ABSTRACT
Brief review of current indications, materials, techniques, complications, results, and controversies around percutaneous procedures for the management of pulmonary valve and arterial branches disease. This article gives the interventional cardiologist a perspective on the material currently available.
Keywords: Valvuloplasty. Angioplasty. Percutaneous valve.
RESUMEN
Se presentan las indicaciones actuales, el material, las técnicas, las complicaciones, los resultados y las controversias de los procedimientos percutáneos que permiten abordar la patología valvular y de las ramas pulmonares. El presente artículo ofrece una perspectiva del material actualmente disponible de forma clara para el cardiólogo intervencionista.
Palabras clave: Valvuloplastia. Angioplastia. Valvulas percutaneas.
Abbreviations
CHD: congenital heart disease. CPC: cavopulmonary connection. PB: pulmonary branches. PR: pulmonary regurgitation. PS: pulmonary stenosis. PVA: pulmonary valve atresia. RV: right ventricle. RVOT: right ventricular outflow tract.
PULMONARY VALVE STENOSIS
The origin of pulmonary valve stenosis (PVS) is almost exclusively congenital. It amounts to 7% to 10% of all congenital heart diseases (CHD). Although it is often an isolated defect, it can be associated with other congenital malformations.
Acquired stenosis is extremely rare and is associated with carcinoid syndrome or rheumatic fever. An emergent form is the stenosis of surgical bioprosthesis or valved conduits.
PVS can coexist with infundibular or supravalvular pulmonary stenosis, the latter often associated with Noonan, Williams or Alagille syndromes as well as with congenital rubella.
Clinical presentation is varied and goes from critical stenosis or pulmonary valve atresia (PVA) in the newborn baby to mild stenosis that can go untreated.
Although its presentation in the adult life is often asymptomatic, in cases of severe stenosis, exertional dyspnea, ventricular dysfunction, arrhythmias or sudden death have been reported. In this group, it can have a native presentation after previous surgery or valvuloplasty.
Etiology
PVS can have 3 anatomopathological presentations1 (figure 1):
-
– Typical PVS. It is the most common: a typical tricommissural valve with mild thickening of the leaflets and commissural fusion. The valve annulus is normally developed, and post-stenotic dilatation often occurs. Valve opening is typically dome-shaped with a central stenotic orifice. It rarely presents calcification.
-
– PVS due to dysplastic valve. It represents almost 20% of all cases pf PVS, although it is common of Noonan syndrome. Valve leaflets are thickened and myxomatous with limited opening and scarce commissural fusion. It can be associated with annular hypoplasia and even with proximal pulmonary trunk.
-
– PVS associated with other CHD such as interatrial communication, interventricular communication, transposition of great arteries, double outlet right ventricle (RV) o tetralogy of Fallot. The valve is often bicuspid or even unicuspid. It can be associated with infundibular or pulmonary supraventricular stenosis and annular hypoplasis.
Figure 1. A: Valve stenosis due to dysplastic valve (left). B: Typical valve stenosis.
Pulmonary valvuloplasty
Since 1982, percutaneous pulmonary valvuloplasty has been the technique of choice to treat pulmonary valve stenosis in newborn babies until adult life. The goal here is to overextend and tear the leaflets at commissural raphe level.
This technique is often curative and has a low rate of restenosis at the follow-up. It can often be treated with a second procedure.
The degree of immediate residual pulmonary regurgitation (PR) does not usually go from severe to mild; instead, it can progress with the passing of time. Despite of this, the need for valve replacement is not usually the case.
Indications
The natural history of pulmonary valve stenosis is associated with the degree of obstruction. Although the Doppler-derived mean pressure gradient is most reliably associated with the peak-to-peak hemodynamic gradient, the international guidelines2-3 establish the degree of obstruction based on the instantaneous Doppler-derived peak pressure gradient:
-
– Mild stenosis (instantaneous Doppler-derived peak pressure gradient < 36 mmHg or peak velocity < 3 m/sec). The course of the disease is often benign and it can be compatible with living a normal life. In the adult patient, evaluations every 5 years are advised.
-
– Moderate stenosis (instantaneous Doppler-derived peak pressure gradient of 36 mmHg to 64 mmHg or peak velocity of 3-4 m/sec). Although often asymptomatic, the limited RV cardiac output can give rise to the appearance of exertional dyspnea or fatigue; 20% of cases can progress towards a greater degree of obstruction. Evaluations every 2 years are advised.
-
– Severe stenosis (instantaneous Doppler-derived peak pressure gradient > 64 mmHg, peak velocity > 4 m/sec or Doppler mean gradient > 40 mmHg. It is associated with the presence of symptoms, RV dysfunction or cyanosis. Treatment is always indicated here.
The indications for the management of pulmonary valve stenosis are shown on table 1.4,5
Table 1. Treatment indications in pulmonary valve stenosis
| Critical stenosis of the newborn baby |
| Severe pulmonary valve stenosis (Doppler-derived peak pressure gradient > 60 mmHg or Doppler-derived mean pressure gradient > 40 mmHg) in asymptomatic patient |
| Moderate pulmonary valve stenosis (instantaneous Doppler-derived peak pressure gradient > 50 mmHg or Doppler-derived mean pressure gradient > 30 mmHg) in symptomatic patient |
| Surgery will be indicated in association with: |
| Moderate or severe pulmonary regurgitation. |
| Subvalvular or supravalvular stenosis. |
| Severe tricuspid regurgitation. |
| Symptomatic dilatation of pulmonary artery due to extrinsic compression of nearby structures. |
| Need for surgical correction of other associated anomalies or arrhythmia surgery (the Maze technique). |
| Percutaneous valvuloplasty can be the first option in case of dysplastic valves compared to surgery. Still, the rate of success can be lower. |
Technique and material
The percutaneous pulmonary valvuloplasty technique has been reported extensively1 and it can be performed under conscious sedation, local anesthesia or even general anesthesia in the pediatric patient. A total of 100 IU/Kg of sodium heparin are administered up to a maximum of 5000 IU. Transthoracic or transesophageal echocardiography are not often used here.
Femoral vein access is the most common of all, although other alternative accesses can also be used such as the jugular vein or the transhepatic access. In cases of large pulmonary valve annulus, 2 simultaneous venous accesses may be necessary to perform the double balloon technique. Arterial access is optional.
After the baseline registry of pressures, a right ventriculography will be performed preferably in the lateral and posterolateral projections with a 30º cranial inclination. The measurements of the pulmonary annulus are taken during systole at valve-leaflet junction level.
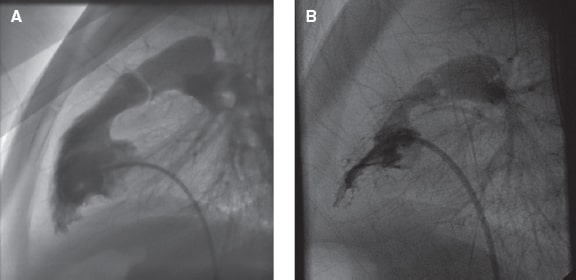
After crossing the pulmonary valve with a catheter, the exchange guidewire will be in position to provide the distal pulmonary artery with high support (preferably the inferior lobar artery). Different types of balloon catheter can be used and early diameters 1.2-1.25 times larger compared to the pulmonary annulus diameter are advised (figure 2A). If the hemodynamic gradient remains > 30 mm Hg and in the absence of significant PR, it is recommended to repeat the procedure with a new balloon catheter until reaching a 1.4 ratio. The 1.5 ratio should be respected except for cases of dysplastic valves. The recommended length of the balloon is 20 mm in newborns and infants, 30 mm in pediatric patients, and 40 mm in adults.
Figure 2. A: Balloon pulmonary valvuloplasty. B: Double balloon pulmonary valvuloplasty.
In case of large valve annulus, the double balloon technique can be used (figure 2B); in this case, both balloons should have the same length. The effective diameter of the combined 2 balloon catheters6 is shown on table 2, and can be estimated as follows:
Table 2. Effective diameter using the double balloon technique
| Diameter | 6 mm | 7 mm | 8 mm | 10 mm | 12 mm | 14 mm | 15 mm | 16 mm | 18 mm | 20 mm | 22 mm | 24 mm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 mm | 9.0 | |||||||||||
| 7 mm | 10.7 | 11.5 | ||||||||||
| 8 mm | 11.5 | 12.3 | 13.1 | |||||||||
| 10 mm | 13.3 | 14.0 | 14.8 | 16.4 | ||||||||
| 12 mm | 15.1 | 15.8 | 16.5 | 18.0 | 19.6 | |||||||
| 14 mm | 16.9 | 17.6 | 18.3 | 19.7 | 21.3 | 22.9 | ||||||
| 15 mm | 17.8 | 18.5 | 19.2 | 20.6 | 22.1 | 23.7 | 24.5 | |||||
| 16 mm | 18.7 | 19.4 | 20.1 | 21.5 | 23.0 | 24.6 | 25.4 | 26.2 | ||||
| 18 mm | 20.6 | 21.2 | 21.9 | 23.3 | 24.7 | 26.3 | 27.0 | 27.8 | 29.5 | |||
| 20 mm | 22.5 | 23.1 | 23.7 | 25.1 | 26.5 | 28.0 | 28.8 | 29.5 | 31.1 | 32.7 | ||
| 22 mm | 24.4 | 25.0 | 25.6 | 26.9 | 28.3 | 29.7 | 30.5 | 31.2 | 32.8 | 34.4 | 36.0 | |
| 24 mm | 26.3 | 26.9 | 27.5 | 28.8 | 30.1 | 31.5 | 32.2 | 33.0 | 34.5 | 36.1 | 37.7 | 39.3 |
Effective diameter = 0.82 (diameter 1 + diameter 2)
No significant differences have been reported in terms of effectiveness between the percutaneous pulmonary valvuloplasty with single or double balloon.
Results and follow-up
The rate of immediate procedural success is close to 90% with a very low mortality rate (0.24%) and scarce major complications (0.35%). In the dysplastic pulmonary valve, the rate of success is even lower.1 Surgery can be spared as a second option in this type of valvular anatomy.
The rate of restenosis seen at the follow-up is 21% in the historic series and between 8% and 10% in the most recent clinical trials.7-9 Risk factors are the presence of dysplastic valve, residual hemodynamic gradient ≥ 30 mmHg, and use of a balloon-to-annulus ratio < 1.2.
In the absence of severe-to-mild PR, repeating the percutaneous valvuloplasty is the selection of choice except for the management of valve dysplasia where surgery can be indicated.
At the follow-up, PR was present in 40% to 90% of the patients with an increase seen at the follow-up. Risk factors are a higher degree of early stenosis, younger age at the moment of the valvuloplasty, and a greater balloon-to-annulus ratio.
Despite this, valve replacement is rarely indicated with indications that will be based on the presence of symptoms like ventricular volumes and RV function parameters is rare. Studies suggest that the same indication parameters as in the corrected TOF with residual PR could be used.10
Special situations
Pulmonary valve atresia
PVA is a complex CHD characterized by the complete obstruction of pulmonary flow and observed within the first days of life following the ductus arteriosus physiological closure; it is incompatible with life if left to its natural progression.
The basic anatomical marker is valve atresia, often membranous, with fused leaflets and valve annulus hypodevelopment. Other lesions are often associated with this main anomaly, among them, the variable RV and tricuspid valve hypodevelopment and coronary circulation anomalies. Both the pulmonary trunk and arteries often appear normal.
The management of these patients includes early stabilization by keeping the temporal maintenance of ductal patency with prostaglandin E1 infusion. The ultimate therapeutic approach will depend on the severity of the associated lesions. The early opening of the valve is indicated in patients with the most favorable prognosis in terms of sufficiently developed RV and tricuspid valve and lack of RV-dependent coronary circulation.
This technique is often performed via percutaneous access11,12 (videos 1-8 of the supplementary data) by accessing the RV and the right ventricular outflow tract (RVOT) or mammary artery via venous access and inserting a right coronary curved (JR) catheter under the imperforate valve plane. Once its correct position has been secured it is advanced through a system of microcatheter and radiofrequency guidewire, with which the valve plane is pierced. Afterwards, a sequential valvuloplasty with balloon catheter will be performed.
Although after this procedure, antegrade flow is established from the RV, this flow is rarely enough to keep an adequate level of arterial oxygen saturation due to different factors: persistent RVOT obstruction at valvular/infundibular level, restrictive behavior of the RV, and tricuspid valve hypodevelopment. All of it conditions an insufficient pulmonary flow through the natural pathway and a significant right-to-left interatrial shunt with the corresponding desaturation. For this reason, an accessory source of pulmonary flow is often required that should remain beyond the neonatal period.
Over the last few years, a new alternative has been implanting a coronary stent into the ductus arteriosus. This can be performed during the same procedure or during a second procedure via venous antegrade or arterial retrograde access. The goal here is to implant a 3 mm to 4 mm coronary stent in such a way that ductal length is fully covered, thus avoiding stent protrusion into the aortic or pulmonary borders.
This technique facilitates keeping enough pulmonary flow and arterial oxygen saturation until the RV is properly developed. Ductal stent usually closes spontaneously by endoluminal proliferation within the first year of life. In some cases, a new in-stent stent implantation may be required at the follow-up.
Fetal pulmonary valvuloplasty
Fetal pulmonary valvuloplasty is a rare technique applicable to fetuses with prenatal diagnosis of critical pulmonary valve stenosis or PVA and risk of progression towards RV hypoplasia. It is often performed between the 21st and 28th weeks of pregnancy. The goal here is to promote a better intrauterine development of right heart structures, thus favoring biventricular physiology after birth.
Through simultaneous ultrasound guidance and after achieving a proper fetal position, a transuterine, fetal transthoracic, and cardiac puncture is performed by accessing the RVOT with a 22-G Chiva needle. The pulmonary valve is then punctured with the needle in case of atresia and a 0.014 inch coronary guidewire is distally placed. Mounted over this guidewire and inside the needle, a very low-profile coronary balloon catheter is advanced and valve dilatation is performed.
Regarding results, this is a complex technique that requires multidisciplinary experience and collaboration. One of the difficulties is achieving the right needle orientation since the size of the RV cavity is so small and its geometry so complex. There is a high rate of complications including fetal arrhythmias, pericardial effusion or even fetal death.
A recent international multicenter clinical trial13 documented this procedure in 58 fetuses and reported a 55% rate of complications including 7 deaths. Compared to the disease progression of patients treated with a similar cohort of treatment-naive fetuses, a greater tendency towards biventricular physiology was confirmed after birth in the first group (87% vs 43%). Despite this, to this date, no criteria have been established with indications for this technique. Therefore risks, the group experience, and the possible benefits should all be assessed in each particular case.
PERCUTANEOUS PULMONARY VALVES
Many CHD require RVOT reconstruction using a patch, a bioprosthesis or a conduit between the RV and the pulmonary artery. In the tetralogy of Fallot, 90% of the patients who undergo surgery during childhood will reach the adult age and a significant number of them will develop regurgitation or PS following the annulus section or use of conduits. When and how to treat these conditions is still controversial since there is no expert consensus, and the American, Canadian, and European guidelines5 establish general rules with suboptimal levels of evidence. Also, each particular case shows characteristics unforeseen by the algorithms proposed.
Surgeons use different materials to solve these dysfunctions: allografts, porcine pericardium based heart valves—with and without support—, mechanical valves, and valved (hand-made) and non-valved conduits. Over the last 2 decades, percutaneous coronary interventions have broken into our setting pushed by the development of bioprosthesis mounted on stents; still, we don’t know when to treat asymptomatic patients: precocity in replacement is associated with faster deterioration and more procedures being performed—each one with its own risk—while late procedures may no longer stop or revert the deterioration of ventricular function and volumes.
The morphology of a dysfunctional RVOT is very complex, determines the therapeutic approach (the “pyramid” morphology is not very compatible with self-expanding valves), and behaves dynamically. Echocardiography is not enough here. Instead, the computed tomography scan is required to see the coronary anatomy. Also, the cardiac magnetic resonance imaging (that cannot be performed in all cases due to problems with clips, pacemakers or heart valves causing interferences) facilitates the assessment of volumes and function, and the visualization of such dynamic behavior. Currently, the RV function is more important than the RV size both to indicate the implant and to make follow-up assessments. The coronary anatomy does not always show the behavior during and after implantation. As a matter of fact, the different RVOT measure changes during implantation don’t cause linear changes in coronary arteries, which is why coronary angiography still plays an essential role. The length of self-expanding valves is significantly greater compared to balloon-expandable (and surgical) valves, which is why the total distance in the area left for implantation becomes crucial. We have known for years that the pulmonary and the aortic valves don’t share the same architecture and work differently. Also, that the same heart valves operate differently in the aortic and pulmonary positions (actually, they may even not close effectively).14
Indications and historical perspective
The indications for percutaneous pulmonary valves included in the European5 and American15 guidelines are similar to surgical replacement. In symptomatic patients the indication is well established. Asymptomatic patients, instead, require ECG data (absolute prolonged QRS interval duration > 180 or progression), hemodynamic data (like a correlation of pressures between the RV and the left ventricle > 0.7), and Doppler-derived peak and mean pressure gradients > 50 and > 30, respectively. But, above all, magnetic resonance imaging data in cases of significant pulmonary regurgitation (regurgitation fraction > 30%): right ventricular end-diastolic volumes > 160 mL/m2, double right end-diastolic volume compared to the left one, end-diastolic volume > 80 mL/m2, and RV ejection fraction < 0.40-0.45 (or negative progression).
The first heart valve ever implanted percutaneously was the Bonhoeffer pulmonary valve back in 2000. It was based on the idea of suturing a bovine jugular vein with the valve in a vascular stent. The valve was given the name Melody (Medtronic Inc, United States) and obtained the CE marking and the Canadian marking in 2006 and the United States Food and Drug Administration marking back in 2010. It is indicated for elderly patients with dysfunctional surgical conduits. Its off-label use has increased and it is used in patients of up to 20 kg of weight and in native RVOTs,16 with technical modifications (previous stent implantation in the implant area) to minimize some of the most common complications reported (stent fracture), but with precautions due to the significant numbers of infectious endocarditis reported (a problem shared with the surgical bovine heart valve Contegra (Medtronic, United States). The limitation of valve sizes available (18 mm, 20 mm, and 22 mm) is also a problem because many patients with regurgitation have large-caliber pulmonary trunks, which has led to imaginative solutions for extended uses.
In 2008 The Edwards SAPIEN heart valve (Edwards Lifesciences LLC, United States) for aortic positioning started being used in the right position thanks to the COMPASSION clinical trial that proved it safe and effective for conduits with moderate or severe pulmonary regurgitation with or without stenosis.17 Its sizes are larger (23, mm 26 mm, and 29 mm), it does not fracture, and the incidence rate of infectious endocarditis is lower (although, on this regard, the literature available is not that “solid”). In 2016 the Edwards SAPIEN XT heart valve was approved by the European and American regulatory agencies for use in children and adults with regurgitation or PS; the current SAPIEN 3 heart valve is approved for the aortic position only, but numerous off-label implants have been reported in the pulmonary position.
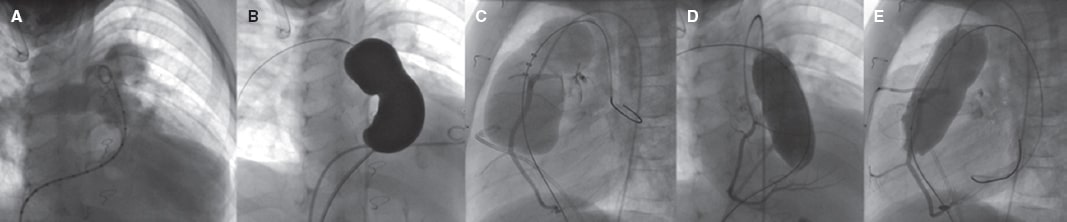
The procedure requires a meticulous prior preparation. An arterial access and 2 venous accesses, preferably femoral, are required. It can also be implanted via jugular vein access. The angiographic study (figure 3) serves 2 purposes: a) study the anatomy and sizes of the “trunk” or conduit and pulmonary branches (PB) for which different projections are needed (the RV outflow tract is better seen on the right anterior oblique projection at 20º + cranial at 20º and in the lateral position; still, changes need to be made in this particular case), and inject contrast agents both in the trunk and in the RV while the trunk is occluded with a balloon catheter; and b) study the anatomical relations of proximity since the adjacent structures can be compressed (the ascending aorta or the coronary arteries). To that end, while the balloon catheter remains inflated in the pulmonary artery, an aortogram or coronary angiography or both is performed. A 34 mm or 35 mm very compliant cutting balloon for interatrial communication can be used. However, at times, the balloon compliance exceeds the target diameter of the implant causing coronary or aortic compression. When this happens, other balloons of identical diameter to the target implant will be required. In the presence of a calcified conduit, the approach should be gradual given the risk of rupture. If the Melody valve is used, a previous stent should always be implanted—covered if the conduit is calcified—which is not required in the remaining heart valves.
Figure 3. Angiography examples. A: in pulmonary trunk, individualized, in right anterior oblique projection at 20º + cranial at 40º. B: with balloon catheter for interatrial communication measurement and morphologic assessment, in the same projection. C: in lateral projection showing how over-dilatation occludes the left anterior descending coronary artery that originates from the right coronary artery. D: selective coronary angiography with a balloon of the same diameter as the implantable valve diameter, without coronary obstruction, the same projection as in A. E: with the same balloon as in D, in lateral projection.
There is no consensus as to whether the entire procedure should be performed in 1 or 2 stages leaving the first stage to anatomical/physiological study and stent implantation.
Pulmonary prosthetic valves available
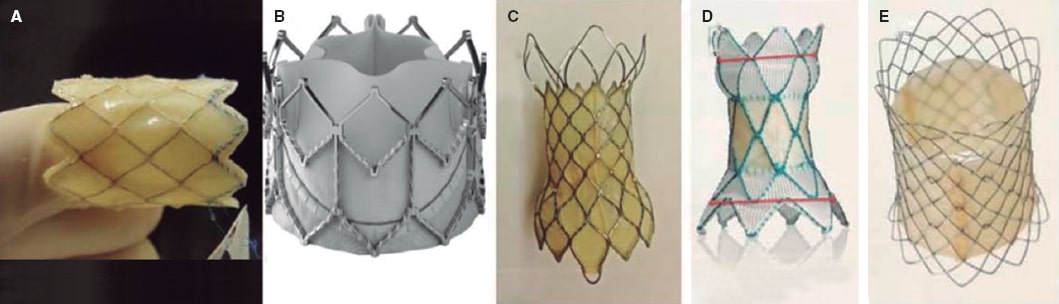
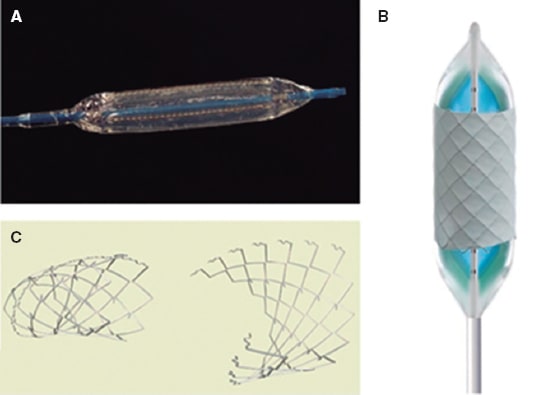
There are 2 large groups of heart valves available, balloon-expandable valves and self-expanding valves. The former have been around a little longer, are approved by regulatory agencies, over 10 000 of them have already been implanted worldwide, have greater radial strength, allow a better control of the diameter to reach, shorten when dilated, and are extremely demanding from the technical point of view. Self-expanding valves are more modern, are in the pipeline in several clinical trials still pending approval, reach larger diameters, and are longer; still, there can be problems in the pulmonary trunk distal portion as they don’t shorten, there is no control over their diameter (they reach their nominal value), and no re-dilatations are possible. Figure 4 shows the heart valves currently available for pulmonary use.
Figure 4. Percutaneous pulmonary valves. A: Melody valve (Medtronic, United States). B: Edwards XT valve (Edwards Lifesciences LLC, United States). C: Venus valve (Venus MedTech, China). D: Harmony valve (Medtronic, United States). E: Pulsta valve (Taewoong Medical, South Korea).
The Melody TPV valve
The Melody TPV valve (Medtronic Inc, United States)18 is a balloon-expandable valve. It is built from a bovine jugular vein with an 18 mm native valve sutured to a platinum-iridium CP stent (NuMED, Canada). The overall length of the entire system is 28 mm and shortens in relation to the final diameter. It can expand from 16 mm to 22 mm in diameter (it probably also works up to 24 mm). Valve crimping is manual and it is delivered and released using Medtronic Ensemble patented system—a version of the double balloon angioplasty BIB (NuMED Inc., United States)—with a 22-Fr profile in its distal portion and a 16-Fr profile in its proximal portion, of 100 mm in length; it is advanced through a high-support guidewire allocated in a pulmonary branch (preferably the left one).
The Edwards SAPIEN valve
The Edwards SAPIEN valve (Edwards Lifesciences LLC, United States)18 is a balloon-expandable valve with porcine pericardium leaflets mounted on a cobalt chromium stent. Its height is smaller compared to the Melody valve. The SAPIEN XT THV model has been approved for the pulmonary position and is built in 23 mm (height of 14.3 mm), 26 mm (height of 17.2 mm), and 29 mm (height of 19.1 mm). Valve crimping is performed with a specific device, proximal to the position of the balloon, “mounted” on it, and once in the inferior vena cava, delivered and released using Novaflex patented system (Edwards Lifesciences, United States) with 18-Fr, 19-Fr or 20-Fr profiles depending on the valve diameter. It is very rigid and not easy to advance or retrieve especially with a previous stent already implanted, which can end up damaging the tricuspid valve. The SAPIEN 3 THV model is built of the same diameters (in heights of 18 mm, 20 mm, and 22.5 mm) but it is an evolution whose internal covered portion is fairly shorter (9.3 mm, 10.2 mm and 11.6 mm). It has an outer protection of polyethylene terephthalate to minimize the possibility of leaks. Its stent has different geometries in the proximal and distal portions so that it shortens even more in its proximal border. It is delivered through the “deflectable” Edwards Commander Delivery System of 14-Fr for the 23 mm and 26 mm valves, and 16-Fr for the 29 mm valve with a patented introducer sheath (Edwards eSheath). It has been reported that through greater volume inflations compared to the nominal volume, the SAPIEN 3 valve can reach 30 mm.
The Venus P-valve
The Venus P-valve (Venus MedTech, China) is designed for native tracts. It is a self-expanding, trileaflet valve of porcine pericardium with a 30 mm in length nitinol stent covered by porcine pericardium—except for its distal portion—that dilates in both borders (1 cm in each border), with radiopaque marks to facilitate it location; the diameter of the valve is that of the nondilated portion (18 mm to 34 mm). Delivery system is MedTech patented with a 20-to-22-Fr distal caliber, a 16-Fr proximal caliber, and a 22-to-24-Fr introducer sheath. Valve crimping is manual by submerging it in a cold saline solution.19
Two clinical trials have been conducted to achieve the CE marking, one in China and the other one in Europe. Both have been completed and are in the data evaluation stage with inclusion criteria similar to those of the remaining valves.
The Harmony mTPV 25 valve
The Harmony mTPV 25 valve (Medtronic, United States) is a self-expanding nitinol structure with a polyester covering and a porcine pericardium valve stitched in the middle. Its conceptual design is vast: the first clinical trials started in the United States in 2013, but, to this date, the valve is still under constant modification (Harmony TPV 22, Harmony TPV 25, and modified Harmony TPV 25). It is available for research purposes only.
The PULSTA valve
The PULSTA valve20 (Taewoong Medical, South Korea) is designed for native tracts. It is a nitinol, trileaflet, self-expanding valve of porcine pericardium also covered with porcine pericardium except for its proximal and distal (major) portions. It has radiopaque marks to outline the area covered. Numbering corresponds to the narrowest area— commissure level—from 18 mm to 32 mm. A larger diameter (1 mm to 2 mm) compared to the pulmonary trunk is selected; the dome-shaped area measures 4 mm more compared to the narrow area. It is built in 2 different lengths: 33 mm and 38 mm. The delivery system caliber is 18-Fr for up to 28 mm and 20-Fr for larger sizes. Its radial strength is lower compared to that of the Melody or SAPIEN valves, That is why it is not the heart valve of choice for stenotic conduits.
Currently, most implants have been performed in Asia, but since December 2019 there is an ongoing clinical trial being conducted in Europe, in which Spain participates. The inclusion criteria are similar to those of any other valve.
Complications and limitations
The complications and limitations of the heart valves described above are:
-
– Compressions: in approximately 5% of the patients there is risk of coronary compression during the procedure. Large heart valves can distort the aortic root with regurgitation.
-
– Ruptures: of the conduit, especially if calcified, during predilatation requiring immediate implantation of a covered stent; navigating heart valves is not easy and requires high-support guidewires capable of perforating the PB; during advance and retrieval maneuvers, the tricuspid valve can be damaged causing regurgitation.
Still, mortality rate during the procedure is around 1.4%.21
Fractures were a common thing at the follow-up (12.4%) with the old Melody implants without previous stent implantation and when the risk factors were young age, greater pre- and postprocedural residual gradient, smaller conduit size, proximity to sternum, and presence of recoil after delivery.22 The second most common complication is infectious endocarditis (4.9%), mostly with the Melody, with a higher incidence rate compared to surgical cases. Several hypotheses have been proposed to explain this: damage to the valve during the assembly, no strict observance of sterility measures, previous endocarditis, unsatisfactory hemodynamic results, poor dental hygiene, piercings, tattoos, etc.23
Data from registries24 and multicenter clinical trials on the mid-term evolution of the Melody heart valve and further reinterventions of the valve.25 During a > 5-year follow-up period it is expected that in up to 14.4% to 15% of the cases some procedure will be performed, mostly due to fractures. There is a great variety of indications among the different centers. Actually, in up to 65% of all procedures a valve-in-valve procedure has been performed. This confirms that infectious endocarditis is still the Achilles heel of the Melody valve with a 2.4% incidence rate per patients-year. There are no data available on other types of valves.
PERCUTANEOUS TREATMENT OF PULMONARY BRANCHES
The percutaneous management of PB stenosis has become widely used after the arrival of new materials and technologies. Currently, it is the first option because surgical outcomes are poor. PB stenosis can be congenital, associated with syndromes (Williams-Beuren, Alagille, etc.) or connatal infections like rubella or be part of complex CHD like tetralogy of Fallot, pulmonary atresia or pulmonary artery sling. However, stenosis is the evolutionary or residual result of surgery in these same and other CHD as in the aftermath of the Jatene arterial switch procedure (the Lecompte maneuver) or when it is necessary to place a conduit between the RV and the PB. Percutaneous treatment improves cardiac output and alleviates pressure to the RV, re-balances the distribution of flow to both lungs, improves functional class, exercise capacity (VO2 and VE/VCO2), and eventually the prognosis of patients with univentricular and biventricular physiology. The following are considered criteria for hemodynamic repercussions: gradient ≥ 20mmHg, angiographic stenosis ≥ 50%, RV or pulmonary artery pressure ≥ 60% of systemic blood pressure (biventricular) or asymmetry (≥ 30%) in pulmonary reperfusion as see on the magnetic resonance imaging or the scintigraphy. This flow asymmetry can stop unilateral non-significant stenosis from translating into pressure gradients since we are dealing with a parallel flow.26
Generalities
Balloon angioplasty
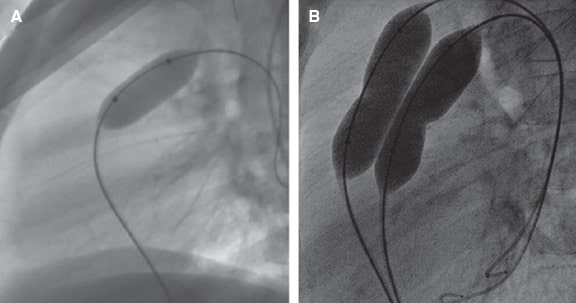
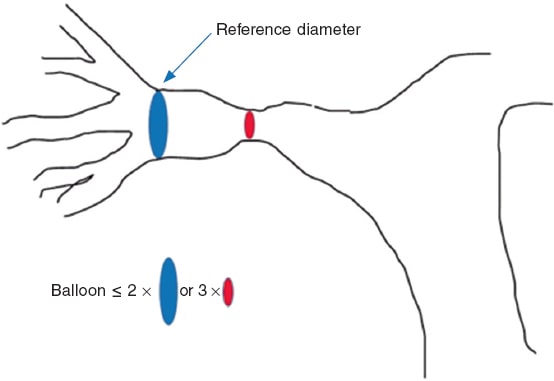
Balloon angioplasty is spared for patients with low body weight when the anatomy is not suitable for stent implantation or when the segments will be involved in future surgeries. Immediate restenosis, of up to 50%, due to recoil or extrinsic compression is more common compared to stents. It is a safe procedure with a < 1% rate of major complications. Taking the hilar diameter as the vessel reference, balloons whose critical diameter is 3 times larger compared to the diameter of stenosis are selected without exceeding the double of the reference diameter (figure 5). To be effective the notch needs to go away, and a controlled intimal-medial tear needs to occur for eccentric remodeling, which means that results may not be immediate. The procedure is considered successful with lumen increases ≥ 50% of the minimum diameter, reductions ≥ 20% of RV or systemic pressure or flow increases ≥ 20% towards the target lung.27 Using the latest noncompliant high-pressure balloons and microtome or cutting balloons improves the results of angioplasties that do not respond to conventional balloons or that are especially resistant like those of lobar branches in patients with genetic syndromes. Common cutting balloons are 8 mm in diameter and they need to be placed in the target area through a sheath to avoid damaging the tricuspid or pulmonary valve when advanced or retrieved (figure 6).
Figure 5. Reference diameters in the percutaneous treatment of pulmonary branches.
Figure 6. A: Cutting balloon. B: BIB balloon with mounted covered CP stent. C: BeGrow stent.
Stent angioplasty
Stent angioplasty, described by Mullins in the 1980s, provides structural support and avoids immediate restenosis due to recoil or folding. Its mid- and long-term results are superior to conventional angioplasty. Better profiles and semi-open or open cell designs that allow re-dilatation have made this technique available for younger and thinner patients. However, this has come at the expense of successive re-dilatations needed to adapt to the size of the vessel due to growth or to dilate the origin of jailed branches. Several studies prove that these successive re-dilatations are safe and effective. Closed-cell stents are more stable in the manual crimping and they usually have greater radial strength. They normally navigate on the balloon inside a sheath towards the stenosis to avoid damaging the right valves, stent migration so they can be repositioned before implantation.28 The profile of this sheath is proportional to the diameter of the balloon on which it is mounted. Same sized premounted balloons have a better profile; some models do not even require the use of long introducers for implantation purposes. One of the most commonly used balloons is the BIB double balloon, currently 8 mm to 30 mm in diameter. It consists of 2 concentric balloons where the inflation of the internal one allows us to predict how the stent will behave in the stenosis facilitating sequential expansion, and repositioning if necessary (figure 6). Considering a normal Nakata index of 250 mm2/m2 to 300 mm2/m2, an adult patient of 2 m2 of body surface will need a stent capable of reaching an ideal diameter of 18 mm to 20 mm in each branch. Self-expanding stents improve the profile because they don’t need a balloon for implantation purposes and are not re-dilatable. To this date, they have been approved for other locations like the biliary route or the femoropopliteal axis.29
In general, bare-metal stents are implanted leaving the covered ones for cases where it is necessary to repair the damaged vessel wall or regulate flow using a diabolo-shaped configuration towards one branch or the other or else through a systemic-pulmonary artery shunt (table 3).
Table 3. Stents currently used in pulmonary branches
New technologies
Some of these new technologies are:
-
– Drug-eluting balloons: noncompliant balloons with an antiproliferative substance covering like rapamycin analogues or placlitaxel approved to treat peripheral arterial stenosis, but particularly useful to treat in-stent restenosis due intimal proliferation.30
-
– Bioresorbable stents: they avoid further re-dilatations in growing patients. They can be built with organic polymers or corrodible metal alloys like iron or magnesium. Some studies show inflammatory responses in the vessel wall and doubts surrounding significant restenosis. Still pending approval by the United States Food and Drug Administration, the Pediatric Bioresorbable Stent (480 Biomedical Stent Inc., United States) is the design in the most advanced clinical trial stages so far and has been specifically designed to treat PB stenosis in pediatric CHD.
-
– Breakable stents: cells with hinges programmed to break with a balloon like the BeGrow (Bentley, Germany) or ready for resorption like the Growth Stent (QualiMeD, Germany) consisting of 2 halves joined by biodegradable sutures that disappear within 5 months, thus facilitating blood vessel growth (figure 6).
Complications
Percutaneous procedures on pulmonary branches are associated with moderate (angioplasty) or high (stent) risk. They are often performed under general anesthesia, with unfractionated heparin at 100 IU/Kg (to a maximum of 5000 IU) and with an activated clotting time ≥ 250 seconds. The most common complications and risk factors are shown on table 4; and they are more common when the procedure is an emergency procedure and when the patient is of a younger age reaching 38% in newborn babies. As a general rule, the support guidewire should be placed in the inferior lobar branch and further guidewires should not be used. Once removed, advancing the sheath again through the recently dilated segment is also ill-advised. Tears or dissections are more common in the traditional balloon, tipically without any clinical repercussions. When there are repercussions, anticoagulation reversal is required by re-inflating the balloon in the leak area, implanting a stent or through surgery. In-stent restenosis is often due to intimal proliferation but also to stent fractures that cause it to lose its structural integrity or due to extrinsic compression. It is less common with semi-open cell designs and with the greater flexibility of self-expanding stents. There are no clear recommendations on antithrombotic treatment after implantation. Endothelization occurs 6 months after implantation and, although thrombosis is rare, the routine clinical practice is using antiplatelet therapy during that time.31
Table 4. Complications and most common risk factors
| Complications | Risk factors |
|---|---|
| Vascular damage | Overdilatation, too rigid guidewire, surgical patches, recent surgery |
| Thrombosis | Cavopulmonary connection, cyanosis, small branches, short activated clotting time, no antiplatelet therapy |
| Stent embolization | Low weight, disproportion, bifurcation, malapposition, emergency, manual crimping |
| Reperfusion edema | Chronic cyanosis, older age |
| Mismatch | Early implantation, non-dilatable design |
| Restenosis | Short overlapping, genetic syndrome, cavopulmonary connection, bifurcation, residual waist, oversizing, external compression, self-expanding stent |
Specific situations
Bifurcations
There are 2 technical options to repair these stenoses without compromising the contralateral branch flow:
-
– Through the simultaneous implantation of 2 stents, each one mounted on its own guidewire and sheath. Both stents should be of the same size and both balloons should be inflated simultaneously and progressively (figure 1A of the supplementary data).
-
– By implanting a long open-cell stent from one of the branches towards the pulmonary trunk to later recross towards the contralateral branch with a different guidewire and then implant a second shorter stent (figure 1B of the supplementary data). This technique is useful when we only have 1 venous access or when the implantation of a pulmonary valve with pre-stenting is intended.
Cavopulmonary connection
In the fragile Fontan-type univentricular physiology—a pumpless non-pulsatile pulmonary circuit—the criteria upon which the indication is based are in a lower threshold compared to biventricular physiology. Also, almost any degrees of angiographic stenosis or hemodynamic gradient are significant since they can significantly increase central venous pressure and large asymmetries in flow distribution towards both lungs. Securing the most symmetrical flow distribution possible to both lungs is key to avoiding the formation of arteriovenous fistulas. That is why it may be necessary to reduce the flow of a fistula previously created or towards 1 of the branches through a diabolo-shaped covered stent (figure 2 of the supplementary data). These procedures are complex because of the access routes (femoral, jugular, transhepatic), because patients have been operated on many times, have a higher risk of thrombosis (polyglobulia, non-pulsatile slow flow), sometimes they even have recent surgical beds, are young patients, etc. The PB angioplasty is the most commonly performed procedure—only second to the extracardiac conduit angioplasty—in an extensive modern cohort of patients with total cavopulmonary connection (CPC) both after superior CPC and after completing the inferior CPC (figure 3 of the supplementary data). Stenosis most often occurs in the left PB (the right PB proximal segment originally) due to its longer course, compression of the neighboring ascending aorta, and the presence of lower flow in the stage between the pulsatile Glenn and the total CPC.32
The final size of PB and therefore their flow is directly associated with the long-term success of this physiology and with the patient’s functional class. However, it is often necessary to perform periodic invasive reassessments of the state of the stents previously implanted to adjust them to growth. This is because non-invasive methods may not be sensitive enough. After stent implantation, the clinical practice guidelines published by the American Heart Association recommend anticoagulant therapy for 3 to 6 months.
Postoperative state
The repair of certain CHD is associated with a risk of residual or evolutionary PB stenosis. With the Lecompte maneuver, associated with the arterial switch for the repair of the d-transposition of great arteries, PB stenosis occurs early in up to 28% of patients. On the other hand, pulmonary supravalvular stenosis is the leading cause of reintervention during childhood. Percutaneous treatment is technically challenging because we are dealing with small patients with sometimes recent sutures, in bifurcation, and a compromised space with the SVC and the ascending aorta. (figures 4 and 5 of the supplementary data). This technique is useful when we only have 1 venous access or when the implantation of a pulmonary valve with pre-stenting is intended.
Other CHD with common residual PB stenosis after surgery are the tetralogy of Fallot, truncus arteriosus, the pulmonary artery sling, etc. and all those that require having to place a conduit between the RV and the PB like the Ross, Rastelli, Yasui, Sano procedures, etc.
The current technological advances made in imaging techniques, materials, and devices has revolutionized the possibilities regarding the percutaneous management of pulmonary trunk, valve, and PB lesions. That is why the indications published in the clinical practice guidelines are being changed.
FUNDING
No funding.
AUTHORS’ CONTRIBUTION
F. Gutiérrez-Larraya Aguado, coordination and final draft revision; C. Abelleira Pardeiro and E.J. Balbacid Domingo partial drafts and provision of figures.
CONFLICTS OF INTEREST
None reported.
SUPPLEMENTARY DATA
Vídeo 1. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 2. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 3. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 4. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 5. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 6. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 7. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
Vídeo 8. Gutiérrez-Larraya F. DOI: 10.24875/RECICE.M20000196
REFERENCES
1. Rao PS. Percutaneous Balloon Pulmonary Valvuloplasty:State of the Art. Catheter Cardiovasc Interv. 2007;69:747-763.
2. Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis:EAE/ASE recommendations for clinical practice. American Society of Echocardiography, European Association of Echocardiography. J Am Soc Echocardiogr. 2009;22:1.
3. Silvilairat S, Cabalka AK, Cetta F, et al. Echocardiographic assessment of isolated pulmonary valve stenosis:which outpatient Doppler gradient has the most clinical validity. J Am Soc Echocardiogr. 2005;18:1137.
4. Warnes CA, Williams RG, Bashore, et al. TMACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease:a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:e714.
5. Baumgartner H, De Backer J, Babu-Narayan S, et al. 2020 ESC Guidelines for the management of adult congenital heart disease (ACHD). Eur Heart J. 2021;42:563-645.
6. Narang R, Das G, Dev V, et al. Effect of the balloon-annulus ratio on the intermediate and follow-up results of pulmonary balloon valvuloplasty. Cardiology. 1997;88:271-276.
7. McCrindle BW. Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation. 1994;89:1751-1759.
8. Voet A, Rega F, Van de Bruaene A, et al. Long-term outcome after treatment of isolated pulmonary valve stenosis. Int J Cardiol. 2012;156:11-15.
9. Devanagondi R, Peck D, Sagi J, et al. Long-Term Outcomes of Balloon Valvuloplasty for Isolated Pulmonary Valve Stenosis. Pediatr Cardiol. 2017;38:247-254.
10. Ruckdeschel E, Kim YY. Pulmonary valve stenosis in the adult patient:pathophysiology, diagnosis and management. Heart. 2019;105:414-422.
11. Petit CJ, Glatz AC, Qureshi AM, et al. Outcomes After Decompression of the Right Ventricle in Infants With Pulmonary Atresia With Intact Ven-tricular Septum Are Associated With Degree of Tricuspid Regurgitation:Results From the Congenital Catheterization Research Collaborative. Circ Cardiovasc Interv. 2017;10:e004428.
12. Morgan GJ, Narayan SA, Goreczny S, et al. A low threshold for neonatal intervention yields a high rate of biventricular outcomes in pulmonary atresia with intact ventricular septum. Cardiol Young. 2020;30:649-655.
13. Hogan WJ, Grinenco S, Armstrong A, et al. Fetal Cardiac Intervention for Pulmonary Atresia with Intact Ventricular Septum:International Fetal Cardiac Intervention Registry. Fetal Diagn Ther. 2020;47:731-739.
14. Pragt H, van Melle JP, Verkerke GJ, