INTRODUCTION
Therapies for the management of heart failure have been responsible for the great benefit experienced by the population in terms of hope and quality of life. However, they are nothing more than palliative measures that have not resolved the tissue destruction due to this problem whose malignity causes 20 million cardiac deaths worldwide every year.1 Reparative and regenerative medicine was born over 2 decades ago as a biological response to the pressing need for innovation in this field. Its objective is to orchestrate diagnostic tools and therapeutic strategies to restore the molecular, cellular, and tissue health of the cardiac organs damaged.2
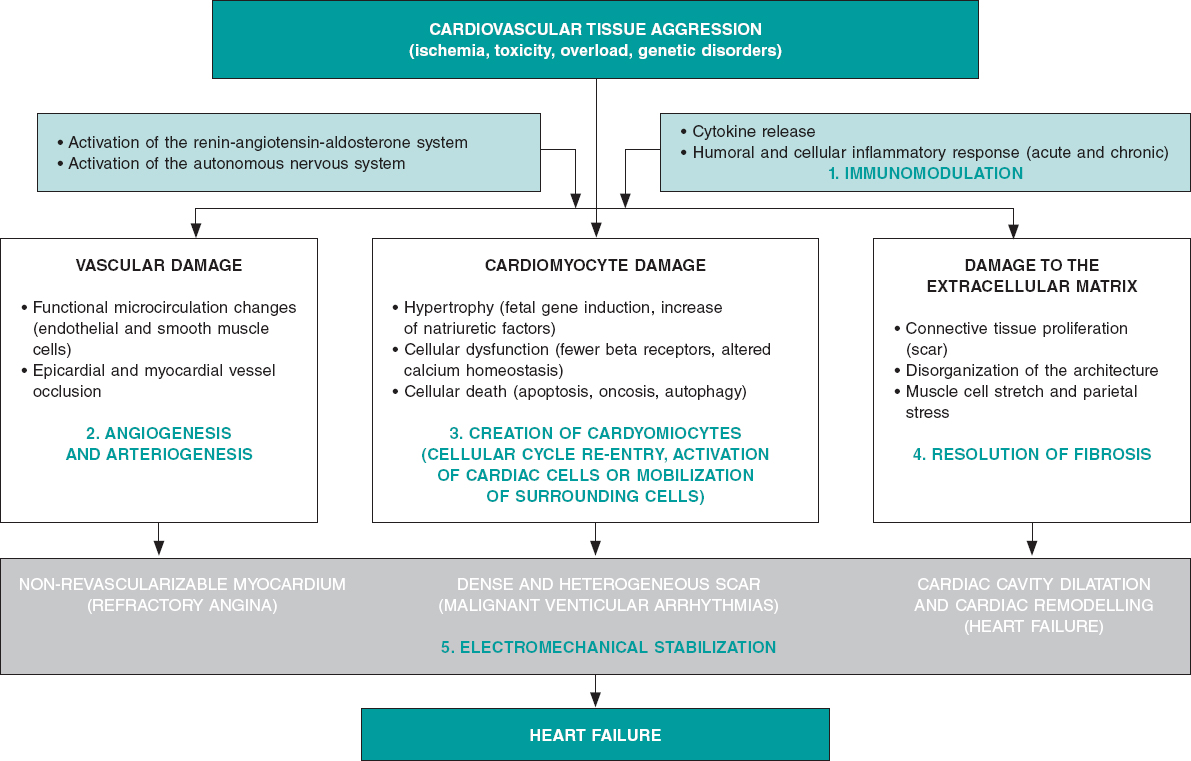
Although we can categorically say that the adult heart has a limited capacity for regeneration that depends on the formation of new cardiac muscle, endothelial and smooth muscle cells from a reservoir of existing heart stem cells,3 we can also say that this capacity is marginal and insufficient to repair cardiac organs after sustaining ischemic, toxic, valvular or inherited damage,4 even after physiological aging.5 On the contrary, the myocardium major repair response consists of cellular hypertrophy, in some cases, and replacement of damaged functional tissue by more or less dense fibrous tissue (scar) in most cases. Process that causes the adverse ventricular remodeling that defines the advanced stages of heart failure (systolic ventricular disfunction and cardiac cavity dilatation) of both ischemic and non-ischemic origin (figure 1).
Figure 1. Common biopathological mechanisms indicative of myocardial damage in its vascular, muscular, extracellular components due to different types of tissue damage including the role of neurohormonal compensatory mechanisms and inflammatory response. The bottom shows the 3 main componentes of heart failure (angina, ventricular arrhythmias and ventricular remodelling). In green, the 5 beneficial action mechanisms confirmed by basic, preclinical research through which reparative and regenerative therapies work.
FUNDAMENTALS OF PATHOBIOLOGY AND PRECLINICAL EXPERIENCES
Ever since the 1990s, different types of cells have been studied in the lab in small and large animal models; in chronological order: skeletal myoblasts, hematopoietic end endothelial cells (in most cases harvested from the bone marrow), mesenchymal cells (harvested from bone marrow or adipose tissue), cardiac cells and, recently, embryonic or adult somatic induced pluripotent stem cells.6 All these types of cells have been studied mostly in ischemic heart disease models. In some cases, they have been explored in their allogenic origin of healthy donors of the same species (unlike autologous stem cells that are harvested from the same recipient). As years have gone by, new products have appeared with regenerative or reparative capabilities. Added to gene therapy that has coexisted with cell therapy almost since the beginning, “non-cellular” products have been developed (growth factors, cytokines, proteins or types of ribonucleic acid—microARN). Many of them contained in microvesicles or exosomes that release stem cells or adult cells when they suffer an aggression. These products together with tissue engineering platforms (nanoparticles, gels, and matrices), have recently become part of regenerative medicine; and clinical research is still in its infancy.
Lab experiments and animal model experimentation have been useful because they have proved that: a) the process of myocardial and vascular repair is incredibly complex, both on the molecular, cellular, and tissue levels, which to this day, it is mostly unknown; b) the contribution made by cardiac stem cells to this process is marginal as it is the re-entry process of mature cardiomyocytes to the cellular cycle; c) the beneficial effects of the different products in the myocardium are due to the effect of the proteins and cytokines released by the cells administered (paracrine effect). They are not due to cellular fusion, proliferation or differentiation of these cells into cardiomyocytes, endothelial cells or smooth muscle cells (this has only been confirmed with pluripotent and embryonic cells that are still not used in clinical research); and d) these positive effects include protection from apoptosis, reduced fibrous tissue resulting from myocardial damage, modulation of the inflammation that precedes or is associated with such damage, creation of new blood vessels or formation of small amounts of cardiomyocytes (figure 1).
However, as in other areas of cardiovascular research,7 there has also been a significant “translational gap” in regenerative medicine, and the closer animal research has been to human clinical features the lower the impact of the therapies applied. For example, a meta-analysis of 80 studies showed that in small animal models, the average reduction of the infarction size with different products was 11%.8 However, in large animals, this reduction was only 5%, and it is well-known that in humans it is between 2% and 4%.9 This discrepancy in the results obtained in animals and humans is partly explained by the complexity of cellular processes that regulate cardiac repair, the enormous size difference among species, and the amount of cells (or products) needed to revert it. Other areas with room for improvement and other concepts that require further study regarding preclinical research are doses, administration times, and combination of products. Lastly, the rigor of clinical research is not always thoroughly applied to animal research. This means that it is required to standardize animal models and protocols to obtain and prepare repair products and develop multicenter, randomized and previously registered studies.2
CLINICAL EVIDENCE
The pressing need for innovation in the management of heart failure promoted research in human regenerative therapies early on. Phase I and pilot studies would soon be followed by clinical trials with small numbers of patients. Some products reached phase III in clinical research, particularly in patients with acute myocardial infarction, refractory angina or ischemic and non-ischemic heart failure. Of all the evidence available to this day these conclusions can be drawn: a) except for exceptional well-identified cases (ventricular arrhythmias with initial cellular types), all products administered in humans have proven safe and no cases of rejection (not even with allogenic products), oncogenesis, worsening of the patients’ cardiovascular status or major complications during the administration have been reported; b) the real clinical efficacy of these therapies has not been undeniably confirmed through hard endpoints. While some studies have shown neutral results, others have confirmed a reduction of the infarction size, increased myocardial perfusion or ejection fraction, and better soft endpoints; c) the most promising scenarios are heart failure with systolic ventricular dysfunction and refractory angina; and d) a large and very sophisticated array of administration strategies, including surgical, has been developed (mostly percutaneous) to allow the injection of biological materials in certain cardiac regions using conventional or special catheters and navigational systems to accurately guide the administration.
