Abstract
The antithrombotic treatment after percutaneous revascularization in patients with chronic indication for oral anticoagulation has always been a matter of great interest and complexity, basically because of the high ischemic and thromboembolic risk of this population and high hemorrhagic risk associated with combination therapy with antiplatelet and anticoagulant drugs. The actual invasive management of ischemic cardiomyopathy has made this population of patients grow and raised concerns on which the optimal drugs and therapeutic strategies really are. Yet despite the scarce scientific evidence available, different antithrombotic regimens have been studied over the last few years in an attempt to reduce hemorrhagic events without affecting the efficacy of the new combination therapies. The strategies studied have been based on shortening the duration of triple anticoagulation therapy, and even on the use of double anticoagulation therapy (anticoagulation plus one single antiplatelet drug) prioritizing clopidogrel. But it has been the arrival of direct-acting anticoagulants, with important clinical trials conducted on this population, that has provided us with relevant and fundamental information that will undoubtedly contribute to change the actual clinical practice.
Keywords: Atrial fibrillation. Percutaneous coronary intervention. Stent. Oral anticoagulation.
Resumen
El tratamiento antitrombótico tras una revascularización percutánea en los pacientes con indicación de anticoagulación oral crónica ha sido siempre un tema de máximo interés y de gran complejidad, debido sobre todo al alto riesgo isquémico y tromboembólico intrínseco de esta población, y al elevado riesgo hemorrágico que comporta la combinación de fármacos antiagregantes y anticoagulantes. El manejo invasivo actual de la cardiopatía isquémica hace que esta población esté en crecimiento, aspecto que incrementa el interés por definir cuáles son los mejores fármacos y estrategias terapéuticas. A pesar de la escasa evidencia científica, a lo largo de los últimos años se han estudiado diferentes regímenes antitrombóticos, buscando fundamentalmente una reducción de los eventos hemorrágicos, sin que esto repercutiera en la eficacia de las nuevas combinaciones. Las estrategias estudiadas se han basado en el acortamiento de la duración del tratamiento triple e incluso en el uso del tratamiento doble (anticoagulación más un único antiagregante) priorizando el clopidogrel. Sin embargo, ha sido la llegada de los anticoagulantes de acción directa, con la realización de importantes ensayos clínicos en esta población, lo que está aportando información relevante y trascendente que, sin lugar a dudas, contribuirá a modificar la práctica clínica.
Palabras clave: Anticoagulación oral. Fibrilación auricular. Intervencionismo coronario percutáneo. Stent.
Abbreviations: ACS: acute coronary syndrome. AF: atrial fibrillation. ASA: acetylsalicylic acid. DAPT:dual antiplatelet therapy. DAT: dual antithrombotic therapy. DOAC: direct oral anticoagulation. NOAC: non-vitamin K antagonist oral anticoagulant PCI: percutaneous coronary intervention. P2YI12: P2YI12 receptor inhibitor. TAT: triple antithrombotic therapy. VKA: vitamin K antagonist.
Introduction
The antithrombotic management of patients with atrial fibrillation (AF) who undergo percutaneous coronary interventions (PCI) has been, is, and will always be cause for study discussion, and research. The complexity of this population with high comorbidity leads to a poor prognosis in the mid and long term with a high rate of ischemic events. On the other hand, the use of combined antithrombotic therapies (dual or simple antiplatelet and anticoagulant drugs) aimed at improving the ischemic prognosis of these patients generates a number of hemorrhagic complications we should not overlook that has made us have to look for safer antithrombotic regimens (both in intensity and time) without affecting the efficacy.
The complexity of these patients and the difficulty when trying to include them in clinical trials that are actually representative of the real world has led to us obtain the information on the optimal antithrombotic regime from the information provided by registries, meta-analyses, and expert and work group recommendations.
It is precisely the arrival of direct-acting oral anticoagulants (DOAC), safer drugs and, at least, as efficient as vitamin K antagonists (VKA) for the management of AF, that has brought us new evidence on this regard. The existence of an important number of patients with AF treated with stents has produced four large clinical trials that compare the safety and efficacy of DOAC and VKA with the use of different antithrombotic regimens.
Throughout this article we will be reviewing the evidence available on this fundamental population so important for its elevated prevalence, poor prognosis and amazing advances we have witnessed over the last few years and the new advances that are still to come.
MAGNITUDE OF THE PROBLEM AND PROGNOSIS
The prevalence of patients with AF treated with PCI goes from 6% to 10% depending on the different registries, the populations included, and the syndromes treated. There seems to be a higher prevalence of AF in patients revascularized due to stable angina compared to those due to acute coronary syndrome. Thus, Rohla et al.1 describe a 10.2% prevalence in stable patients versus 6.5% in patients revascularized due to ACS. This prevalence grows bigger in the Spanish registries with ACS up to 8%-9%2,3. We are, therefore, talking about very well-known patients in the cardiology units and interventional suites.
However, what is really important here is to be able to recognize how the presence of AF in patients who have been percutaneously revascularized is one of the predictors of the worst prognoses possible. In general, and without evaluating what the influence of the treatment is in all this, in the mid-term (20-month follow-up) one out of every three patients (32.3%) will have a major adverse event and almost 1 in out of every 4 (22.6%) will die because of it4. When comparing this population to patients without AF, we can see how long-term mortality (56 months) triples for just having AF (41% versus 13%), being the presence of arrhythmia one of the greatest predictors of mortality.1.
Another aspect that we should take into consideration is the appearance of de novo AF in patients hospitalized due to ACS. The information from the ARIAM registry (Analysis of delay in acute myocardial infarction)2 describes how patients with de novo AF can amount to 55% of all patients with AF who are hospitalized due to ACS. What is important here is not only the high percentage of this presentation, but also the poor hospital prognosis associated with it, which happens to be an independent predictor of hospital mortality and is associated with a higher presence of reinfarction, malignant arrhythmia, and heart failure.
