ABSTRACT
Introduction and objectives: A better positioning of left atrial appendage closure (LAAC) requires assessment of its clinical benefits to reduce thromboembolic and bleeding events in a real-word population.
Methods: Single-center retrospective study of our consecutive LAAC activity for 9 years. Both the device success and procedural success were registered as well as the reduction of the expected rates of thromboembolic and major bleeding events.
Results: A total of 260 LAAC procedures were performed in a population with nonvalvular atrial fibrillation with CHA2DS2-VASc and HAS-BLED scores of 4.3 ± 1.6 and 3.7 ± 1.2, respectively. Procedural success was 98.8%, and the rate of serious adverse events within the first 7 days was 2.3%. At a median follow-up of 2.5 ± 1.9 years and an estimated population of 637.9 patients-year, the thromboembolic event rate was 1.4 per 100 patients-year (75.5% risk reduction) and the rate of major bleeding was 3.0 per 100 patients-year (58.5% risk reduction), which was significantly lower than anticipated. The thromboembolic and major bleeding events per 100 patients-year showed a lower tendency for patients with very long follow-up (over 4 years) compared to the remaining of the population (0.7 vs 2.0 with P = .17, and 1.7 vs 4.0 with P = .09, respectively).
Conclusions: In our population, the LAAC showed high procedural success and a low rate of periprocedural adverse events. LAAC induced a significant reduction in the rate of predicted thromboembolic and hemorrhagic events, and this reduction was maintained even at very long follow-up.
Keywords: Percutaneous closure. Arterial embolism. Cerebral ischemia.
RESUMEN
Introducción y objetivos: Conocer el beneficio clínico del cierre percutáneo de la orejuela izquierda (OI) en nuestro medio; en concreto, la reducción de eventos tromboembólicos y hemorrágicos, que permitiría un mejor posicionamiento de esta intervención.
Métodos: Estudio retrospectivo que recoge la actividad del cierre de OI en un centro durante 9 años. Se registraron la tasa de éxito del dispositivo y del procedimiento, así como las tasas de eventos tromboembólicos y de hemorragia mayor.
Resultados: Se evaluaron 260 procedimientos de cierre de OI en una población con fibrilación auricular no valvular y puntuación en las escalas CHA2DS2-VASc de 4,3 ± 1,6 y HAS-BLED de 3,7 ± 1,2. El éxito del procedimiento fue del 98,8%, y la tasa de eventos adversos graves en los primeros 7 días fue del 2,3%. Con un seguimiento medio de 2,5 ± 1,9 años y una población de 637,9 pacientes-año, la tasa de eventos tromboembólicos fue de 1,4 por 100 pacientes-año (75,5% de reducción del riesgo) y la de hemorragia mayor fue de 3,0 por 100 pacientes-año (58,5% de reducción del riesgo), ambas significativamente menores que las predichas. Las tasas de eventos por 100 pacientes-año en los pacientes con seguimiento muy largo (más de 4 años) mostraron tendencia a ser menores que en el resto de la población (0,7 frente a 2,0, con p = 0,17, para evento tromboembólico, y 1,7 frente a 4,0, con p = 0,09, para hemorragia mayor).
Conclusiones: En nuestra población, el cierre de la OI mostró un elevado éxito del procedimiento y una baja tasa de eventos inmediatos. El cierre de la OI indujo una significativa reducción en la tasa prevista de eventos tromboembólicos y hemorrágicos, y dicha reducción se mantuvo a muy largo plazo.
Palabras clave: Cierre percutáneo. Embolia arterial. Isquemia cerebral.
INTRODUCTION
Percutaneous left atrial appendage closure (LAAC) has been extensively studied in clinical trials. Despite the excellent results of efficacy and safety regarding the LAAC from randomized clinical trials,1 these studies are limited by their design, which is still not applicable to our routine clinical practice. Maybe this is the reason why in our setting, the LAAC program is still far from reaching its full potential.2 Without detriment to the current level and grade of clinical recommendation for the LAAC,3 the medical community will only gain confidence in this procedure when further studies are presented assessing its performance in our routine clinical practice.
