ABSTRACT
Introduction and objectives: The strategy of the percutaneous treatment of patients with multivessel disease associated with chronic total coronary occlusion (CTO) lesions is not well defined. Also, the functional significance of lesions located in the collateral donor artery has not been fully addressed. Using the fractional flow reserve (FFR) the objective was to evaluate the amount of ischemia related to the angiographically intermediate stenosis of collateral donor vessels before and immediately after successful percutaneous coronary intervention (PCI) of a CTO. Also, to assess any changes operated in the amount of ischemia using cardiovascular magnetic resonance imaging prior to the PCI and at 1-month follow-up.
Methods: Prospective pilot study including 14 patients with stable angina and a CTO receiving collateral circulation from a blood vessel with intermediate stenosis (50%-70% diameter stenosis measured using quantitative angiography). In order to indicate recanalization by PCI all patients were referred for magnetic resonance assessment of the presence of myocardial viability.
Results: Seven (50%) of the 14 patients included showed FFR values ≤ 0.80 before the PCI. FFR measures of the donor artery significantly increased after the revascularization of the CTO (0.75 [0.73-0.78] vs 0.83 [0.81-0.84]; P = .017). Eventually, only 3 patients showed hemodynamically significant FFR values after the recanalization of CTO requiring further revascularization. There was a tendency towards a reduction of the number of ischemic segments (2.5 [0-4] vs 0 [0-0.25]; P = .066) assessed using magnetic resonance imaging before and after the PCI. No major adverse cardiovascular events were reported at the 2-year follow-up.
Conclusions: Our data suggest that FFR measurements in intermediate stenoses of collateral donor vessels of a CTO may be misleading. Therefore, the strategy of focusing primarily on the revascularization of the CTO and then on the assessment of the intermediate lesion in a collateral donor vessel may be recommended.
Keywords: Chronic total coronary occlusion. Collateral donor vessel. Fractional flow reserve. Cardiovascular magnetic resonance imaging.
RESUMEN
Introducción y objetivos: La estrategia de tratamiento percutáneo de los pacientes con enfermedad multivaso y oclusión total crónica (OTC) no está bien definida. La importancia funcional de las lesiones localizadas en arterias donantes de colaterales no se ha abordado por completo. Nuestro objetivo fue evaluar mediante reserva fraccional de flujo (RFF) la cantidad de isquemia dependiente de una lesión angiográfica intermedia en un vaso donante de colaterales antes y después de la recanalización de la OTC, y valorar el cambio en la cantidad de isquemia por resonancia magnética cardiaca (RMC) antes y 1 mes después de la recanalización.
Métodos: Estudio piloto prospectivo en 14 pacientes con angina estable y una OTC que recibía circulación colateral de un vaso con una estenosis intermedia (50-70% por angiografía coronaria cuantitativa). Para indicar la revascularización, todos los pacientes presentaban viabilidad miocárdica por RMC.
Resultados: De los 14 pacientes, 7 (50%) evidenciaron una RFF ≤ 0,80 antes de la recanalización. Los valores medios de RFF de la arteria donante aumentaron significativamente tras la revascularización de la OTC (0,75 [0,73-0,78] frente a 0,83 [0,81-0,84]; p = 0,017). Solo 3 pacientes mostraron valores de RFF hemodinámicamente significativos después de la recanalización de una OTC que requirió revascularización adicional. Hubo una tendencia hacia una reducción del número de segmentos isquémicos (2,5 [0-4] frente a 0 [0-0,25]; p = 0,066) evaluados por RMC antes y después del intervencionismo. No se observaron eventos cardiacos adversos mayores durante el seguimiento de 2 años.
Conclusiones: Las mediciones de RFF en estenosis intermedias de vasos donantes de colaterales de una OTC pueden ser engañosas. En estos casos podría plantearse la estrategia de centrarse primero en la revascularización de la OTC y después en la evaluación de la lesión intermedia del vaso donante.
Palabras clave: Oclusión total crónica. Reserva fraccional de flujo. Resonancia magnética cardiaca. Vaso colateral donante.
Abreviaturas: CMR: cardiovascular magnetic resonance imaging. CTO: chronic total coronary occlusion. FFR: fractional flow reserve. PCI: percutaneous coronary intervention.
INTRODUCTION
The prevalence of chronic total coronary occlusions (CTO) is around 16% to 52% in patients with significant coronary artery disease on the angiography.1 In the presence of a CTO, collateral blood supply is often enought to maintain resting perfusion and contractility in the collateral-dependent myocardium.2 Restoration of antegrade flow by the percutaneous coronary intervention (PCI) of a CTO is associated with a rapid reduction in the collateral supply received in the treated vessel.3
Randomized trials support the use of fractional flow reserve (FFR) to guide the PCI with an established treatment threshold of ≤ 0.8.4-8 Although the FFR is reported to be independent of hemodynamic changes,9 it is intimately related to total coronary flow through a stenosis, which in turn is related to perfused myocardial mass.10 In keeping with this, there have been several reports of normalization of FFR values from collateral donor vessel after successful recanalization of a CTO.11 By removing nutrient flow to the collateralized territory by CTO recanalization, the collateral network almost immediately increased its resistance, thus favoring flow to the donor territory during maximal hyperemia.12
In patients with Rentrop grade-2 or grade-3 collateral flow, the FFR value of the donor artery increased at least 0.10 after revascularization of the recipient artery. However, the FFR value did not change significantly in patients with Rentrop grade-0 or grade-1 collateral flow following revascularization. This suggests that well-developed collateral circulation might overestimate the FFR value in the donor artery with mild stenosis.13
The assessment of myocardial-perfusion through cardiovascular magnetic resonance imaging (CMR) is a noninvasive imaging modality for the detection of coronary artery disease with a high degree of concordance with the FFR for ischemia detection.14-16 Also, the CMR has emerged as robust and reproducible method to assess the ischemia and viability of the myocardium related to the CTO.17-19 The MR-INFORM trial showed that in patients with stable angina and risk factors for coronary artery disease, the CMR of myocardial perfusion was associated with a lower incidence of coronary revascularization compared to the FFR and was noninferior to the FFR regarding major adverse cardiovascular events (all-cause mortality, non-fatal myocardial infarction or target-vessel revascularization) at 12 months.20 However, it is uncertain whether opening a CTO can modify the amount of ischemia related to an angiographically intermediate lesion of the collateral donor vessel. It could also be possible to diagnose microvascular dysfunction using CMR.21
Therefore, in this pilot study, using the FFR we assessed changes in the amount of ischemia related to the angiographically intermediate stenosis of collateral donor vessel before and immediately after the successful PCI of a CTO. We also tried to determine any changes in the amount of ischemia using the CMR prior to the PCI and 1 month after recanalization.
METHODS
In this prospective pilot study, we included patients with stable angina and CTO with collateralization of the distal vascular bed, and collateral donor vessel with a single angiographically intermediate lesion (50%-70% diameter stenosis by quantitative coronary angiography). In order to indicate recanalization through PCI all patients were referred for CMR evaluation to assess the presence of myocardial viability. During the procedure, the FFR of the donor vessel was measured before the PCI of the CTO (figure 1). Only with FFR values ≤ 0.80, the measure was reassessed after the procedure (figure 2). A second CMR was performed 1 month after the index PCI. All patients gave their informed consent, the local ethics committee approved the study, and all procedures were performed in accordance with the Helsinki Declaration. The study population was clinically followed for 2 years. The rate of major adverse cardiovascular events was established. This was defined as a composite of all-cause mortality, non-fatal acute myocardial infarction (AMI), clinically-driven target vessel revascularization or rehospitalization due to unstable or progressive angina according to Braunwald Unstable Angina Classification. The exclusion criteria were: prior IAM; failed recanalization of the CTO, inability to obtain signed written informed consents; severity of valvular heart disease; acutely decompensated chronic heart failure; asthma or obstructive sleep apnea; high risk of bleeding; known hypersensitivity or contraindication to aspirin; nursing subjects; patients with pacemakers/implantable cardioverter- defibrillators.
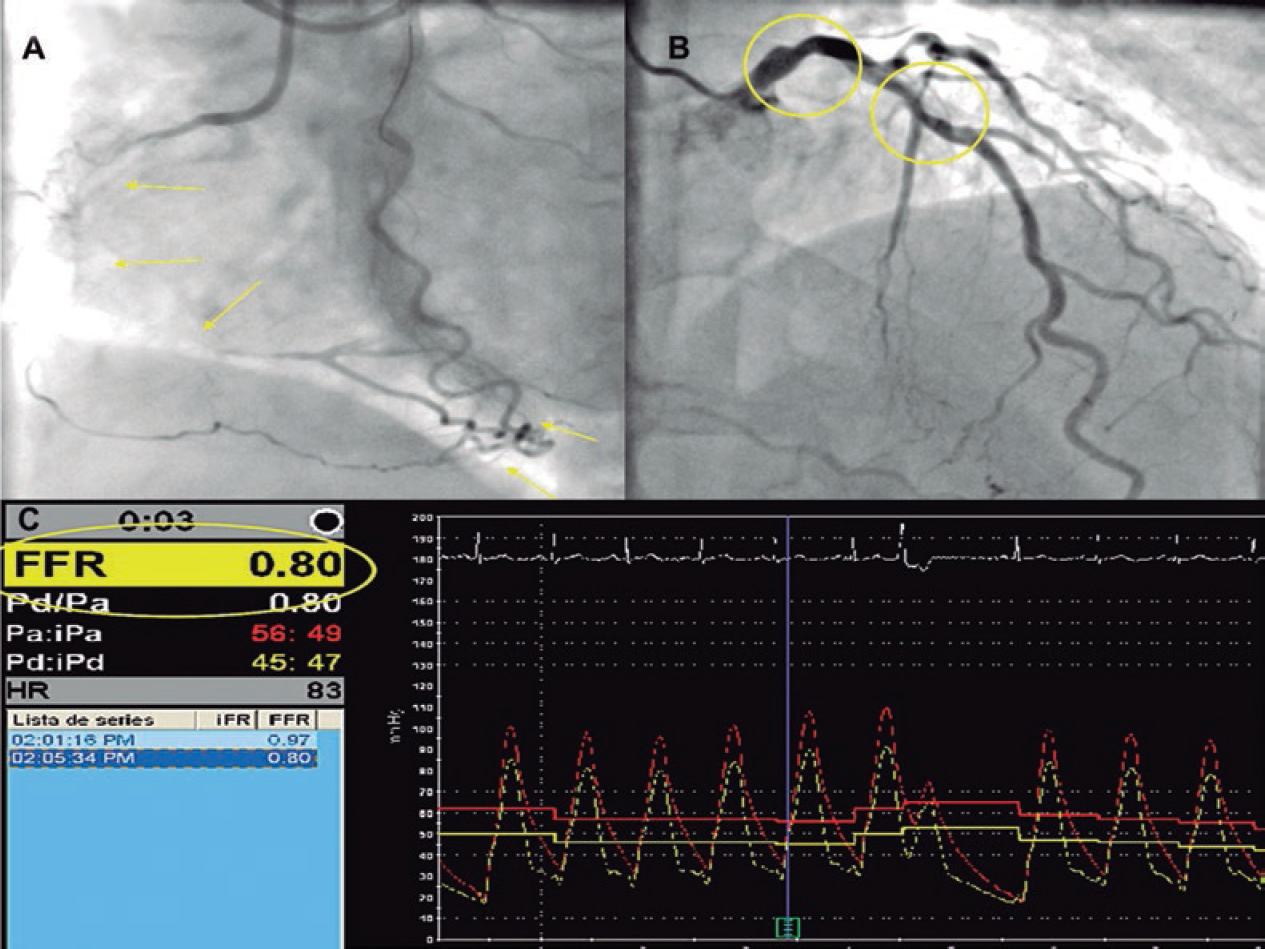
Figure 1. Example of chronic total coronary occlusion (CTO) of right coronary artery (panel A, yellow arrows) with collateralization of distal vascular bed, and left main and left anterior descendent artery (LAD) as the collateral donor vessel shows an angiographically intermediate lesion (panel B, yellow circles). During the procedure, the fractional flow reserve (FFR) of the donor vessel was measured before the percutaneous coronary intervention of the CTO (panel C).
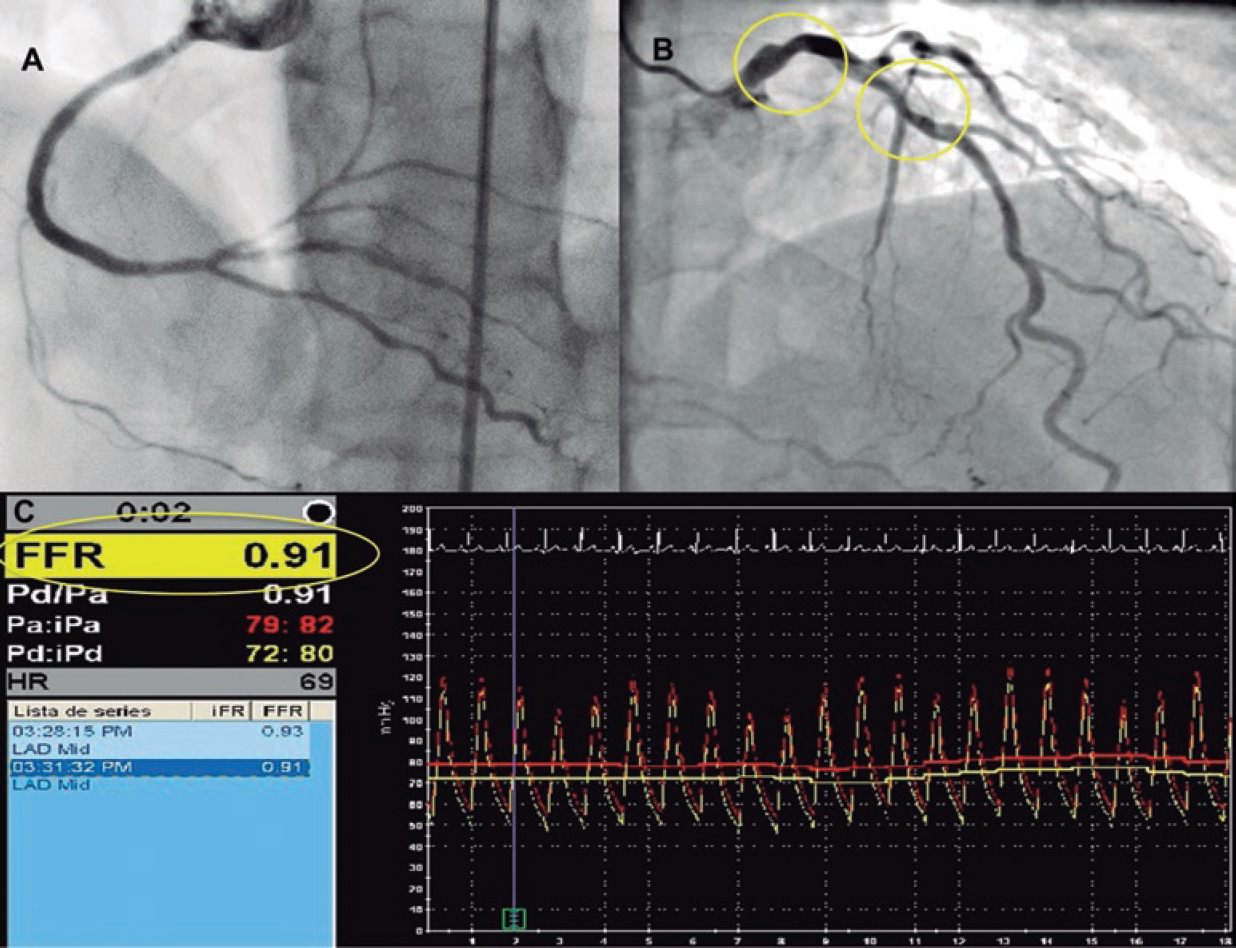
Figure 2. Example of the recanalization of chronic total coronary occlusion (CTO) of the right coronary artery (panel A) with left anterior descendent artery (LAD) as the collateral donor vessel shows an angiographically intermediate lesion (panel B, yellow circles). Panel C: after the CTO repermeabilization, the fractional flow reserve (FFR) value of the LAD increased (FFR value = 0.91).
The percutaneous coronary intervention
The PCI was performed using bilateral femoral artery access and 7-Fr sheaths and guide catheters. Anticoagulation was achieved with 100 U/Kg of unfractionated heparin to maintain activated clotting times of 250-300 msec. All the procedures on the CTO were performed using the antegrade wire escalation technique. All patients were treated with drug-eluting stent implantation. The J-CTO score was calculated for each CTO lesion and assessed taking the following parameters into consideration: occlusion length, stump morphology, presence of calcification, presence of tortuosity and prior attempt to open the CTO.22 Collateral flow was graded in accordance with Rentrop collateral flow classification.23 Procedural success was defined as achievement of residual post-PCI stenosis < 30% in the target lesion associated with TIMI grade-3 flow without mortality, IAM or new lesion revascularization during the index hospitalization.
Assessment using fractional flow reserve
To measure FFR in the intermediate coronary lesions a 0.014-inch pressure-monitoring guidewire (Prime Wire Volcano Therapeutics, Inc, Rancho Cordova, CA, United States) was used. After calibration of both the aortic and wire pressures, the FFR wire was advanced until the tip of the guiding catheter. Equalization of both pressures was performed. Then, the wire was advanced and positioned distally at least 15 mm from the stenotic lesion followed by the administration of 0.2 mg of nitroglycerin to avoid any form of epicardial vasoconstriction. Maximal hyperemia was induced through the IV infusion of adenosine (180 µg/kg/min). After reaching the steady state we measured the FFR as the ratio between mean distal coronary pressure and mean aortic pressure. Values < 0.80 were considered significant from the hemodynamical standpoint. After FFR measurement and under maximal hyperemia, the pressure wire was pulled back until the sensor was close to the tip of the guiding catheter to make sure that no drift had occurred.
Cardiovascular magnetic resonance imaging
All CMR studies were performed using a General Electric Signa HDxt 1.5-T scanner equipped with an 8-channel coil and cardiac-dedicated software. Perfusion studies were conducted using a gradient-echo turbo-field sequence prescribed in the left ventricular short-axis orientation, at the basal, mid-ventricular and apical levels after 4 min of IV administration of adenosine (Atepo-din) at a dose of 180 µg/kg/min and simultaneous administration of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Hispania) at a 5 mL/s rate. The functional and volumetric assessment of the left ventricle (LV) was conducted using the conventional Steady State Free Precession (SSFP) cine sequence, prescribed in sequential short-axis slices, and encompassing the entire LV and the 2-, 3-, and 4-chamber views. The typical temporal and in-plane spatial resolution of these images was 40 ms and 1.4 × 1.4 mm, respectively. Rest perfusion images were obtained at least 10 min after the stress perfusion study using the same sequence, location, and contrast injection protocol. Ten minutes after administering the dose of gadolinium for the rest perfusion study, late gadolinium-enhanced images were obtained using a segmented inversion-recovery spoiled gradient echo sequence in the same location and identical spatial resolution as the cine images. To calculate left ventricular ejection fraction (LVEF), the LV mass and left ventricular end-systolic and end-diastolic volumes, the endocardial and epicardial borders were manually traced at end-systole and end-diastole in the cine short-axis images using a dedicated software package (ReportCard, GE). The regional wall motion analysis was performed by visual grading of the cine images according to the 17-segment model proposed by the American Heart Association.17 The pre- and post-PCI image analysis was conducted by 2 independent experienced operators masked to the patient’s coronary anatomy and the PCI results; the disparities in their evaluation were resolved by consensus with a third independent operator. The appropriate allocation between the involved myocardial segments and the correspondent coronary anatomy in each case was evaluated according to previously reported criteria.18
Statistical analysis
The distribution of continuous variables was assessed by visual inspection of frequency histograms and using the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) when they followed a normal or non-normal distribution, respectively. The continuous variables were compared using the unpaired Student t test or Mann–Whitney U test and the categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Correlations between variables were conducted using the Pearson test. The software SPSS 17.0 (SPSS Italy, Florence, Italy) was used for statistical analyses.
RESULTS
We screened 23 patients with stable angina and CTO with collateralization of distal vascular bed, and collateral donor vessel with angiographically intermediate lesion. We excluded 9 patients who showed some exclusion criteria. Fourteen patients were finally included in the study (figure 3). The clinical characteristics and angiographic details are shown on table 1. Seven intermediate lesions (50%) of the collateral donor vessels showed FFR values ≤ 0.80 before the recanalization of the CTO. On average, FFR measures significantly increased after CTO revascularization (0.75 [0.73-0.78] vs 0.83 [0.81-0.84]; P = .017) (table 2 and figure 4). Four patients normalized their FFR values, while in the other 3 the FFR remained hemodynamically significant and required subsequent PCI. There was a tendency towards a reduction of the number of ischemic segments assessed through CMR before and after the recanalization of the CTO (2.5 [0-4] vs 0 [0-0.25]; P = .066). No differences were found in other parameters including the number of hypokinetic segments, left ventricular ejection fraction, left ventricular end-diastolic and end-systolic volumes; left ventricular mass; and necrotic mass before and after the PCI (table 2). In addition, the number of ischemic segments did not significantly correlate with the FFR values before or after PCI (R2 = -0.31, P = .328; R2 = -0.68, P = .20, respectively). Finally, no major adverse cardiovascular events were reported during the 2-year follow-up.
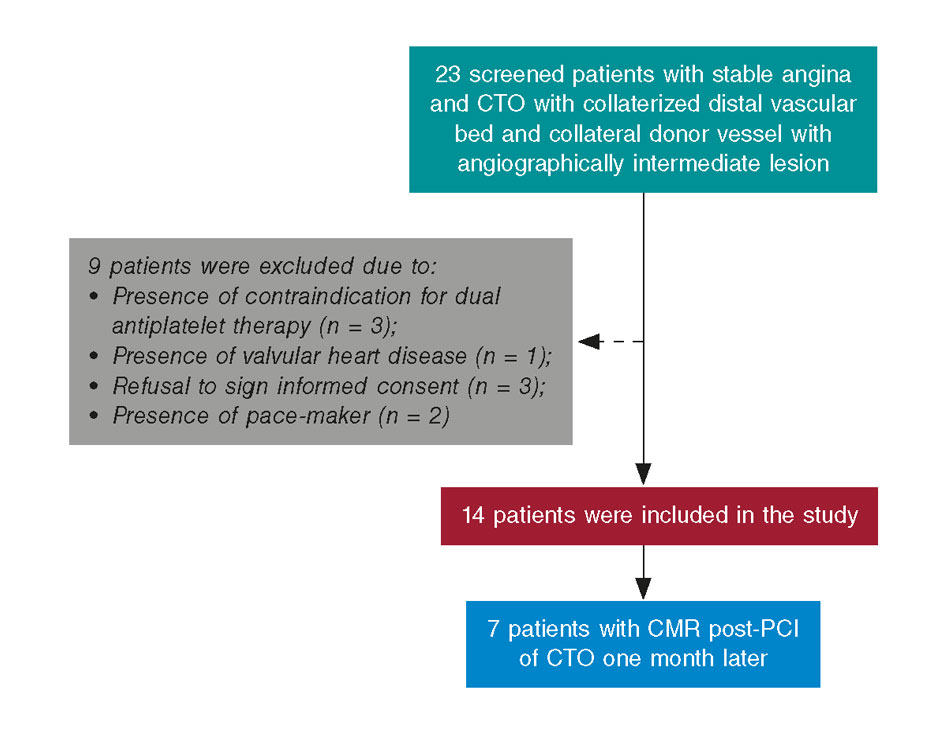
Figure 3. We screened 23 patients with stable angina and chronic total occlusion (CTO) with collateralization of distal vascular bed, and collateral donor vessel with angiographically intermediate lesion; 9 of them were excluded after meeting the exclusion criteria. In particular, 3 contraindications for dual antiplatelet therapy, 1 valvular heart disease requiring surgery, 3 refusals to sign the informed consent, and 3 pacemakers. CMR, cardiovascular magnetic resonance; PCI, percutaneous coronary intervention.
