounted on a nitinol frame, preloaded, and deployed through a controlled mechanical expansion system. It measures 72 mm before expansion and 19 mm after implantation. There are 3 diameters available: 23 mm, 25 mm, and 27 mm. The Lotus Edge valve available today has a more flexible deployment catheter, an easier implantation system, and can be implanted through a 14-Fr expandable introducer.
The delivery system and the introducer sheath have been designed to facilitate a precise and predictable delivery to guarantee the valve early functionality, and the possibility of non-traumatic repositioning and retrieval at any time prior to the definitive delivery of the valve. The device has a sealing system (urethane membrane) designed to minimize the rate of paravalvular regurgitation.
Procedure
Implantation was performed according to the method described in the medical literature.13 It was performed in the cardiac catheterization laboratory under general anesthesia or deep sedation, in a sterile environment, following the operator’s preferences, and with or without transesophageal echocardiography guidance.
Transfemoral access was used in all cases using a fully percutaneous technique or surgical exposure. An 18-Fr introducer was advanced for the 23 mm-valve (minimum diameter required: 6 mm) and a 20-Fr introducer for the 25 mm and 27 mm-valves (minimum diameter required: 6.5 mm) towards the descending aorta. The native aortic valve was crossed using the routine technique. Before using the guidewire to cross to the left ventricle, a temporary transvenous pacemaker was implanted.
The Safari high-support guidewire was used (0.035 in guidewire, 260 cm) (Boston Scientific). The decision to perform a prior balloon valvuloplasty was left to the operator’s discretion.
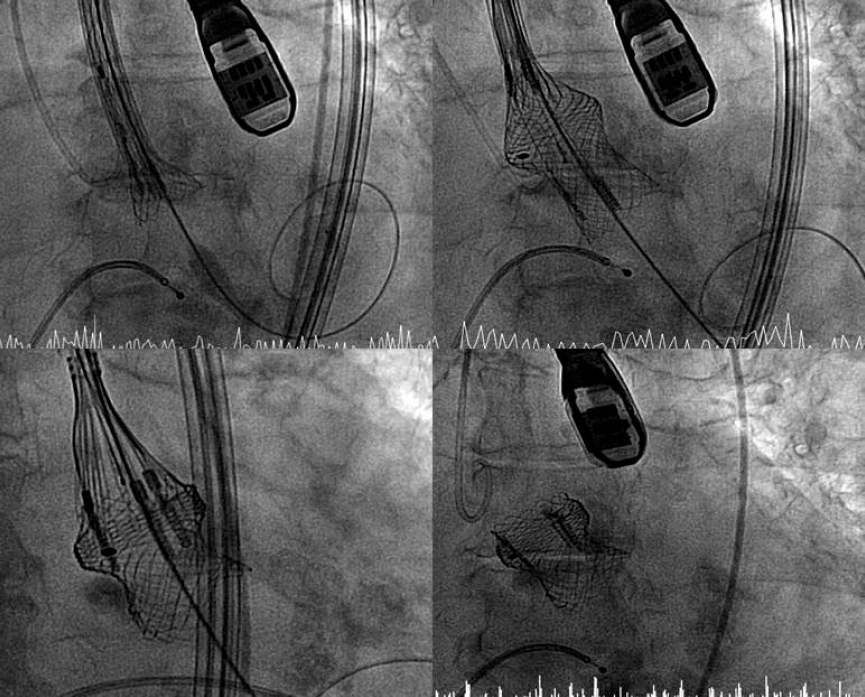
To implant the device, the delivery system is steered, and the radiopaque marker is positioned towards the aorta external region to facilitate the advancement of the catheter thanks to its adapted morphology. After crossing the native aortic valve and without the need for cardiac pacing, the valve is expanded. The proper anchoring of the valve support systems and positioning of the valve are confirmed. Finally, it is delivered and the system removed (figure 1).
Figure 1. Lotus valve implantation procedure.
