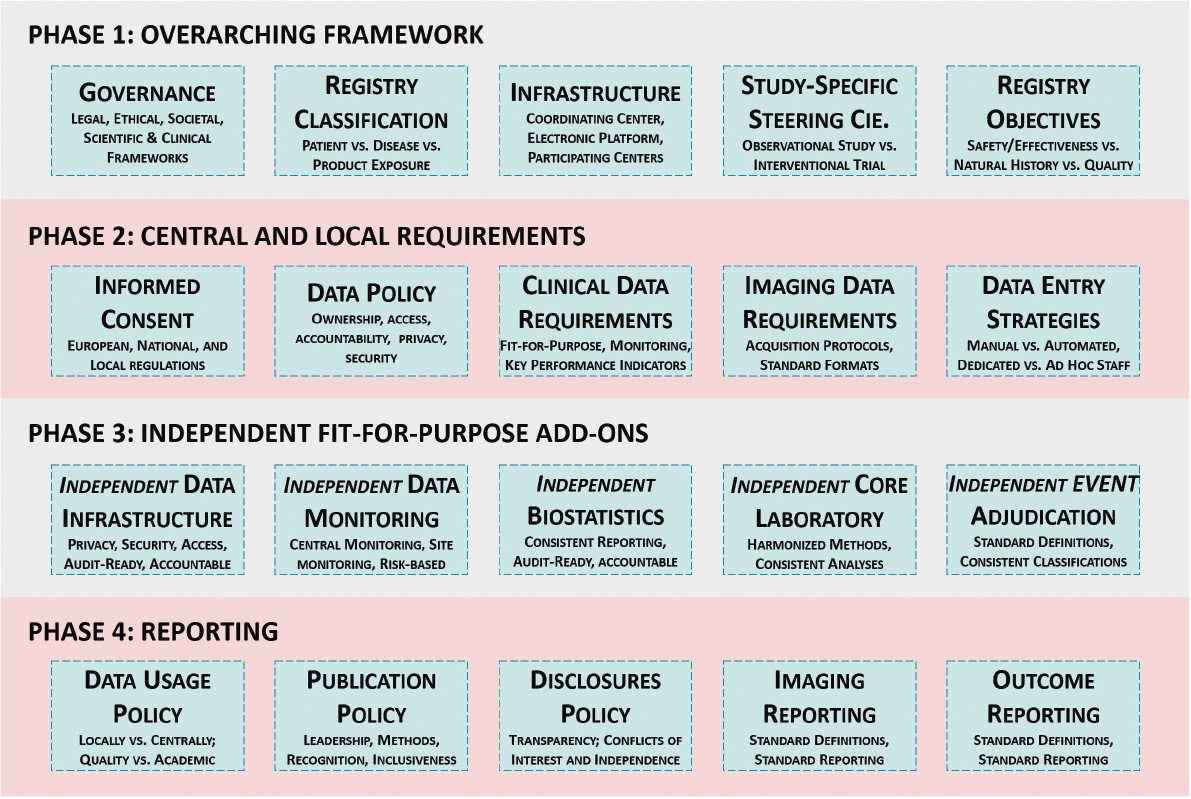
To the Editor,
The excimer laser coronary angioplasty technique was first developed in the early 1980s. However, its safety and effectiveness have only improved in recent years, allowing its integration in an increasing number of cath labs. The technique has a triple mechanism of action: photochemical, photothermal, and photokinetic. When a mixture of hydrogen chloride and a noble gas such as xenon is exposed to a high-voltage electric field, an extremely unstable bond of chlorine and xenon atoms occurs. The separation of these atoms emits a photon which, when amplified, creates a high-energy laser.1
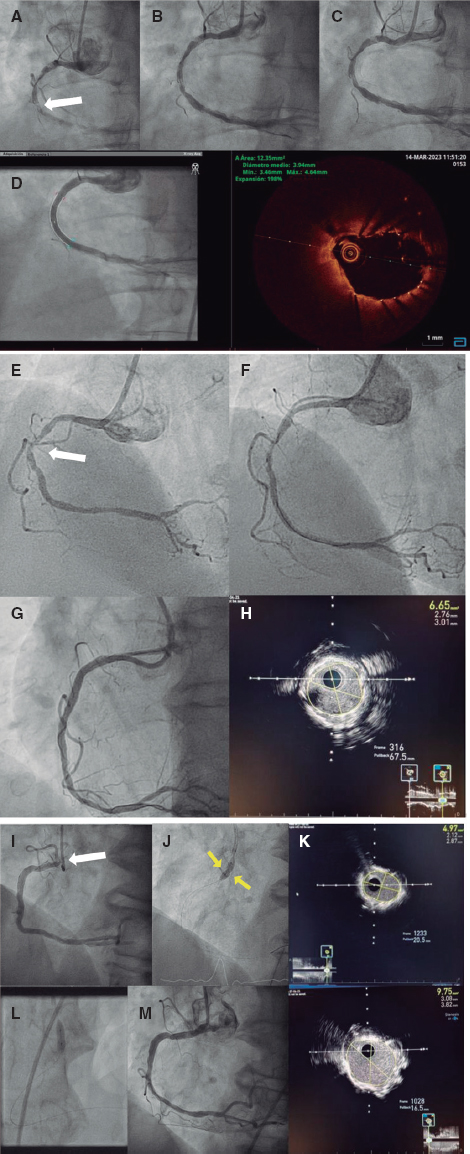
We report the cases of 3 patients, 2 with uncrossable lesions and 1 with rebel stent underexpansion, treated in our unit. Laser atherectomy, along with simultaneous intracoronary infusion of a physiological saline solution (using a 0.9 mm ELCA catheter, Spectranetics, United States), was unsuccessful at the first attempt. This was because, after its application, no other angioplasty devices (predilatation balloons, low-profile microcatheters, or rotational atherectomy guidewires) could be advanced in any of the uncrossable lesions. In the patient with underexpansion, effective dilatation with high-pressure noncompliant balloons after lithotripsy was also unfeasible. These are the only cases treated in our unit with failed laser therapy and a new angioplasty attempt in the culprit lesion. All patients signed a prior written informed consent form approved by the research ethics committee of our center and accepted to participate in the registry. The 3 patients were scheduled for lesion re-evaluation 7 to 8 days after the index procedure. Angiographic images showed a slight improvement in case #1 compared with the index procedure, unlike cases #2 and #3 whose angiographic images resembled those of the previous procedure. When we attempted to complete the coronary interventions, the previously uncrossable lesions proved perfectly accessible to treatment with rotational atherectomy, allowing the passage of the rotablator wire and use of appropriate plaque modification balloons. Both cases ended with successful drug-eluting stent implantation. In the case of stent underexpansion, effective postdilatation was performed with a noncompliant balloon (minimum luminal area gain from 4.7 mm2 to 9.7 mm2). The patients’ good outcomes were confirmed using intracoronary imaging modalities. All the patients were eventually discharged the day after the procedure. The patients’ clinical, anatomical, and baseline and follow-up procedural characteristics are shown in table 1. Figure 1 shows the angiograms and intravascular images of the 3 patients.
Table 1. Clinical, anatomical, and procedural characteristics at baseline and at the follow-up
| Patient | Case #1 | Case #2 | Case #3 |
|---|---|---|---|
| Sex | Man | Woman | Man |
| Age (years) | 54 | 72 | 72 |
