ABSTRACT
Introduction and objectives: Transcatheter aortic valve implantation (TAVI) has proven safe and effective in low-to-high risk patients, but emergency procedures have been excluded from the landmark trials. We aimed to assess the current outcomes and main factors conditioning the prognosis during emergency TAVI.
Methods: A systematic search in PubMed and Google Scholar was conducted for all studies comparing elective vs emergency TAVI. Searched terms were “emergency” and/or “urgent”, “elective”, and “transcatheter valve replacement” and/or “heart failure” and/or “cardiogenic shock”. Emergency TAVI was considered as any unscheduled TAVI performed to treat refractory heart failure or cardiogenic shock. A random-effects model was used.
Results: A total of 7 studies with 84 427 TAVI patients were included (14 241 emergency procedures; 70 186 elective TAVIs). Emergency cases presented higher risk scores (logistic EuroSCORE 65.9% ± 21% vs 29.4% ± 18%, P < .001; Society of Thoracic Surgeons Risk Score 29.4% ± 27.4% vs 13.7% ± 11.6%, P < .001). More advanced heart disease was observed with deterioration of left ventricular (LV) function (39.5% ± 17.8% vs 52.5% ± 12.8%; P < .001) and larger LV end-diastolic diameters (55 ± 9 mm vs 48 ± 7 mm; P < .001) despite similar aortic valve areas and gradients. Elective TAVIs presented a greater success rate (93.6% vs 92.5%; odds ratio [OR] = 0.84; 95%CI, 0.74-0.95; P = .005), less acute kidney injury, and a lower need for dialysis and mechanical circulatory support. Overall, non-emergency cases had lower in-hospital (3.3% vs 5.7%; P < .001), 30-day (4.4% vs 8.8%; P < .001) and 1-year mortality rates (19.7% vs 34.75%; P = .0001). The main determinants of mortality were need for new dialysis (OR = 2.26; 95%CI, 1.84-2.76; P < .001) or mechanical circulatory support (OR = 2.55; 95%CI, 1.14-5.67; P < .001).
Conclusions: Emergency TAVI recipients presented worse baseline risk and more advanced cardiac disease that determined greater in-hospital, 30-day, and 1-year mortality rates. The early identification of patients at risk for requiring mechanical circulatory support or dialysis may contribute to a better indication of TAVI in emergency scenarios.
Keywords: Cardiogenic shock. Heart failure. Transcatheter aortic valve replacement. Aortic stenosis.
RESUMEN
Introducción y objetivos: El implante percutáneo de válvula aórtica (TAVI) ha demostrado ser seguro y eficaz en pacientes tanto de bajo como de alto riesgo, pero los procedimientos emergentes se han excluido en los principales estudios. El objetivo fue determinar los resultados actuales y los condicionantes del pronóstico durante el TAVI emergente.
Métodos: Se realizó una búsqueda sistemática en PubMed y Google Scholar de cualquier estudio que comparara el TAVI electivo frente al emergente. Los términos empleados fueron «emergent» y/o «urgent», «elective», y «transcatheter valve replacement» y/o «heart failure» y/o «cardiogenic shock». Se consideró TAVI emergente todo procedimiento no programado realizado para tratar la insuficiencia cardiaca refractaria o el shock cardiogénico. Se utilizó un modelo de efectos aleatorios.
Resultados: Se incluyeron 7 estudios (84.427 pacientes) tratados con TAVI (14.241 emergentes y 70.186 electivos). Los casos electivos presentaron una mayor puntuación de riesgo (EuroSCORE logístico 65,9 ± 21 frente a 29,4 ± 18%, p < 0,001; Society of Thoracic Surgeons Risk Score 29,4 ± 27,4 frente a 13,7 ± 11,6%, p < 0,001). Presentaron una enfermedad cardiaca más avanzada, con peor función ventricular izquierda (39,5 ± 17,8 frente a 52,5 ± 12,8%; p < 0,001) y mayor diámetro telediastólico del ventrículo izquierdo (55 ± 9 frente a 48 ± 7 mm; p < 0,001), pese a tener similar área valvular aórtica y gradientes. El TAVI electivo tuvo mayor tasa de éxito (93,6 frente a 92,5%; odds ratio [OR] = 0,84; IC95%, 0,74-0,95; p = 0,005), con menor tasa de fallo renal agudo y menos necesidad de diálisis y de soporte circulatorio mecánico. En conjunto, los casos no emergentes tuvieron menor mortalidad intrahospitalaria (3,3 frente a 5,7%; p < 0,001), a 30 días (4,4 frente a 8,8%; p < 0,001) y a 1 año (19,7 frente a 34,75%; p = 0,0001). Los principales determinantes de mortalidad fueron la nueva necesidad de diálisis (OR = 2.26; IC95%, 1,84-2,76; p < 0,001) o requerir soporte circulatorio mecánico (OR = 2,55; IC95%, 1,14-5,67; p < 0,001).
Conclusiones: Los receptores de TAVI emergente presentaron peor riesgo basal y enfermedad cardiaca más avanzada, que determinaron una mayor mortalidad intrahospitalaria, a 30 días y a 1 año. La identificación precoz del riesgo de precisar soporte circulatorio mecánico o diálisis podría ayudar a una optimización de la indicación de TAVI emergente.
Palabras clave: Shock cardiogenico. Insuficiencia cardiaca. Implante percutaneo de valvula aortica. Estenosis aortica.
Abbreviations
AS: aortic stenosis. CKD: chronic kidney disease. CS: cardiogenic shock. HF: heart failure. SAVR: surgical aortic valve replacement. TAVI: transcatheter aortic valve implantation.