In the absence of significant atrioventricular conduction disturbances, the temporary pacemaker was removed 24-48 hours after the procedure. The indications for the definitive pacemaker were established by the local protocols of each center. Antithrombotic treatment at discharge was dual antiplatelet therapy with acetylsalicylic acid and clopidogrel during the first 3-6 months, except for cases with indications for chronic oral anticoagulation.
Statistical analysis
Statistical analysis was performed using the SPSS 22 statistical software package (SPSS Inc., United States). Categorical variables were expressed as percentages, and quantitative variables as mean ± standard deviation or median (interquartile range). Continuous variables were compared using the Student t test for paired data, and categorical variables were compared using the chi-square test.
RESULTS
Baseline characteristics of the patients
A total of 102 patients were included from 5 Spanish centers and 3 Portuguese centers (table 1). Baseline characteristics are shown on table 2. Mean age was 80.4 ± 6.1 years, 52.9% were women, and the STS score was 5.4% (3.7-7.7).
Table 1. Participant hospitals in the study and number of patients per hospital
| Hospital Universitario La Paz, Madrid, Spain | 33 (32.4%) |
| Policlínica Gipuzkoa, San Sebastián, Spain | 19 (18.6%) |
| Hospital Universitari Vall d’Hebron, Barcelona, Spain | 7 (6.9%) |
| Hospital Virgen de las Nieves, Granada, Spain | 8 (7.8%) |
| Hospital Puerta del Mar, Cádiz, Spain | 6 (5.9%) |
| Centro Hospitalar de Vila Nova de Gaia, Oporto, Portugal | 8 (7.8%) |
| Centro Hospitalar de Lisboa Central, Lisbon, Portugal | 10 (9.8%) |
| Hospital Santa Cruz, Lisbon, Portugal | 11 (10.8%) |
Table 2. Baseline characteristics of patients (N = 102)
| Age (years) | 80.4 ± 6.1 |
| Feminine sex | 54 (52.9%) |
| Coronary artery disease | 44 (43.1%) |
| Percutaneous revascularization | 24 (54.5%) |
| Surgical revascularization | 11 (25%) |
| No revascularization | 9 (20.5%) |
| Cerebrovascular disease prior to TAVI | 8 (7.8%) |
| Chronic kidney disease (CrCl < 60 mL/min) | 37 (36.3%) |
| Without dialysis | 33 (32.4%) |
| With dialysis | 4 (3.9%) |
| Atrial fibrillation prior to TAVI | 42 (41.2%) |
| Paroxysmal | 13 (12.7%) |
| Permanent | 29 (28.5%) |
| Ventricular function prior to TAVI (N = 82) | |
| > 50% | 65 (79.3%) |
| 30%-50% | 9 (11%) |
| < 30% | 8 (9.8%) |
| STS score | 5.4% (3.7-7.735) |
| Pacemaker prior to TAVI | 11 (10.8%) |
| CrCl, creatinine clearance; TAVI, transcatheter aortic valve implantation. | |
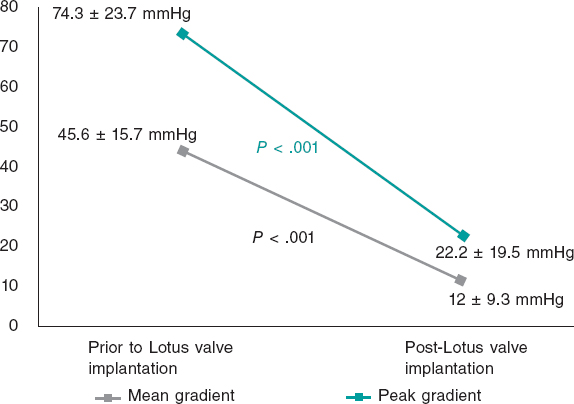
Most patients had preserved systolic function and they were all diagnosed with severe AS with a mean indexed valve area of 0.66 ± 0.17 cm2/m2 and peak and mean aortic gradients of 74.3 ± 23.7 and 45.6 ± 15.7 mmHg, respectively; 22% of the patients had moderate AR too (≥ 2).