The LAAC is a solid structural procedure that in Spain is only second to transcatheter aortic valve implantation (TAVI).4 The experience gained with the LAAC has moved the focus of attention from the early aspects of success and safety towards other issues still not properly addressed such the performance of LAAC reducing long-term cardiovascular events or its lingering benefits over time.
To this day, there are very few papers gathering the long-term experience gained with the LAAC with a median follow-up of 2.5 years.1,5-9 It is only from this long-term perspective that we will understand the value of a procedure largely based on the prophylaxis of the thromboembolic complications occurred during the patient’s life.
The objective of this study was to present our own experience in the follow-up of the population treated with LAAC from the beginning of this program to assess its overall performance and, especially, the reduction of long and very long-term thromboembolic and bleeding events.
METHODS
Our study is a retrospective analysis of the LAAC activity developed consecutively in a teaching hospital from March 2011 through February 2020. This procedure was indicated by different large volume hospital units including the internal medicine, neurology, and cardiology units. Our unit has included the LAAC as a strategic program within our structural heart procedures.
Left atrial appendage closure: the procedure and the device implanted
All procedures were performed in an identical working setting (facilities and personnel). However, 3 different modalities were used: on the one hand, general anesthesia and conscious sedation, both with transesophageal ultrasound guidance, and a third modality with fluoroscopy guidance only while the patient remained awake.
Although at the beginning of our experience only general anesthesia was used, 2.5 years later the possibility of conscious sedation administered by our personnel started to become a reality; the criterion to choose between general anesthesia or conscious sedation was logistical due to the discretional participation of the anesthesiology unit in structural heart procedures. In both modalities, the type of probe used for the transesophageal ultrasound was the exact same one.
The protocol of conscious sedation consisted of sedoanalgesia through the IV administration of 50 mg of pethidine followed by a bolus of 0.5 mg/kg of propofol with slow infusion in 3 min. with continuous monitorization of saturation and hemodynamics. After the introduction of the transesophageal probe several boluses of 10 mg of IV propofol were administered on demand based on the patient’s discomfort or rejection.
The procedure guided by fluoroscopy only was spared for cases with absolute or relative contraindication for transesophageal ultrasound use (in our unit we do not have intracavitary ultrasound) and for patients considered very frail for anesthetic induction; however, it became a reality 4 years after we started our experience. In these patients, a coronary computed tomography angiography was recommended to assess the left atrial appendage and discard the presence of an inner thrombus; in any case, an angiography was performed via transseptal access through a pigtail catheter from the left atrial appendage ostium without selective cannulation to discard the presence of thrombus. After catheterizing the left atrial appendage, a 180º rotational acquisition was performed through the injection of contrast at a flow rate of 8 mL/s with a total of 48 mL; by doing this a 3D image of the left atrial appendage was obtained (software i-Pilot, Siemens, Germany) that fused with the real fluoroscopy.
The 2 most popular devices in the market today were used: the WATCHMAN device in its WATCHMAN 2.5 and WATCHMAN Flex versions; Boston Scientific, United States) and the AMPLATZER ACP/Amulet device (Abbott, United States); the LAmbre device (Lifetech Scientific, China) was implanted anecdotically. The selection of one or the other did not follow any clinical or anatomical criteria and the alternate use of both devices was well-balanced. Only in fluoroscopy-guided procedures the AMPLATZER Amulet device was preferential since its delivery criteria are basically fluoroscopic.
Performance of left atrial appendage closure and follow-up
The definitions were based on the Munich consensus document regarding the LAAC.10 Successful LAACs were defined as successful devices (successful implantation of the first device selected) and successful procedures (uneventful final successful implantation within the first 24 hours). The device was released after confirming the suitability of ultrasound and fluoroscopic parameters. In cases performed under fluoroscopy guidance, position and stability were assessed over the fusion imaging as well as the lack of uncovered lobes in the angiography.
Regarding treatment after the implant, there was no pre-specified criterion and the patient’s bleeding risk was adjusted. All patients were assessed using a thoracic ultrasound within the first 24 hours prior to hospital discharge. Adherence to the transesophageal ultrasound control 1.5 months after the procedure was very irregular.