The worst prognosis of these patients is associated with their advanced age, greater comorbidity and, on many occasions, because they go undertreated both with recommended strategies (fewer catheterizations and percutaneous revascularizations) and drugs2,5.
Another fundamental aspect in this poor prognosis situation is the use of the recommended antithrombotic therapies. The high ischemic risk of these patients requires regimens that are based on a combination of dual or simple antiplatelet and anticoagulant drugs. The quest for reducing ischemic events increases the number of drug-induced severe hemorrhages that have been correctly prescribed. Eventually, these hemorrhagic complications end up being determinant in the patient’s prognosis.
So, it is essential to identify this population and its associated risk to be able to come up with the optimal treatment strategies and measures during the hospital stay, discharge, and follow-up, in an attempt to improve the prognosis that is severe per se.
Anthitrombotic therapy with Vitamin K antagonists: dual or triple therapy
Chronic oral anticoagulation (OAC) is superior to antiplatelet therapy (whether monotherapy or dual therapy) when it comes to the prevalence of thromboembolic complications (stroke and systemic embolism) of AF6,7, while the dual antiplatelet therapy (DAPT) with acetylsalicylic acid (ASA) and a P2Y12 receptor inhibitor (P2YI12) is the antithrombotic therapy of choice to prevent atherothrombotic ischemic events (myocardial infarction and stent thrombosis) in patients who undergo PCI (in the context, or not, of an ACS)8,9. When both situations occur picking the antithrombotic therapy becomes a clinical issue because it is well-known that the easiest choice, that is, the use of triple antithrombotic therapy (TAT plus OAC and dual antiplatelet therapy) increases the risk of major hemorrhages at least 2 to 3-fold compared to any other antithrombotic regimens, whether dual antiplatelet therapy or dual antithrombotic therapy (DAT plus OAC and one antiplatelet drug)10-12. Therefore, controversy arises on whether or not using TAT due to the increase of hemorrhagic complications and the possible increase of ischemic events when using less aggressive therapies like DAT.
The higher hemorrhagic risk associated with the use of TAT has been consistently confirmed by numerous observational studies (including large registries), while findings on the prevention of antithrombotic events are not that clear, although, in general, no significant differences have been found between the TAT and the DAT when it comes to reducing ischemic events12-14. It is important to say here that the higher hemorrhagic risk associated with TAT is kept throughout the entire duration of the TAT regimen12, which is why, if we decide to give it a go, the evidence available tells us we should keep it the shortest time possible to obtain the benefit of less atherothrombotic events. On the other hand, we should look back at studies conducted in the 1990s that started talking about dual antiplatelet therapies in the context of PCI with coronary stents15-18. In these studies, the dual antiplatelet therapies (ASA plus thienopyridine) was beneficial for reducing ischemic events, particularly the first month after the PCI compared to a DAT strategy (ASA plus VKA) (table 1). Also, we should mention here that the ischemic events (myocardial infarction or stent thrombosis) that happen the first month after the PCI have worse prognosis when it comes to mortality compared to those that happen later in time19,20.
Table 1. Randomized clinical trials comparing dual antiplatelet therapy (ASA and VKA) and dual antithrombotic therapy after coronary stenting
| Clincal trial | Treatment groups | Major cardiac adverse events | Hemorrhagic events | ||
|---|---|---|---|---|---|
| Definition | Results | Definition | Results | ||
| ISAR15(n = 517) | DAPT (ASA + ticlopidine) versus DAT (ASA + VKA) | Cardiac death, MI, revascularization surgery or reintervention at 30 days | 1.6% versus 6.2%; P = .01 |
Any bleeding at 30 days | 0% versus 6.5%; P < .01 |
| STARS16(n = 1.653) | ASA versus DAPT (ASA + ticlopidine) versus DAT (ASA + VKA)* | Death, MI, ST or target lesion revascularization at 30 days | 0.5% versus 2.7%; P = .01 |
Any bleeding at 30 days | 5.5% versus 6.2%; P = .99 |
| MATTIS17(n = 350) | DAPT (ASA + ticlopidine) versus DAT (ASA + VKA) | CV death, MI or new revascularization at 30 days | 5.6% versus 11.0%; P = .07 |
Major hemorrhage or major vascular complication at 30 days | 1.7% versus 6.9%; P = .02 |
| FANTASTIC18(n = 485) | DAPT (ASA + ticlopidine) versus DAT (ASA + VKA) | Death, MI, or stent occlusion at 6 weeks | 5.7% versus 8.3%; P = .37 |
Any bleeding at 6 weeks | 13.5% versus 21.0%; P = .03 |
|
ASA, acetylsalicylic acid; CV, cardiovascular; DAPT, dual antiplatelet therapy; DAT, dual antithrombotic therapy; MI, myocardial infarction; ST, stent thrombosis; VKA, vitamin K antagonist. Only the results from the comparison between dual antiplatelet therapy and dual antithrombotic therapy are reported here. |
|||||
However, we should be cautious when drawing conclusions or making comments in favor or against TAT based on observational studies since, down the road, they are limited and non-randomized which can produce significant biases (such as, in this case, confusion bias by indication of different antithrombotic regimens based on the characteristics of the patients). For all this, we should emphasize the results from randomized clinical trials although, as we will see below, they also have limitations especially when it comes to the assessment of efficacy (prevention of ischemic events).