Procedural characteristics
Procedural characteristics are shown on table 3. General anesthesia was used, and the procedure was transesophageal echocardiogram-guided in most patients. Implantation was performed using transfemoral access; in 91.2% of the cases x-ray-guided percutaneous punctures and closures were performed.
Table 3. Procedural characteristics
| Procedural characteristics (N = 102) | |
|---|---|
| General anesthesia | 94 (92.1%) |
| Perioperative transesophageal echocardiogram | 94 (92.1%) |
| Transfemoral access | 102 (100%) |
| Surgical exposure | 8 (7.8%) |
| Percutaneous | 94 (92.1%) |
| ProGlide closure system | 38 (37.2%) |
| Prostar closure system | 56 (54.9%) |
| Pre-dilation | 20 (19.6%) |
| Repositioning | |
| Partial | 12 (11.8%) |
| Complete | 1 (1%) |
| Post-dilation | 0 |
| Valve size | |
| 23 mm | 43 (42.1%) |
| 25 mm | 27 (26.5%) |
| 27 mm | 32 (31.4%) |
The size of the valve was decided based on the dimensions of the annular area and perimeter based on the computed tomography scan performed in each center. Valve pre-dilation was performed in 19.6% of the patients, and no patient was post-dilated.
Before the definite implantation the device had to be repositioned in 12 procedures (11.8%) and the valve fully recaptured in 1 occasion because the patient showed severe periprosthetic regurgitation due to valve malapposition; the same device was successfully re-implanted in this patient.
Procedural results
The valve was successfully implanted in 100 patients (98%), except for 2 patients due to major vascular complications: one case of a ruptured iliac artery that required surgical intervention (with good progression) and another case of aortic rupture prior to device implantation (the patient eventually died). In all the cases where the native aortic valve was accessed, the device was successfully implanted.
After the implant there was a significant reduction of transvalvular mean and peak gradients and the percentage of significant AR (P < .001) (figure 2). There was paravalvular leak grade 2 in 1 case, but no serious leaks whatsoever. In this case, a large annulus is described (a 27 mm diameter measured through CAT scan exceeding the upper limits recommended by the manufacturer). The main cause for the moderate paravalvular leak reported may have been a moderate oversized valve with respect to the annular size.
Figure 2. Gradients before and after valve implantation.
There were no complications associated with the valve malapposition and there was only 1 case of perioperative thromboembolic coronary occlusion. It soon resolved rusing coronary thromboaspiration and balloon angioplasty without any major adverse events (with intraoperative infarction but no death or worsening of the left ventricular ejection fraction after the procedure).
Complications are shown on table 4. In-hospital mortality was 3.9% (4 patients). As reported, 1 patient died of a ruptured aorta prior to device implantation. This patient had a porcelain aorta and the perioperative transesophageal echocardiogram performed showed plaque ulceration in the aortic wall. The second patient died of cardiogenic shock 4 days after admission; he showed ventricular dysfunction and left bundle branch block prior to device implantation. However, there were no complications during the procedure. The third patient suffered a perioperative ischemic stroke, and had a long hospital stay. He eventually died 74 days after admission of nosocomial infection. Finally, the fourth patient had a past medical history of hepatic failure and presented with liver failure and hemodynamic instability. He died within the first 30 days following the intervention.