Follow-up was conducted back in February 2020 by reviewing the Andalusian (Diraya) electronic health record system. The appearance and dates of the following events were registered: death and causes, ischemic stroke/systemic embolism, major bleeding (incapacitating and major hemorrhages), and medical therapy at the follow-up. The futility of the LAAC was defined as mortality rate due to non-cardiac causes reported within the first year.
The performance of the LAAC at the follow-up was assessed using the risk reduction rate of thromboembolic (ischemic stroke/systemic embolism) or bleeding events (major bleeding) while taking into account the risk estimates from the CHA2DS2-VASc11 and HASBLED scores,12 respectively.
A 4-year follow-up limit has been established to start taking about «very long evolution» since this was the follow-up period of the Protect AF clinical trial1 that confirmed the superiority regarding mortality of LAAC over anticoagulation.
Statistical analysis
The estimates were obtained using IBM SPSS v26.0 and Epidat 4.2 statistical software. Initially, a descriptive analysis of data was conducted by generating means and standard deviations of numerical variables, and frequency and percentage distributions of qualitative variables.
The comparison between the demographic and clinical quantitative variables was conducted using the ANOVA test after verifying the hypotheses of normality using the Shapiro-Wilks test; when significant differences were seen, multiple comparisons were conducted using the Bonferroni correction.
The comparison among the different qualitative variables was conducted using contingency tables and the chi-square test.
The comparison between event incidence rates was conducted using the Rothman index score and 95% confidence intervals (95%CI) were estimated using Rosner’s method.
Finally, Kaplan-Meier curves were generated and then compared using the log rank test.
RESULTS
Population
The population studied included 260 patients with nonvalvular atrial fibrillation aged between 42 and 92 years old. The clinical characteristics of the population are shown on table 1.
Table 1. Clinical characteristics of the population
| Age (years) | 74.8 ± 8.1 |
| Males | 160 (61.5%) |
| Risk factors | |
| Arterial hypertension | 238 (91.5%) |
| Diabetes (types 1 and 2) | 118 (45.4%) |
| Smoking | 93 (35.8%) |
| Dyslipidemia | 130 (50%) |
| Kidney disease | 64 (24.6%) |
| Ischemic heart disease | 89 (34.2%) |
| Previous stroke | |
| Ischemic stroke | 38 (14.6%) |
| Hemorrhagic stroke | 57 (21.9%) |
| CHA2DS2-VASc | 4.3 ± 1.6 |
| CHA2DS2-VASc ≥ 4 | 176 (67.7%) |
| HAS-BLED | 3.7 ± 1.2 |
| HAS-BLED ≥ 3 | 222 (85.4%) |
|
Data are expressed as no. (%) or median ± standard deviation. |
|
The most common indication for LAAC was the absolute contraindication for anticoagulant therapy due to hemorrhagic events in 229 cases (88.1%) or high-risk (2 patients with brain tumors and 1 patient with an aortic dissection; 1.1%). In 28 patients (10.8%) indication was due to the inability to take oral anticoagulants due to different bleeding risks: in 13 due to rejection to anticoagulant therapy, in 6 due to previous psychiatric history that did not recommend it, in 3 due to higher risk of falling, in 3 due to poor control of the international normalized ratio, and in other 3 due to cardioembolic stroke yet despite the proper anticoagulant therapy.
When the LAAC was indicated, therapy was mostly anticoagulation (68.8% of the patients): vitamin K antagonists (83 cases, 31.9%), direct anticoagulants (71 cases, 27.3%) or dual therapy (anticoagulation and single antiplatelet therapy, 25 cases, 9.6%). Regarding patients who were not on anticoagulant therapy prior to the LAAC, 48 of them (18.5%) were on antiplatelet therapy and 33 (12.7%) did not use any antiplatelet/anticoagulant drugs.
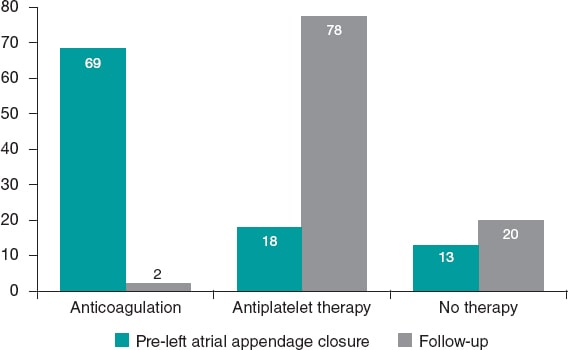
While follow-up was being conducted (February 2020 or prior to the patient’s death), our population was being treated with absence of antiplatelet/anticoagulant therapy (51 patients, 19,9%), single antiplatelet therapy with acetylsalicylic acid (135 patients, 52.5%), single antiplatelet therapy with clopidogrel (51 patients, 19,9%), dual antiplatelet therapy (14 patients, 5.4%) or anticoagulation (6 patients, 3%) (figure 1).
Figure 1. Evolution of antithrombotic therapy prior to the left atrial appendage closure (LAAC) until the final follow-up (%).
Procedural characteristics
Procedures were performed mostly under general anesthesia and monitored under transesophageal ultrasound guidance (59.6%). Conscious sedation, also monitored under transesophageal ultrasound guidance, was performed in 27.3% of all procedures. Only 13.1% of all procedures were performed under fluoroscopy guidance only.
The most commonly used device was the WATCHMAN (142 patients, 54.6%) followed by the AMPLATZER ACP/Amulet (116 patients, 44.6%), and occasionally the LAmbre (2 patients, 0.8%). Given the extension of the follow-up period, 2 models of the WATCHMAN (generation 2.5 in 125 patients and WATCHMAN Flex in 17 patients) and 2 models of the AMPLATZER device (ACP in 16 patients and Amulet in 100 patients) were used.
The device success rate was 98.5% (failed in 4 patients). Failed cases were due to the device not meeting the sealing criteria for the left atrial appendage so it had to be recaptured; after choosing a different device (different size, and in 1 case, also a different model), the procedure ended satisfactorily.
Procedural success was 98.8%; 1 oropharyngeal hemorrhage due to traumatic intubation and 2 tamponades were the reason for the lack of success. Tamponades (0.77%) were due, in the first case, to a perforation of the left atrial appendage in the recapture maneuver of the WATCHMAN device; the second case, after 24 hours, was due to the perforation of the left pulmonary artery possibly eroded by the LAmbre device. These 2 patients had a good clinical progression, the first one after pericardiocentesis and the second one after surgery with pericardial patch interposition between the pulmonary artery and the left atrial appendage. No deaths, strokes, or systemic embolisms were reported during the procedure or within the first 24 hours.
The number of serious adverse events reported within the first week was 6 (2.3%) as shown on table 2.
Table 2. Serious adverse events within the first 7 days after the implant
| Day | Event | Description | Death |
|---|---|---|---|
| Procedure | Hemorrhage | Traumatic intubation for general anesthesia | No |
| Procedure | Tamponade | Pericardiocentesis | No |
| 1 day | Tamponade | Perforation of pulmonary artery Surgery | No |
| 4 days | Bronchial aspiration | Bronchial aspiration while eating | Yes |
| 4 days | Hemorrhage | Upper gastrointestinal bleeding | Yes |
| 6 days | Hemorrhage | Upper gastrointestinal bleeding | No |
The comparative analysis between the results of the first 50 LAACs and the remaining ones give us a glimpse of the existence of a learning curve that can be seen in the procedural variables that assess the operator’s technical skills (significant reduction of fluoroscopy time and radiation dose from the first 50 procedures): 13.6 ± 5.5 min vs 18.7 ± 18.2 min and 18 413 µGym ± 11 622 µGym vs 24 798 µGym ± 18 802 µGym,2 respectively with P values = .03). However, no differences were found in the procedural success rate (98% for the first 50 cases and 99% for the remaining ones).
The procedural characteristics of the left atrial appendage closure are shown on table 3.
Table 3. Procedural characteristics
| Procedural modality | |
| General anesthesia | 155 (59.6%) |
| Conscious sedation | 71 (27.3%) |
| Fluoroscopy | 34 (13.1%) |
| Device | |
| ACP-Amulet | 116 (44.6%) |
| WATCHMAN | 142 (54.6%) |
| LAmbre | 2 (0.8%) |
| Device size (mm) | 25.2 ± 3.4 |
| Fluoroscopy time (min) | 14.6 ± 9.7 |
| Radiation (µGym2) | 19 636 ± 13 488 |
| Device success | 98.5% |
| Procedural success | 98.8% |
|
Data are expressed as no. (%) or median ± standard deviation. |
|
Follow-up and events
With a median follow-up of 2.5 years ± 1.9 years (median, 1.4 years; 95%CI, 1.1 to 1.9 years) our series included 637.9 patients-year.
A total of 58 deaths were reported at the follow-up (22.3% of the sample, 9.1% patients-year). Half of them were due to cardiac causes (4.6% patients-year). A total of 6 deaths were due to noncardiac causes within the first year, which means that LAAC futility rate was 2.3%.
Events such as ischemic strokes/systemic embolisms were reported in 9 patients (1.4% patients-year, 95%CI. 0.6-2.7); compared to the estimated risk of 5.7% patients-year, the reduction of relative risk was 75.2% (P < .001). A total of 19 major hemorrhages were reported (3.0% patients-year. 95%CI. 1.8-4.7), which is a 58.5% reduction of relative risk compared to the estimated risk of 7.2% patients-year (P < .001)
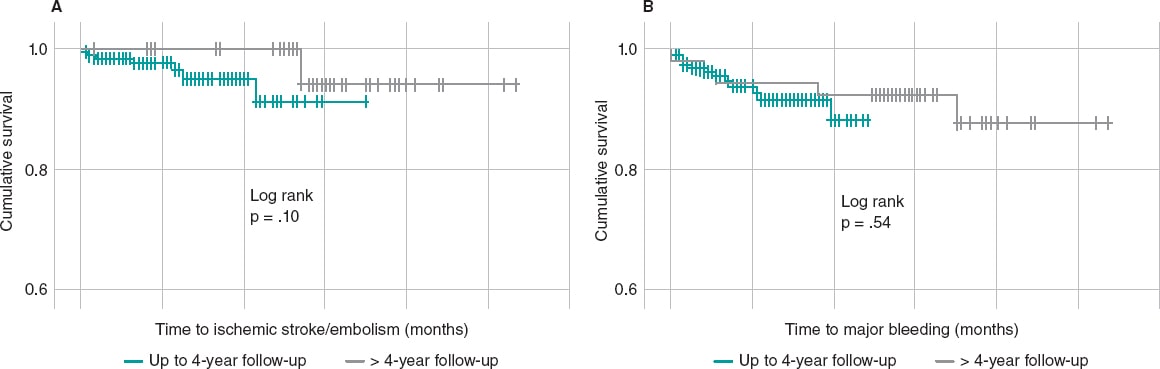
The assessment of the protective capacity of LAAC to avoid long-term thromboembolic phenomena and major hemorrhages is very relevant. Events were compared in patients with follow-ups of up to 4 years (N = 206; 346.7 patients-year) and in patients beyond this 4-year follow-up mark (N = 54; 291.3 patients-year). It was confirmed that, over time, protection against thromboembolic and hemorrhagic events still remains, and there is even a decreasing tendency: annual rate per 100 patients-year for ischemic stroke/embolism of 2.0 vs 0.7 (P = 0.17) and for major hemorrhages of 4.0 vs 1.7 (P = .09) in patients with up to 4-year follow-ups and longer follow-ups, respectively. The comparison of event-free survival rates for thromboembolism and major bleeding between the different populations based on the duration of the follow-up did not show any significant results (log rank with P = .10 for thromboembolisms and P = .54 for hemorrhages) (figure 2).
Figure 2. Thromboembolic event-free (A) and major bleeding-free (B) survival curves of patients with < 4 year (green) and > 4-year follow-up (gray). The curve comparison does not show any significant differences for either one of the events.
