ABSTRACT
Introduction and objectives: Female sex is believed to be a significant risk factor for mortality among patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary interventions (pPCI).
Methods: We collected data on all consecutive STEMI patients treated with pPCI within 12 hours and compared the males vs the females. The primary endpoint was long-term mortality one month after hospital discharge. The secondary endpoint was 30-days mortality.
Results: From March 2006 to December 2016, 1981 patients underwent pPCI at our hospital, 484 (24.4%) were females. Compared with men, women were older (mean age 71.3 ± 11.6 vs 62.9 ± 11.8 years, P < .001), less smokers (26.7% vs 72.7%; P < .001), more diabetic (28.0% vs 22.3%; P < .002), more hypertensive (69.6% vs 61.3%; P < .001), presented more often with shock at baseline (13.2% vs 9.0%; P = .006), had longer symptoms-to-balloon time frames (5.36 ± 3.97 vs 4.47 ± 3.67 hours; P < .001). Also, women were less likely to receive glycoprotein IIb-IIIa inhibitors (59.5% vs 71.4%; P < .001) and stents (79.5% vs 86.6%; P = .01). During the 30-day and long-term follow-up (mean 4.9 ± 3.2 years) the female sex was associated with a higher mortality rate (8.9% vs 4.0%, P < .001 and 23.8% vs 18.4%, P = .01, respectively). After propensity score matching, 379 men and 379 women were selected. Female sex continued to be associated with a higher death rate at 30 days (9.5% vs 5.5%; P = .039) but not in the long term among survivors (25.6% vs 21.4%; P = .170).
Conclusions: Compared to men, women with STEMI undergoing pPCI had higher 30-day mortality rates. However, among survivors, the long-term mortality rate was similar. Even if residual confounding cannot be ruled out, this difference in the outcomes may be partially explained by biological sex-related differences.
Keywords: ST-segment elevation myocardial infarction. Primary angioplasty. Sex differences. Outcomes.
RESUMEN
Introducción y objetivos: El sexo femenino se considera un importante factor de riesgo de mortalidad en el infarto agudo de miocardio con elevación del segmento ST (IAMCEST) tratado con intervención coronaria percutánea primaria (ICPp).
Métodos: Se analizó a todos los pacientes consecutivos con IAMCEST tratados con ICPp dentro de las primeras 12 horas, y se compararon varones y mujeres. El objetivo principal fue la mortalidad a largo plazo en los supervivientes después del primer mes del alta, y el objetivo secundario fue la mortalidad a los 30 días.
Resultados: Desde marzo de 2006 hasta diciembre de 2016 se trató con ICPp 1.981 a pacientes, de los cuales 484 (24,4%) eran mujeres. En comparación con los varones, las mujeres tenían mayor edad (edad media 71,3 ± 11,6 frente a 62,9 ± 11,8 años, p < 0,001) y la frecuencia de fumadoras era más baja (26,7 frente a 72,7%; p < 0,001), mientras que era más alta la frecuencia de diabetes (28,0 frente a 22,3%; p < 0,002), hipertensión arterial (69.6 frente a 61,3%, p < 0,001) y shock al ingreso (13,2 frente a 9,0%; p = 0,006), y más largo el tiempo desde el comienzo de los síntomas hasta la intervención con balón (5,36 ± 3,97 frente a 4,47 ± 3,67 horas; p < 0,001). Además, la frecuencia de tratamiento con inhibidores de la glucoproteína IIb-IIIa (59,5 frente a 71,4%; p < 0,001) y stent (79,5 frente a 86,6%, p = 0,01) fue inferior. Tanto a los 30 días como a largo plazo (media 4,9 ± 3,2 años), el sexo femenino se asoció con una mortalidad más alta (8,9 frente a 4,0%, p < 0,001, y 23,8 frente a 18,4%, p = 0,01, respectivamente). Se seleccionaron 379 mujeres y 379 varones emparejados por puntuación de propensión. Se mantuvo la asociación entre sexo femenino y mayor mortalidad a los 30 días (9,5 frente a 5,5%; p = 0,039), pero no a largo plazo (25,6 frente a 21,4%; p = 0,170).