| Procedural results (VARCS2 criteria) (N = 102) | |
|---|---|
| Successful implantation | 100 (98%) |
| Hospital stay (days) | |
| Mean | 12.8 ± 16.5 |
| Median | 8.5 ± 4.5 |
| Device malapposition | 0 |
| Migration | 0 |
| Embolization | 0 |
| Valve-in-valve | 0 |
| Coronary occlusion | 1 (1%) |
| Periprosthetic aortic regurgitation (grade) | |
| 0 | 81 (79.4%) |
| 1 | 20 (19.6%) |
| 2 | 1 (1%) |
| 3 | 0 |
| In-hospital mortality | 4 (3.9%) |
| Stroke | 3 (2.9%) |
| Disabling | 2 (1.9%) |
| Non-disabling | 1 (1%) |
| Bleeding | 5 (4.9%) |
| Life-threatening | 3 (2.9%) |
| Major | 1 (1%) |
| Minor | 1 (1%) |
| Renal failure | |
| Stage 2 | 5 (4.9%) |
| Stage 3 | 2 (2%) |
| Vascular complications | 11 (10.8%) |
| Major | 4 (3.9%) |
| Minor | 7 (6.9%) |
| Conversion to open surgery | 1 (1%) |
| Definitive pacemaker implantation | 33 (36.3%) |
| Combined efficacy parameter | 95 (93.1%) |
| Combined safety parameter | 92 (90.2%) |
|
VARC2, Valve Academic Research Consortium 2. |
|
The rate of perioperative stroke was 2.9%, and the rate of major vascular complications was 3.9%. There were 2 ruptured aortas with cardiac tamponade. In 1 case the patient died and in the other, the patient required conversion to sternotomy and surgery with good disease progression. The other 2 major complications were a ruptured iliac artery and a retroperitoneal hematoma that required intervention with good disease progression.
The rate of successful implantation defined according to the Valve Academic Research Consortium 2 criteria, was 93.1% (95 out of the 102 patients included), since 4 patients died. In 1 patient the device was not implanted due to a major vascular complication, another patient had a mean gradient > 20 mmHg after implantation, and another showed moderate AR. The combined initial safety parameter (until hospital discharge) reached 90.2% of the cases (92 out of the 102 patients included).
A definitive pacemaker was implanted prior to hospital discharge in 33 out of the 91 patients who did not carry a pacemaker prior to device implantation (36.3%).
Follow-up
Out of the 92 patients who reached the combined initial safety parameter, it was possible to analyze the 3-year follow-up results in 57 of them (62%) with a mean age of 80 ± 6 years and a median follow-up of 37 months (22-47).
The 1-year mortality was 10.5% (6 patients). Two patients died of endocarditis: 1 case of mitral valve endocarditis (a patient with severe mitral regurgitation prior to TAVI) 10 months after the procedure, and another case of aortic valve endocarditis 3 months after the implant. The 4 remaining patients died of non-cardiac causes (1 patient died of metabolic encephalopathy and 3 of sepsis of a different origin). The 3-year mortality rate was 35.1% (20 patients): 6 patients (10.5% of the total) died of cardiac causes, 10 of non-cardiac causes, and 4 for unknown reasons.
Regarding the echocardiographic parameters, the persistence of good long-terms results was seen without significant variations of the valvular gradients post-TAVI (mean gradient of 12 ± 9.3 mmHg at discharge vs 12.4 ± 6.8 mmHg at the 3-year follow-up; peak gradient of 22 ± 19.5 mmHg at discharge vs 24.5 ± 13.2 mmHg at the 3-year follow-up). However, valve thrombosis was seen in 2 patients (3.5%), both diagnosed in a routine echocardiographic examination without any associated clinical events. One case was an early thrombosis that occurred 2 months after device implantation in an 87-year-old patient with severe ventricular dysfunction and implantation of a 27-mm Lotus valve on dual antiplatelet therapy at hospital discharge. The other was a case of very late thrombosis —46 months after device implantation— in a 71-year-old patient with moderate ventricular dysfunction and implantation of a 23-mm Lotus valve. Both patients improved with anticoagulant medication. No cases of periprosthetic aortic regurgitation grade > 1 were reported at the follow-up, and no patient required reintervention.