Follow-up based on the type of device implanted
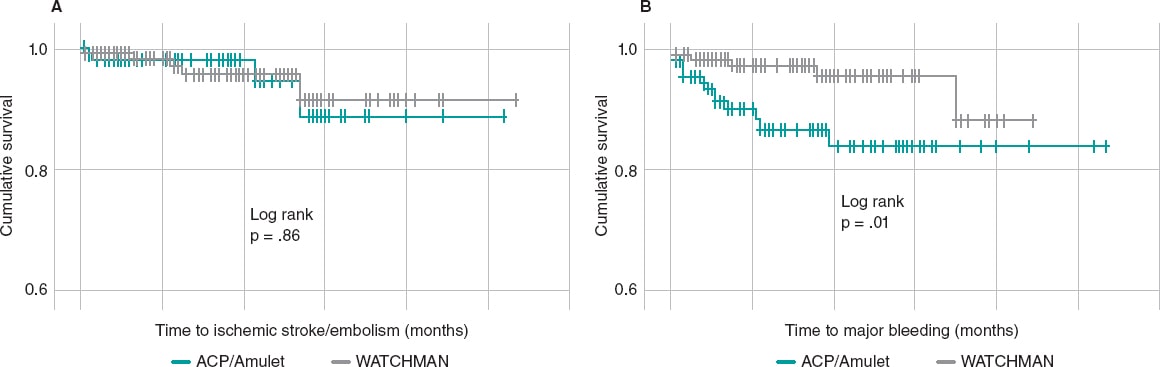
A comparative analysis of the event-free survival rate in patients treated with the WATCHMAN and AMPLATZER devices found no significant differences between the 2 regarding their protective capabilities against ischemic strokes/systemic embolisms (log rank P = .86); however, the WATCHMAN showed a major hemorrhage-free cumulative incidence rate superior to the AMPLATZER device (log rank P = .01) (figure 3).
Figure 3. Thromboembolic event-free (A) and major bleeding-free (B) survival curves of patients with the AMPLATZER (green) and the WATCHMAN device (gray). No differences were seen in the devices used for thromboembolic events, but there were differences regarding major bleeding with a higher event-free survival rate in patients treated with the WATCHMAN device.
DISCUSSION
This real-world single-center registry shows our experience performing left atrial appendage closure in 260 consecutive patients with nonvalvular atrial fibrillation over the last 9 years. Results have been exposed in an attempt to answer the following questions: what were the results of LAAC in our population? what is the actual performance of LAAC reducing thromboembolic or hemorrhagic events compared to the estimated risk rates? and finally, is this this event reduction maintained at the follow-up?
The clinical characteristics of our population are consistent with those of the LAAC target population in the routine clinical practice. Thus, our population showed clinical characteristics of thromboembolic risk that were similar to those published in large registries: the CHA2DS2-VASc score of 4.3 was intermediate between the AMPLATZER Amulet registry13 with a CHA2DS2-VASc score of 4.2 and the NCDR registry14 with a CHA2DS2-VASc score of 4.6. Regarding the risk of bleeding, in our population the mean HAS-BLED score was 3.8, slightly higher compared to the numbers already published, and situated between the EWOLUTION registry with a HAS-BLED score of 2.315,16 and the AMPLATZER Amulet registry with a HAS-BLED score of 3.3.13
This high risk of bleeding of our population may be explained by the fact that the indication for LAAC for almost 90% of the patients was a past medical history of bleeding (mostly gastrointestinal followed by cerebral); for the remaining 10%, the indication for LAAC was the «inability to take oral anticoagulants due to different risks of bleeding»,10 that is, by a number of reasons that forced the patient (5% of the population) or the doctor to make the decision of choosing mechanical local therapy over the anticoagulant therapy. Although in our case the volume of elective decisions regarding the LAAC is far from the volume reported in the German registry LAARGE,17 where patient selection was essential to propose the indication in a fourth of the population, a reflection can be made on to what extent information brought to the patient is decisive to generalize this therapy.