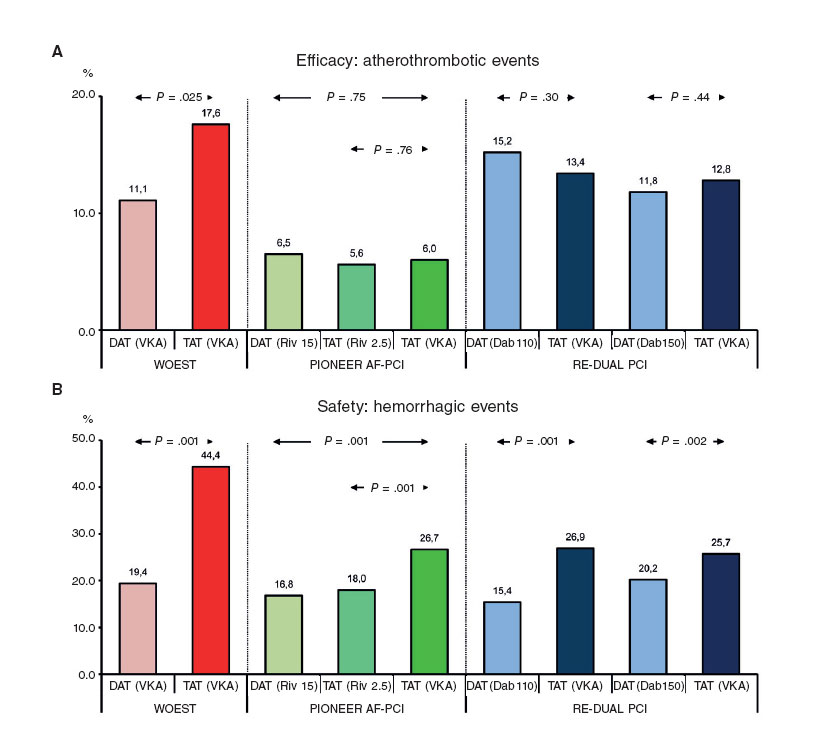
Before jumping into what we could call the era of the DOAC, two randomized clinical trials that assessed the safety of different antithrombotic strategies with VKA were conducted: the WOEST and the ISAR-TRIPLE.21,22. The WOEST trial included 573 patients with need for OAC (for several indications, 69% with AF or atrial flutter) who were randomized to receive TAT with VKA versus DAT consistent of clopidogrel plus VKA, and confirmed a significant reduction of the risk of bleeding (all bleeding events) at one year-follow-up in patients who received DAT (19.4% versus 44.4%; hazard ratio [HR], 0.36; 95% confidence interval [95%CI], 0.26- 0.50; P < .001) 21, although this benefit was mainly due to the occurrence of less minor bleeding events, while no increase of atherothrombotic events was confirmed in the group on DAT(figure 1). The study has been criticized for its numerous limitations among which we find the lack of statistical power to assess the variables of efficacy, the inclusion of patients with relative low risk of ischemic events (little more than 25% with ACS) and the presence of other aspects that may favor an increase of bleeding such as an excessive duration of the course of TAT in the control group (1 year, the most highly recommended), the femoral access in most cases, and the low use of proton pump inhibitors (PPI)23. Yet despite all this, the relevance of the WOEST trial is evident since it was the very first trial to ever question the need for TAT and hypothesized whether using DAT right after the PCI in this scenario was a real possibility.
Figure 1. Results from randomized clinical trials of triple therapy versus dual antithrombotic therapy in patients with an indication for chronic oral anticoagulation undergoing percutaneous coronary interventions. Safety: hemorrhagic events (A). Efficacy: major adverse cardiac events (B). The definitions of the main variable of safety vary depending on the study: WOEST, any hemorrhagic event at 12 months; PIONEER AF-PCI, clinically significant hemorrhage (TIMI) at 12 months; RE-DUAL PCI, major bleeding or clinically relevant bleeding (ISTH) at follow-up (average, 14 months). The definitions of major adverse cardiac events vary from one study to the next: WOEST, death, myocardial infarction, stroke, target vessel revascularization, or stent thrombosis at 12 months; PIONEER AF-PCI, cardiovascular mortality, myocardial infarction or stroke at 12 months; RE-DUAL PCI, death, thromboembolic event (myocardial infarction, stroke, or systemic embolism) or unplanned revascularization at follow-up. Dab 110, dabigatran at 110 mg/12 hours; Dab 150, dabigatran at 150 mg/12 hours; DAT, dual antithrombotic therapy; Riv 2.5, rivaroxaban 2.5 mg/12 hours; Riv 15, rivaroxaban at 15 mg/24 hours; TAT, triple antithrombotic therapy; VKA, vitamin K antagonist.
The ISAR-TRIPLE trial randomized 614 patients with an indication for OAC (83.9% with AF) who underwent PCIs with drug-eluting stents and 2 different courses of TAT: 6 months (long) versus 6weeks (short) (both including ASA, clopidogrel plus VKA followed by ASA plus VKA) 22. No differences between the 2 courses of TAT were reported when it comes to the main variable (a composite endpoint of death, myocardial infarction, definitive stent thrombosis, and major bleeding according the Thrombolysis in Myocardial Infarction [TIMI]) classification at 9 months (9.8% versus 8.8%; HR, 1.14; 95%CI, 0.68-1.91; P = .63). No significant differences were found either when it comes to the ischemic events and the hemorrhagic events separately. However, the analysis of the events that occurred at 6 weeks (once clopidogrel was withdrawn in the arm that received the short TAT course) confirmed a slight increase of all bleeding events according to the classification established by the Bleeding Academic Research Consortium (not the TIMI classification) in the arm that received the long TAT course. Yet despite the limitations of both studies, and the fact that the main absence was the lack of statistical power for an adequate assessment of the efficacy of the different strategies analyzed on the prevention of atherothrombotic events, its results suggest that the duration of the TAT should not be extended for no reason to avoid increasing the hemorrhagic risk beyond necessary.
Where do direct-acting oral anticoagulants stand?
Among the strategies used to reduce hemorrhagic complications due to the use of antithrombotic drugs and on top of reducing the courses of TAT or withdrawing ASA in certain high-risk groups, the use of DOAC is another strategy to take into consideration here.
The best safety profile of these new anticoagulant drugs, together with the logical interest to make them the leading therapy in large populations and their room for improvement, fostered a clinical trial for each and every DOAC already approved.