INTRODUCTION
Aortic stenosis (AS) is the most commonly treated valvular heart disease in Western countries.1 In a relatively small but growing proportion of patients (from 3.5% to 12%), AS may present as cardiogenic shock (CS) with an estimated short-term mortality as high as 70% if definitive surgical or percutaneous treatment is not provided.2 CS is characterized by an inadequate tissue perfusion as a result of a decompensated cardiac disease that translates into a low-output state. Early management is directed toward keeping a steady hemodynamic profile and ensuring tissue oxygenation through medication or advanced support.3 However, specific therapies are required to ensure a complete resolution, yet conventional surgical aortic valve replacement (SAVR) is often associated with a very high risk of mortality.2
Several trials have shown that transcatheter valve implantation (TAVI) is a safe alternative to SAVR in low-to-high risk patients in stable situations and it is currently considered the preferred alternative in those of high prohibitive surgical risk.4-7 Nevertheless, the risk scores for the main studies that settled the evidence for TAVI procedures were estimated after excluding patients with CS. As a consequence, the main outcomes in this challenging scenario have not been randomly compared to surgery. Actually, such a comparison is unlikely to be performed due to the highly variable baseline profile and differential availability of resources such as mechanic circulatory assist devices. In addition, the different outcomes in emergency TAVIs and planned interventions have been scarcely researched; still, they are key to improve results in what stands as the worst possible clinical scenario. We aimed to assess the current outcomes of emergency/urgent TAVI and the main factors conditioning its prognosis through a systematic review and meta-analysis.
METHODS
Literature search strategy
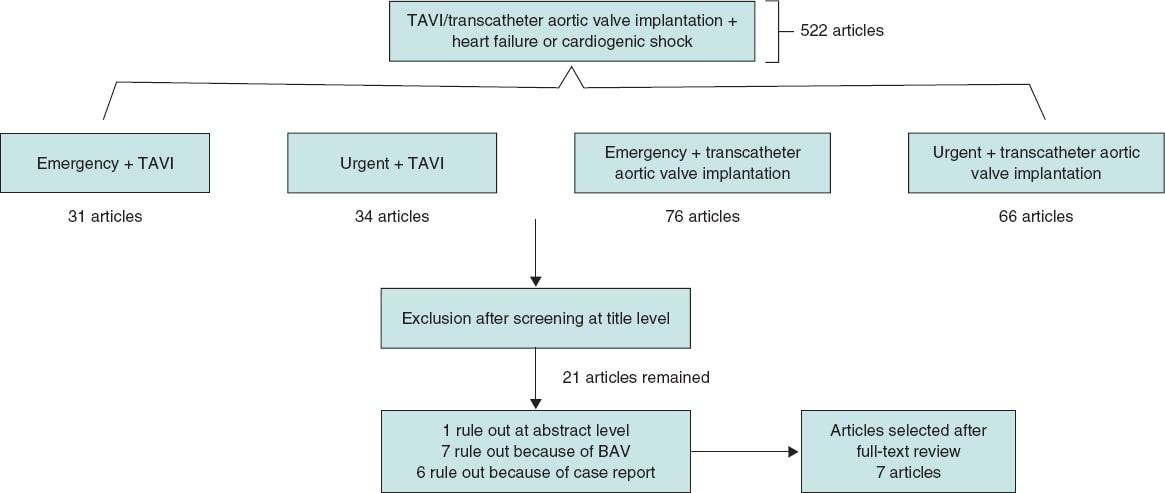
A systematic review of all published articles in PubMed and Google Scholar databases between January 2014 and January 2020 regarding emergency/urgent versus elective TAVI in severe AS was independently performed by 2 of the authors (A. Aparisi and M. Carrasco-Moraleja). Searched terms were “emergency” and/or “urgent”, “elective”, AND “transcatheter valve replacement” or “TAVR” (transcatheter aortic valve replacement) or “heart failure” and/or “cardiogenic shock”. Definition of emergency/urgent procedures was variable, but the consensus reached for this article was to include patients who required an unscheduled TAVI procedure to treat their refractory heart failure or CS to correct this condition within the next 72 hours after admission. A total of 7 studies8-14 were chosen, and the inclusion criteria established by our group were: a) the study population included patients with aortic stenosis who underwent TAVI; b) only cohort studies that compared emergency or urgent to elective TAVI were included; c) only full English peer-reviewed papers with enough data of outcomes were chosen. The selected exclusion criteria were: a) abstracts; b) case reports; c) editorials; d) experts’ opinions; and e) repetitive studies. Discrepancies between reviewers were resolved through discussion, and consensus was reached. Flowchart is shown on figure 1 and the main features of the studies included are shown on table 1 of the supplementary data.
Figure 1. Flowchart showing search results and selection of the studies included in the meta-analysis.