| Hypertension | Yes | Yes | Yes |
| Diabetes | Yes | Yes | No |
| Dyslipidemia | Yes | Yes | Yes |
| Smoking | Yes | No | Yes |
| Kidney disease | No (Cr, 0.82) | No (Cr, 0.41) | No (Cr, 1.26) |
| Indication | STEACS | Silent ischemia | Silent ischemia |
| Index procedure | |||
| Vascular access | Radial 6-Fr | Radial 6-Fr | Femoral 6-Fr |
| Lesion location | Mid-right coronary artery | Mid-right coronary artery | Ostial right coronary artery |
| Initial TIMI grade flow | 1 | 3 | 3 |
| No. of laser pulses | 38 812 | 11 780 | 28 654 |
| Overall time of laser therapy (s) | 501 | 169 | 365 |
| Peak laser frequency and energy | 80 Hz 80 mJ/mm² | 80 Hz, 80 mJ/mm² | 80 Hz, 80 mJ/mm² |
| Other plaque modification techniques | – | – | Noncompliant balloon Intracoronary lithotripsy (3.5 mm and 4 mm balloons) |
| Final TIMI grade flow | 3 | 3 | 3 |
| Follow-up procedure | |||
| Days between procedures | 7 | 8 | 7 |
| Vascular access | Femoral 7-Fr | Femoral 6-Fr | Femoral 6-Fr |
| Initial TIMI grade flow | 3 | 3 | 3 |
| Other plaque modification techniques | Noncompliant balloon Cutting balloon Intracoronary lithotripsy (3.5 mm balloon) Rotational atherectomy (1.5 mm and 1.75 mm burrs) | Noncompliant balloon Rotational atherectomy (1.25 mm burr) | Noncompliant balloon |
| Intracoronary imaging modalities | OCT | IVUS | IVUS |
| After dilatation after stenting | Yes | Yes | Yes |
| Final TIMI grade flow | 3 | 3 | 3 |
|
Cr, creatinine; IVUS, intracoronary ultrasound; OCT, optical coherence tomography; STEACS, ST-segment elevation acute coronary syndrome; TIMI, thrombolysis in myocardial infarction. |
|||
Figure 1. Case #1. A: extreme calcification of the right coronary artery with an uncrossable lesion in its middle third (white arrow). Initial angiograpm. B: angiogram after laser therapy and failed angioplasty during the index procedure. C: Initial angiogram of the second procedure. D: final angiographic and OCT results after the second procedure. Case #2. E: severely calcified uncrossable lesion in the middle of the right coronary artery (arrow). Initial angiogram. F: angiogram after laser therapy and failed index procedure. G: final angiographic result after the second procedure. H: final result according to intracoronary ultrasound. Case #3. I: significant stent underexpansion at the level of the right coronary artery ostium. Initial ostial lesion (arrow). J: after laser therapy in the index procedure, the noncompliant balloon failed to fully expand, showing a waist in its middle third (arrows). K: final result of the index procedure according to the intracoronary ultrasound, with a minimal luminal area of 4.97 mm² (50% expansion). L: complete expansion of the noncompliant balloon after the second procedure. M: final angiographic result after the second procedure according to angiography and intracoronary ultrasound, with an almost 2-fold increase of minimal luminal area compared with baseline and an expansion rate of nearly 100%.
