ABSTRACT
Introduction and objectives: The PSP (pre-dilation, sizing and post-dilation) score, derived from the GHOST-EU registry, has evaluated the relationship between the implantation technique of bioresorbable scaffolds and the clinical outcomes. The objective was to perform an external validation of the PSP technique and to determine its effect on adverse cardiac events in various clinical and anatomical scenarios.
Methods: Data from the REPARA registry (2230 patients) were used for external validation, whereas a common database combining REPARA and GHOST-EU (3250 patients) data was used to evaluate the effect of PSP technique in various clinical and anatomical scenarios. PSP-1 and PSP-3 were used to score the appropriateness of pre-dilation, scaffold sizing, and post-dilation. The primary endpoint was 1-year device-oriented composite endpoint of cardiac death, target-vessel myocardial infarction, and target-lesion revascularization. The definite/probable scaffold thrombosis according to the Academic Research Consortium criteria was also evaluated.
Results: A total of 303 (18.2%) patients were treated with an optimal PSP-1, and 182 (8.2%) with an optimal PSP-3. The external validation showed that PSP has a very high negative predictive value for device-oriented composite endpoint and scaffold thrombosis (91.8% and 89.1% for PSP-1; 98.4% and 97.3% for PSP-3, respectively). Patients with an optimal PSP-3 had a numerically lower rate of device-oriented composite endpoint and scaffold thrombosis compared to those without it (0.5% vs 2.9%; P = .085 and 0.5% vs 1.8%; P = .248, respectively). In the merged database, PSP benefits were seen on many scenarios, except in the ST-segment elevation myocardial infarction where a trend towards no benefit of an optimal PSP technique was present (Pinteraction = .100).
Conclusions: In the REPARA registry, at 1-year follow-up, an optimal PSP technique was not associated with a lower rate of device-oriented composite endpoint. Further research is necessary to assess the impact of the PSP technique in longer follow-ups.
Keywords: Coronary artery disease. Percutaneous coronary intervention. Bioresorbable scaffolds. Bioresorbable vascular scaffolds.
RESUMEN
Introducción y objetivos: La escala de puntuación PSP (pre-dilation, sizing and post-dilation), derivada del registro GHOST-EU, evalúa la relación entre la técnica de implante de los armazones bioabsorbibles y los resultados clínicos. El objetivo fue realizar una validación externa de la escala PSP y determinar su efecto en eventos cardiacos adversos en diversos escenarios clínicos y anatómicos.
Métodos: Para la validación externa se emplearon los datos del registro REPARA (2.230 pacientes), mientras que se utilizó una base de datos común que combina datos de REPARA y GHOST-EU (3.250 pacientes) para evaluar el efecto de la técnica PSP en varios escenarios clínicos y anatómicos. Se usó PSP-1 y PSP-3 para calificar la calidad de la predilatación, el dimensionamiento de los armazones y la posdilatación. El objetivo primario fue la variable compuesta orientada al dispositivo (muerte cardiaca, infarto de miocardio del vaso diana y revascularización de la lesión diana) a 1 año. También se evaluó la trombosis definitiva o probable del armazón según los criterios del Academic Research Consortium.
Resultados: Se trató a 303 (18,2%) pacientes con una PSP-1 óptima y a 182 (8,2%) con una PSP-3 óptima. La validación externa mostró que la escala PSP tiene un valor predictivo negativo muy alto para el objetivo primario compuesto orientado al dispositivo y la trombosis del armazón (91,8 y 89,1% para PSP-1; 98,4 y 97,3% para PSP-3, respectivamente). En pacientes con PSP-3 óptimo, el objetivo primario compuesto orientado al dispositivo y la trombosis del armazón fueron numéricamente inferiores en comparación con los pacientes sin PSP-3 óptimo (0,5 frente a 2,9%; p = 0,085; y 0,5 frente a 1,8%; p = 0,248, respectivamente). En la base de datos combinada, los beneficios de la escala PSP se observaron en diversos escenarios, excepto en el de infarto de miocardio con elevación del segmento ST, en el que se observó una tendencia hacia la ausencia de beneficios de una técnica de PSP óptima (pinteracción = 0,100).
Conclusiones: Una técnica de PSP óptima no se asoció con una tasa más baja del objetivo primario compuesto orientado al dispositivo. Se necesitan nuevos estudios para evaluar el impacto de la técnica de PSP con un seguimiento más prolongado.
Palabras clave: Enfermedad coronaria. Intervencion coronaria percutanea. Armazon bioabsorbible. Armazon vascular bioabsorbible.
Abreviaturas: BVS: bioresorbable vascular scaffolds. DOCE: device-oriented composite endpoint. PSP: pre-dilation, sizing and post-dilation. STEMI: ST-segment elevation myocardial infarction.
INTRODUCTION
Recent meta-analyses of randomized clinical trials have rised concerns about the safety of first-generation bioresorbable vascular scaffolds (BVS).1 Specifically, a higher than expected scaffold thrombosis rate compared to drug-eluting stents was found.1-4
Optimization of the implantation technique was proposed to improve clinical outcomes of patients treated with BVS.5,6 The PSP (pre-dilation, sizing and post-dilation) score is a simple model designed to assess the quality of the BVS implantation technique, evaluate the preparation of the lesion, the size of the scaffold, and post-dilation. This score has been developed and internally validated in the GHOST-EU registry and is associated with the occurrence of adverse cardiac events at 1-year follow-up.7 However, this score has not been externally validated, and no data are available on whether the effect of the PSP implantation technique is different in various clinical and anatomical scenarios.
Therefore, we tried to perform an external validation of the PSP technique and evaluate its effect on the adverse cardiac events of patients treated with BVS in various clinical and anatomical scenarios.
METHODS
Population
The REPARA registry is an investigator-initiated, prospective, multicenter registry conducted at 58 Spanish and Portuguese centers. The registry included consecutive patients who underwent single or multivessel percutaneous cardiac intervention with at least one everolimus-eluting BVS device (Absorb BVS; Abbott Vascular, Santa Clara, CA, United States). Patients who underwent percutaneous cardiac intervention for one or two new native coronary artery lesions –up to four lesions– in separate epicardial coronary vessels were eligible for enrollment. Patients with acute myocardial infarction and specific complex lesion features were also included. Data from the REPARA registry were used for external validation of the PSP score. This is a retrospective not pre-specified analysis.
Data from REPARA and GHOST-EU registries were pooled in a single database by one investigator (L. Ortega-Paz) and used to evaluate the effect of the PSP technique in various clinical and anatomical scenarios. Details of the GHOST-EU registry have been described above.7
Procedures and follow-up
All interventions were performed according to the actual guidelines on the management of percutaneous cardiac intervention. Briefly, balloon pre-dilation was not mandatory but highly recommended. Scaffold implantation at pressures not exceeding the burst pressure rate was mandatory. Use of post-dilation was left at operator discretion and, if performed, one non-compliant balloon was recommended according to the protocol. Quantitative coronary angiography analysis pre-BVS implantation was analyzed in a centralized CORE Lab, and only patients with complete quantitative coronary angiography data were included in this analysis.
The PSP evaluation of the BVS implantation technique was applied according to the models previously detailed.7 Overall, 3 steps of scaffold implantation were evaluated in the PSP models shown on table 1. The PSP-2 model was not assessed because the quantitative coronary angiography after pre-dilation was not available.
Table 1. PSP models for the evaluation of implantation
| Implantation steps | PSP-1 | PSP-2 | PSP-3 |
|---|---|---|---|
| Pre-dilation | - Not performed - Performed |
- Either not performed or performed with a QCA residual stenosis ≥ 30% - Performed with a QCA residual stenosis < 30% |
- Not performed - Performed |
| Scaffold sizing | Correct sizing, defined as the following: - implantation of a 2.5 mm diameter scaffold in a vessel with a proximal/distal RVD ≥ 2.5 mm and < 2.75 mm - implantation of a 3.0 mm diameter scaffold in a vessel with a proximal/distal RVD ≥ 2.75 mm and < 3.25 mm; or - implantation of a 3.5 mm diameter scaffold in a vessel with a proximal/distal RVD ≥ 3.25 mm and ≤ 3.75 mm - if proximal and distal RVD differed, the mean value was used Incorrect sizing |
||
| Post-dilation | - Either not performed or performed with a compliant or non-compliant balloon with diameter 0.5 mm greater than the scaffold diameter or performed with a NC balloon with a diameter less than or equal to the scaffold diameter - Performed with an NC balloon of larger diameter than the scaffold, up to a maximum of 0.5 mm |
- Either not performed or performed with a compliant or non-compliant balloon with diameter 0.5 mm greater than the scaffold diameter or performed with a NC balloon with a diameter less than or equal to the scaffold diameter at a pressure < 16 atmospheres. - Performed with a NC balloon of larger diameter than the scaffold, up to a maximum of 0.5 mm and at a pressure ≥ 16 atmospheres. |
|
|
BVS, bioresorbable vascular scaffolds; NC, non-compliant; PSP, pre-dilation, sizing and post-dilation; QCA, quantitative coronary angiography; RVD, reference vessel diameter. |
|||
Clinical follow-up was obtained through clinical visits or phone calls at 12-month in both registries. In the REPARA registry, the process of data mining was externally monitored, and events were adjudicated by an independent committee. The occurrence of periprocedural myocardial infarction was not systematically assessed.
Outcomes and definitions
The primary endpoint was device-oriented composite endpoint (DOCE) of cardiac death, target-vessel myocardial infarction, and clinically driven target lesion revascularization (CD-TLR). Secondary outcomes were the individual components of the primary endpoint and definite/probable scaffold thrombosis, defined according to the Academic Research Consortium (ARC) criteria.8 REPARA and GHOST-EU registries used the same endpoints definitions according to the ARC criteria.8 Optimal PSP technique was defined as the highest PSP score value.7 In patient with more than one lesion treated, all the lesions should fulfill the optimal PSP criteria; otherwise the patient was classified as non-optimal. All endpoints were analyzed at 1-year follow-up.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation or median and interquartile range, as appropriate. Categorical variables are reported as absolute number and percentage. Differences in proportions were tested with chi-square or Fisher exact test and differences in continuous variables were tested with Student t-test.
External validation of the PSP score was performed according to TRIPOD type 4 validation.9 The PSP scores were evaluated in terms of overall performance, calibration, and discrimination, as previously shown.10 The overall performance of the models was assessed by Nagelkerke’s R2.10 Calibration was measured by the Hosmer-Lemeshow test.10 Discrimination was measured with the area under the receiver-operator characteristic curves (AUCs).10 Predictive values and likelihood ratios were also calculated.10 In the external validation population of the REPARA registry, weight of PSP technique and each component was evaluated by a Cox regression, adjusting for those variables predictors of DOCE at univariate analysis: diabetes, prior myocardial infarction or revascularization, multivessel disease, severely calcified lesion, bifurcations, and scaffold overlapping.
In the pooled database (REPARA and GHOST-EU data), the effect of the PSP technique on the DOCE was evaluated with formal interaction testing in various clinical and anatomical scenarios. These analyses were performed only for the model that performed the best.
A Kaplan-Meier method was used to derive the event rates at follow-up and to plot time-to-event curves, dividing the population according to the optimal PSP technique or each implantation step score. The Kaplan-Meier curves were compared using the log-rank test.
A 2-tailed P-value < .05 was considered significant. All data were processed using the Statistical Package for Social Sciences, version 22 (SPSS Inc., Chicago, IL, United States).
RESULTS
External validation population
A total of 2448 patients (3370 lesions) were included in the REPARA registry. Due to missing data for the evaluation of the PSP-1 and PSP-3 scores, only 2230 patients (2553 lesions) were included in this analysis (figure 1 of the supplementary data). The PSP-2 score was not evaluable due to missing residual percentage stenosis after pre-dilation in all patients, and therefore not considered for this analysis. There were no differences between patients included and those excluded in terms of clinical outcomes (data not shown).
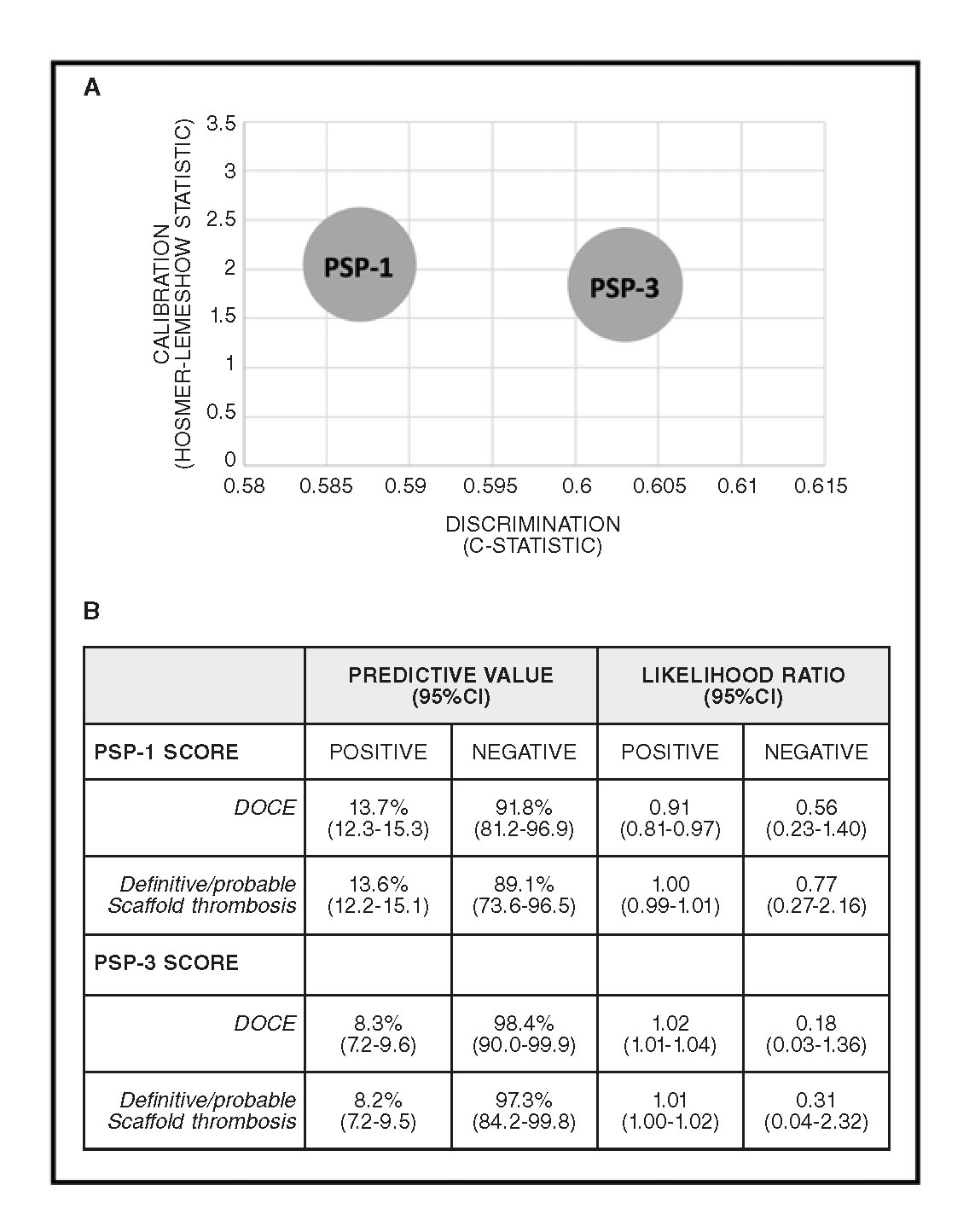
Figure 1. PSP score models performance. A: Rule-in performance: PSP-1: R2: 0.02, HL: X2=2.04 (0.727), and AUCs: 0.587 (0.511-0.664); P = .020. PSP- 3: R2: 0.03, HL: X2=1.84 (0.606), and AUCs: 0.603 (0.528-0.677); P = .006. B: Rule-out performance. 95%CI, 95% confidence interval; UCs, area under the receiver-operator characteristic curve (95%CI); DOCE, device-oriented composite endpoint; HL, Hosmer-Lemeshow (P-value); R2, Nagelkerke’s R2.
Patients treated with an optimal PSP-1 and PSP-3 techniques were 303 (13.6%), and 182 (8.2%), respectively (table 2; figure 2 of the supplementary data). The clinical and procedural data according to the optimal PSP score are shown on table 1 of the supplementary data, table 2 of the supplementary data, table 3 of the supplementary data, and table 4 of the supplementary data.