Conclusiones: En comparación con los varones, las mujeres con IAMCEST tratadas con ICPp tuvieron mayor mortalidad a los 30 días. Sin embargo, entre los supervivientes, la mortalidad a largo plazo fue similar. Aunque no puede descartarse el efecto de variables residuales de confusión, las diferencias en el pronóstico podrían explicarse en parte por diferencias biológicas relacionadas con el sexo.
Palabras clave: Infarto agudo de miocardio con elevacion del segmento ST. Angioplastia primaria. Diferencias por sexo. Pronostico.
Abreviaturas
pPCI: primary percutaneous coronary interventions. STEMI: ST-segment elevation myocardial infarction.INTRODUCTION
Primary percutaneous coronary interventions (pPCI) have proven superior to fibrinolytic therapy for the management of patients with ST-segment elevation myocardial infarction (STEMI)1-3 becoming the treatment of choice in this field.4 However, the question of whether there are any prognostic differences between women and men is still under discussion. Yet despite the fact that in recent studies women exhibit higher mortality rates,5 it is not clear if these differences are associated with worse risk profiles or with a sex-related frailty. Indeed, some studies have not shown any significant relationships between sex and mortality in STEMI, even after adjusting for age and other risk factors.6 Actually, only a limited number of studies have described medium or long-term mortality outcomes and differences in the inclusion criteria (ie, the entire acute coronary syndrome spectrum or only the STEMI subset) and in the therapeutic strategies (ie, medical or interventional) might explain these different findings. The goal of this large, single-center registry was to assess whether in consecutive patients with STEMI undergoing pPCI there were any differences between men and women in the clinical, angiographic, procedural characteristics, and clinical outcome at 30 days or in the long-term.
METHODS
All consecutive patients admitted to our center between January 2006 and December 2016 with a diagnosis STEMI treated with pPCI within 12 hours of symptom onset were recruited. The baseline features (age, sex, burden of cardiovascular risk factors, time from symptoms onset to balloon) were collected along with the procedural characteristics (target vessel, site and type of lesion, pharmacological treatment, thrombus aspiration, type of stent). All interventions were performed following the actual standards of PCI, and the treatment choice was left at the discretion of the operator who performed the procedure. All patients were routinely treated with aspirin (325 mg upon arrival, and then 100 mg daily indefinitely), and an IV bolus of unfractionated heparin (5000 IU). The use of bivalirudin (0.75 mg/kg and 1.75 mg/kg/h at least to the end of the procedure), or unfractionated heparin (100 U/kg or 60 U/kg if abciximab was used) or abciximab was left at the operator’s discretion. When used, the infusion of abciximab was extended for another 12 hours after the procedure. A loading dose of clopidogrel (600 mg), prasugrel (60 mg), or ticagrelor (180 mg) was administered before or immediately after the PCI, unless patients were already on chronic maintenance therapy, and then followed by a maintenance dose of clopidogrel (75 mg once a day), prasugrel (10 mg once a day), or ticagrelor (90 mg twice a day) for 12 months when possible. Repeated revascularization was only performed in the presence of symptom recurrence or proven ischemia related to the treated lesion.
Data on the 30-day follow-up were available for all patients at our center database. Information beyond the first month was collected through outpatient visits, telephone calls or by reviewing any available medical records to obtain the longer follow-up for each patient. All data were entered into a dedicated database.
The men vs women outcomes before and after the propensity score matching were compared. The primary endpoint was long-term mortality after hospital discharge. The secondary endpoint was 30-day mortality rate, 30-day and long-term Bleeding Academic Research Consortium bleeding type ≥ 2.7 Long-term events were evaluated starting from day 31 after discharge until the longer available follow-up. The rate of procedural efficiency (defined a Thrombolysis in Myocardial Infarction [TIMI] III grade flow and residual stenosis < 30%) and ST-segment resolution of more than 50% 60–90 minutes after the PCI was also collected and reported.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median (Q1-Q3), according to the normality of their distribution. Qualitative variables were expressed as frequencies and percentages. The Fisher’s exact test or the chi-square test were used for qualitative variables while the Student’s t test or the Mann-Whitney U test were used for quantitative variables as appropriate. Survival data were represented and analyzed using the Kaplan-Meier curves and the Cox regression analysis. All statistical tests were 2-sided. Results were considered significant if P values < .05. Given the baseline differences between female and male patients and in order to reduce selection bias, we used propensity score matching. The logistic regression model was used based on the baseline and peri-percutaneous coronary intervention characteristics. Thus, P values < .20 were defined to include the selected variables in the final model. The selected variables were age, smoking habit, hypertension, dyslipidemia, diabetes mellitus type 2, obesity, severe chronic renal failure, shock at presentation, cardiac resuscitation at presentation, ejection fraction < 35%, anterior wall myocardial infarct location, femoral access, use of bivalirudin, use of glycoprotein IIb-IIIa inhibitors, percutaneous transluminal coronary angioplasty on the left main coronary artery, use of counterpulsation, thrombectomy, use of drug-eluting stents and total ischemic time. Using these covariates, propensity score was calculated for each patient. Each female patient was matched using 1:1 nearest neighbor matching with a patient from the control group (male) with the same propensity score. The maximum difference (caliper) in the match propensity scores was < 0.15. The standardized mean differences were estimated before and after the matching and balance between both matched cohorts was assessed using the Hotelling T-squared test. Using this technique, 2 comparable groups of 379 patients each were obtained for final analysis. All standardized mean differences after matching were below 10%. Calibration was tested using the Hosmer-Lermeshow test and accuracy was assessed using the area under the ROC curve. Statistical analyses were performed using SPSS 21 statistical software package (IBM software). Propensity score matching was performed using the MatchIt package of R software (version 3.0.2).
RESULTS
From March 2006 to December 2016, among the 1981 patients who underwent pPCIs at our hospital, 484 (24.4%) were females (table 1). Compared to men, women were older (mean age 71.3 ± 11.6 vs 62.9 ± 11.8 years; P < .001), there were fewer female smokers (26.7% vs 72.7%; P < .001), they were more diabetic (28.0% vs 22.3%; P < .002), more hypertensive (69.6% vs 61.3%; P < .001), and they presented more frequently with cardiogenic shock at admission (13.2% vs 9.0%; P= .006). They also had longer symptoms-to-balloon time (5.36 ± 3.97 vs 4.47 ± 3.67 hours; P < .001) and lower left ventricular ejection fractions (46.8 ± 10% vs 48.5 ± 10%; P = .007). Also, as shown on table 2, women were less likely to be treated with glycoprotein IIb-IIIa inhibitors (59.5% vs 71.4%; P < .001), thrombus aspiration devices (48.3% vs 58.0%, P < .001) and stents (79.5% vs 86.6%; P = .01). Procedural efficiency and ST-resolution were significant lower in the female cohort (93.0 vs. 97.1%, P < .001 and 60.0 vs 65.8%, P = .033, respectively, and table 3).
| Raw | Matched | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 1981) | Female (n = 484; 24.4%) | Male (n = 1497; 75.6%) | P | Female (n = 379) | Male (n = 379) | P | |
| Age | 65.0 ± 12.3 | 71.3 ± 11.6 | 62.9 ± 11.8 | < .001 | 68.5 ± 11.9 | 69.2 ± 11.6 | .43 |
| Age > 80 years | 262 (13.2) | 134 (27.7) | 128 (8.6) | < .001 | 69 (18.2) | 75 (19.8) | .58 |
| Diabetes mellitusa | 469 (23.7) | 135 (27.9) | 334 (22.3) | .002 | 63 (16.6) | 74 (19.5) | .29 |
| Hypertensionb | 1254 (63.3) | 337 (69.6) | 917 (61.3) | .001 | 227 (59.9) | 240 (63.3) | .33 |
| Dyslipidemiac | 742 (37.5) | 182 (37.6) | 560 (37.4) | .93 | 134 (35.4) | 142 (37.5) | .54 |
| Obesityd | 307 (15.5) | 113 (23.3) | 194 (13.0) | < .001 | 64 (16.9) | 66 (17.4) | .85 |
| Chronic kidney failuree | 53 (2.7) | 17 (3.5) | 36 (2.4) | .19 | 23 (6.1) | 23 (6.1) | 1.00 |
| Smoking (current or former smoker) | 1217 (61.4) | 129 (26.7) | 1088 (72.7) | < .001 | 129 (34.0) | 129 (34.0) | 1.00 |
| Cardiogenic shock at presentation | 198 (10.0) | 64 (13.2) | 134 (9.0) | .006 | 56 (14.8) | 43 (11.3) | .16 |
| Oral intubation | 116 (5.9) | 31 (6.4) | 85 (5.7) | .56 | 26 (6.9) | 23 (6.1) | .66 |
| Cardiac resuscitation at presentation | 21 (1.1) | 8 (1.7) | 13 (0.9) | .14 | 7 (1.8) | 8 (2.1) | .79 |
| Left ventricular ejection fraction (%) | 48.4 ± 10.0 | 46.8 ± 10.0 | 48.5 ± 10.0 | .007 | 47.5 ± 9.4 | 47.3 ± 9.6 | .83 |
| Left ventricular ejection fraction < 35% | 202 (10.2) | 69 (14.3) | 133 (8.9) | .001 | 43 (11.3) | 44 (11.6) | .91 |
| Total ischemia time | 4.7 ± 3.8 | 5.4 ± 4.0 | 4.5 ± 3.7 | < .001 | 4.4 ± 3.7 | 4.6 ± 4.0 | .13 |
| Anterior wall infarct location | 877 (44.3) | 217 (44.8) | 660 (44.1) | .82 | 165 (43.5) | 165 (43.5) | 1.00 |
|
a American Heart Association Guidelines definition. |
|||||||
Table 2. Angiographic and periprocedural features
| Raw | Matched | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 1981) | Female (n = 484; 24.4%) | Male (n = 1497; 75.6%) | P | Female (n = 379) | Male (n = 379) | P | |
| Multivessel disease | 1055 (53.3) | 259 (53.5) | 796 (53.2) | .90 | 194 (51.2) | 195 (51.5) | .94 |
| Graft disease | 6 (0.3) | 2 (0.4) | 4 (0.3) | .62 | 1 (0.3) | 2 (0.5) | .56 |
| Radial access | 371 (18.7) | 82 (16.9) | 289 (19.3) | .25 | 58 (15.3) | 57 (15.0) | .92 |
| Use of GP IIb-IIIa | 1357 (68.5) | 288 (59.5) | 1069 (71.4) | < .001 | 251 (66.2) | 242 (63.9) | .49 |
| Bivalirudin | 210 (10.6) | 61 (12.6) | 149 (9.9) | .12 | 35 (9.2) | 45 (11.9) | .24 |
| Multivessel PCI | 93 (4.7) | 25 (5.2) | 68 (4.5) | .57 | 20 (5.3) | 18 (4.7) | .74 |
| PCI on left main coronary artery | 64 (3.2) | 17 (3.5) | 47 (3.1) | .69 | 13 (3.4) | 13 (3.4) | 1.00 |
| Aortic counterpulsation | 251 (12.7) | 69 (14.3) | 182 (12.2) | .23 | 57 (15.0) | 49 (12.9) | .40 |
| Thrombus aspiration | 1102 (55.6) | 234 (48.3) | 868 (58.0) | < .001 | 203 (53.6) | 193 (50.9) | .47 |
| Stent implantation | 1682 (84.9) | 385 (79.5) | 1297 (86.6) | .01 | 314 (82.9) | 318 (83.9) | .58 |
| Drug-eluting stent implantation | 832 (42.0) | 194 (40.0) | 658 (43.9) | .09 | 148 (39.0) | 152 (40.1) | .77 |
|
Values are expressed as mean ± standard deviation or frequencies (percentages). |
|||||||
| Raw | Matched | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 1981) | Female (n = 484; 24.4%) | Male (n = 1497; 75.6%) | P | Female (n = 379) | Male (n = 379) | P | |
| Procedural efficacy | 1903 (96.1) | 450 (93.0) | 1453 (97.1) | < .001 | 342 (90.2) | 358 (94.4) | .039 |
| ST-segment resolution > 50% | 1086 (64.4) | 243 (60.0) | 843 (65.8) | .033 | 180 (47.5) | 205 (54.1) | .07 |
| 30-day BARC bleeding type ≥ 2 | 27 (2.1) | 22 (4.5) | 21 (1.4) | .002 | 16 (4.2) | 6 (1.6) | .007 |
| Long-term BARC bleeding type ≥ 2 | 41 (2.1) | 21 (4.3) | 20 (1.3) | < .001 | 13 (3.4) | 7 (1.8) | .257 |
| 30-day mortality rate | 103 (5.2) | 43 (8.9) | 60 (4.0) | < .001 | 36 (9.5) | 21 (5.5) | .039 |
| Overall mortality at long-term follow-up | 390 (19.7) | 115 (23.8) | 275 (18.4) | .01 | 97 (25.6) | 81 (21.4) | .170 |
|
Values are expressed as frequencies (percentages). |
|||||||
At the 30-day and long-term follow-up (mean 4.9 ± 3.2 years, completed in 1634, 82.5% patients) the female sex was associated with a higher mortality rate (8.9% vs 4.0%, P < .001 and 23.8% vs 18.4%, P = .01, respectively) and a higher rate of major bleedings at 30 days (4.5% vs 1.4%; P = .002).
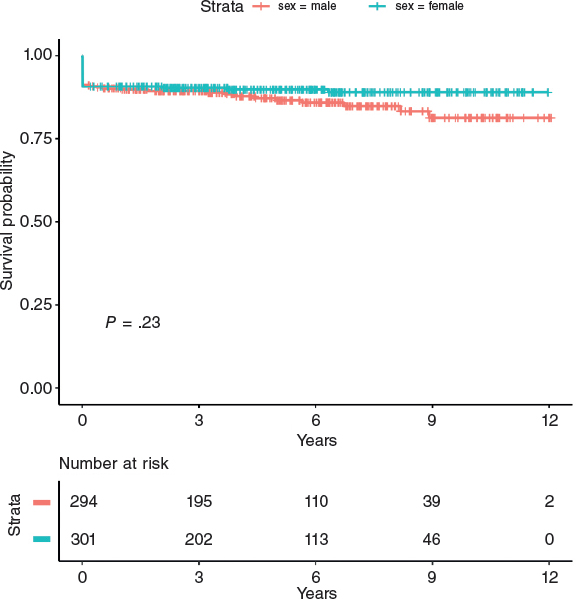
After propensity score matching, 379 men and 379 women were selected. The baseline and peri-procedural characteristics of the propensity-matched pairs were identical (table 1, table 2, figure 1, figure 2, and table 1 and table 2 of the supplementary data). In this cohort, female sex continued to be associated with a lower procedural efficiency (94.4% vs 90.2%; P = .039) and a higher rate of major bleedings and death at 30 days (9.5% vs 5.5%, P = .039 and 4.2% vs 1.6%, P = .007). Conversely, in the matched cohort no significant differences were found in the long-term mortality rate among survivors (25.6% vs 21.4%, P = .170, table 3; and log-rank P = .23, figure 3). The multiple Cox regression analysis revealed that age (hazard ratio [HR], 1.09; (1.06 – 1.12); P < .001), cardiogenic shock at presentation (HR, 6.82 (3.84 – 12.12); P < .001), the left ventricular ejection fraction < 35% (HR, 1.98 (1.11 – 3.54); P = .022) and procedural efficacy (HR, 0.46 (0.23 – 0.89); P = .022) have an effect on mortality when included together with female sex (HR, 0.68 (0.42 – 1.09); P = .106), which is not significant as stated before (table 4).
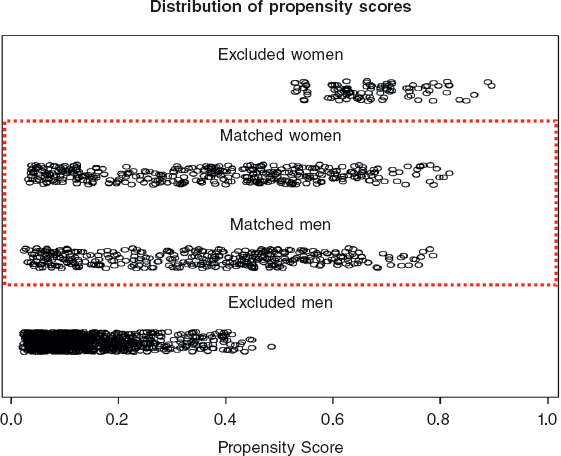
Figure 1. Distribution of propensity scores. Propensity score goes between 0 and 1 and several variables are included in its estimation. It serves as a tool for measuring the similarities among patients. Therefore, matched female and male patients show similar distributions of propensity scores and a wide range, covering all types of patients. Unmatched propensity score units lay on opposite extremes, as expected.
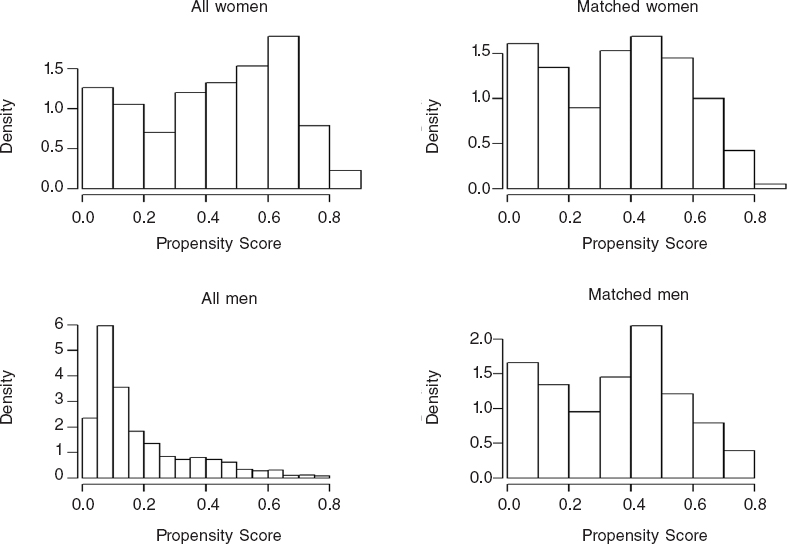
Figure 2. Distribution of propensity score before and after the matching.
Figure 3. Kaplan-Meier curve for long-term mortality based on sex. There is no difference on the long-term mortality rate between female and male patients in the matched population.
Table 4. Multiple regression analysis
| HR (95%CI) | P | |
|---|---|---|
| Female sex | 0.68 (0.42 – 1.09) | .106 |
| Age | 1.09 (1.06 – 1.12) | < .001 |
| Cardiogenic shock at presentation | 6.82 (3.84 – 12.12) | < .001 |
| Left ventricular ejection fraction < 35% | 1.98 (1.11 – 3.54) | .022 |
| Procedural efficacy | 0.46 (0.23 – 0.89) | .022 |
|
Cox proportional hazard model for overall mortality at long term follow-up. |
||
Main outcome analysis was performed based on a 2-time period (2006-2010 and 2011-2016) without underlining any differences among the groups (table 2 of the supplementary data).