The hypothesis generated after these findings is that excimer laser therapy could induce a subacute molecular change (presumably due to the photochemical mechanism), which would cause internal changes to the plaque that would take a few days to fully establish and facilitate the subsequent treatment of the lesions with a failed first attempt at laser therapy.
Low-intensity laser therapy has demonstrated stimulating effects on various types of cells involved in wound healing and tissue regeneration through the photochemical mechanism. Although the onset of the process is immediate after tissue photon absorption, the cascade of biological responses triggered extends over time, with angiogenesis being an essential part of this process. Phototherapy has been extensively investigated to determine its effect on vessel formation. This therapy has demonstrated its ability to stimulate endothelial cells, fibroblasts, smooth muscle cells, and lymphocytes in vitro, in vivo, and in clinical settings. By triggering the activation of cytochrome c oxidase, leading to the production of nitric oxide, reactive oxygen species, and adenosine triphosphate in mitochondria, these molecules seem to act as secondary messengers that initiate the ERK/Sp1 and PI3K signaling pathways, which in turn leads to proliferation, migration, and proangiogenic factor synthesis.2,3
In the uncrossable lesions, changes were made to access in the second procedure (from radial to femoral access, and from a 6-Fr to a 7-Fr guide catheter in 1 procedure), which should have facilitated the advancement of materials (which would be the main limitation to the hypothesis raised in uncrossable lesions). However, in all 3 cases, the guide catheter support in the initial procedures was correct (aided in one of them with a Guideliner extension, Teleflex, United States). This, along with the limited relevance of support in the case of stent underexpansion, makes it unlikely that all the above can account for the dramatic change seen in the final outcomes.
In a recent registry of 126 uncrossable lesions treated with excimer laser,4 primary success was achieved in 81.8% of cases and the success rate rose to nearly 90.5% when other techniques were used as bailout (mainly rotational atherectomy).4 Similar success rates were reported in older registries,5 where 90% seemed to be the ceiling for the effectiveness of the technique, both alone and when used as part of a hybrid strategy. Other techniques have been described, such as applying laser therapy with simultaneous contrast injection instead of a physiological saline solution, to significantly increase the released energy. This can be useful in extreme cases like those reported above, although the risk of complications increases significantly.
Although the data referred to here may be considered of interest, they are, however, purely observational and are not based on previous experimental work, with their main value being their possible utility as hypothesis generators.
In patients with very unfavorable lesions and an apparently failed first attempt at laser atherectomy, it would be appropriate to consider a more conservative strategy (if allowed by the patient’s clinical status), consisting of a second attempt at laser therapy, or another alternative plaque modification technique a few days later to achieve final procedural success. This could result in a lower risk of complications compared with some highly aggressive interventional procedures.
In addition, experimental and clinical trials should be conducted, with larger registries and even randomized clinical trials, in patients treated with failed attempts at the laser technique to confirm or refute the hypothesis raised.
FUNDING
None declared.
ETHICAL CONSIDERATIONS
All patients signed the written informed consent forms approved by the research ethics committee of our center and accepted to participate in the registry. Possible biases related to sex and gender have been considered while drafting this manuscript.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence was not used in the development of this work.
AUTHORS’ CONTRIBUTIONS
J. Valencia was the lead author of the manuscript. All the authors from Hospital General Universitario Dr. Balmis medical team contributed to the preparation of the case reports. A. Jurado-Román was also involved in critical review of the manuscript.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Egred M, Brilakis ES. Excimer laser coronary angioplasty (ELCA):fundamentals, mechanism of action, and clinical applications. J Invasive Cardiol. 2020;32:E27-35.
2. Chaudary S, Rieger S, Redl H, Dungel P. Stimulation by Light. En:Holnthoner W, Banfi A, Kirkpatrick J, Redl H, ed. Vascularization for Tissue Engineering and Regenerative Medicine. Reference Series in Biomedical Engineering. Cham:Springer;2017. 273-303.
3. Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann N Y Acad Sci. 2005;1056:486-493.
4. Ojeda S, Azzalini L, Suárez de Lezo J, et al. Excimer laser coronary atherectomy for uncrossable coronary lesions. A multicenter registry. Catheter Cardiovasc Interv. 2021;98:1241-1249.
5. Fernandez JP, Hobson AR, Mckenzie D, et al. Beyond the balloon:excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expandable lesions. EuroIntervention. 2013;9:243-250.