Table 1. Baseline clinical and echocardiographic characteristics of patients undergoing elective or emergency TAVIs
| Variable | No. of patients | Overall TAVI population N = 84 427 | Elective TAVI N = 70 186 (83.1%) | Emergency/urgent TAVI N = 14 241 (16.9%) | P |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Sex (male) (%) | 84 427 | 43 735/84 427 (51.8%) | 36 576/70 186 (52.11%) | 7 159/14 241 (50.27%) | < .001 |
| Age (years) | 44 385 | 81.12 ± 8.47 | 81.16 ± 8.27 | 80.96 ± 9.08 | .041 |
| EuroSCORE (%) | 1387 | 31.24 ± 18.15 | 29.42 ± 17.99 | 68.88 ± 20.97 | < .001 |
| STS score (%) | 985 | 14.76 ± 13.34 | 13.66 ± 11.61 | 29.39 ± 27.39 | < .001 |
| Anemia (%) | 42 524 | 11 415/42 524 (26.84%) | 8004/32 382 (24.71%) | 3411/10 142 (33.63%) | < .001 |
| Atrial fibrillation (%) | 41 185 | 17 373/41 885 (41.47%) | 15 304/37 780 (40.51%) | 2069/4105 (50.40%) | < .001 |
| CAD (%) | 41 329 | 25 723/41 329 (62.24%) | 23 178/37 308 (62.13%) | 2545/4021 (63.29%) | .147 |
| CKD (%) | 83 308 | 17 948 /83 308 (21.54%) | 13 368/69 187 (19.32%) | 4580/14 121 (32.43%) | < .001 |
| COPD (%) | 84 398 | 25 081/84 398 (29.72%) | 20 315/70 157 (28.96%) | 4766/14 241 (33.47%) | < .001 |
| Diabetes (%) | 84 040 | 29 670/84 040 (35.30%) | 24 571/69 820 (35.19%) | 5099/14 220 (35.86%) | .130 |
| Hypertension (%) | 83 308 | 70 608/83 308 (84.75%) | 59 117/69 187 (85.44%) | 11 491/14 121 (81.38%) | < .001 |
| NYHA III-IV (%) | 41 143 | 33 056/41 143 (80.34%) | 29 297/37 065 (79.04%) | 3759/4078 (92.17%) | < .001 |
| PAD (%) | 84 069 | 25 236/84 069 (30.02%) | 20 933/69 849 (29.96%) | 4303/14 220 (30.26%) | .490 |
| Porcelain aorta (%) | 40 669 | 2158/40 669 (5.3%) | 1914/36 669 (5.22%) | 244/4000 (6.1%) | .018 |
| Previous AVR (%) | 40 658 | 1599/40 658 (3.93%) | 1292/36 664 (3.53%) | 307/3994 (7.69%) | < .001 |
| Previous CABG (%) | 83 656 | 20 924/83 656 (25.01%) | 18 000/69 442 (25.92%) | 2924/14 214 (20.57%) | < .001 |
| Previous MI (%) | 83 040 | 15 173/83 040 (18.27%) | 12 597/68 868 (18.29%) | 2576/14 172 (18.18%) | .747 |
| Previous PCI (%) | 83 029 | 22 118/83 029 (26.64%) | 18 979/68 863 (27.56%) | 3139/14 166 (22.16%) | < .001 |
| Previous PM/ICD | 40 774 | 8304/40 774 (20.36%) | 7401/36 723 (20.15%) | 903/4051 (22.29%) | .001 |
| Previous stroke/TIA (%) | 42 244 | 8815/42 244 (20.87%) | 7884/38 118 (20.68%) | 931/4126 (22.57%) | .005 |
| Ecocardiographic characteristics | |||||
| Aortic valve area (cm²) | 2 230 | 0.7 ± 0.23 | 0.7 ± 0.23 | 0.66 ± 0.21 | .308 |
| LVEDD (mm) | 616 | 48.98 ± 7.34 | 48.53 ± 7.20 | 55.05 ± 9.03 | < .001 |
| LVEF (%) | 1861 | 51.51 ± 13.24 | 52.23 ± 12.71 | 29.58 ± 14.89 | < .001 |
| Mean gradient (mmHg) | 1398 | 43.71 ± 16.42 | 43.91 ± 16.31 | 40.26 ± 18.29 | .061 |
| AR III-IV (%) | 41 032 | 8156/41 032 (19.88%) | 7159/37 033 (19.33%) | 997/3999 (24.93%) | < .001 |
| PHT (%) | 43 251 | 2003/43 251 (4.63%) | 1536/33 088 (4.64%) | 467/10 163 (4.6%) | .843 |
|
AR, aortic regurgitation; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA; New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PHT; pulmonary hypertension; PM, pacemaker; STS, Society of Thoracic Surgeons score; TIA, transient ischemic attack; TAVI, transcatheter aortic valve implantation. |
|||||
Primary endpoints
Primary endpoints were short-term mortality and procedural success. Secondary outcomes were perioperative complications. Complications were mostly reported by using the definitions established by the Valve Academic Research Consortium-2.15
Statistical analysis
Qualitative variables were expressed as absolute frequency and percentage. Continuous variables were expressed as mean ± standard deviation unless specified otherwise. To compare the demographic variables and the risk factors between thew groups, the chi-square test or Fisher’s exact test were used for the categorical variables. The Student t test was used for the continuous variables, when applicable.
As a measure of the combined effect, the studies included the odds ratio (OR), a 95% confidence interval, and statistical significance. The homogeneity among the studies was compared using the QH statistic. With regard to the low sensitivity of this test, P values < .10 were considered significant. To somehow overcome this limitation, the I2 statistic was estimated as well, which measures the percentage of the overall variation of the studies explained by the heterogeneity and its 95%CI. A random effects model was used for cases in which the I2 statistic was > 50% and a fixed effects model was used for the opposite cases. The potential publication bias was assessed using a funnel plot, Egger’s test, and Begg and Mazumdar rank correlation test. In the presence of publication bias, the trim-and-fill method was used to reassess the pooled OR. Sensitivity analyses sequentially eliminating dissimilar studies were also conducted.
All P values were 2-tailed. Statistical analyses were conducted using the R software, version 3.6.1 (R Project for Statistical Computing) and Review Manager 5.3.
RESULTS
Patient distribution and baseline characteristics
Seven studies were selected including a total of 84 427 patients who underwent TAVIs, with 70 186 elective procedures (83.1%) and 14 241 emergency ones (16.9%). The main baseline characteristics according to the elective or emergency character of the intervention are shown on table 1 and sensitivity and asymmetry analyses are shown on table 2 of the supplementary data and figure 1 of the supplementary data. Asymmetry was detected for acute kidney injury and, therefore, the trim-and-fill method had to be used to reassess the odds ratio. The percentage of men who underwent elective procedures (52.1%) was higher compared to emergency interventions (50.27%, P < .001). Overall, patients treated urgently showed more comorbidities as summarized by the logistic EuroSCORE (65.9% ± 21% vs 29.4% ± 18%, P < .001) and the Society of Thoracic Surgeons Risk Score (STS) (29.4 ± 27.4 vs 13.7 ± 11.6, P < .001). However, the classical cardiovascular risk factors did not differ among groups (hypertension and diabetes mellitus) and the rates of myocardial infarction and percutaneous coronary intervention were similar. On the contrary, those treated urgently more often had undergone a previous aortic valve replacement. Regarding the main echocardiographic characteristics, emergency procedures were performed in patients with left ventricular (LV) function deterioration (39.5% ± 17.8% vs 52.5% ± 12.8%; P < .001), larger LV end-diastolic diameters (55 ± 9 vs 48 ± 7; P < .001), but similar aortic valve areas (0.66 ± 0.21 vs 0.70 ± 0.23; P = .308), and transaortic mean gradients (40.3 ± 18.3 vs 43.9 ± 16.3; P = .061).
Table 2. Procedural characteristics of patients undergoing elective or emergency/urgent TAVIs
| Variable | No. of patients | Overall TAVI population | Elective TAVI | Emergency/urgent TAVI | P |
|---|---|---|---|---|---|
| Success rate (%) | 41 140 | 38 765/41 440 (93.54%) | 35 038/37 413 (93.65%) | 3727/4027 (92.55%) | .007 |
| Device migration (%) | 40 042 | 105/40 042 (0.26%) | 90/36 090 (0.25%) | 15/3952 (0.38%) | .129 |
| General anesthesia (%) | 40 669 | 34 419/40 669 (84.6%) | 31 004/36 669 (84.55%) | 3415/4000 (85.37%) | .170 |
| Transapical (%) | 83 953 | 14 742/83 953 (17.56%) | 12 194/69 790 (17.47%) | 2548/14 163 (18%) | .139 |
| Transfemoral (%) | 83 811 | 66 526/83 811 (79.38%) | 55 196/69 612 (79.29%) | 11 330/14 199 (79.79%) | .177 |
| Transsubclavian (%) | 40 813 | 643/40 813 (1.57%) | 573/36 834 (1.55%) | 70/3979 (1.76%) | .327 |
| Mechanical circulatory support (%) | 83 326 | 1858/83 326 (2.29%) | 1355/69 211 (1.96%) | 503/14 115 (3.56%) | < .001 |
|
TAVI, transcatheter aortic valve implantation. |
|||||
Perioperative characteristics
Procedural results from the studies included are shown on table 2. Transfemoral access (79.3% vs 76.8%; P = .177) and use of general anesthesia (84.5% vs 85.4%; P = .17) were the preferred approaches in both groups. Elective TAVIs showed a higher procedural success rate (93.6% vs 92.5%; P = .007) and a lower need for mechanical circulatory support (1.96% vs 3.56 %; P < .001). Other procedural outcomes were comparable between both cohorts.
Postoperative outcomes
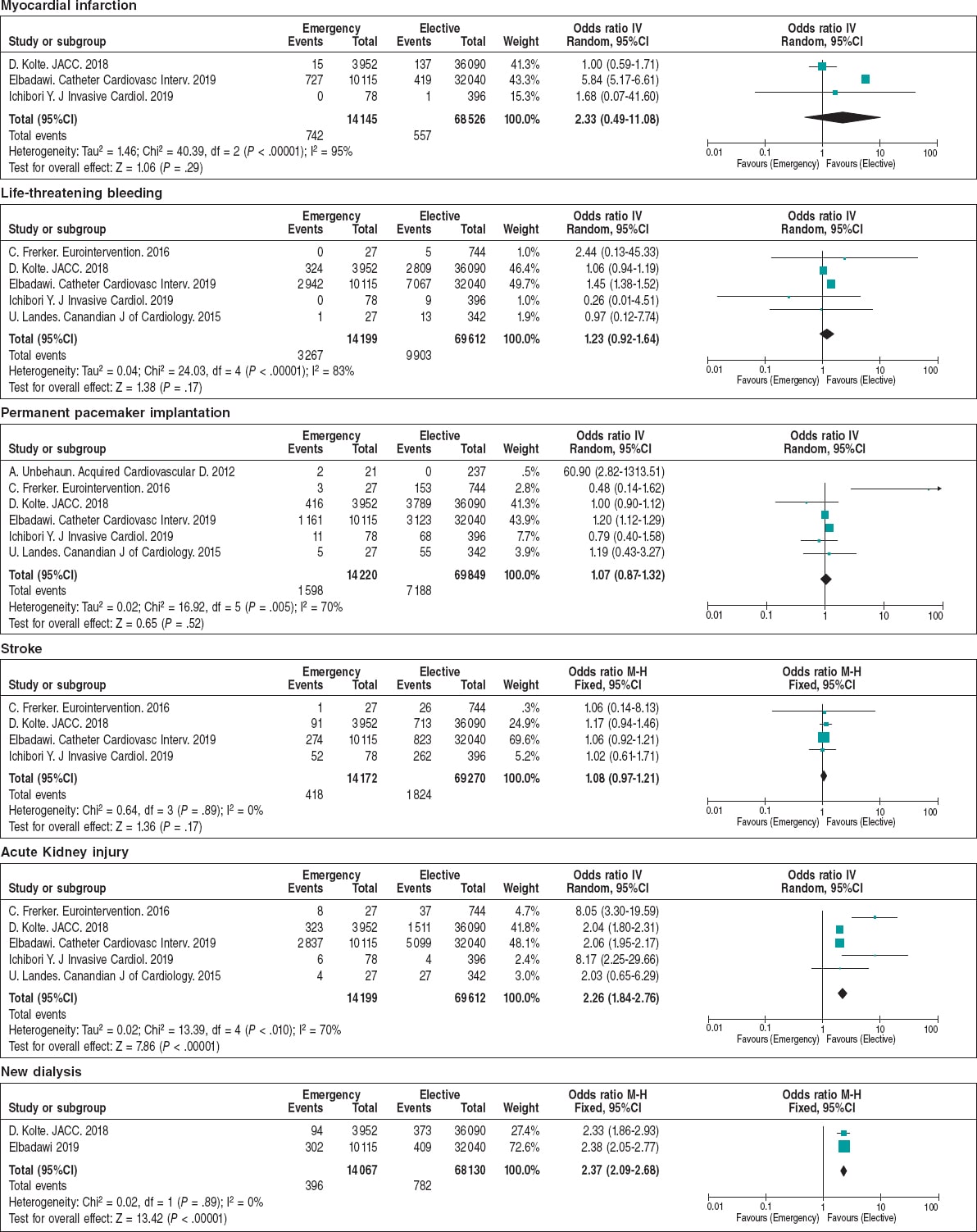
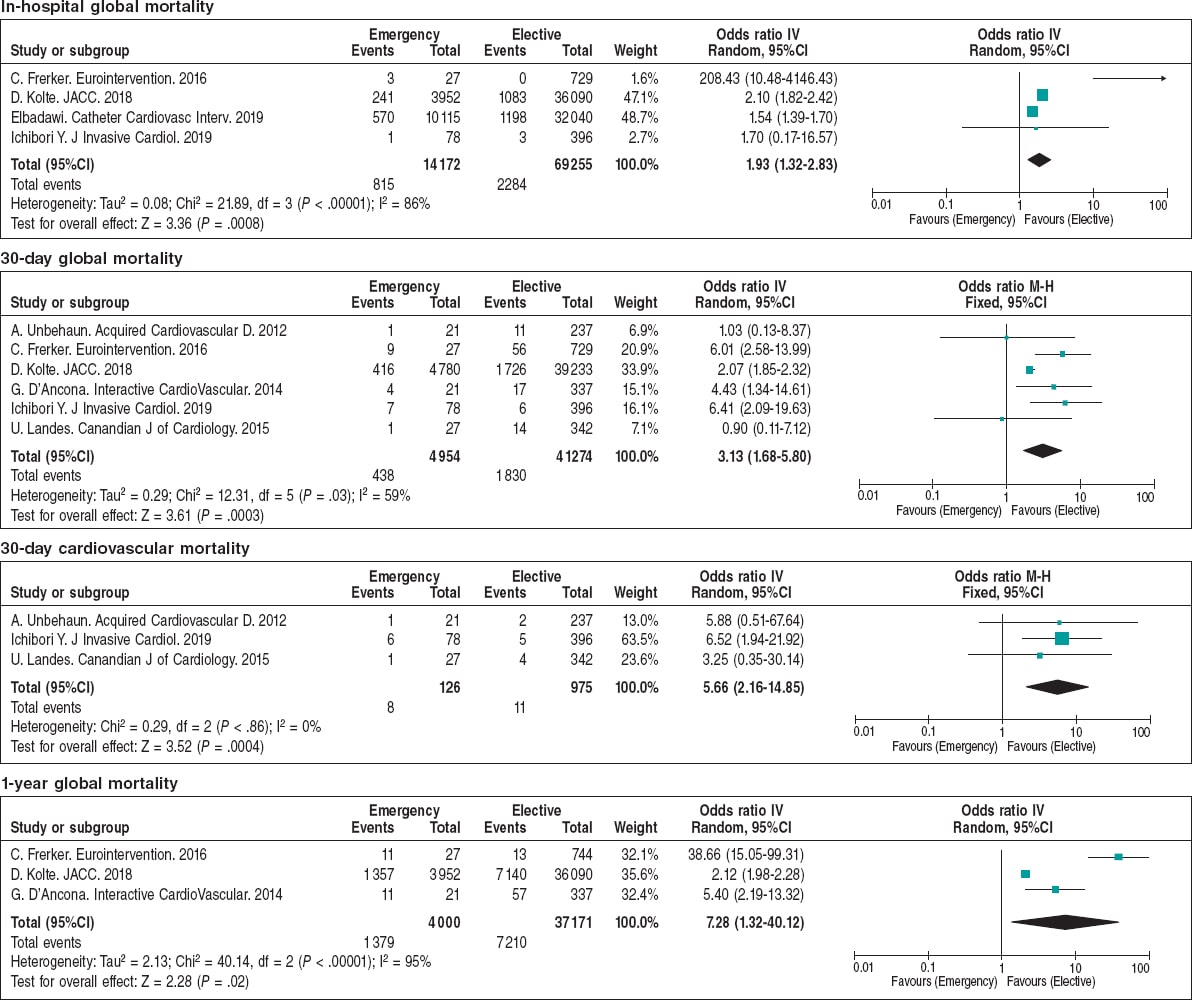
The main postoperative outcomes are shown on table 3 and figure 2. The ORs for perioperative myocardial infarction, life-threatening bleedings, need for permanent pacemaker implantation, and stroke were similar regardless of the planned or emergency setting. On the contrary, the elective cohort showed a smaller rate of acute kidney injury (9.6% vs 22.4%; OR = 2.26; 95%CI, 1.84-2.76; P < .001), and need for dialysis (1.1% vs 2.8%; OR = 2.37; 95%CI, 2.09-2.68; P < .001). Overall, this translated into shorter hospital stays for elective cases, lower in-hospital (3.3% vs 5.75%; OR = 1.32; 95%CI, 1.32-2.83; P < .001), 30-day (4.43% vs 8.84%; OR = 3.13; 95%CI, 1.68-5.80; P < .001), and 1-year mortality rates (19.7% vs 34.47%; OR = 2.87; 95%CI, 1.67-4.94; P = .0001) for elective TAVI (figure 3).