Table 2. Distribution of PSP models
| PSP-1 (n = 2553)a | PSP-3 (n = 2553)a | |
|---|---|---|
| Optimal PSP technique (%) | 357 (14.0) | 219 (8.6) |
| 1:1 pre-dilation, n (%) | ||
| No | 497 (19.5) | 497 (19.5) |
| Yes | 2056 (80.5) | 2056 (80.5) |
| Scaffold sizing, n (%) | ||
| Incorrect | 507 (19.9) | 507 (19.9) |
| 2.50 mm | 135 (26.6) | 135 (26.6) |
| 3.00 mm | 193 (38.1) | 193 (38.1) |
| 3.5 mm | 179 (35.3) | 179 (35.3) |
| Correctb | 2046 (80.1) | 2046 (80.1) |
| Post-dilation, n (%) | ||
| No | 1313 (51.4) | 1313 (51.4) |
| Over-expandedb | 39 (1.5) | 39 (1.5) |
| NC balloon > 1:1b | 623 (24.4) | NA |
| NC balloon > 1:1 and pressure ≥ 16 atm | NA | 393 (15.4) |
| QCA analysis pre-BVS implantation | ||
| RVD proximal (mm) | 3.10 ± 0.42 | 3.10 ± 0.42 |
| RVD distal (mm) | 2.92 ± 0.55 | 2.92 ± 0.55 |
| Mean RVD (mm) | 3.02 ± 0.51 | 3.02 ± 0.51 |
| Lesion length (mm) | 18.15 ± 9.32 | 18.15 ± 9.32 |
| Stenosis (%) | 84.10 ± 13.1 | 84.10 ± 13.1 |
| MLD (mm) | 0.98 ± 1.15 | 0.98 ± 1.15 |
|
Lesion-level analysis. aDefined as in the development and internal validation.7 bATM, atmospheres; MLD, minimal lumen diameter; NA, not applicable; NC, non-compliant; PSP, pre-dilation, sizing and post-dilation; QCA, quantitative coronary angiography; RVD, reference vessel diameter. |
||
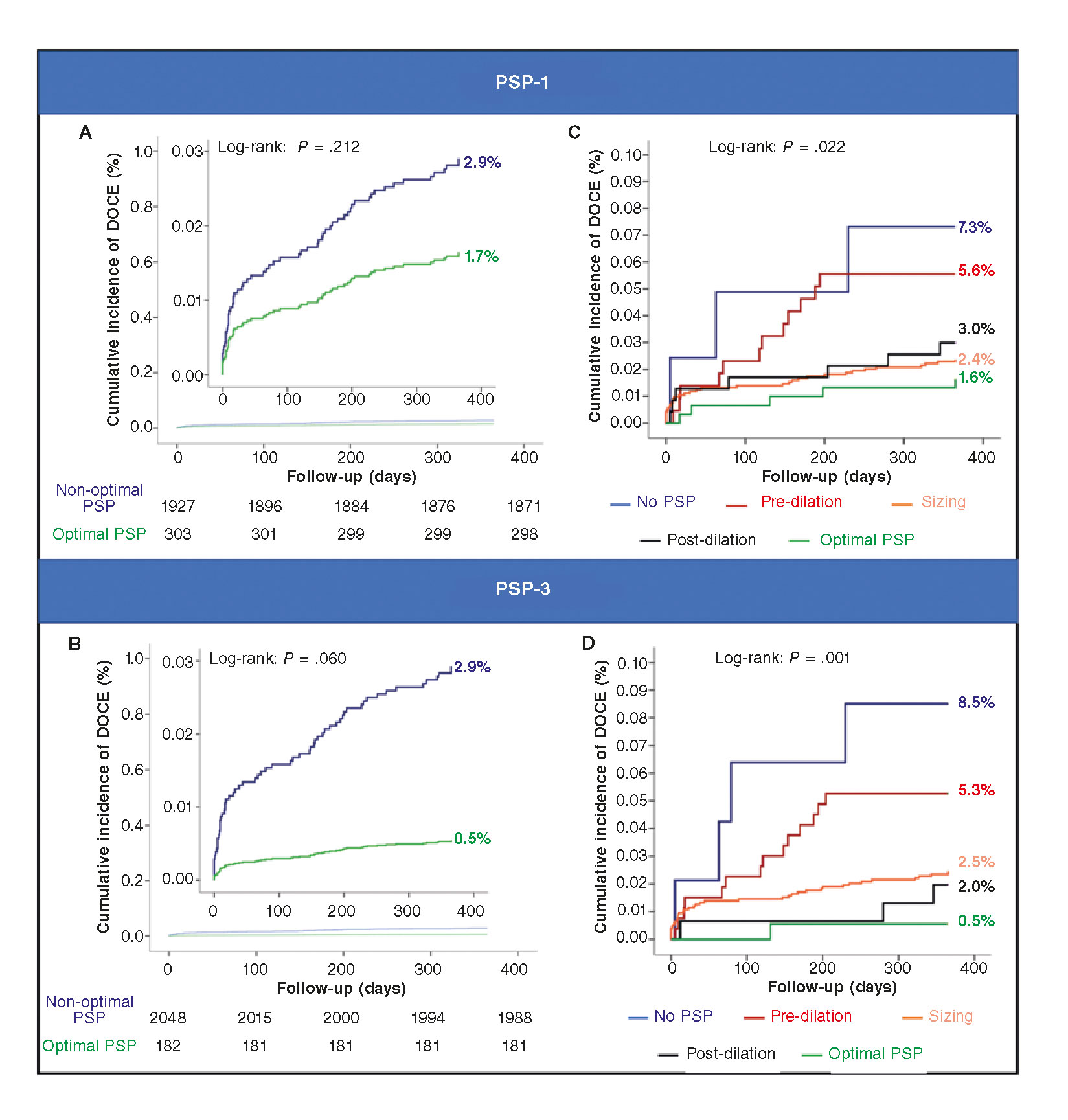
Figure 2. PSP technique: Kaplan-Meier analysis. Panel A: PSP-1: acute events (< 24h): 0% (0/303) vs 0.3% (6/1927), log-rank P = .331. Sub-acute events (1-30 d) 0.3% (1/303) vs 0.9% (18/1921), log-rank P = .286. Late events (30-235 d) 1.3% (4/302) vs 1.7% (32/1903), log-rank P = .650. Panel B: PSP-3: Acute events (<24h): 0% (0/182) vs 0.3% (6/2048), log-rank P = .465. Sub-acute events (1-30 days) 0% (0/182) vs 0.9% (19/2042), log-rank P = .192. Late events (30-365 days) 0.5% (1/182) vs 1.8% (36/2023), log-rank P = .230. Panel C: pre-dilation: HR, 1.07; 95%CI, 0.58-2.00; P = .824. Scaffold sizing: HR, 0.43; 95%CI, 0.25-0.75; PP = .003. Post-dilation: HR, 0.66; 95%CI, 0.34-1.26; P = .208. Panel D: pre-dilation: HR, 1.10; 95%CI, 0.59-2.05; P = .766. Scaffold sizing: HR, 0.42; 95%CI, 0.24-0.72; P = .002. Post-dilation: HR, 0.33; 95%CI, 0.12-0.92, P = .035. At a patient-level analysis, DOCE includes TV-MI, and target lesion revascularization. 95%CI, 95% confidence interval; DOCE, device-oriented composite endpoint; HR, hazard ratio; PSP, pre-dilation, sizing and post-dilation; TV-MI, target vessel myocardial infarction.
Table 3. Clinical outcomes at 1-year follow-up stratified according to the optimal PSP technique
| PSP-1 model | ||||
|---|---|---|---|---|
| Optimal PSP (n = 303)a | Non-optimal PSP (n = 1927)a | HR (95%CI) | P | |
| DOCEb | 5 (1.7) | 56 (2.9) | 1.75 (0.69-4.45) | .219 |
| Cardiac death | 1 (0.3) | 14 (0.7) | 2.21 (0.30-16.87) | .444 |
| TV-MI | 3 (1.0) | 31 (1.6) | 1.64 (0.50-5.38) | .419 |
| TLR | 3 (1.0) | 42 (2.2) | 2.23 (0.69-7.23) | .182 |
| Definite/probable scaffold thrombosis | 4 (1.3) | 33 (1.7) | 1.30 (0.46-3.70) | .620 |
| PSP-3 model | ||||
| Optimal PSP (n = 182)a | Non-optimal PSP (n = 2048)a | HR (95%CI) | P | |
| DOCEb | 1 (0.5) | 60 (2.9) | 5.73 (0.78-41.88) | .085 |
| Cardiac death | 0 | 15 (0.7) | NA | .627 |
| TV-MI | 1 (0.5) | 33 (1.6) | 2.96 (0.40-21.80) | .286 |
| TLR | 1 (0.5) | 44 (2.1) | 3.97 (0.54-29.01) | .174 |
| Definite/probable scaffold thrombosis | 1 (0.5) | 36 (1.8) | 3.24 (0.44-23.76) | .248 |
|
aPatient-level analysis. bMultivariate adjusted Cox model. DOCE includes cardiac death, TV-MI, and TLR. 95%CI, 95% confidence interval; DOCE, device-oriented composite endpoint; HR, hazard ratio; NA, not applicable; PSP, pre-dilation, sizing and post-dilation; TLR, target lesion revascularization; TV-MI, target vessel myocardial infarction. |
||||
External validation
The PSP-3 score displayed the best calibration (X 2= 1.84, P = .606 using the Hosmer-Lemeshow statistic test) and the best discrimination (AUC, 0.603; 95% confidence interval [95%CI], 0.528–0.677; P = .006) (figure 1A). Both PSP-1 and PSP-3 scores displayed a high negative predictive value with a low negative likelihood ratio either for DOCE or scaffold thrombosis (figure 1B).
At 1-year follow-up, there was no difference in the rate of DOCE between patients with an optimal PSP-1 technique and those without it (1.6% vs 2.9%; hazard ratio [HR], 1.75; 95%CI, 0.69–4.45]; P = .239, adjusted analysis) (table 3). A trend towards a lower rate of DOCE was found in patients with an optimal PSP-3 technique compared to those without it (0.5% vs 2.9%; HR, 5.73; 95%CI, 0.78–41.88; P = .085, adjusted analysis) (table 3). Figure 2A and figure 2B show the Kaplan-Meier curves for DOCE of the PSP scores.
Within the PSP-1 score, correct scaffold sizing was associated with a reduction in DOCE (HR, 0.43; 95%CI, 0.25–0.75; P = .003). Within the PSP-3 score, either the correct scaffold sizing (HR, 0.42; 95%CI, 0.24–0.72; P = .002) or the correct post-dilation (HR, 0.33; 95%CI, 0.12–0.92; P = .035) were associated with fewer DOCE (figure 2C,D).
At 1-year follow-up, simplified strategies considering only the pre-dilation and post-dilation as defined according to the models PSP-1 (HR, 1.50; 95%CI, 0.70–3.19; P = .294) and PSP-3 (HR, 1.80; 95%CI, 0.65–5.02; P = .260) were not associated with a lower DOCE either.
Effect of an optimal PSP technique on various clinical and anatomical scenarios
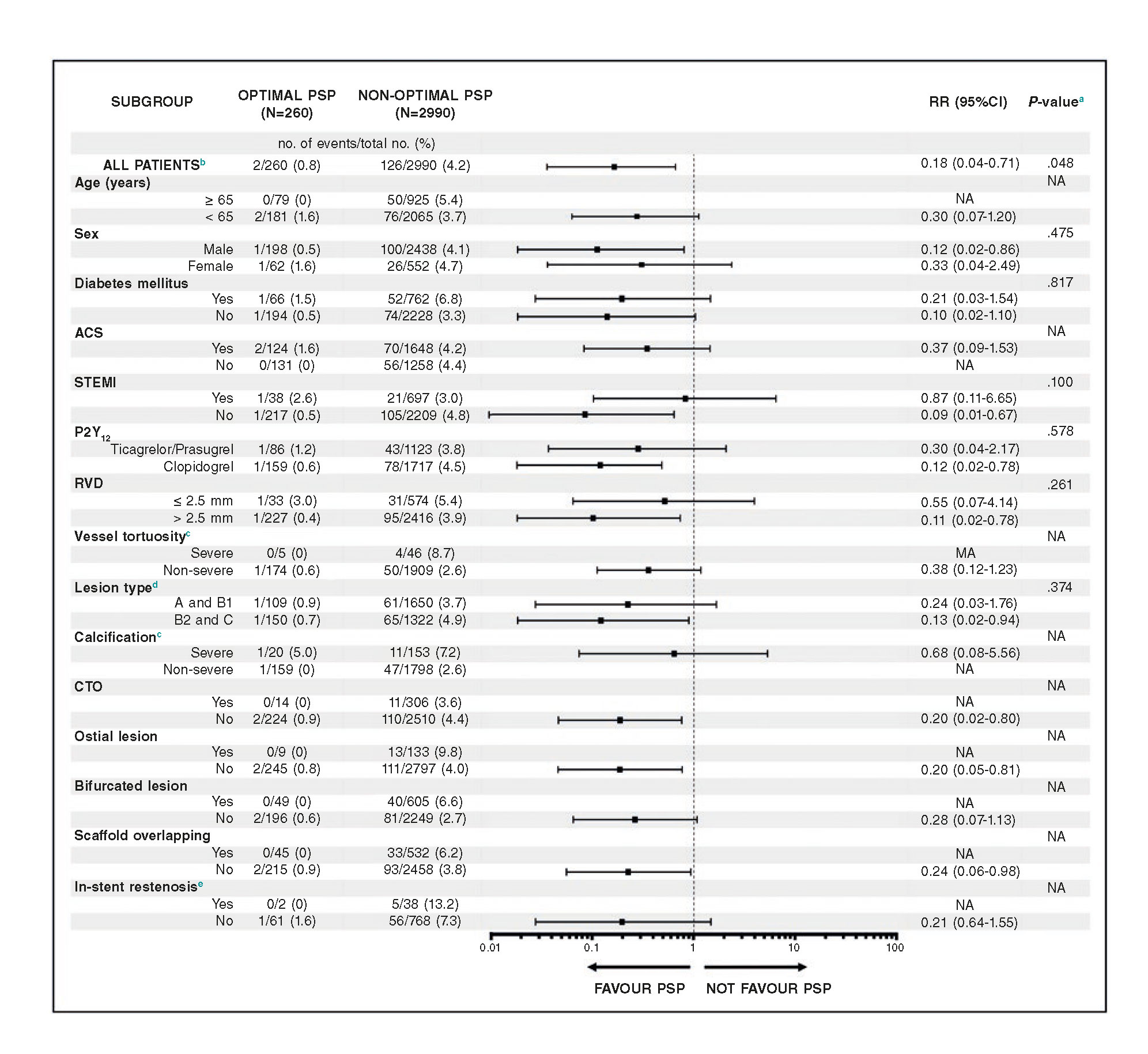
In the combined REPARA and GHOST-EU database, patients with the optimal PSP technique have lower rates of DOCE compared to those without it in almost all clinical and angiographic scenarios analyzed. In STEMI patients, there was a trend towards no benefit of an optimal PSP technique (Pinteraction = .100) (figure 3).
Figure 3. Effect of an optimal PSP technique on DOCE in various clinical and anatomical scenarios at 1-year follow-up Pooled analysis of the GHOST-EU and REPARA registries.
a The P-value for interaction represents the likelihood of interaction between the variable and a maximum PSP score.
b Multivariate adjusted model.
c Data were only available from the REPARA registry.
d According to the criteria of the American College of Cardiology-American Heart Association.
e Data were only available from the GHOST-EU registry.
Patient-level analysis.
95%CI, 95% confidence interval; ACS, acute coronary syndrome; CTO, chronic total occlusion; NA, not applicable; PSP, pre-dilation, sizing and post-dilation; RR, risk ratio; RVD, reference vessel diameter; STEMI, ST-segment elevation myocardial infarction.
DISCUSSION
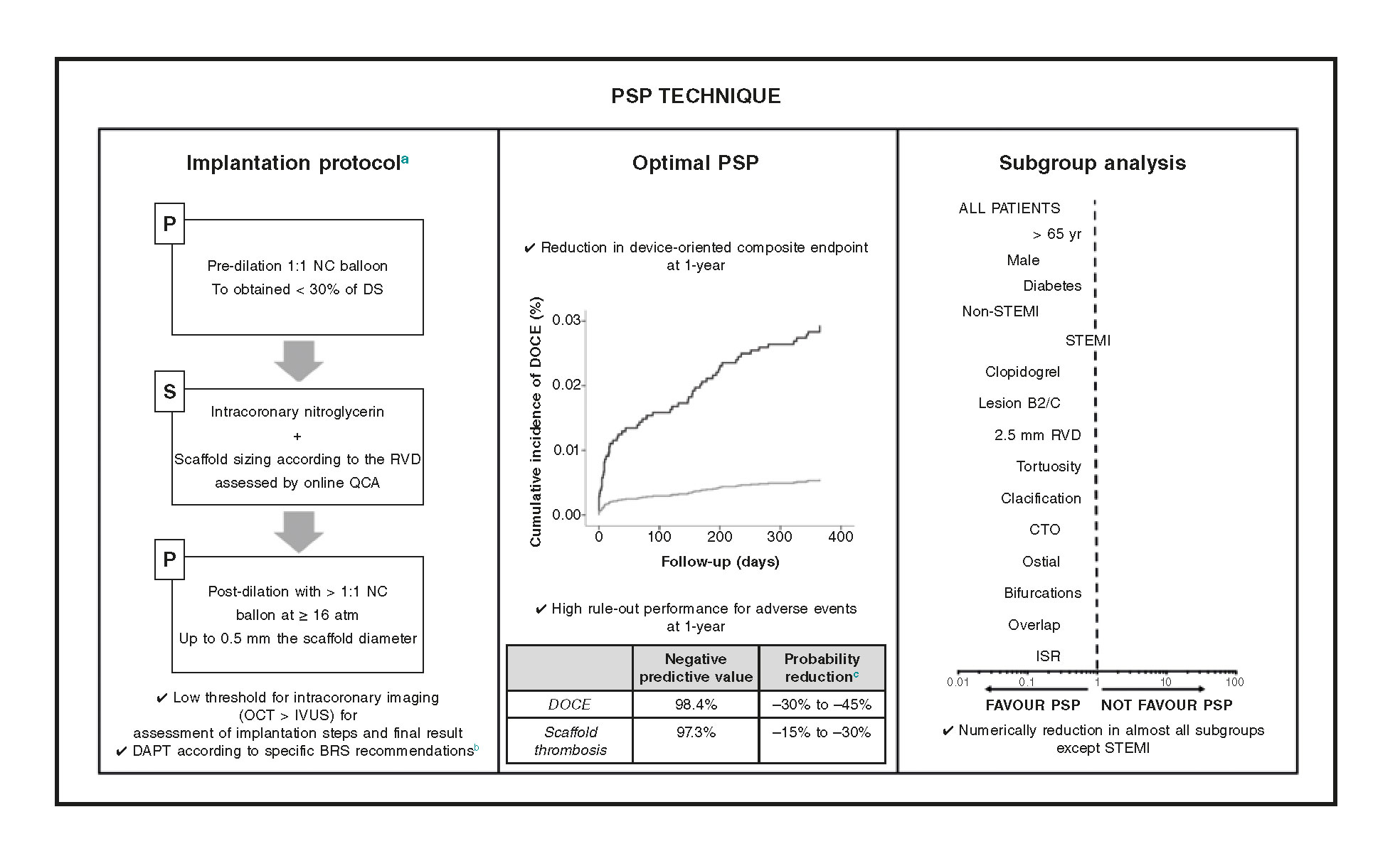
The main findings of our study are: a) an optimal PSP technique was not associated with a lower rate of DOCE; b) a correct scaffold sizing and post-dilation were associated with fewer DOCE; c) the effect of an optimal PSP technique seems to be less important in the ST-segment elevation myocardial infarction (STEMI) compared to other clinical and anatomical scenarios (figure 4).