DISCUSSION
This is the first study to report on real-life results of the Lotus valve (Boston Scientific) in the Iberian Peninsula. They are similar to the results published in former studies and registries (table 5),17-23 in particular the results of the RESPOND study.23 It should be mentioned the low rate of periprosthetic regurgitation (1% moderate and 0% severe), the lack of complications related to the valve malapposition, and no need for post-dilation despite a low rate of pre-dilation. Three factors are responsible for these results:
Table 5. Studies published on the Lotus valve
| Series | Number of patients | Successful implantation | Mortality | Periprosthetic aortic regurgitation ≥ 2 | Pacemaker |
|---|---|---|---|---|---|
| Reprise II17,18 | 120 | 100% | 4.2% | 1% | 28.6% |
| Rampat et al.19 | 228 | 99.1% | 1.8% | 0.8% | 31.8% |
| De Backer et al.20 | 154 | 100% | 1.9% | 0.6% | 27.9% |
| Wöhrle et al.21 | 26 | 100% | 0 | 0 | 26.9% |
| RESPOND23 | 1014 | 98.1% | 2.9% | 0.3% | 34.6% |
| Current series | 102 | 98% | 3.9% | 1% | 36.3% |
-
- The possibility of fully or partially repositioning and recapturing the device, thus facilitating a more accurate positioning of the valve.
-
- The presence of great valvular radial strength. It has a controlled mechanical expansion mechanism, not a self-expanding one (while the device is released from the delivery system, the nitinol frame shortens and expands always in a totally reversible way).
-
- The presence of a new sealing system (urethane membrane) adapted to the annulus irregular surface to minimize perivalvular regurgitation and also in heavily calcified and irregular annuli.
In our population in-hospital mortality (3.9%) is similar to that of the Reprise II trial15 and a little higher compared to that of the landmark registry published to this day of 1014 patients: the RESPOND clinical trial.23 However, results are hardly comparable due to the different populations included, especially the high-risk population of the Reprise II like that of recruitment centers. This is so because in the RESPOND trial the participant centers had a huge experience in Lotus valve implantation. Our registry included an intermediate-risk population (STS score of 4%-8%), similar to that of the PARTNER 2,6 and in our study all-cause mortality was consistent with the one reported in such trial (3.9% at 30 days). If we take into account the importance of the learning curve when analyzing the results of new devices and the fact that our registry included centers with < 10 years of experience, in-hospital mortality was relatively low. In our series, cardiovascular mortality was 3%.
One of the advantages of this device is that it guarantees the patient’s hemodynamic stability during the entire procedure. First, no cardiac pacing is required during implantation. Second, the leaflets start to function very early on and before the valve shortens because they are attached to the device most distal portion, thus avoiding hypotension periods. Third, it can be implanted directly without pre-dilation with certain frequency because it has tremendous radial strength. In our registry, only 19.6% of the patients were pre-dilated, fewer patients compared to the RESPOND trial (53.9%).
The rate of significant periprosthetic AR (grade ≥ 2) was fairly low with similar results to those of former studies published on the Lotus valve (table 5). Moderate periprosthetic regurgitation was seen in one patient only, but it was not serious. Moderate-severe periprosthetic AR (grade ≥ 2) has been associated with worst post-TAVI results and higher short and long-term mortality rate.9-12 The PARTNER 2 clinical trial revealed a 30-day rate of moderate-severe periprosthetic AR of 3.7%. The 2-year mortality rate in these patients was higher compared to those with grade 0-1 periprosthetic regurgitation (P < .001).6 Our registry and the Reprise II trial 1-year follow-up confirmed that the results seen during the first 30 days are kept in time including the low 1-year rate of significant periprosthetic regurgitation.18 The possibility of valve repositioning and retrieval prior to the device implantation reduces other valve malapposition-related complications. No cases of device embolization were seen in our population, and no patient required valve-in-valve implantation, which increases the device safety profile.
In our registry the rate of strokes was 2.9% (3 patients, of these 2 suffered disabling strokes) similar to that of the RESPOND trial23 (overall strokes: 3%; disabling strokes: 2.2%). However, due to the lack of a systematic neurological exam before and after the pro- cedure and an event adjudication committee we cannot draw definitive conclusions or compare the rate of this complication between our registry and other studies.
The rate of major vascular complications is not different from the one published in other series and with other devices.
The issue that still needs to be addressed with the Lotus valve is the rate of definitive pacemaker implantation. As previous studies report, around 30% of the cases require a definitive pacemaker, yet the reason for it is still not clear. Several factors have been proposed in association with this complication. The Reprise II trial suggested overstretching—defined as a ≥ 10% ratio between the valve theoretical area and the annular area or left ventricular outflow tract measured through CAT scan21—as the main independent predictive factor of pacemaker implantation. This, added to the higher rate of pacemaker implantation of some self-expandable valves24 and cases of valve deeper implants25 leads us to think that the occurrence of conduction disturbances may be associated with excessive mechanical stress in areas where the conduction system passes through like the aortomitral junction.26 Also, better valve size selection based on data from the CAT scan, technique modifications for higher valve implantation depths, and the arrival of the LOTUS Edge device may reduce the rate of this complication. Compared to the former Lotus valve system generation, the LOTUS Edge valve is easier to deliver, has a more flexible catheter, and is easier to follow-up. The Depth Guard delivery technology and the radiopaque markers added contribute to simplify the release. Depth Guard technology has been designed to minimize valve implantation depths, thus reducing its interaction with the left ventricular infundibulum. By reducing contact with the left ventricular infundibulum, the rates of definitive pacemaker implantation go down.
In conclusion, the results already published of the REPRISE III trial on a randomized comparison between the Lotus valve and the CoreValve self-expandable aortic valve (Medtronic, United States) are very interesting.27 The results available validate those from our registry regarding the safety and efficacy profile of the Lotus valve. No significant differences were seen at the 2-year follow-up either regarding the mortality and stroke rates compared to the CoreValve. Also consistent with our results, a lower rate of moderate-severe periprosthetic AR with the Lotus valve at the 2-year follow-up (0.3% with Lotus vs 3.8% with CoreValve; P < .01) and device embolization (0.0% with Lotus vs 2.0% with CoreValve; P < .01) was seen. However, there was a higher need for pacemaker implantation (41.7% with Lotus vs 26.1% with CoreValve; P < .01) and a higher rate (3%) of valve thrombosis at the long-term follow-up in our registry and in the REPRISE III trial.
CONCLUSIONS
This is the first study to describe the safety and functioning data of the Lotus valve in Spain and Portugal. Our results confirm those obtained by former studies and indicate that the Lotus valve is a safe and effective alternative for patients with symptomatic and severe AS. In particular, a low rate of periprosthetic AR after device implantation at the expense of a high rate of pacemaker implantation was reported.
Limitations
The study main limitations are probably the lack of a comparison group, and the non-negligible percentage of patients lost to follow-up since the study main initial objective was to assess the in-hospital results of device implantation. Second, the device available today is the LOTUS Edge valve that still has the advantages of the original Lotus valve plus an improved catheter and delivery system. Lastly, another limitation is the lack of a common predefined protocol for patient inclusion and result collection although it was prospective in each center.
CONFLICTS OF INTEREST
R. Moreno is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. R. Moreno is also a proctor for Boston Scientific.
WHAT DOES THIS STUDY ADD?
- The experience with this kind of device in our setting is still limited. The relevance of this study is that this is the first description of the safety and functioning data of the Lotus valve in Spain and Portugal. Our results confirm those of former studies: high successful implantation rate, low mortality, and low rate of periprosthetic aortic regurgitation at the expense of a high rate of pacemaker implantation. Also, our registry reported on the long-term follow-up results (3 years), making it even more relevant because there are very few data in the medical literature on the durability of this valve.
WHAT IS KNOWN ABOUT THE TOPIC?
- Despite the always growing experience in the percutaneous management of severe aortic stenosis, we still face TAVI-related complications. They can have a negative impact on the short and mid-long-term results, in particular periprosthetic aortic regurgitation. The Lotus is a fully retrievable and repositionable second-generation, transcatheter, aortic valve with good initial efficacy and safety results in former studies and registries. Also, it showed fewer major complications like periprosthetic aortic regurgitation or device malapposition.