With the information available today, the last European guidelines on clinical practice recommend the use of DOAC in this population of patients to the detriment of VKA.8,24.
Although the 4 clinical trials conducted compared patients with AF who were revascularized with PCI, the safety of the newanticoagulant drugs versus TAT (VKA, ASA and clopidogrel) and showed very similar inclusion and exclusion criteria, there are significant differences in the antithrombotic regimens and drug dosage used, which in turn could influence the conclusions of the trials and eventually have practical repercussions. We hereby present, in chronological order, the 4 large clinical trials already published or in follow-up stages today, including the most relevant aspects and most controversial issues. Table 225 shows the design, goals, and main findings of each and every one of these trials.
Table 2. Differential characteristics and fundamental findings of the 4 clinical trials comparing direct-acting oral anticoagulants versus antivitamin K anticoagulants in patients with atrial fibrillation percutaneously revascularized
| Study | Population | Patients | Study groups | Control groups | Safety primary endpoint | Main findings | Efficacy | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PIONEER AF-PCI26 | Patients with AF undergoing PCI | 2.124 | Ribaroxaban at 15 mg/24 h + P2YI12 | Ribaroxaban at 2.5 mg/12 h + P2YI12 + ASA | Warfarin + P2YI12 + ASA | Clinically significant bleeding (TIMI classification) | TIMI major (12%), minor (7%) bleeding requiring medical attention (85%) | The percentage of bleeding was lower in the two rivaroxaban groups (16.8% and 18%) compared to the control group (26.7%); P < .0001 |
Non inferior. Study not statistically powered to detect efficacy differences | |
| RE-DUAL PCI29 | Patients with AF undergoing PCI | 2.725 | Dabigatran at 150 mg/12 h + P2YI12 | Dabigatran at 110 mg/12h + P2YI12 | Warfarin + P2YI12 + ASA | Major bleeding or clinically relevant bleeding (ISTH classification) | ISTH major bleeding (32%) or non-major clinically relevant bleeding (68%) | The percentage of bleeding was lower in the two dabigatran groups (20.2% and 15.4%) compared to the control group (25.7% and 26.9%, respectively); P < .0001 |
Non inferior. Study not statistically powered to detect efficacy differences | |
| AUGUSTUS31 | Patients with AF or PCI | 4.614 | Apixaban at 5 or 2.5 mg/12 h + P2YI12 + ASA | Apixaban at 5 or 2.5 mg/12 h + P2YI12 | Warfarin + P2YI12 + ASA | Warfarina + IP2Y12 | Major bleeding or clinically relevant non-major bleeding (ISTH classification) | The percentage of bleeding was lower with apixaban compared to VKA (10.5% and 14.7%; P < .001) and with placebo versus ASA (9% and 16.1%; P < .001) |
Less hospital admissions with apixaban, and a similar incidence of ischemic events | |
| ENTRUST-AF PCI32 | 1.500 | Edoxaban at 60 mg/24 h + P2YI12 | Warfarin + P2YI12 + ASA | Major bleeding or clinically relevant non-major bleeding (ISTH classification) | In the follow-up stage | In the follow-up stagewww | ||||
|
ASA, acetylsalicylic acid; ACS, acute coronary syndrome; AF, atrial fibrillation; ISTH, International Society on Thrombosis and Hemostasis; P2YI12, P2Y12 receptor inhibitor; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; VKA, vitamin K antagonist. Modified with permission from Lip et al.25 |
||||||||||
The PIONEER AF-PCI trial
The PIONEER AF-PCI trial was the first trial published. It is a multicenter international trial that randomized 2124 patients with AF revascularized with stents into three treatment strategies: rivaroxaban at 15 mg/day and P2YI12; rivaroxaban at 2.5 mg/12 hours and ASA plus P2YI12; or TAT (VKA, ASA plus clopidogrel).26. The primary endpoint was the occurrence of clinically significant bleeding (major or minor bleeding according to the TIMI classification) or hemorrhages requiring medical attention.
The goal of this study was achieved for both groups on rivaroxaban (16.8% and 18% versus 26.7%; P < .001). When it comes to efficacy, ischemic events (cardiovascular death, infarction, or stroke) and global mortality were similar in the 3 groups, yet the study did not have enought statistical power to be able to assess differences when it comes to efficacy. The authors concluded that the treatment with rivaroxaban at 15 mg/day plus clopidogrel with or without rivaroxaban at 2.5 mg/12 hours plus clopidogrel and ASA is safer than TAT with VKA, clopidogrel and ASA.
This was the first study that brought DOAC to this population, but many more things should be said on this regard. We do not know if these two doses of rivaroxaban are enough to prevent strokes from happening in patients with AF compared to VKA or rivaroxaban in doses of 20 mg/day in patients with normal renal function27,28. The dose of rivaroxaban at 15 mg/24 hours has not been studied widely in patients with AF in thromboembolic prevention and can be controversial when it comes to recommending it in all kinds of patients. And the same thing happens with doses at 2.5 mg/12 hour studied for the management of ischemic heart disease but not for the management of atrial fibrillation, but still presumed clearly insufficient.
The use of these doses not approved for the management of AF has placed rivaroxaban as the first studied DOAC in this population of patients with a positive safety profile and has generated an indication with a level IIbB evidence only in the European guidelines on dual antiplatelet drugs (rivaroxaban at 15 mg/day)8, strangely enough lower to the one assigned to the use of doses of rivaroxaban at 20 or 15 mg/day (based on the renal function), based on its general study and sub-studies (IIaC)8,27.