DISCUSSION
Our large single-center registry showed that in a high PCI volume center, women admitted with STEMI undergoing pPCI have a higher 30-day and long-term mortality rate compared to men. This difference persists after propensity score adjustment regarding the 30-day mortality.
A drop in cardiovascular mortality has been observed over the last few decades, but cardiovascular disease is still the leading cause of mortality in women worldwide. Cardiovascular mortality remains higher in women compared to men.5 So far, the reasons of this difference have been mainly justified by the higher prevalence of traditional risk factors (higher mean age, hypertension, diabetes, and renal failure) in the female cohort.6 Also, women who experience myocardial infarction often present with atypical symptoms like dyspnea, fatigue, nausea/vomiting and atypical chest pain, which can lead to delayed diagnosis and treatment.8 Another factor associated with a higher mortality rate to be taken into account is represented by bleeding and mechanical complications, more common in women compared to men.9-11 Our study confirmed all these data: in our population, women were significantly older, with more traditional risk factors (except for smoking), longer ischemic time frames and higher-risk presentations. Also, they had a significantly higher rate of bleeding and mechanical complications.
After propensity score adjustment, out study showed that female sex was independently associated with 30-day but not long-term mortality. These findings are similar to those of a recent large meta-analysis led by Conrotto et al.,12 that included 98 778 patients (73 559 men and 25 219 women) and could be explained, at least in part, by the different pathophysiology of coronary disease in women: the rupture of the plaque surface, the leading cause of coronary occlusion in men, happens only in around 50% of women,13 the remaining percentage being represented by the erosion of the plaque,14 coronary spasm leading to thrombus generation,15 and spontaneous coronary artery dissection.16 Particularly spontaneous coronary artery dissection seems to play an important role in younger women (< 60 years of age) and is associated with a high rate of major adverse cardiac events.17 Therefore, these findings may explain why, in our population, women had lower rate of thrombus aspiration, use glycoprotein IIb-IIIa inhibitors, and stent implantation, which in turn may justify, along with the clinical features, the lower procedural success and ST-segment resolution observed in our study. These factors, associated with the higher rate of bleedings and mechanical complications and other psychological factors, like depression, more prevalent in women compared to men in the general population,18,19 may be phenotypes of a higher frailty in the female sex, and may definitely explain the worse outcomes, at least in the short-term. At long-term, other factors, like the lower in-stent restenosis rate and the resulting lower need for target vessel revascularization observed in women, and already shown in some studies,20-23 may explain the similar outcomes observed in the propensity score analysis.
Limitations
This study has some important limitations; first, even though is a retrospective analysis, it is based on a prospective and dedicated database with propensity score matched analysis. Secondly, data are derived from a single center, which limits their applicability. For example, the use of drug-eluting stents was lower than it is actually is, mainly because the time frame of the study is wide. Actually, from 2006 to 2010 the percentage of drug-eluting stents was about 15% while during the second period (2011-2016), we found 65% of cases with drug-eluting stent implantation. Finally, due to the time frame of the data collection, most procedures were performed via femoral artery access, so we can assume that radial access could have lowered the rate of access bleeding complications, but we need further randomized studies targeted at the female population before being able to validate this hypothesis. However, this is probably a representative sample of an all-comers STEMI population who undergo pPCI in the real world.
CONCLUSIONS
In conclusion, in our single-center cohort of patients and after propensity score adjustment, women with STEMI undergoing pPCI seem to have a higher 30-day mortality rate but similar long-term outcomes after discharge compared to men. Even if residual confounding cannot be ruled out, this difference in the outcomes may be partially explained by biological sex-related differences.
CONFLICT OF INTERESTS
None of the authors have declared any conflicts of interests related to the present article.
WHAT IS KNOWN ABOUT THE TOPIC?
- Women have a higher ACS-related mortality rate but there is no consensus on whether to consider female sex as a risk factor of poor outcomes. This is due to the fact that many authors explain this sex difference by the atypical onset of symptoms in women and the lower recurrence to cath. lab procedures and angioplasties performed in females. However, only a limited number of studies have reported medium or long-term mortality results and even fewer studies have had clear inclusion criteria (ie, the entire acute coronary syndrome spectrum or only the STEMI subset) or reported on the treatment strategies used.