REFERENCES
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Adams DH, Popma JJ, Reardon MJ, et al. Core Valve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
5. Salinas P, Moreno R, Calvo L, et al. Long term follow-up after Transcatheter Aortic Valve Implantation for severe aortic stenosis. Rev Esp Cardiol. 2016;69:37-44.
6. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374: 1609-1620.
7. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705.
8. Popma JJ, Deeb GM, Yakubov SJ, et al.;Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715.
9. Moreno R, Calvo L, Salinas P, et al. Causes of peri-operative mortality after transcatheter aortic valve implantation:a pooled analysis of 12 studies and 1223 patients. J Invasive Cardiol. 2011;23:180-4.
10. Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299-308.
11. SabatéM, Cánovas S, García E, et al. Predictores de mortalidad hospitalaria y a medio plazo tras el reemplazo valvular aórtico transcatéter:datos del registro nacional TAVI 2010-2011. Rev Esp Cardiol. 2013;66:949-958.
12. Sinning JM, Vasa-Nicotera M, Chin D, et al. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:11-20.
13. Meredith I, Kristin L, Haratani N, Allocco D, Dawkins K. Boston Scientific Lotus Valve. EuroIntervention. 2012;8(Suppl Q):Q70-74.
14. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496.
15. O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models:part-2 –isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23-42.
16. Kappetein AP, Head SJ, Genereux P, et al. Updated standardized end point definitions for transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418.
17. Meredith IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system:30-day primary end point results from the REPRISE II study. J Am Coll Cardiol. 2014;64:1339-1348.
18. Meredith IT, Walters DL, Dumonteil N, et al. 1-Year Outcomes With the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients With Severe Aortic Stenosis:Results of the REPRISE II Study. JACC Cardiovasc Interv. 2016;9:376-384.
19. Rampat R, Khawaja MZ, Byrne J, et al. Transcatheter Aortic Valve Replacement Using the Repositionable LOTUS Valve:United Kingdom Experience. JACC Cardiovasc Interv. 2016;9:367-372.
20. De Backer O, Götberg M, Ihlberg L, et al. Efficacy and safety of the Lotus Valve System for treatment of patients with severe aortic valve stenosis and intermediate surgical risk:Results from the Nordic Lotus-TAVR registry. Int J Cardiol. 2016;219:92-97.
21. Wöhrle J, Gonska B, Rodewald C, et al. Transfemoral aortic valve implantation with the repositionable Lotus valve compared with the balloon- expandable Edwards Sapien 3 valve. Int J Cardiol. 2015;195:171-175.
22. Larman Tellechea M, Telleria Arrieta M, Lasa Larraya G, Sanmartin Pena JC, Gaviria Molinero K. Transcatheter aortic valve replacement with Lotus valve:initial experience. Rev Esp Cardiol. 2014;67:956-958.
23. Falk V, Wöhrle J, Hildick-Smith D, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice:the RESPOND Study. Eur Heart J. 2017;38:3359-3366.
24. Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement:the CHOICE randomized clinical trial. JAMA. 2014;311:1503-1514.
25. De Torres-Alba F, Kaleschke G, Diller GP, et al. Changes in the Pacemaker Rate After Transition From Edwards SAPIEN XT to SAPIEN 3 Transcatheter Aortic Valve Implantation:The Critical Role of Valve Implantation Height. JACC Cardiovasc Interv. 2016;9:805-813.
26. Moreno R, Dobarro D, López de Sá E, et al. Cause of complete atrioventricular block after percutaneous aortic valve implantation:insights from a necropsy study. Circulation. 2009;120:e29-30.
27. Reardon MJ, Feldman TE, Meduri CU, et al. Two-Year Outcomes After Transcatheter Aortic Valve Replacement With Mechanical vs Self-expanding Valves:The REPRISE III Randomized Clinical Trial. JAMA Cardiol. 2019;4:223-229.
Corresponding author: Playa de Sitges 39, 28290 Las Rozas de Madrid, Madrid, Spain.
E-mail address: daniele.gemma@hotmail.com (D. Gemma).