Figure 4. Effect of the PSP technique in various clinical and anatomical scenarios. In the routine clinical practice, the PSP score is a simple score model designed to assess the quality of BVS implantation technique by assessing the 3 fundamental steps of the PSP technique. At 1-year follow-up, an optimal PSP technique is related to fewer DOCE and a very high negative predictive value for DOCE and definite/probable scaffold thrombosis. The effect of an optimal PSP technique on STEMI patients seems to be less significant.
aDetailed procedural considerations were reported during the development and internal validation.7
bExpert recommendations on DAPT in patients treated with BVS.4
cThe probability reduction was estimated by means of negative likelihood ratios.13
ATM, atmospheres; BVS, bioresorbable scaffolds; CTO, chronic total occlusion; DAPT, dual antiplatelet therapy; DOCE, device-oriented composite endpoint; DS, diameter stenosis; ISR, in-stent restenosis; IVUS, intravascular ultrasound; NC, non-compliant balloon; OCT, optical coherence tomography; PSP, pre-dilation, sizing and post-dilation; QCA, quantitative coronary angiography; RVD, reference vessel diameter; STEMI, ST-segment elevation myocardial infarction.
Clinical value of the PSP technique in the validation cohort
The PSP technique has been proposed to analyze the quality of the BVS implantation technique with clinical outcomes.7 The present analysis applied the PSP score to the population of the REPARA registry in external validation. An optimal PSP technique was not associated with a lower rate of DOCE. Specifically, an optimal PSP-1 technique was not associated with a lower rate of DOCE, meanwhile there was a trend to a lower rate of DOCE in patients treated with an optimal PSP-3 technique. The low rate of DOCE and the improvement of the technique may be related with this finding. Even though we did not confirm the effect of the PSP score on clinical outcomes, we believe that most of the medical literature suggests that an optimal implantation technique may improve the outcomes. In the analysis of the ABSORB trials, the sizing of the vessel and operator technique were strongly associated with the outcomes at a 3-year follow-up.11 Nevertheless, other authors had found no relationship between the PSP technique and the outcomes when the analysis was done at lesion-level.12
Also, in the derivation cohort or this validation cohort, the rate of patients treated with optimal PSP technique was very low (13.3% and 8.2%, respectively).7 Patients herein treated with an optimal PSP technique exhibited a trend towards a lower rate of DOCE compared to those without it. We should mention here that the PSP technique exhibited a high rule-out performance, with a very high negative predictive value and a low likelihood ratio: this shows that a patient with an optimal PSP technique has a probability of being DOCE-free at 1-year that is close to 100%.10 Likelihood ratios are used for assessing the value of performing a diagnostic test or score model, with a lower value associated with a lower probability of an endpoint. Therefore, the very low negative likelihood ratios found in this analysis means that an optimal PSP technique was associated with a large to moderate reduction in DOCE occurrences (-30% to -45%).13
Within the individual steps of the PSP technique, the correct scaffold sizing was performed in a higher percentage of patients in this validation cohort compared to the derivation cohort (80% versus 50%, respectively). This improvement could be related to the publication of the ABSORB III trial in between the GHOST-EU and REPARA registries, which showed a higher incidence of events in small vessels.14 The importance of the correct sizing of the vessels for BVS implantation was further highlighted in our analysis, together with correct post-dilation (figure 2).
Effect of an optimal PSP technique in various clinical and anatomical scenarios
Current data support the use of the pre-specified implantation technique for BVS implantation, but it is unknown if this should be applied to all patients or lesions or if some subgroup may benefit most from it.5,7 Whereas calcified lesions may, for example, require a perfect PSP technique for BVS outcome optimization, soft lesions may not. For this reason, in the pooled database, we explored the effect of an optimal PSP technique on DOCE in different clinical and anatomical scenarios. Interestingly, we found that in all situations, the analyzed patients treated with an optimal PSP technique have a lower rate of DOCE compared with patients without it. However, in STEMI patients, there was a trend towards no benefit of an optimal PSP technique. This could be related to the specific characteristic of the STEMI lesions, which are usually soft and thrombotic with less need for lesion preparation or post-dilation in order to reduce distal embolization.15 In patients with abundant thrombotic material or coronary vasoconstriction, the vessel diameter can be underestimated; use of manual thrombus aspiration and intracoronary nitroglycerin could be useful in these situations, thus allowing the implantation of bigger and shorter stents.16 A former study suggested, for example, that in STEMI patients a slight scaffold oversizing could help achieve better acute outcomes.17 It should also be noted here that the percentage of STEMI patients was lower in the derivation than in the validation cohort (15% vs 26%, respectively): this difference could have affected the performance of the score in this specific situation. This finding is supported by the results of the BVS STEMI STRATEGY-IT Study, in which a pre-specified Absorb BVS implantation strategy in STEMI was evaluated. In this study, a low rate of DOCE was observed during the short and mid-term follow-up.18 Furthermore, in a sub study of the STRATEGY-IT, we found that an optimal PSP technique was not associated with improved outcomes. Interestingly, thrombectomy before optimal BVS implantation showed a trend towards higher post minimal lumen diameter and lower scaffold footprint.19
Even though the Absorb BVS is no longer available in the clinical practice, there are several BVS ongoing clinical and preclinical evaluation. In these new devices the effect of the implantation technique is unknown, but due to the similarities of these technologies, it seems probable that the implantation technique should also have an effect on the clinical outcomes.