Table 3. Main postoperative outcomes of patients undergoing elective or emergency/urgent TAVIs
| Variable | No. of patients | Overall TAVI population | Elective TAVI | Emergency/urgent TAVI | P |
|---|---|---|---|---|---|
| Clinical outcomes | |||||
| Life-threatening bleeding (%) | 83 811 | 13 170/83 811 (15.71%) | 9903/69 612 (14.22%) | 3267/14 199 (23.01%) | < .001 |
| Major bleeding (%) | 43 400 | 14 725/43 400 (33.93%) | 11 065/33 180 (33.35%) | 3660/10 220 (35.81%) | < .001 |
| Major vascular complications (%) | 41 656 | 513/41 656 (1.23%) | 460/37 572 (1.22%) | 53/4084 (1.29%) | .686 |
| Myocardial infarction (%) | 82 671 | 1299/82 671 (1.57%) | 557/68 526 (0.81%) | 742/14 145 (5.24%) | < .001 |
| Acute kidney injury (%) | 83 811 | 9856/83 811 (11.75%) | 6678/69 612 (9.59%) | 3178/14 199 (22.38%) | < .001 |
| Need for dialysis (%) | 82 197 | 1178/82 197 (1.43%) | 782/68 130 (1.15%) | 396/14 067 (2.81%) | < .001 |
| PPMI (%) | 84 069 | 8786/84 069 (10.45%) | 7188/69 849 (10.29%) | 1598/14 220 (11.24%) | < .001 |
| Stroke (%) | 83 442 | 2242/83 442 (2.69%) | 1824/69 270 (2.63%) | 418/14 172 (2.94%) | .034 |
| In-hospital mortality rate | 83 427 | 3099/83 427 (3.71%) | 2284/69 255 (3.3%) | 815/14 172 (5.75%) | < .001 |
| 30-day mortality rate | 46 228 | 2268/46 228 (4.9%) | 1830/41 274 (4.43%) | 430/4954 (8.84%) | < .001 |
| 1-year mortality rate | 41 156 | 8706/41 156 (21 15%) | 7327/37 156 (19.72%) | 1379/4000 (34.75%) | < .001 |
| Echocardiographic outcomes | |||||
| Mean gradient (mmHg) | 369 | 7.75 ± 4.15 | 7.82 ± 4.22 | 6.9 ± 3.2 | .269 |
| AR III-IV (%) | 17 977 | 1465/17 977 (8.15%) | 1299/16 125 (8.05%) | 166/1852 (8.96%) | .176 |
|
AR, aortic regurgitation; PPMI, permanent pacemaker implantation; TAVI, transcatheter aortic valve implantation. |
|||||
Figure 2. Forest plot showing the main postoperative complications of patients included in the meta-analysis.* * Vertical line represents “no difference” point between the emergency and the elective TAVI groups. Horizontal lines represent the 95%CI. Squares represent the OR for each study (the size of each square shows the amount of information given by each study). Diamonds represent pooled OR from all studies.
Figure 3. Forest plot showing the in-hospital to 1-year mortality rates of patients included in the meta-analysis.* * Vertical line represents “no difference” point between the emergency and the elective TAVI groups. Horizontal lines represent the 95%CI. Squares represent OR for each study (the size of each square shows the amount of information given by each study). Diamonds represent pooled OR from all studies.
DISCUSSION
When patients with AS present with severe acute heart failure (HF) or CS the 5-year all-cause mortality is above 60% despite the implementation of therapies to treat valvular heart disease, which poorly compares to this rate in patients free of HF (~20%) or with chronic HF symptoms (~30%) in this setting (16). Determining the factors that condition such a high mortality rate is the key to improve the management of this growing group of patients. The main findings of this study are: a) patients who required emergency TAVIs had a higher baseline risk compared to planned procedures, not only due to the emergency setting, but also to a high burden of comorbidities and deterioration of LV function; b) although procedural success rate was significantly higher in planned cases, this difference was small (93.6% vs 92.5%; P = .007) suggestive that the higher short- and mid-term mortality rates seen in emergency cases were mainly due to postoperative complications, not to the intervention per se; c) need for mechanical circulatory support and dialysis was higher after emergency cases. The early identification of patients at risk who may require these therapies might be useful for a better indication of TAVI in emergency settings.