WHAT DOES THIS STUDY ADD?
- Our large single-center registry showed that in a high PCI volume center, women admitted with STEMI to undergo pPCI have a higher rate of 30-day and long-term mortality compared to men. This difference persisted even after propensity score adjustment on the the 30-day mortality, which may be justified by the higher frailty of the female sex, which could in turn explain the worse outcomes seen, at least, in the short-term.
REFERENCES
1. Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328:673-679.
2. Schömig A, Kastrati A, Dirschinger J, et al. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction Study Investigators. N Engl J Med. 2000;343:385-391.
3. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction:a quantitative review of 23 randomized trials. Lancet. 2003;361:13-20.
4. Steg PG, James SK, Atar D, et al. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
5. Mozzafarian D, Benjamin EJ, Go AS, et al. On behalf of the American Heart Association Statistics Committee and Stroke Statistics subcommittee. Heart disease and stroke statistics-2015 update:a report from the American Heart Association. Circulation. 2015;131:e29-e322.
6. Otten AM, Maas AH, Ottervanger JP, et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent?Gender difference in STEMI stratified on age. Eur Heart J Acute Cardiovasc Care. 2013;2:334-341.
7. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials:a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747.
8. Wenger NK. Angina in women. Curr Cardiol Rep. 2010;12:307-314.
9. Lansky AJ, Pietras C, Costa RA, et al. Gender differences in outcomes after primary angioplasty versus primary stenting with and without abciximab for acute myocardial infarction:results of the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. Circulation. 2005;111:1611-1618.
10. Yu J, Mehran R, Grinfeld L, et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction:three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85:359-368.
11. Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556-2566.
12. Conrotto F, D'Ascenzo F, Humphries KH, et al. A Meta-Analysis of Sex-Related Differences in Outcomes After Primary Percutaneous Intervention for ST-Segment Elevation Myocardial Infarction. J Intervent Cardiol. 2015;28:132-140.
13. Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes:the pathologists'view. Eur Heart J. 2013;34:719-728.
14. Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core:a frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354-1363.
15. Kawana A, Takahashi J, Takagi Y, et al. Japanese Coronary Spasm Association. Gender differences in the clinical characteristics and outcomes of patients with vasospastic angina:a report from the Japanese Coronary Spasm Association. Circ J. 2013;77:1267-1274.
16. Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010;96:801-808.
17. Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579-588.
18. Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study):case-control study. Lancet. 2004;364:937-952.
19. Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health:Mental Health Findings. 2012. Available at:http://www.samhsa.gov/data/sites/default/files/2011MHFDT/2k11MHFR/Web/NSDUHmhfr2011.htm. Consulted 1 Jun 2019.
20. Alfonso F, Hernández R, Bañuelos C, et al. Initial results and long-term clinical and angiographic outcome of coronary stenting in women. Am J Cardiol. 2000;86:1380-1383.
21. Mehilli J, Kastrati A, Bollwein H, et al. Gender and restenosis after coronary artery stenting. Eur Heart J. 2003;24:1523-1530
22. Presbitero P, Belli G, Zavalloni, et al. “Gender paradox“in outcome after percutaneous coronary intervention with paclitaxel eluting stents. EuroIntervention. 2008;4:345-350.
23. Regueiro A, Fernández-Rodríguez D, Brugaletta S, et al. Sex-related Impact on Clinical Outcome of Everolimus-eluting Versus Bare-metal Stents in ST-segment Myocardial Infarction. Insights From the EXAMINATION Trial.Rev Esp Cardiol. 2015;68:382-389
Corresponding author: Interventional Cardiology, Infermi Hospital Rivoli and San Luigi Gonzaga Hospital, 10043 Orbassano, Turin, Italy.
E-mail addresses: enrico.cerrato@gmail.com (E. Cerrato).
◊ These authors equally contributed to the realization of the paper.