The LAAC is a procedure with high device and procedural success rates in most of the series already published. In our series the device success rate in the implant was 98.5%; 1.5% of failed procedures were due to an erroneous selection of the size of the device. However, the left atrial appendages of all the patients from the series were eventually sealed, which contrasts with up to 7% of the procedures cancelled due to inaccessible left atrial appendage anatomies;14 this may have to do with our capacity to approach this procedure using different modalities (general anesthesia, conscious sedation, and fluoroscopy without ultrasound guidance) and different types of occluder devices (yet the device had to be changed for a different one only in 1 patient in order to finish the procedure). Our procedural success rate was 98.8%, which is higher compared to the rate reported in other registries with similar populations regarding their baseline clinical characteristics.14 Regarding procedural safety, our adverse event rate within the first 7 days after the procedure was 2.3%, which is consistent with the rate reported by large registries.13-16 Overall, this speaks of the progressive decline seen in the rate of adverse events reported during the early stage after the procedure.
In the consecutive analysis of procedural results like the left atrial appendage closure, right from the beginning of our experience and until today, the presence of a learning curve may be anticipated. However, beyond procedural variables like the radiation duration and dose, no differences were reported regarding the procedural success rate between the early period and the rest of the experience. Standardizing procedures and training the operators may be the reasons of the high success rate reported in left atrial appendage closure despite the poor early experience reported.18
Our registry, with a median follow-up of 2.5 years and a fifth of the patients with follow-up periods > 4 years allows us to assess the efficacy of the LAAC with a certain perspective. In the first place, mortality rate is surprisingly high since 22.3% of the patients included died at the follow-up. This is an annual mortality rate of 9.1%, 3 times higher compared to the 4-year follow-up of the Protect AF,1 but it is nearly identical as other registries with a similar risk population compared to ours.9 The highest mortality risk seen at the follow-up has been associated with factors such as age, male sex, history of stroke or intracranial hemorrhage, low ejection fraction, and chronic kidney disease;8,9 in any case, this high mortality rate seen at the follow-up shows how frail this diseased population really is, which would justify an interesting debate on the futility of the LAAC in some patients19 (2.3% in our series).
The primary endpoint of LAAC is to reduce the risk of cardiac embolism in a population with nonanticoagulated atrial fibrillation. In our case, the annual rate of ischemic stroke and embolism was 1.4%, which was a significant reduction of the relative risk of 75%, which is consistent with the best data reported in the medical literature.20 Regarding major bleeding, in a population with an estimated rate of bleeding > 7%, our rate was 3.0%, that is, half the rate reported by other authors.9
To this day, very few studies have been conducted on the long-term efficacy profile of the left atrial appendag-e closure. In the Ibérico II registry,8 the rate of thromboembolic events remained low while the rate of major bleeding was lower compared to the early rates at the 2-year follow-up. In our population, the analysis of patients with very long clinical courses (implantation times > 4 years) revealed that the efficacy of the LAAC still remains. Also, that thromboembolic and bleeding events showed a tendency towards a lower incidence rate compared to the earliest stage.
Limitations
This study has some limitations. In the first place, no systematic antithrombotic pattern was followed after implantation. Instead, it was left to the operator’s discretion, which may have impacted the short-term bleeding rate. On the other hand, no systematic imaging follow-up was arranged 45 days to 3 months after implantation, which means that an important piece of information was lost: the rate of thrombosis associated with the device, lack of residual sealing…
CONCLUSIONS
In our setting, left atrial appendage closure is an effective therapy for patients with nonvalvular atrial fibrillation and coagulation issues. It significantly reduces the rates of thromboembolic and hemorrhagic events that remain consistent in the very long term.
FUNDING
No funding related for this work.
AUTHORS' CONTRIBUTION
R.J. Ruiz-Salmerón: author of the manuscript; M. Ronquillo-Japón, C. Robles-Pérez, M. Iglesias-Blanco, C. Rubio-Iglesias,R. García de la Borbolla, C. Carrascosa-Rosillo, S. Rodríguez de Leiras, M. Vizcaíno-Arellano, and I. Méndez-Santos: critical review and J. Polo-Padillo: statistical analysis.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- It is estimated that only 5% of the patients with nonvalvular atrial fibrillation and inability to use oral anticoagulant therapy have benefited from the left atrial appendage closure. The evidence from randomized clinical trials is based on a population that is not similar to the one considered eligible for LAAC in the real world, which is a limitation. Relevant real-world registries do not have long-term follow-ups either.