The RE-DUAL PCI trial
The RE-DUAL PCI clinical trial randomized 2725 patients into 3 groups (TAT plus VKA, dual therapy with dabigatran at 150 at mg/12 hours and dual therapy with dabigatran at 110 mg/12 hours) based on the hypothesis that dual therapy with dabigatran and P2YI12 may be safer than the standard therapy with TAT in patients with AF who undergo PCI.29. The incidence of the primary event (major or clinically relevant bleeding according to the International Society on Thrombosis and Haemostasis [ISTH]) classification was 15.4% for doses of dabigatran at 110 mg and 26.9% for TAT (HR, 0.52; P < .0001 for non-inferiority and superiority), and 20.7% for doses of dabigatran at 150 mg versus 25.7% for TAT (HR, 0.72; P < .0001 for non-inferiority). The incidence of the composite event of efficacy was similar in all 3 groups. The study concludes that in patients with AF who undergo PCI, the risk of bleeding was lower in both groups of dabigatran compared to TAT, with no significant differences of efficacy.
The most relevant issue is the safety profile dabigatran brings to the table with a reduction of 48% in patients treated with dabigatran at doses of 110 mg and 24% in those treated with dabigatran at doses of 150 mg. In defense of this study, we should mention that the doses used were the same ones than the doses used in the RELY trial30, that proved its efficacy and safety for the management of non-valvular AF which would later be confirmed in wide real world registries.
The main doubt following this study came with the finding of an incidence of stent thrombosis close to 1.5% in the dabigatran group at 110 mg versus 0.8% in the TAT group (P = .15), and a higher infarction rate (4.5% versus 3.0%; P = .09). We should mention here that these rates of thrombosis and infarction were not observed with the highest possible dose of 150 mg/12 hour. And even though the differences were not statistically significant, these data should make us think whether we are going from very powerful antithrombotic regimens to too light antithrombotic regimens in an attempt to prioritize safety over efficacy. And the second question here that still remains is that we still need to know whether the best safety profile of dabigatran is so due to the greater safety of the drug or to the non-exposure to the ASA. At the end of the day, these two clinical trials do not compare similar strategies (triple versus dual therapy) which means that they will not be able to solve issues that are absolutely critical.
In spite of everything, the REDUAL-PCI trial should be considered a landmark study that accurately assessed this new therapeutic option in patients with AF treated with stents and even more accurately in patients with high hemorrhagic risk.
The AUGUSTUS trial
The AUGUSTUS31 trial is undoubtfully the one that was best structured and answered a lot of questions. This trial was designed with a factorial 2 x 2 design to compare apixaban with VKA plus ASA versus placebo in patients with AF and ACS undergoing PCI or receiving P2YI12. This is the largest study and included 4614 patients.
The primary endpoint is a composite of major bleeding and non-clinically relevant bleeding following the ISTH classification. The study secondary endpoints are all-cause mortality, all-cause hospitalizations, and ischemic events.
The primary event incidence rate was 10.5% in the apixaban group vs 14.7% in the VKA group (HR = 0.69; P < .001 for inferiority and superiority) and 16.1% in patients who received ASA vs 9% in those who received placebo (HR = 1.89; P < .001). The patients in the apixaban group had a lower incidence rate of hospitalization compared to those from the KVA group and a similar number of ischemic events.
It seems clear that this study confirms the superiority of DOACs in this population of patients thanks to their more evident safety profile. However, it probably does not generate enough evidence to withdraw ASA and make it the standard routine in these patients. We should emphasize here the presence of more thromboembolic events in the placebo group compared to the ASA group with twice as much stent thrombosis. This difference was not statistically significant and can generate doubts due to the lack of statistical power to detect ischemic events. Also, we should remember that patients were randomized days or weeks after the ischemic event happened and that they had ASA until the randomization stage.
The ENTRUST-AF PCI trial
The ENTRUST-AF PCI clinical trial32 still in the follow-up stage, randomized 1500 patients with AF on anticoagulant therapy and revascularized with PCI, to 2 therapeutic strategies: edoxaban at 60 mg/24 hours (or 30 mg if the necessary criteria are met for this dose) plus one P2YI12 versus TAT with VKA. The primary safety endpoint is the incidence of major bleeding and non-clinically relevant bleeding following the ISTH classification, and the primary efficacy endpoint is a composite of cardiovascular mortality, stroke, embolism, infarction, or definite stent thrombosis.
The small size of the study will only answer to whether a dual therapy with edoxaban is safer than a therapy with TAT and VKA. It is easy to hypothesize that this will be the case since, at the end of the day, identical conditions are not being compared since ASA is being withdrawn from the edoxaban group, same as it happened with the REDUAL-PCI trial. Also, the lack of statistical power should not be able to show any differences in efficacy between both strategies.
Considerations of clinical practice guidelines
Over the last few years, different clinical practice guidelines have been designed by different scientific societies and consensus documents have been established by different expert groups that have brought a series of recommendations for the management of antithrombotic therapy in patients with a need for chronic OAC who undergo PCI7,8,24,25,33,34. The fact that the recommendations established by these documents are not always coincidental added to the fact that most of them show low levels of evidence only tells us how complex this clinical scenario is.
When it comes to antithrombotic therapy post-PCI, the most recent recommendations (that incorporate results from studies on DOAC) from the European scientific societies can be summed up in the following items8,24,25:
- TAT with ASA, clopidogrel and OAC for a month is the strategy of choice in patients who receive a stent, regardless of the type of stent being implanted.
- TAT at least 1 month in patients with high ischemic risk due to ACS or other anatomical or procedural characteristics that exceeds their hemorrhagic risk.
- Initial DAT with OAC and clopidogrel (the preferred one) or ASA in patients with high hemorrhagic risk that exceeds their ischemic risk.
- In patients with non-valvular AF, the use of DOAC should be recommended.
- DOAC should be administered in their lowest effective dose to avoid strokes as assessed by AF trials.
- The use of a 15 mg dose of rivaroxaban (instead 20 mg) can be considered, although its efficacy in the prevention of stroke has not been appropriately assessed yet.
- When DAT is used the preferred dose of dabigatran is 150 mg/12 hours.
- In case of using VKA, the objective International Normalized Ratio should be in the low range while time in the therapeutic range should be > 65%.
- The use of ticagrelor or prasugrel as part of the TAT is not recommended.
- Withdrawing antiplatelet therapy should be considered and only leave the OAC 12 months after the procedure, although we can still add antiplatelet drugs in particular cases based on ischemic risk.
On this regard, we should mention a consensus document written by US experts that has been controversial because it recommends, among other things, DAT as the strategy of choice in most cases plus withdrawing TAT 1 entire month, at most, in patients with high ischemic risk and low hemorrhagic risk34. In sum, these differences emphasize how difficult it is to manage these patients, how varied the different interpretations of the studies available are, and how important it is to have more scientific evidence available on this regard.
Optimize the balance between efficacy and safety. Practical considerations
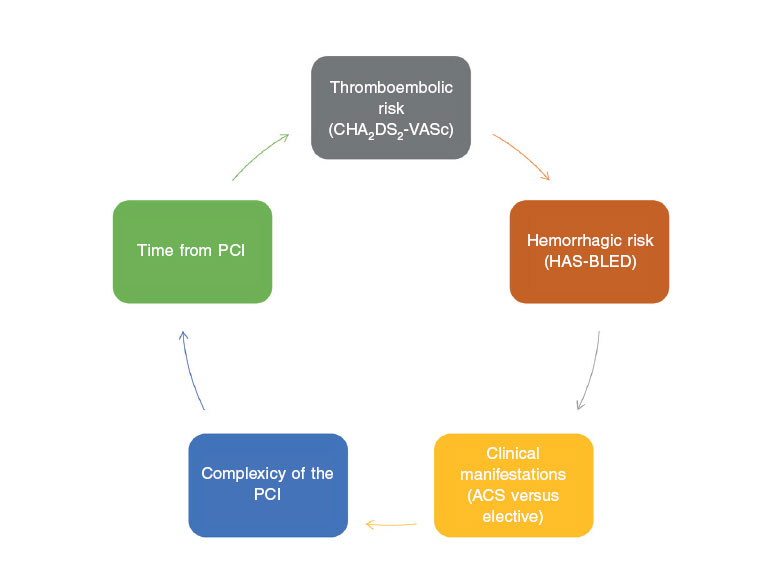
Once the evidence generated by the studies and the clinical practice guidelines have been analyzed, it seems evident that we need to make thorough assessments of the individual risk of each patient to suffer ischemic, thromboembolic, and hemorrhagic events(figure 2). These individual assessments should incorporate the evaluation of factors associated with the patient and the procedure being conducted.
Figure 2. Variables that should be taken into consideration when deciding what the optimal antithrombotic therapy is in patients with atrial fibrillation undergoing percutaneous coronary interventions. AF, atrial fibrillation; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
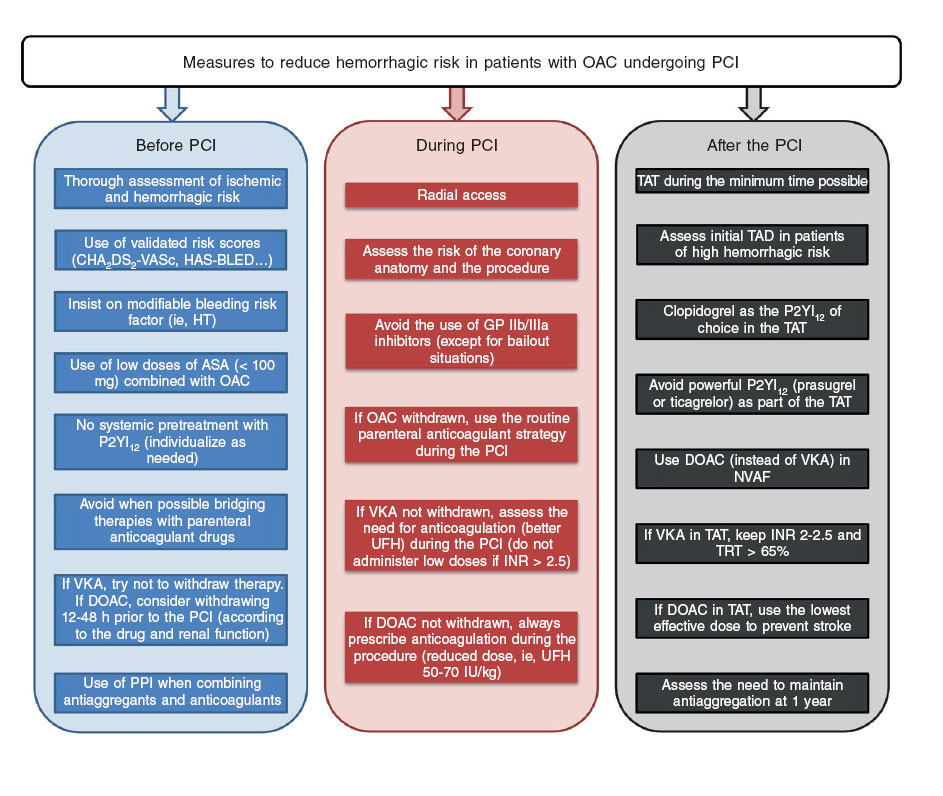
Therefore, there are clinical characteristics (presentation as ACS, prior history of myocardial infarction or stent thrombosis, presence of comorbidity such as diabetes mellitus, renal failure, or peripheral artery disease, etc.), coronary anatomy characteristics (multivessel diffuse disease) and PCI characteristics (complex procedures with treatment of various lesions, implantation of several stents or stents of a significant length, 2-stent techniques in bifurcations, chronic occlusions, etc.) that suggest higher ischemic risk, and that should be taken into consideration when choosing more powerful and longer regimens8,35. The other side of this story would be those factors that may condition higher hemorrhagic risk (prior history of major bleeding or hemorrhagic stroke, short life expectancy, anemia, older age, active neoplasm, severe renal failure, frailty, etc.) that should also be taken into consideration when choosing less powerful shorter antithrombotic strategies. When it comes to the type of stent being implanted, the greater safety profile that last generation drug-eluting stents bring is a reality these days in patients of high hemorrhagic risk24,36, and their use should be generalized. In any case, from the practical point of view, the first step in all patients is to consider and apply a series of measures that contribute to minimize the risk of bleeding, when possible, before, during, and after the PCI(figure 3).
Figure 3. Measures that should be taken into consideration to reduce the hemorrhagic risk of patients with an indication for chronic oral anticoagulation undergoing percutaneous coronary interventions. AAS, acetylsalicylic acid; DAT, dual antithrombotic therapy; DOAC, direct-acting oral anticoagulants; GP, glycoprotein; HT, hypertension; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulation; PCI: percutaneous coronary intervention; PPI: proton pump inhibitor; P2YI12, P2Y12 receptor inhibitor; TAT, triple antithrombotic therapy; TTR, time in the therapeutic range; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Both the aforementioned clinical trials that compared TAT plus VKA versus strategies of DAT plus VKA or DOAC21,26,29, and some meta-analyses that grouped the data of such trials37,38, showed higher hemorrhagic risks with the use of TAT without finding a clear benefit of this regimen when it comes to reducing ischemic events. However, with the actual evidence available, ruling out the TAT does not seem justified in this scenario for several reasons: 1) none of the clinical trials had enough statistical power to assess adequately the variables of efficacy (ischemic and thromboembolic events); 2) another limitation of these clinical trials was the inclusion of patients with relatively low risk of atherothrombotic events, which means that we do not have enough information to ensure the efficacy of DAT regimens in individuals with high risk of ischemic events or strokes; and 3) the ischemic events (myocardial infarction and stent thrombosis) that occur early after the PCI have worse prognosis, which would also suggest ruling out the TAT, at least, in the initial period after the procedure. For all these reasons, the authors’ opinion (which is consistent with the European guidelines) is that, with the actual evidence available, after an individualized thorough assessment of the patient’s ischemic and hemorrhagic risk, it seems advisable to implement an initial TAT strategy during the shortest time possible (when each patient has the highest probability of suffering from an ischemic adverse event) in order to not increase, unnecessarily, the risk of bleeding, thus leaving DAT as an alternative in those individuals with high hemorrhagic risk -actually higher than their ischemic risk. In sum, patients in whom there is not clear leverage from using TAT for the prevention of atherothrombotic events. As a practical, though empirical recommendation, one month of TAT seems enough in most patients with PCI and in the context of stable ischemic disease, while in patients with ACS, it is recommended to implement individual courses of TAT (1 through 6 months) and always based on the aforementioned balance of risks. However, the 6-month duration of TAT seems advisable for patients with a very high risk of ischemic events only.
When it comes to choosing the anticoagulant drug, the DOAC, due to their safety profile, seem the optimal drugs in a clinical context where the risk of bleeding is very high due to the necessary combination of antiplatelet drugs24. When it comes to the dose of DOAC used, in the TAT we should be using the minimum dose possible that has proven effective for the prevention of strokes in landmark trials on AF8,24. And this is a particularly relevant aspect since there are more and more evidences that tell us that using unnecessary reduced doses of DOAC (not meeting the adjustment criteria specified in the label) is associated with more thromboembolic events at follow-up39. If we choose dabigatran, it is recommended to use doses of 110 mg during the TAT and doses of 150 mg if the patient is on DAT.
And even though data from clinical trials on the combination of OAC plus a powerful P2YI12 are really scarce (< 6% in the PIONEER AF-PCI trial and approximately 12% of ticagrelor in the RE-DUAL PCI trial), several observational studies have shown very high rates of bleeding with the use of prasugrel or ticagrelor as part of the TAT40,41. Choosing ASA or clopidogrel as part of the DAT can be somehow controversial; even though the clinical guidelines advocate for the use of clopidogrel as the antiplatelet drug of choice in this context, the combination of ASA plus OAC is also valid and can be an option especially if we take into account that a significant number of patients (around 15%-30% in our context) show inadequate answers to clopidogrel42.
Conclusions
The patient treated with anticoagulant drugs who is percutaneously revascularized has a poor prognosis in the mid and long-term and a high incidence rate of ischemic, thromboembolic, and hemorrhagic events.
As a summary to this paper, we could make the following overall recommendations while taking into consideration that the complexity of these patients will always require individual therapies in each case:
- The intensity and duration of antithrombotic therapy should be determined by the patient’s clinical manifestations which established the revascularization and by the patient’s ischemic, thromboembolic, and hemorrhagic risks. The type of stent is no longer a variable that will influence the decisionmaking process on the antithrombotic therapy of this population, and most patients will require drug-eluting stents because they are safer and more effective compared to conventional stents.
- When choosing TAT, its duration should be reduced as much as possible and it should focus on the period of highest ischemic risk and stent thrombosis in order to minimize hemorrhagic risk. With the information available today, the use of TAT seems justified in patients where ischemic risk is prioritized and in whom hemorrhagic risk is somehow acceptable.
- In patients where hemorrhagic risk is prioritized over ischemic risk, DAT may be indicated (anticoagulation plus one single antiaggregant agent, being clopidogrel the one preferred by the clinical practice guidelines) from revascularization.
- The use of new antiaggregant agents (prasugrel or ticagrelor) is clearly not recommended in this population. They should be contraindicated as part of the TAT (except for certain exceptions) and there is little evidence on their use in DAT regimens.
- The use of DOAC in this context seems especially beneficial and is recommended by the most recent clinical practice to the detriment of VKA. Although we do not have enough statistically powerful trials to be able to determine differences of efficacy, the evidence we have so far speaks to us about the superiority of DOAC as safer drugs of a similar efficacy. It is important to emphasize that we should indicate DOAC doses that have proven their efficacy in the prevention of thromboembolic events in general studies of patients with AF.
- Regardless of the antithrombotic recommendations, general measures should always apply associated with the procedure in an attempt to reduce hemorrhagic events (radial access, avoid bridging therapies when possible, or contraindicate the use of glycoprotein IIb/IIIa inhibitors except when in bailout situations.
Conflicts of interest
J.M. Ruiz-Nodar declares to have received fees for lectures given on behalf or AstraZeneca, Biosensor, Boston Scientific, Medtronic, and Terumo. J.L. Ferreiro declares to have received fees for lectures given on behalf or Eli Lilly Co., Daiichi Sankyo, Inc., Astra- Zeneca, Roche Diagnostics, Pfizer, and Boehringer Ingelheim; and fees received in his capacity of consultant for AstraZeneca, Eli Lilly Co. and Ferrer declares to have received research grants from AstraZeneca.
References
1. Rohla M, Vennekate CK, Tentzeris I, et al. Long-term mortality of patients with atrial fibrillation undergoing percutaneous coronary intervention with stent implantation for acute and stable coronary artery disease. Int J Cardiol.2015;184;108-114.
2. Almendro-Delia M, Valle-Caballero MJ, Garcia-Rubira JC, et al. Prognostic impact of atrial fibrillation in acute coronary syndromes: results from the ARIAM registry. Eur Heart J Acute Cardiovasc Care. 2014;3:141-148.
3. Ruiz-Nodar JM, Cequier A, Lozano T, et al. Impacto del tipo de hospital en el tratamiento y evolución de los pacientes con síndrome coronario agudo sin elevación del ST. Rev Esp Cardiol. 2010;63:390-399.
4. Ruiz-Nodar JM, Marín F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation: implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008;51:818-825.
5. Lopes RD, Li L, Granger CB, et al. Atrial fibrillation and acute myocardial infarction: antithrombotic therapy and outcomes. Am J Med. 2012:125:897-905.
6. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912.
7. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962.
8. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260.
9. Lugo LM, Ferreiro JL. Dual antiplatelet therapy after coronary stent implantation: individualizing the optimal duration. J Cardiol. 2018;72:94-104.
10. Rubboli A, Agewall S, Huber K, Lip GY. New-onset atrial fibrillation after recent coronary stenting: warfarin or non-vitamin K-antagonist oral anticoagulants to be added to aspirin and clopidogrel? A viewpoint. Int J Cardiol. 2015;196:133-138.
11. Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967-1974.
12. Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126:1185-1193.
13. Wustrow I, Sarafoff N, Haller B, et al. Real clinical experiences of dual versus triple antithrombotic therapy after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2018;92:1239-1246.
14. Kawai H, Watanabe E, Yamamoto M, et al. Major bleeding complications related to combined antithrombotic therapy in atrial fibrillation patients 12 months after coronary artery stenting. J Cardiol. 2015;65:197-202.
15. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
16. Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665-1671.
17. Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the multicenter aspirin and ticlopidine trial after intra-coronary stenting (MATTIS). Circulation. 1998;98:2126-2132.
18. Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation. 1998;98:1597-1603.
19. Brener SJ, Kirtane AJ, Stuckey TD, et al. The Impact of Timing of Ischemic and Hemorrhagic Events on Mortality After Percutaneous Coronary Intervention: The ADAPT-DES Study. JACC Cardiovasc Interv. 2016;9:1450-1457.
20. Secemsky EA, Matteau A, Yeh RW, et al. Comparison of Short- and Long-Term Cardiac Mortality in Early Versus Late Stent Thrombosis (from Pooled PROTECT Trials). Am J Cardiol. 2015;115:1678-1684.
21. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107-1115.
22. Fiedler KA, Maeng M, Mehilli J, et al. Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol. 2015;65:1619-1629.
23. Capodanno D, Lip GY. Triple therapy for atrial fibrillation and ACS with or without PCI: don’t drop aspirin just yet. J Am Coll Cardiol. 2015;65:515-516.
24. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
25. Lip GYH, Collet JP, Haude M, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. 2018. https://doi.org/10.1093/europace/euy174
26. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-2434.
27. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
28. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018:39:1330-1393.
29. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
30. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
31. Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019. https://doi.org/10.1056/NEJMoa1817083
32. Vranckx P, Lewalter T, Valgimigli M, et al. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: rationale and design of the ENTRUST-AF PCI trial. Am Heart J. 2018;196:105-112.
33. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Am Coll Cardiol. 2016;68:1082-1115.
34. Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention. A North American Perspective – 2018 Update. Circulation. 2018;138:527-536.
35. Giustino G, Chieffo A, Palmerini T, et al. Efficacy and Safety of Dual Anti-platelet Therapy After Complex PCI. J Am Coll Cardiol. 2016;68:1851-1864.
36. Urban P, Meredith IT, Abizaid A, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038-2047.
37. Cavallari I, Patti G. Meta-Analysis Comparing the Safety and Efficacy of Dual Versus Triple Antithrombotic Therapy in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2018;121:718-724.
38. Golwala HB, Cannon CP, Steg PG, et al. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2018;39:1726-1735a.
39. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-Vi-tamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol. 2017;69:2779-2790.
40. Sarafoff N, Martischnig A, Wealer J, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol. 2013;61:2060-2066.
41. Verlinden NJ, Coons JC, Iasella CJ, Kane-Gill SL. Triple Antithrombotic Therapy With Aspirin, IP2Y12 Inhibitor, and Warfarin After Percutaneous Coronary Intervention: An Evaluation of Prasugrel or Ticagrelor Versus Clopidogrel. J Cardiovasc Pharmacol Ther. 2017;22:546-551.
42. Angiolillo DJ, Ferreiro JL. Platelet adenosine diphosphate P2Y12 receptor antagonism: benefits and limitations of current treatment strategies and future directions. Rev Esp Cardiol. 2010;63:60-76.