Like the preclinical setting, the clinical evidence available allows us to identify some of the variables that should be confirmed and better defined before conducting large-scale clinical trials; among them, the selection of the type of product, the total dose, and the optimal administration time for every particular condition. Also, comparative studies of products, repeated administration, and improvement of myocardial retention in the products infused or injected. All these aspects should be rigorously analyzed through basic and preclinical research before conducting new studies in humans. At this point, the rigorous design of clinical trials with well-defined endpoints, adequate sample sizes, and strict regulatory control should be mentioned here.
INTERNATIONAL ALLIANCES AND SPECIFIC WORKING GROUPS
As part of the development of regenerative medicine, and to go deeper in its study and organize and structure a still marginal research, 2 main organizations have been created:
-
– The international consortium TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes).10 This consortium includes over top 100 international research working groups in this field. It is an worldwide, collaborative consortium network for the writing of position papers and recommendations, the rigorous promotion in all settings (scientific, institutional, and social), and for the design and development of coordinated and efficient clinical and preclinical research projects.
-
– The Working Group on Cardiovascular Regenerative and Reparative Medicine,11 is part of the European Society of Cardiology (ESC). It is a dynamic body founded on the pillars of training, education, research, congress participation, and field support as defined by the ESC rules and regulations.
The common initial objective of both associations is to analyze the evidence available on cardiovascular regenerative and reparative medicine, establish future research lines, and ultimately, facilitate the development of therapies to improve the patients’ cardiovascular health.
CONCLUSIONS
Although the clinical efficacy of regenerative and reparative medicine has not been confirmed yet to be able to include it in the routine clinical practice, it has overwhelmingly contributed to broaden our knowledge on the molecular biological, cellular, and tissue processes that govern functional loss, homeostasis, and cardiovascular system repair. By analyzing and planning future studies using multicenter, multidisciplinary, coordinated, evidence-based, rigorous methodology we will be closer to obtaining therapies capable of partially or totally reversing irreversible myocardial tissue damage and improving cardiovascular health.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever.
REFERENCES
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017:a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788.
2. Fernández-Avilés F, Sanz-Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017;38:2532-2546.
3. Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911-921.
4. Bergmann O, Zdunek S, Felker A, et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566-1575.
5. Climent AM, Sanz-Ruiz R, Fernández-Avilés F. Cardiac rejuvenation:a new hope in the presbycardia nightmare. Eur Heart J. 2017;38:2968-2970.
6. Buja LM. Cardiac repair and the putative role of stem cells. J Mol Cell Cardiol. 2019;128:96-104.
7. Roolvink V, Ibáñez B, Ottervanger JP, et al. Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J Am Coll Cardiol. 2016;67:2705-2715.
8. Zwetsloot PP, Végh AM, Jansen of Lorkeers SJ, et al. Cardiac Stem Cell Treatment in Myocardial Infarction:A Systematic Review and Meta-Analysis of Preclinical Studies. Circ Res. 2016;118:1223-1232.
9. Fisher SA, Doree C, Mathur A, et al. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361-1377.
10. TACTICS Alliance. Available online:https://www.tacticsalliance.org. Accessed 7 Oct 2019.
11. ESC Working Group on Cardiovascular Regenerative and Reparative Medicine. Available online:https://www.escardio.org/Working-groups/Working-Group-on-Cardiovascular-Regenerative-and-Reparative-Medicine. Accessed 7 Oct 2019.
Corresponding author: Servicio de Cardiología, Hospital General Universitario Gregorio Marañón, Dr. Esquerdo 46, 28007 Madrid, Spain.
E-mail address: (F. Fernández-Avilés).