Abstract
Introduction and objectives: Mitral regurgitation is one society’s most prevalent valvular diseases. Transcatheter mitral valve repair with the MitraClip system has become more widely used for the management of this condition. The endpoints of the study were the changes in the mitral annular morphology, the recurrent grade III-IV mitral valve regurgitation, and a composite endpoing of heart failure readmission and all-cause mortality.
Methods: Single-centre, prospective and observational study. We included patients admitted due to transcatheter mitral valve repair between October 2015 and October 2018. The three-dimensional analysis of the mitral valve annulus was performed using the MVQ QLAB mitral valve quantification software (Philips; Amsterdam, The Netherlands).
Results: Fifty procedures were performed on 48 patients. A significant decrease of both annular diameters, perimeter and area was observed after the procedure. The antero-posterior diameter reduction was more significant in patients with functional mitral regurgitation compared to patients with organic mitral regurgitation (13.2 ± 8.8 vs 8.6 ± 7.5; P = .05). The posterior leaflet grasping was the only parameter associated with less chances of significant recurrent mitral regurgitation (OR = 0.89; 95CI%, 0.79-0.98).
Conclusions: Mitral annular morphological changes occur after MitraClip implantation. The magnitude of these changes varies depending on the etiology of mitral regurgitation. Posterior leaflet grasping is the main factor associated with these changes and prevents the recurrence of significant mitral regurgitation.
Keywords: Transcatheter mitral valve repair. MitraClip. Severe mitral regurgitation. Mitral annulus.
Resumen
Introducción y objetivos: La insuficiencia mitral es una de las enfermedades valvulares más prevalentes en nuestro medio. La reparación mitral transcatéter con el sistema MitraClip es un procedimiento cada vez más utilizado en este contexto. Los objetivos del estudio fueron evaluar los cambios morfológicos anulares, la recurrencia de la insuficiencia mitral significativa y un objetivo combinado de reingreso por insuficiencia cardiaca y mortalidad global.
Métodos: Estudio prospectivo, observacional y unicéntrico. Se incluyeron pacientes tratados con reparación mitral transcatéter entre octubre de 2015 y octubre de 2018. Se realizó un análisis tridimensional del anillo con el software de cuantificación mitral MVQ QLAB 10.0 (Philips; Amsterdam, Países Bajos).
Resultados: Se realizaron 50 procedimientos en 48 pacientes. Tras el procedimiento se observó una disminución significativa de ambos diámetros anulares, así como del perímetro y del área, y una mayor reducción del diámetro anteroposterior en los pacientes con insuficiencia mitral funcional con respecto a aquellos con insuficiencia mitral orgánica (13,2 ± 8,8 frente a 8,6 ± 7,5; p = 0,05). El porcentaje de grasping sobre el velo posterior fue el único parámetro que se asoció estadísticamente a una menor probabilidad de desarrollar insuficiencia mitral significativa (OR = 0,89; IC95%, 0,79-0,98).
Conclusiones: Tras el implante de MitraClip se producen cambios morfológicos en el anillo mitral. La magnitud de estos cambios es diferente según la etiología de la insuficiencia mitral. El grasping del velo posterior es el principal factor asociado a dichos cambios y previene la recurrencia de la insuficiencia mitral significativa.
Palabras clave: Anillo mitral. Insuficiencia mitral grave. MitraClip. Reparación mitral transcatéter.
Abbreviations:
MR: mitral regurgitation. TVMR: transcatheter mitral valve repair.
Introduction
Mitral regurgitation (MR) is the most prevalent valvular disease in the United States and the second most prevalent in Europe1,2. The transcatheter mitral valve repair (TMVR) treated with the MitraClip system (Abbott Vascular, Menlo Park, California, United States) imitates the edge-to-edge approach surgical technique proposed by Alferi to achieve an effective reduction of the degree of MR3,4. This technique is more widely used, particularly in patients of high or prohibitive surgical risk because it is less invasive and has shown good efficacy and safety results in the mid-term5-7.
This procedure is thought to be able to operate changes in the anatomy of the mitral annulus beyond the edge-to-edge approach of the valvular leaflets, but there is very little information on this regard. Some studies speak about a significant change of anteroposterior diameters in RM of functional etiology8, while others describe changes of diameter, in non-constant areas and in etiologydependent areas9.