Baseline characteristics and predicted mortality
In our study, emergency/urgent TAVI patients had a more significant number of comorbid conditions compared to those who underwent elective procedures. We should mention that the Society of Thoracic Surgeons (STS) score has been widely used to assess mortality risk in SAVR patients.17 Nevertheless, the score developed by the Transcatheter Valve Therapy (TVT) group to evaluate in-hospital and 30-day mortality rates18 may be more accurate. According to that score, the prognosis is strongly influenced by the presence of chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and need for emergency TAVI. Of note, AS with concomitant CKD has been linked to higher all-cause and cardiovascular mortality rates compared to patients with AS and without this condition; indeed, the increase of all-cause mortality exponentially correlates with a decline in the glomerular filtration rate.19 In addition, the higher rate of anemia20 and increased bleeding risk in CKD patients is well-known, which conditions a higher need for red blood cell transfusion21 parallel to a deterioration of renal function and survival rate.
The LV function is a well-known prognostic factor of valvular heart disease and its deterioration conditions surgical or transcatheter aortic valve treatment even in asymptomatic patients.22 We should mention that the similar transaortic gradient, despite a reduced LV function in the emerging baseline cases, suggests a more severe valve disease, probable more calcified and degenerated native valves, as also suggested by the higher rate of aortic regurgitation of this cohort. Therefore, the multidisciplinary and multi-imaging approach might be particularly useful for procedural planning and outcome improvement.23
Procedural complications and mortality
Most procedural complications were similar in elective and emergency TAVIs. Although this may be partially explained by the growing operators’ experience worldwide and the lack of differences in the rate of transfemoral approach,24 the greater use of mechanical circulatory support devices may have been particularly relevant in emergency/urgent cohorts. Indeed, the more limited LV contractile reserve of this group of patients can lead to rapid deterioration in the presence of complications like periannular shunts, severe aortic regurgitation or coronary obstruction. Therefore, the presence of risk factors for these complications may suggest the need for circulatory support devices in certain cases before valve implantation as a potential strategy to avoid dreadful prognoses if they occur in the emergency setting.25-27 Prior experience with the Impella device and extracorporeal membrane oxygenation is shown on table 3 of the supplementary data; however, whether there are mortality differences between those with and without mechanical support requires further research. Since procedural success was similar to that of the standard setting, the clinical translation of this is that, even if these cases can be performed successfully in all centers by implanting TAVI, this profile of patients should only be treated in centers with mechanical circulatory support devices available (particularly ECMO), which would exclude low volume or non-surgical centers.
In the present meta-analysis, the cases treated with isolated balloon aortic valvuloplasty were not included. This strategy bears a class IIb-C level of evidence in the last iteration of the guidelines, but it is often used as a bridging therapy to definitive TAVI in hemodynamically unstable patients.28,29 A single-center retrospective study found that TAVI may be superior to a stand-alone balloon aortic valvuloplasty and medical therapy in patients with severe AS and CS, since the isolated balloon aortic valvuloplasty is not free of complications (~25%) and has higher mortality rates.30 Despite of this, large randomized controlled trials exploring this scenario with TAVI are lacking.
Postoperative complications associated with a higher mortality rate
In this systematic review and meta-analysis, we found that emergency/urgent TAVIs had a significantly higher rate of AKI, hemodialysis, and mortality. This is consistent with previous reports that found that patients with post-TAVI AKI were more likely to die. Besides, AKI is a predictor of sepsis, which is also an independent predictor of mortality. The main factors increasing the risk of AKI include CKD, peripheral artery disease, diabetes mellitus, and deterioration of LV function.31,32 A prophylactic strategy may vary from simple hydration with a normal saline solution to forced diuresis with early supportive measures;33 indeed, the use of prophylactic dialysis has been explored in TAVI patients with a high risk of AKI and may be particularly useful in the emergency setting.
Study limitations
There are several limitations related to this systematic review and meta-analysis. First, the studies included were observational since no multicenter randomized studies specifically addressing this topic could be found. Secondly, the definition of emergency/urgent procedures was variable in the studies although an inclusive definition was reached by the study team. Finally, the results may not be generalizable and should be interpreted with caution due to the high heterogenicity reported, which may relate to variability in the study samples and designs.
CONCLUSIONS
In conclusion, the association between emergency/urgent TAVIs and a higher short-to-mid-term mortality rate is mainly due to a high-risk baseline profile, advanced stage of the cardiac disease, and higher rate of acute renal failure. The early identification and referral of patients at high risk for circulatory collapse or AKI need to be properly identified to reduce the TAVI related mortality rate. Further research is needed to elucidate the role of TAVI in emergency or urgent scenarios.
FUNDING
No funding to declare.
CONFLICTS OF INTEREST
I. J. Amat-Santos is a proctor for Boston Scientific.
WHAT IS KNOWN ABOUT THE TOPIC?
- TAVI is performed mainly in hemodynamically stable patients, otherwise aortic balloon valvuloplasty is empirically preferred as a bridging therapy to TAVI. However, few studies have addressed TAVI in life-threatening scenarios and multicenter randomized controlled trials are still lacking.
WHAT DOES THIS STUDY ADD?
- In this large pooled meta-analysis (n = 84 427) emergency TAVI was not rare but associated with higher in-hospital, 30-day, and 1-year mortality rates compared to elective procedures. The need for dialysis or mechanical circulatory support conditioned the mortality rate following emergency TAVIs. The early identification of patients at risk of circulatory collapse or acute kidney injury may help to determine if TAVI is futile in this setting.
REFERENCES
1. Thaden JJ., Nkomo VT., Enriquez-Sarano M. The Global Burden of Aortic Stenosis. Prog Cardiovasc Dis. 2014;56:565-571.
2. Akodad M., Schurtz G., Adda J., Leclercq F., Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis. 2019;112:773-780.
3. Castrodeza J, Serrador Frutos AM, Amat-Santos IJ, et al. Prophylactic percutaneous circulatory support in high risk transcatheter aortic valve implantation. Cardiol J. 2019;26:424-426.