WHAT DOES THIS STUDY ADD?
- Our study provides data on the performance of the left atrial appendage closure in our routine clinical practice on procedural success and performance reducing thromboembolic and major bleeding events, which, overall, is significant compared to the estimated rates and also remains consistent over the very long-term.
REFERENCES
1. Reddy VY, Sievert H, Halperin J, et al. Percutaneous Left Atrial Appendage Closure vs Warfarin for Atrial Fibrillation:A Randomized Clinical Trial. JAMA. 2014;312:1988-1998.
2. Fukutomi M, De Backer O, Søndergaard L. Indications, current adoption and future perspectives for percutaneous left atrial appendage closure. EuroIntervention. 2019;14:1707-1709.
3. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373-498.
4. Ojeda S, Romaguera R, Cruz-González I, Moreno R. Registro Español de Hemodinámica y Cardiología Intervencionista. XXIX Informe Oficial de la Sección de Hemodinámica y Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2019). Rev Esp Cardiol. 2020;73:927-936.
5. Wiebe J, Franke J, Lehn K, et al. Percutaneous Left Atrial Appendage Closure With the Watchman Device:Long-Term Results Up to 5 Years. J Am Coll Cardiol Intv. 2015;8:1915-1921.
6. Betts TR, Leo M, Panikker S, et al. Percutaneous left atrial appendage occlusion using different technologies in the United Kingdom:A multicenter registry. Catheter Cardiovasc Interv. 2017;89:484-492.
7. Korsholm K, Nielsen KM, Jensen JM, Jensen HK, Andersen G, Nielsen-Kudsk JE. Transcatheter left atrial appendage occlusion in patients with atrial fibrillation and a high bleeding risk using aspirin alone for post-implant antithrombotic therapy. EuroIntervention. 2017;12:2075-2082.
8. López-Mínguez JR, Nogales-Asensio JM, Infante De Oliveira E, et al. Reducción de eventos a largo plazo tras el cierre de la orejuela izquierda. Resultados del Registro Ibérico II. Rev Esp Cardiol. 2019;72:449-551.
9. Regueiro A, Cruz-Gonzalez I, Bethencourt A, et al. Long-term outcomes following percutaneous left atrial appendage closure in patients with atrial fibrillation and contraindications to anticoagulation. J Interv Card Electrophysiol. 2018;52:53-59.
10. Tzikas A, Holmes DR, Gafoor S, et al. Percutaneous left atrial appendage occlusion:the Munich consensus document on definitions, endpoints and data collection requirements for clinical studies. EuroIntervention. 2016;12:103-111.
11. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach:the euro heart survey on atrial fibrillation. Chest. 2010;137:263-272.
12. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients:The Euro Heart Survey. Chest. 2010;138:1093-1100.
13. Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device:periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867-876.
14. Freeman JV, Varosy P, Price MJ, et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75:1503-1518.
15. Boersma LV, Schmidt B, Betts TR, et al. EWOLUTION investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device:peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465-2474.
16. Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation:1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302-1308.
17. Fastner C, Nienaber CA, Park JW, et al. Impact of left atrial appendage morphology on indication and procedural outcome after interventional occlusion:results from the prospective multicenter German LAARGE registry. EuroIntervention. 2018;14:151-157.
18. Sawant AC, Seibolt K, Sridhara S, et al. Operator experience and outcomes after transcatheter left atrial appendage occlusion with the Watchman device. Cardiovasc Revasc Med. 2020;21:467-472.
19. Asmarats L, Rodés-Cabau J. Resultados a largo plazo tras el cierre de la orejuela izquierda:ampliando la perspectiva en la prevención no farmacológica del ictus en pacientes con fibrilación auricular. Rev Esp Cardiol. 2019;72:440-442.
20. Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion –an update. EuroIntervention. 2020;15:1133-1180.