The goal of this study is to analyze the morphological changes occurring in the mitral valve after the TMVR and its relation to the degree of reduction of MR in the short and mid-terms, and its association with the clinical goals.
Methods
This is an observational, prospective study conducted at Hospital Universitario Central de Asturias de Oviedo, Spain.
Inclusion de patients
Patients were included between October 2015 and October 2018. These were the inclusion criteria: patients with grade III-IV symptomatic mitral failure despite the optimal medical therapy considered of high surgical risk by the multidisciplinary team and who would adequately meet the anatomical criteria needed for the implant4,7. These patients were excluded: patients with prior mitral surgical annuloplasty due to the impossibility of measuring annular anatomic changes. A prior transesophageal ecochardiography was conducted in all patients. The etiology of MR was categorized into organic or degenerative, and functional. Patients with a mixed etiological profile in their MR were recategorized into one of the aforementioned groups based on their predominant component after 2 expert cardiologists studied the transesophageal echocardiography and achieved consensus. All patients received oral written information on the risks and benefits of the procedure, and they all signed a written informed consent according to the Declaration of Helsinki.
Description of the procedure
The TMVR was performed using the MitraClip system, which received the European certificate of conformity (CE mark) in March 2018. The implantation procedure has already been described in prior studies7. In sum, the intervention is conducted under general anesthesia and guided by a 3D transesophageal echocardiography and under the supervision of a MitraClip technical expert. More than one clip was implanted in cases where the reduction of the degree of MR was not of, at least, one grade, and as far as there was no significant residual mitral stenosis estimated through the average diastolic transmitral valve pressure gradient10.
Echocardiographic study
All patients underwent transesophageal echocardiographic studies in 2 and 3 dimensions before and right after the completion of the procedure that was conducted by an expert echocardiography expert using a state-of-the-art echocardiography machine model EPIQ 7 (Philips; Amsterdam, The Netherlands). The patient’s afterload hemodynamic condition was taken into consideration before and after the procedure.
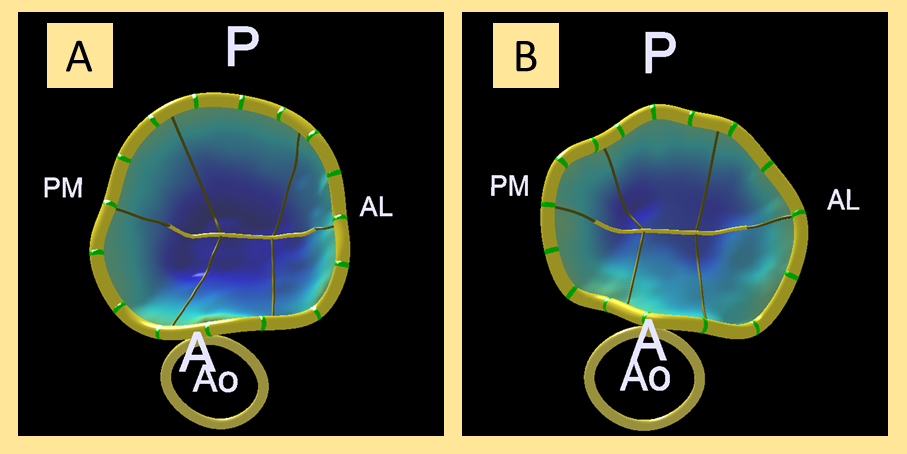
In order to conduct the 3D study of the mitral annulus, 3D images were acquired (Zoom 3D, Philips; Amsterdam, The Netherlands) during the procedure that were later analyzed using the MVQ QLAB 10.0 mitral quantification software (Philips; Amsterdam, The Netherlands). Figure 1 shows an example of 3D reconstruction before and after the procedure.
The analysis of the leaflet grasping was estimated using the lengths of both leaflets before and after the procedure in the same plane of the implant of the device. The length before the clip was measured between the anchor site of the leaflet to the annulus and the leaflet free-edge and, the length after the clip was estimated between the anchor site of the leaflet to the annulus and the leaflet site immediately proximal to the part of the leaflet inside the device:
– Total grasping (mm): pre mitral leaflet length − post mitral leaflet length.
– Per cent grasping (%): ([pre mitral leaflet length − post mitral leaflet length]/pre mitral leaflet length) × 100.
Figure 1. 3D analysis before the clip (A) and after the clip (B) of mitral annulus in frontal view from the left ventricle. A, anterior; AL, anterolateral; Ao, aorta; P, posterior; PM, posteromedial.
Study variables
Echocardiographic variables
The technical success, the device success, and the procedure success were all defined according to the consensus document put together by the Mitral Valve Academic Research Consortium11. Both the etiology and severity of the MR were classified and assessed according to the clinical practice guidelines designed by the European Society of Cardiology12-14, being severity subdivided into four degrees in a similar way to what the EVEREST clinical trial did4,7.
Clinical variables
The patient’ functional capacity was assessed following the New York Heart Association classification. Admission to due heart failure was defined as patients coming back to their hospital floor or being assisted in the ER and having to stay and sleep over. The EuroSCORE II and the Surgeon Thoracic Score were estimated too. Follow-up event was defined as a hospitalization due to heart failure or all-cause mortality.
Study goals
The study goals were the assessment of the annular morphological changes, the recurrence of MR (at least grade III/IV) and a composite endpoint of rehospitalization due to heart failure and global mortality.
Statistical analysis
Qualitative variables were expressed as absolute number and percentage and quantitative variables as mean ± standard deviation. The Student t test for paired data was used to assess morphological changes before and after the procedure. The chi-square and Student t tests were used for different groups as methods to compare categorical and quantitative variables. Linear regression analyses were conducted to assess the predictors of annular quantitative modification, the binary logistics regression analysis was used for the study of MR recurrence, together with the survival analysis using the Kaplan-Meier method. A peak alpha error of 0.05 was assumed. All analyses were conducted using the Stata 14 software (Stata Statistical Software: Release 14. College Station, Texas: Stata- Corp LP).
Results
Fifty TMVR procedures were conducted between October 2015 and October 2018 in 48 patients: 48 MitraClip primary implants and two reinterventions due to the partial detachment of the posterior leaflet. The average age was 74.8 ± 7.2 years and 31.3% of the patients were females. Ten procedures (20.8%) were conducted in patients with organic MR and 38 (79.2%) in patients with functional MR. The baseline characteristics of the population based on the etiology of the MR and the echocardiographic data are shown on table 1 and table 2. An average 1.5 ± 0.5 clips per procedure were implanted. In 43 (86%) cases, the first-generation clip was used, while in 7 (14%) cases, the XTr clip was used. Technical success was 100% and the procedural success was close to 92% (46/50). The four unsuccessful cases were due to partial detachments, one failed reintervention, and persistent grade III/IV MR after the implant.
Table 1. Baseline characteristics of the population
| Global N = 48 (100%) | Organic n = 10 (2.8%) | Functional n = 38 (79.2%) | P | |
|---|---|---|---|---|
| Age, years | 74.8 ± 7.2 | 76.6 ± 2.2 | 74.3 ± 1.2 | .70 |
| Women | 15 (31.3) | 5 (50) | 10 (26.3) | .15 |
| Weight, kg | 74.8 ± 14.2 | 75.3 ± 5.2 | 74.8 ± 2.2 | .90 |
| Height, cm | 164.1 ± 9.1 | 158.6 ± 2.4 | 165.6 ± 2.5 | .03 |
| Hypertension | 36 (75) | 9 (90) | 27 (71.1) | .22 |
| Diabetes mellitus | 16 (33.3) | 5 (50) | 11 (29) | .21 |
| Dyslipidemia | 22 (45.8) | 6 (60) | 14 (36.8) | .24 |
| Renal disease | 20 (41.6) | 3 (30) | 17 (44.7) | .19 |
| Prior stroke | 10 (20.8) | 2 (20) | 8 (21) | .94 |
| Ischemic heart disease | 23 (47.9) | 4 (40) | 19 (50) | .48 |
| PCI | 16 (33.3) | 3 (30) | 13 (24.4) | .34 |
| CABG | 7 (14.6) | 1 (10) | 6 (15.8) | .36 |
| Atrial fibrillation | 30 (62.5) | 6 (60) | 24 (63.2) | .84 |
| Degree of MR | ||||
| III/IV | 8 (16.7) | 2 (20) | 6 (15.8) | .79 |
| IV/IV | 40 (83.3) | 8 (80) | 32 (84.2) | .80 |
| SPAP, mmHg | 43.5 ± 12.4 | 49.5 ± 4.4 | 41.8 ± 2.2 | .06 |
| COPD | 11 (22.9) | 3 (30) | 8 (21) | .55 |
| Functional class | ||||
| NYHA III | 35 (72.9) | 7 (70) | 28 (73.7) | .83 |
| NYHA IV | 13 (27.1) | 3 (30) | 10 (26.3) | .79 |
| EuroSCORE II | 5.4 ± 4 | 4.7 ± 1.6 | 5.6 ± 2.2 | .62 |
| STS mortality | 5.2 ± 3.2 | 7.2 ± 5.4 | 4.7 ± 2.1 | .02 |
|
Data are expressed as n (%) or mean ± standard deviation. CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SPAP, systolic pulmonary artery pressure; STS, Society of Surgeon Thoracic score. |
||||
Table 2. Echocardiographic characteristics
| Global | Organic MR | Functional MR | P | |
|---|---|---|---|---|
| Left ventricular ejection fraction | 41.5 ± 12.8 | 50.46 ± 4.1 | 39.15 ± 1.9 | .01 |
| LVESVi, mL/m2 | 85 ± 32.2 | 61.1 ± 19.9 | 91.4 ± 32 | .01 |
| LVESDi, mm/m2 | 60.5 ± 9.5 | 29.9 ± 4.1 | 33.9 ± 5 | .02 |
| ERO, cm2 | 0.38 ± 0.12 | 0.43 ± 0.12 | 0.36 ± 0.13 | .09 |
| Intercomissural diameter, mm | 39.2 ± 4.7 | 37.8 ± 2.5 | 39.4 ± 5.1 | .17 |
| Anteroposterior diameter, mm | 38.1 ± 5.3 | 35.8 ± 3.2 | 39.2 ± 5.6 | .01 |
| Bidimensional perimeter, mm | 124.6 ± 14.6 | 114.9 ± 10.4 | 126.7 ± 14.6 | .02 |
| 3D perimeter, mm | 130.6 ± 16 | 117.7 ± 10 | 133.8 ± 15.7 | .01 |
| 2D area, cm2 | 12.04 ± 3.1 | 10.1 ± 2.1 | 12.5 ± 3.1 | .02 |
| 3D area, cm2 | 12.45 ± 3.2 | 10.2 ± 1.9 | 12.9 ± 3.2 | .01 |
| Anterior leaflet length, mm | 24.7 ± 3.2 | 25.1 ± 2.8 | 26.2 ± 3.1 | .11 |
| Posterior leaflet length, mm | 13.7 ± 2.4 | 12.6 ± 2.4 | 13.8 ± 2.4 | .12 |
| Anterior annulus-leaflet length, degrees | 27.9 ± 6.3 | 25.8 ± 2.7 | 28.5 ± 6.5 | .12 |
| Posterior annulus-leaflet length, degrees | 43.3 ± 10.8 | 39.7 ± 8.5 | 44.2 ± 11.2 | .13 |
|
Data are expressed as n (%) or mean ± standard deviation. ERO, effective regurgitant orifice; LVESVd, left ventricular end-diastolic volume diameter; LVESVi, left ventricular end-diastolic volume index; MR, mitral regurgitation. |
||||
After the procedure, in the 3D analysis of the mitral annulus, there was a significant reduction of both annular diameters, the perimeter and both 2D and 3D areas(table 3). The comparative analysis based on etiology (table 4) found a greater reduction in the anteroposterior diameter in patients with functional MR compared to those with organic MR (13.2 ± 8.8 versus 8.6 ± 7.5 of per cent reduction, respectively; P = .05) and a greater tendency to a reduced area in the same sense (13.3 ± 12.4 versus a 7.2 ± 11.1 of per cent reduction, respectively; P = .01).
Table 3. Global annular changes
| Absolute reduction | Relative reduction (%) | P | |
|---|---|---|---|
| Intercomissural diameter, mm | 2.4 ± 2.2 | 5.99 ± 5.6 | < .01 |
| Anteroposterior diameter, mm | 4.7 ± 3.8 | 12.1 ± 8.7 | < .01 |
| 2D annular perimeter, mm | 7.6 ± 7.1 | 6.1 ± 5.6 | < .01 |
| 3D annular perimeter, mm | 8.5 ± 6.2 | 6.4 ± 6.1 | < .01 |
| 2D annular area, cm2 | 1.43 ± 1.3 | 11.8 ± 11.4 | < .01 |
| 3D annular area, cm2 | 1.52 ± 1.3 | 11.9 ± 12.2 | < .01 |
|
Data are expressed as n (%) or mean ± standard deviation. 2D, 2 dimensions; 3D, 3 dimensions. |
|||
Table 4. Annular changes and mitral leaflet grasping based on the etiology of mitral regurgitation
| Organic MR | Functional MR | P | |
|---|---|---|---|
| Intercomissural diameter reduction, % | 6.1 ± 5.1 | 5.9 ± 6.3 | .48 |
| Anteroposterior diameter reduction, % | 8.6 ± 7.5 | 13.2 ± 8.8 | .05 |
| 2D annular perimeter reduction, % | 5.4 ± 6.1 | 6.2 ± 5.5 | .35 |
| 3D annular perimeter reduction, % | 5.6 ± 5.5 | 6.7 ± 6.2 | .35 |
| 2D annular area reduction, % | 6.8 ± 11.3 | 13.1 ± 12.4 | .09 |
| 3D annular area reduction, % | 7.2 ± 11.1 | 13.3 ± 12.4 | .10 |
| Anterior leaflet grasping, mm | 9.1 ± 3.8 | 7.3 ± 3.2 | .07 |
| Anterior leaflet grasping, % | 36.6 ± 11.5 | 27.8 ± 11.4 | .02 |
| Posterior leaflet grasping, mm | 4.3 ± 1.4 | 4.8 ± 1.8 | .19 |
| Posterior leaflet grasping, % | 34 ± 8.1 | 34.4 ± 10.6 | .44 |
|
Data are expressed as n (%) or mean ± standard deviation. 2D, 2 dimensions; 3D, 3 dimensions; MR, mitral regurgitation. |
|||
When it comes to both leaflet-grasping it was observed that in patients with organic MR, a greater percentage of anterior leaflet tissue inside the device is approached (36.6 ± 11.5% in the organic MR versus 27.8 ± 11.4% in the functional MR; P = .02), while posterior leaflet grasping is similar in both subtypes (34 ± 8.1% in the organic MR versus 34.4 ± 10.6% in the functional MR; P = .04).
In the simple linear regression analysis conducted of predictor factors of reduction of the annular anteroposterior diameter we observed that the percentage of posterior leaflet grasping and the anteroposterior diameter before the implant were the only factors associated with a greater reduction. After adjusting for the etiology of the MR, the indexed ventricular volumes, the annular diameters before the implant, and the anterior leaflet grasping, this association between the posterior leaflet grasping and the reduction of anteroposterior annular diameter was still statistically significant (ß coefficient = 0.27; 95%CI, 0.05-0.48; P = .02).
After an average 454 days of follow-up (interquartile range, 195- 699), 7 out of the 48 patients (14.6%) and 8 out of the 50 procedures conducted (16%) showed grade III/IV MR. In the binary logistics regression analysis conducted for grade III-IV/IV MR predictors at the echocardiographic follow-up (table 5) it was observed that the percentage of grasping over the posterior leaflet was the only parameter statistically associated with a lower probability to develop significant MR (OR, 0.89; IC95%, 0.79-0.98).
Table 5. Binary logistics regression analysis. Predictors of grade III-IV/IV mitral regurgitation after transcatheter mitral valve repair
| OR (95%CI) | P | |
|---|---|---|
| Left ventricular ejection fraction | 1.03 (0.97-1.1) | .29 |
| LVESVi | 1.01 (0.98-1.03) | .31 |
| Intercomissural diameter relative reduction | 1.02 (0.9-1.16) | .65 |
| Anteroposterior diameter relative reduction | 0.95 (0.86-1.05) | .50 |
| 3D annular perimeter reduction | 0.94 (0.8-1.1) | .47 |
| 3D annular area reduction | 0.99 (0.92-1.06) | .41 |
| Anterior leaflet grasping | 0.99 (0.93-1.06) | .96 |
| Posterior leaflet grasping | 0.89 (0.79-0.98) | .04 |
|
95%CI, 95% confidence interval; LVESVi, left ventricular end-diastolic volume index; OR, odds ratio. |
||
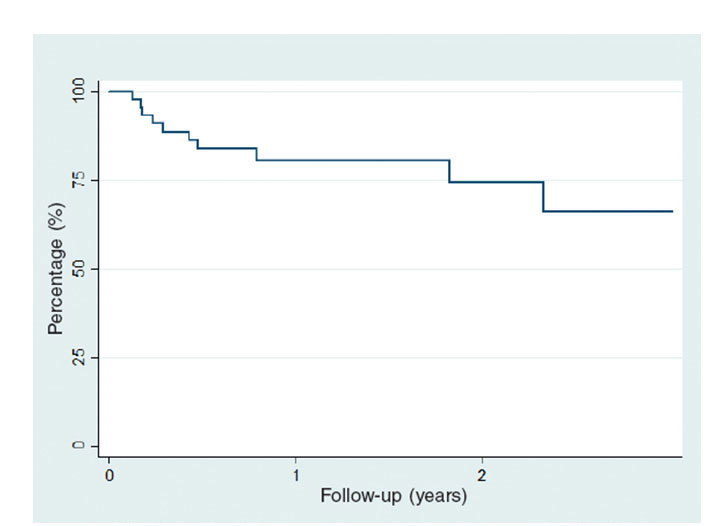
There was a 16% rate of rehospitalizations due to heart failure and a global mortality rate of 12.5% (table 6). The composite endpoint of all-cause mortality or rehospitalization due to hear failure occurred in 10 (20.8%) patients. In the regression analysis for the composite endpoint of mortality or rehospitalization due to heart failure we did not observe an association between the parameters of annular reduction or the leaflet grasping and the endpoint under study. The heart failure-free or all-cause mortality-free survival curve is shown on figure 2.
| Global mortality | n (%) |
|---|---|
| Cardiovascular mortality | 4 (8.33) |
| Sudden death | 1 (2.08) |
| Acute coronary syndrome | 1 (2.08) |
| Non-cardiovascular mortality | 2 (4.17) |
| Sepsis | 1 (2.08) |
| Neoplasm | 1 (2.08) |
| Total | 6 (12.5%) |
Figure 2. Mortality-free or readmission due to heart failure-free survival estimated using the Kaplan-Meier method.
Discussion
The main finding of our study is that after TMVR with MitraClip there are important anatomical changes when it comes to the reduction of anteroposterior and intercomissural diameters, the annular diameters and areas, measured both in 2D and 3D. It was observed that, except for the intercomissural diameter, the remaining annular measurements (anteroposterior diameter, perimeter, and area) were significantly enlarged in patients with functional MR compared to patients with organic MR.
Similar to other studies published8,9, sit has been observed a significant reduction of the anteroposterior diameter after the implant. However, unlike Remy et al.9, describe in patients with functional MR, there was a greater relative reduction of the anteroposterior diameter and a non-significant tendency to a greater reduction of these patients’ area Also, in our series, we saw a reduction of the intercomissural diameter, which may have to do with a significant and sudden reduction of the regurgitation volume and with left intra-articular pressure, rather than with a direct mechanical effect coming from the clip.
With respect to the repercussion of these anatomical changes in the significant clinical results during follow-up, we did not observe any statistically significant correlation between these changes and rehospitalizations due to heart failure or global mortality. There was, however, an inversely proportional correlation between the reduced anteroposterior diameter and the possibility of III/IV MR recurrence (OR, 0.95; 95%CI, 0.89- 1.05). This data has been published in former statistically significant studies15. It is believed that the lack of signification in our study when it comes to these goals, and the non-association between the magnitude of diameters before the implant and MR recurrence may be associated with the number of patients of the overall cohort and the low number of events during follow-up.
There was a greater per cent anterior leaflet grasping in patients with organic MR compared to those with functional MR. This data may be explained by the greater anteroposterior annular diameters of patients with MR of functional etiology and by their association with the tenting phenomenon or apical displacement from the coaptation site, thus making an angle of greater magnitude between the annulus and the leaflet and, therefore, more difficulties to encompass the anterior leaflet during the procedure. On the other hand, it was observed that the posterior leaflet grasping was similar in both groups, which is a particularly important aspect because larger grasping percentages are associated with a greater relative reduction of the anteroposterior diameter with coefficients close to 0.3, which implies that by achieving just a 10% more grasping of the posterior leaflet we would be achieving a 3% reduction in the anteroposterior annular diameter. The posterior grasping was also a protective element against the possibility of significant MR recurrence at follow-up. In this sense, it is believed that patients whose mitral annulus will not allow minimum leaflet coaptation at baseline or will cause excessive tension in the leaflets while grasping with the corresponding risk of tear and break are those patients that may benefit the most from an associated annuloplasty system.
The role that the new generation MitraClip XTr may play in the mitral annular changes of our cohort has not been studied due to the low number of implants of this last device. It would be interesting to publish in the future whether this new device causes changes of different magnitude compared to the previous device, and whether these changes have to do with significant clinical changes at follow-up.
Limitations
This is a single-center study with a modest number of patients (48) and procedures (50). The analysis of predictors of mortality and rehospitalizations due to heart failure may be affected by the small size of the sample and small number of events reported. Also, this is a relatively new technique at our center, meaning that the representation of patients who were followed in the long-term is scarce. Also, no long-term 3D analysis of the mitral annulus after the implant was conducted.
Conclusions
After TMVR with MitraClip there are morphological changes in the mitral annulus. The magnitude of these changes is different based on the MR etiology. The posterior leaflet grasping is the main factor that influences the appearance of changes and is also associated with a lower probability of significant MR recurrence at follow-up.
Funding
This study received no funding whatsoever.
What is known about the topic?
- After a TMVR procedure, there are morphological changes in the mitral annulus.
- Significant reduction of the patients’ anteroposterior diameters with functional MR were confirmed, as well as an inverse relation between the reduction of the anteroposterior diameter and the probability of significant MR recurrence.
What does this study add?
- Morphological changes do not happen in isolation in the mitral anteroposterior diameter, but they also affect other diameters, the area, and the perimeter.
- We hereby state that the TMVR with the MitraClip system may induce the reconfiguration of the 3D structure of the mitral valve, due not only to its direct mechanical effect, but also to the modifications of the intracavitary volumes and pressures. How big these changes are is different based on the etiology of MR.
- Posterior leaflet grasping turned out to be a crucial parameter in the morphological changes observed, and a protective factor of significant MR recurrence.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011.
2. Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231-1243.
3. Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122:674-681.
4. Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686-694.
5. Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37:703–712.
6. Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the Mitraclip therapy in Europe. J Am Coll Cardiol. 2013;62:1052-1061.
7. Feldman T, Foste Er, Glower DD, et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N Engl J Med. 2011;364:2187-2198.
8. Hidalgo F, Mesa D, Ruiz M, et al. Effects of Mitral Annulus Remodeling Following MitraClip Procedure on Reduction of Functional Mitral Regurgitation. Rev Esp Cardiol. 2016;69:1020-1025.
9. Remy T, Bertog SC, Wunderlich N, et al. Change in mitral annular size and geometry after mitraclip implantation in patients with functional and degenerative mitral regurgitation. J Interv Cardiol. 2014;27:516-524.
10. Carrasco-Chinchilla F, Arzamendi D, Romero M, et al. Experiencia inicial del tratamiento percutáneo de la regurgitación mitral con dispositivo MitraClip en España. Rev Esp Cardiol. 2018;67:1007-1012.
11. Stone GW, Adams DH, Abraham WT, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions. J Am Coll Cardiol. 2015;66:308-321.
12. Flachskampf FA, Wouters PF, Edvardsen T, et al. Recommendations for transoesophageal echocardiography: EACVI update 2014. Eur Heart J Cardiovasc Imaging. 2014;15:353-365.
13. Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611-644.
14. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
15. Schueler R, Momcilovic D, Weber M, et al. Acute changes of mitral valve geometry during interventional edge-to-edge repair with the MitraClip system are associated with midterm outcomes in patients with functional valve disease: preliminary results from a prospective single-center study. Circ Cardiovasc Interv. 2014;7:390-399.