Limitations
This study has limitations that should be acknowledged. First, due to the low rate of events and optimal PSP technique, the clinical relevance of the predictive values may be limited. Secondly, the subgroup analyses are statistically underpowered and should be considered hypothesis-generating only. Thirdly, longer-term follow-up is needed to validate the PSP score models beyond 1-year of follow-up. Despite these limitations, this analysis also has important strengths such as being a large multicenter registry with broad inclusion criteria and few exclusion criteria. The REPARA registry, in particular, facilitated a complete temporal and geogra- phical validation of the score, which reinforces the methodology of this analysis.
CONCLUSIONS
In the REPARA, at 1-year follow-up, an optimal PSP technique was not associated with a lower rate of device-oriented composite endpoint. An optimal PSP technique has a very high negative predictive value for BVS DOCE and scaffold thrombosis. It should be noted that, in STEMI patients, there was a trend towards no benefit from using the optimal PSP technique. Studies with longer follow-up are needed to assess the effect of the optimal PSP technique on very-late events and in specific STEMI settings.
FUNDING
The REPARA registry was funded by the Spanish Society of Cardiology.
CONFLICTS OF INTEREST
R. Moreno and J. Sanchis are Associate Editors of REC: Interventional Cardiology.
WHAT IS KNOWN ABOUT THE TOPIC?
- The PSP score has been proposed to assess the quality of bioresorbable scaffolds implantation technique.
- The optimization of BVS implantation can be related to a reduction in adverse events.
WHAT DOES THIS STUDY ADD?
- In patients treated with an optimal PSP technique, there was a reduction of adverse cardiac events. A maximum PSP score was related to a very high negative predictive value of DOCE and scaffold thrombosis.
- The effect of PSP in STEMI seems less important compared to other clinical and anatomical scenarios.
- Future trials with longer follow-up are needed to assess the effect of an optimal PSP technique beyond 1-year follow-up.
- The effect of an optimal PSP technique among different clinical and anatomical scenarios should be confirmed.
- In STEMI patients, further research is necessary to develop and validate a specific implantation protocol.
REFERENCES
1. Ali ZA, Serruys PW, Kimura T, et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease:a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017. 2017;390:760-772.
2. Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II):a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491.
3. Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328.
4. Capodanno D, Angiolillo DJ. Antiplatelet Therapy After Implantation of Bioresorbable Vascular Scaffolds:A Review of the Published Data, Practical Recommendations, and Future Directions. JACC Cardiovasc Interv. 2017;10:425-437.
5. Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis:Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol. 2016;67:921-931.
6. Tamburino C, Latib A, van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds:a European perspective. EuroIntervention. 2015;11:45-52.
7. Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and postdilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events:development and internal validation of the PSP score. EuroIntervention. 2017;12:2110-2117.
8. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials:a case for standardized definitions. Circulation. 2007;115:2344-2351.
9. Collins GS, Reitsma JB, Altman DG, and Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD):the TRIPOD statement. Ann Inter Med. 2015;162:55-63.
10. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models:a framework for traditional and novel measures. Epidemiology. 2010;21:128-138.
11. Stone GW, Abizaid A, Onuma Y, et al. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation:Analysis From the ABSORB Trials. J Am Coll Cardiol. 2017;70:2863-2874.
12. Tijssen RYG, Kraak RP, Elias J, et al. Implantation techniques (predilatation, sizing, and post-dilatation) and the incidence of scaffold thrombosis and revascularisation in lesions treated with an everolimus-eluting bioresorbable vascular scaffold:insights from the AIDA trial. EuroIntervention. 2018;14:e434-e442.
13. McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646-649.
14. Steinvil A, Rogers T, Torguson R, and Waksman R. Overview of the 2016 U.S. Food and Drug Administration Circulatory System Devices Advisory Panel Meeting on the Absorb Bioresorbable Vascular Scaffold System. JACC Cardiovasc Interv. 2016;9:1757-1764.
15. Zhang ZJ, Marroquin OC, Stone RA, et al. Differential effects of post-dilation after stent deployment in patients presenting with and without acute myocardial infarction. Am Heart J. 2010;160:979-986 e1.
16. Fernandez-Rodriguez D, Regueiro A, Brugaletta S, et al. Optimization in stent implantation by manual thrombus aspiration in ST-segment-elevation myocardial infarction:findings from the EXAMINATION trial. Circ Cardiovasc Interv. 2014;7:294-300.
17. Kocka V, Maly M, Tousek P, et al. Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction:a prospective multicentre study 'Prague 19'. Eur Heart J. 2014;35:787-794.
18. Ielasi A, Campo G, Rapetto C, et al. A Prospective Evaluation of a Pre-Specified Absorb BVS Implantation Strategy in ST-Segment Elevation Myocardial Infarction:The BVS STEMI STRATEGY-IT Study. JACC Cardiovasc Interv. 2017;10:1855-1864.
19. Hioki H, Brugaletta S, Ishida K, et al. Impact of Absorb bioresorbable scaffold implantation technique on post-procedural quantitative coronary angiographic endpoints in ST-elevation myocardial infarction:a sub-analysis of the BVS STEMI STRATEGY-IT study. EuroIntervention. 2018. http://dx.doi.org/10.4244/EIJ-D-18-00504.