Table 1. Clinical and angiographic characteristics
| Clinical characteristics | Patients (n = 14) |
|---|---|
| Age, years | 67.44 ± 12.9 |
| Male | 12 (85) |
| Hypertension | 6 (42.8) |
| Smoking | 2 (14.3) |
| Hyperlipidemia | 10 (71.4) |
| Diabetes Mellitus | 5 (35.7) |
| Renal failure | 2 (14.3) |
| Prior CABG | 1 (7.1) |
| Medical treatment | |
| Beta-blockers | 5 (35.7) |
| Calcium antagonist | 2 (14.3) |
| ACE inhibitor | 4 (28.5) |
| Statins | 10 (71.4) |
| Angiographic characteristics | |
| CTO vessel | |
| LAD | 2 (14.3) |
| LCX | 1 (7.1) |
| RCA | 11 (78.6) |
| Calcification | 7 (50%) |
| Bending > 45 degrees | 2 (14.3) |
| Tapered | 8 (57.1) |
| Occlusion length, mm | 24.6 [6-43.3] |
| Rentrop > 1 | 13 (92.8) |
| J-CTO score > 2 | 3 (21.4) |
| Collateral donor vessel | |
| LAD | 7 (50) |
| LCX | 4 (28.6) |
| RCA | 3 (21.4) |
| Stenosis degree | 52 [50-55] |
|
Data are expressed as n (%), mean ± standard deviation or median [interquartile range]. ACE, angiotensin converting enzyme; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; IQR, interquartile range; JCTO, Japanese CTO; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery. |
|
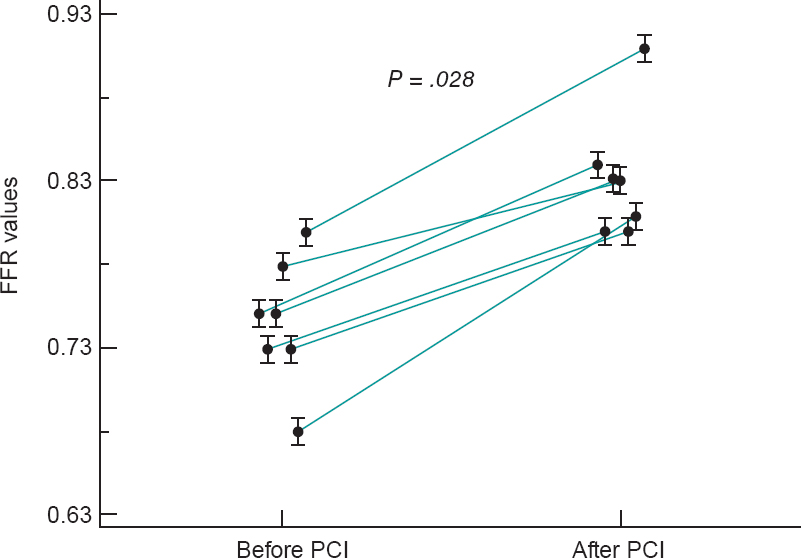
Figure 4. Fractional flow reserve (FFR) values of 7 angiographically intermediate lesions in the collateral donor vessels before and after the percutaneous coronary intervention (PCI) of a chronic total coronary occlusion.
Table 2. FFR and CMR measures in the study population
| Before PCI (n = 7) | After PCI (n = 7) | P | |
|---|---|---|---|
| Pd/Pa | 0.93 (0.88-0.96) | 0.91 (0.89-0.93) | 1.00 |
| FFR | 0.75 (0.73-0.78) | 0.83 (0.81-0.84) | .017 |
| IS | 2.5 (0.0-4.0) | 0.0 (0.0-0.25) | .066 |
| HS | 1.0 (0.0-4.75) | 0.0 (0.0-0.50) | .15 |
| LVEF, % | 60.5 (55.0-63.25) | 63.5 (54.0-65.25) | .41 |
| LVEDV, ml | 111.3 (102.7-451.1) | 109.0 (100.6-139.2) | .50 |
| LVESV, ml | 41.1 (38.6-65.17) | 38.9 (35.2-81.4) | .49 |
| LV mass, gr | 83.4 (56.4-92.1) | 88.5 (69.1-110.2) | .50 |
| NM, gr | 0.83 (0.3-2.3) | 0.92 (0.4-1.5) | 1.0 |
|
CMR, cardiovascular magnetic resonance imaging; FFR, fractional flow reserve; HS, hypokinetic segments; IS, ischemic segments; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NM, necrotic mass; Pd/Pa: resting distal coronary pressure to aortic pressure ratio; PCI, percutaneous coronary intervention. Data expressed as median (interquartile range). |
|||
DISCUSSION
These are the main findings of the study: a) functional assessment of intermediate lesions located in the collateral donor artery showed significantly lower FFR values than it would have in the absence of collateralized CTOs; b) after the recanalization of the CTO, the FFR values of the collateral donor artery normalized in most of patients; c) the amount of ischemia assessed through CMR used to decrease after successful CTO recanalization; d) no major adverse cardiovascular events were reported in our population at the long-term follow-up.
The FFR is a method used to assess the functional significance of coronary stenosis while taking in account the following parameters: severity of stenosis, myocardial territory and viability, and collateral perfusion.19 Results from the FAME trial showed that FFR-guided PCI was superior to the angiography-guided PCI at 1 and 2 years in terms of death or AMI and AMI alone.5,11 In the FAME 2 trial, the FFR-guided PCI reduced the rate of major adverse cardiovascular events compared to medical therapy alone.6 To this day, physiology has been proposed to outline which stenoses should be treated in the context of multivessel disease.24 However, there is uncertainty around what the waiting time is before performing an accurate pressure wire assessment of donor arteries after the successful recanalization of a CTO. Several studies have shown that full collateral regression does not happen immediately after the successful revascularization of a CTO.3 During embryonic development, collaterals derive either from capillary sprouting or pre-existing arteriolar connections.25 Collateral growth occurs through 2 major processes: arteriogenesis and angiogenesis. The former, stimulated by physical forces, consists of the growth, positive remodeling, and expansion of preexisting collateral vessels. The latter, induced by hypoxia, is the de novo growth of new capillaries by sprouting or intussusception from pre-existing vessels.26 Although once established, coronary collaterals are believed to persist and can be re-recruited, this process does not happen immediately. Well-developed collateral vessels close when the pressure gradient across the collateral network disappears. Also, the time needed to reopen the closed collaterals after reestablishing the pressure gradient seems to be directly related to the time interval between coronary occlusions.27 Recently, Mohdnazri et al. have showed that the successful recanalization of a right coronary artery CTO resulted in a modest but statistically significant and immediate increase of instantaneous wave-free ratio (iFR) in the predominant donor vessel following the recanalization of the CTO. At 4 months, both the FFR and the iFR showed significant improvement compared to pre-PCI values together with a concomitant reduction of collateral function.28 Ladwiniec et al. showed that the recanalization of a CTO resulted in a modest FFR increase of the predominant collateral donor vessel associated with a reduced coronary flow, of a similar magnitude at baseline and maximal hyperemia.29 Few patients of our study did not show this improvement. The persistence of non-angiographically visible collateral circulation, the presence of microcirculation dysfunction and type of prior collateral circulation grade,30 and distal embolization or myonecrosis following PCI recanalization may be potential causes of this lack of improvement. In this regard, in a recent study, measurements repeated shortly after the PCI of a CTO showed transient procedural-related changes like microvascular dysfunction secondary to distal embolization, catecholamine release, left ventricular stunning or hyperemic stimulus related to side-branch occlusion.29
Our data suggest that in the setting of CTOs and an angiographically intermediate lesion of the collateral donor vessel, it seems like the FFR measurement may be misleading. Therefore, it seems advisable to postpone the assessment of intermediate stenoses until achieving the successful recanalization of the associated CTO. This approach should avoid overtreating patients who only require the revascularization of their CTOs. On the contrary, if the recanalization of the CTO fails, treating the intermediate stenosis in the donor artery may be necesary to reduce ischemia in this territory. It also still is a good practice to try to re-open the CTO prior to performing any interventions on the donor vessel, due to the risk of extensive acute ischemia in case of troublesome PCIs.
Moreover, we did not find any correlations between the amount of ischemia assessed through CMR and the FFR values before or after the PCI. As far as we know, this is the first comparison between CMR and FFR assessment of an angiographically intermediate lesion in a collateral donor vessel related a CTO. Former studies have suggested that the CMR underestimates or that the FFR overestimates the number of ischemic segments in multi-vessel disease.31-32 This discrepancy seems to highlight the poor accuracy of the FFR method in the presence of collaterals involving territories that are from the target lesion to be assessed.
Finally, after treating the patients according to the FFR measures obtained after the PCI of a CTO, no major adverse cardiovascular events were detected at the 2-year follow-up.
Limitations
Several limitations should be acknowledged. First, due to the small size of the sample our findings should be, at best, hypothesis- generating findings. Secondly, we only used FFR as hyperemic index; other indices (eg. iFR, IMR, etc.) were not assessed. Similarly, we could not assess the influence of microcirculation through CMR or hyperemic microvascular resistance. Third, we did not assess whether collateral circulation originated from a segment proximal or distal to the target stenosis under study. Fourth, in patients with negative FFR before the recanalization of their CTO we did not repeat the FFR after the PCI. Finally, no follow-up CMRs were performed in patients with negative FFR prior to recanalization.
CONCLUSIONS
The FFR assessment of intermediate stenoses in a collateral donor vessel of a CTO may overestimate the severity of the lesion by increasing the territory at risk. Therefore, the strategy of first focusing on the revascularization of the CTO and then re-assess the intermediate lesion in a collateral donor vessel may be recommended to overcome this pitfall.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
WHAT IS KNOWN ABOUT THE TOPIC?
- In patients with CTOs, collateral circulation supplied by donor vessels is often seen.
- The progression of atherosclerosis in donor vessels may compromise the coronary circulation of several territories.
- Angiography is not a reliable technique to assess the hemodynamic compromise of an intermediate lesion located in a vessel that provides collateral circulation to a chronically-occluded vessel.
WHAT DOES THIS STUDY ADD?
- Patients with positive FFR of donor vessels before the recanalization of a CTO may show significant increases of FFR values (even normalization in most of them too) after successful revascularization of the CTO.
- Also, the revascularization of the CTO may lead to a reduction in the number of ischemic segments assessed through CMR before and after the PCI of the CTO.
- These findings support the strategy of recanalizing the CTO first and then performing the functional assessment of donor artery with intermediate lesions.
REFERENCES
1. Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions:the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991-997.
2. Hoebers L, Claessen B, Dangas G, et al. Contemporary overview and clinical perspectives of chronic total occlusions. Nat Rev Cardiol. 2014; 11:458-469.
3. Fujita M, Sasayama, S. Reappraisal of functional importance of coronary collateral circulation. Cardiology. 2010;117:246-252.
4. Bech GJ, De Bruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis:a randomized trial. Circulation. 2001;103:2928-2934.
5. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224.
6. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012; 367:991-1001.
7. Adjedj J, De Bruyne B, Flore V, et al. Significance of intermediate values of fractional flow reverse in patients with coronary artery disease. Circulation. 2016;133:502-508.
8. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease:2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-184.
9. Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367.
10. Christou MA, Siontis GC, Katritsis DG, Ioannidis JP. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol 2007;99:450-456.
11. Sachdeva R, Uretsky BF. The effect of CTO recanalization on FFR of the donor artery, Catheter Cardiovasc Interv. 2011;77:367-369.
12. Sachdeva R, Agrawal M, Flynn SE, et al. Reversal of Ischemia of Donor Artery Myocardium After Recanalization of a Chronic Total Occlusion. Catheter Cardiovasc Interv. 2013;82:E453-E458.
13. Tigen K, Durmus E, Sari I. Recanalization of a Total Occlusion With Marked Retrograde Collateral Supply:Impact of Collateral Circulation on Fractional Flow Reserve Measurements of Donor Artery. J Invasive Cardiol. 2014;26:E70-E75.
14. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CEMARC):a prospective trial. Lancet. 2012;379:453-460.
15. Watkins S, McGeoch R, Lyne J, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120:2207-2213.
16. Takx RAP, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to inva- sive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8:e002666-e6.
17. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542.
18. Ortiz-Pe?rez JT, Rodri?guez J, Meyers SN, Lee DC, Davidson C, Wu E. Correspondence between the 17-segment model and coronary arterial anatomy using contrast-enhanced cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2008;1:282-293.
19. Christou MA, Siontis GC, Katritsis DG, Ioannidis JP. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol. 2007;99:450-456.
20. Nagel E, Greenwood JP, McCann GP, et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med. 2019;380:2418-2428.
21. Liu A, Wijesurendra RS, Liu JM, et al. Diagnosis of Microvascular An-gina Using Cardiac Magnetic Resonance. J Am Coll Cardiol. 2018;71:969-979.
22. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes:the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011; 4:213-221.
23. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-592.
24. Escaned J, Banning A, Farooq V, et al. Rationale and design of the SYNTAX II trial evaluating the short to long-term outcomes of state-of-the-art percutaneous coronary revascularisation in patients with de novo three-vessel disease. EuroIntervention. 2016;12:e224-e234.
25. Werner GS. The role of coronary collaterals in chronic total occlusions. Curr Cardiol Rev. 2014;10:57-64.
26. Zimarino M, D'Andreamatteo M, Waksman R, et al. The dynamics of the coronary collateral circulation . Nat Rev Cardiol. 2014;1:191-197.
27. Zimarino M, Ausiello A, Contegiacomo G, et al. Rapid decline of collateral circulation increases susceptibility to myocardial ischemia:the trade-off of successful percutaneous recanalization of chronic total occlusions. J Am Coll Cardiol. 2006;48:59-65.
28. Mohdnazri SR, Karamasis GV, Al-Janabi F, et al. The impact of coronary chronic total occlusion percutaneous coronary intervention upon donor vessel fractional flow reserve and instantaneous wave-free ratio:Implications for physiology-guided PCI in patients with CTO. Catheter Cardiovasc Interv. 2018;92:E139-148.
29. Ladwiniec A, Cunnington MS, Rossington J, et al. Collateral Donor Artery Physiology and the Influence of a Chronic Total Occlusion on Fractional Flow Reserve. Circ Cardiovasc Interv. 2015;8:e002219.
30. Brugaletta S, Martin-Yuste V, PadróT, et al. Endothelial and smooth muscle cells dysfunction distal to recanalized chronic total coronary occlusions and the relationship with the collateral connection grade. JACC Cardiovasc Interv. 2012;5:170-178.
31. Hussain ST, Chiribiri A, Morton G, et al. Perfusion cardiovascular magnetic resonance and fractional flow reserve in patients with angiographic multi-vessel coronary artery disease. J Cardiovasc Magn Reson. 2016;18:44.
32. Cardona M, Martín V, Prat-Gonzalez S, et al. Benefits of chronic total coronary occlusion percutaneous intervention in patients with heart failure and reduced ejection fraction:insights from a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18:78.