4. Smith CR., Leon MB., Mack MJ., et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. New Engl J Med. 2011;364:2187-2198.
5. Leon MB., Smith CR., Mack MJ., et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl J Medicine. 2016;374:1609-1620.
6. Reardon MJ., Mieghem NMV., Popma JJ., et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl J Medicine 2017;376:1321-1331.
7. Waksman R., Rogers T., Torguson R., et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients with Symptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2018;72:2095-2105.
8. D'Ancona G., Pasic M., Buz S., et al. Transapical transcatheter aortic valve replacement in patients with cardiogenic shock. Interact Cardiov Th. 2012;14:426-430.
9. Unbehaun A., Pasic M., Buz S., et al. Transapical aortic valve implantation in patients with severely depressed left ventricular function. J Thorac Cardiovasc Surg. 2012;143:1356-1363.
10. Landes U., Orvin K., Codner P., et al. Urgent Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis and Acute Heart Failure:Procedural and 30-Day Outcomes. Can J Cardiol. 2016;32:726-31.
11. Frerker C., Schewel J., Schlüter M., et al. Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis. Eurointervention. 2016;11:1530-1536.
12. Kolte D., Khera S., Vemulapalli S., et al. Outcomes Following Urgent/Emergency Transcatheter Aortic Valve Replacement:Insights from the STS/ACC TVT Registry. Jacc Cardiovasc Interventions 2018;11:1175-1185.
13. Elbadawi A., Elgendy IY., Mentias A., et al. Outcomes of urgent versus nonurgent transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2020 Jul;96:189-195.
14. Ichibori Y., Li J., Patel T., et al. Short-Term and Long-Term Outcomes of Patients Undergoing Urgent Transcatheter Aortic Valve Replacement Under a Minimalist Strategy. J Invasive Cardiol. 2019:E30-E36.
15. Kappetein AP., Head SJ., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418.
16. Nagao K., Taniguchi T., Morimoto T., et al. Acute Heart Failure in Patients With Severe Aortic Stenosis- Insights From the CURRENT AS Registry. Circ J. 2018;82:874-885.
17. Yakubov SJ., Adams DH., Watson DR., et al. 2-Year Outcomes After Iliofemoral Self-Expanding Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery. J Am Coll Cardiol. 2015;66:1327-1334.
18. Arnold SV., O'Brien SM., Vemulapalli S., et al. Inclusion of Functional Status Measures in the Risk Adjustment of 30-Day Mortality After Transcatheter Aortic Valve Replacement A Report From the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
19. Patel KK., Shah SY., Arrigain S., et al. Characteristics and Outcomes of Patients With Aortic Stenosis and Chronic Kidney Disease. J Am Heart Assoc. 2019 Feb 5;8:e009980.
20. DeLarochellière H., Urena M., Amat-Santos IJ., et al. Effect on Outcomes and Exercise Performance of Anemia in Patients With Aortic Stenosis Who Underwent Transcatheter Aortic Valve Replacement. Am J Cardiol. 2015;115:472-479.
21. Kanjanahattakij N., Rattanawong P., Krishnamoorthy P., et al. Anaemia and mortality in patients with transcatheter aortic valve replacement:a systematic review and meta-analysis. Acta Cardiol. 2018;74:1-7.
22. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
23. Fassa A-A., Himbert D., Vahanian A. Mechanisms and management of TAVR-related complications. Nat Rev Cardiol. 2013;10:685-695.
24. TerréJA., George I., Smith CR. Pros and cons of transcatheter aortic valve implantation (TAVI). Ann Cardiothorac Surg. 2017;6:444-452.
25. Chieffo A., Ancona MB., Burzotta F., et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly:the IMP-IT registry. EuroIntervention. 2020 Feb 7;15:e1343-e1350.
26. Singh V., Damluji AA., Mendirichaga R., et al. Elective or Emergency Use of Mechanical Circulatory Support Devices During Transcatheter Aortic Valve Replacement. J Interv Cardiol. 2016;29:513-522.
27. Shreenivas SS., Lilly SM., Szeto WY., et al. Cardiopulmonary bypass and intra?aortic balloon pump use is associated with higher short and long term mortality after transcatheter aortic valve replacement:A PARTNER trial substudy. Catheter Cardiovasc Interv. 2015:316-322.
28. Singh V, Damluji AA, Mendirichaga R, et al. Elective or Emergency Use of Mechanical Circulatory Support Devices During Transcatheter Aortic Valve Replacement. J Interv Cardiol. 2016;29:513-522.
29. Baumgartner H., Falk V., Bax JJ., et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
30. Saia F., Marrozzini C., Ciuca C., et al. Emerging indications, in-hospital and long-term outcome of balloon aortic valvuloplasty in the transcatheter aortic valve implantation era. Eurointervention. 2013;8:1388-1397.
31. Ram P., Mezue K., Pressman G., Rangaswami J. Acute kidney injury post-transcatheter aortic valve replacement. Clin Cardiol. 2017;40:1357-1362.
32. Wang J., Yu W., Zhou Y., et al. Independent Risk Factors Contributing to Acute Kidney Injury According to Updated Valve Academic Research Consortium-2 Criteria After Transcatheter Aortic Valve Implantation:A Meta-analysis and Meta-regression of 13 Studies. J Cardiothor Vasc An. 2017;31:816-826.
33. Putzu A, Berto MB, Belletti A, et al. Prevention of contrast-induced acute kidney injury by furosemide with matched hydration in patients undergoing interventional procedures:a systematic review and meta-analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:355-363.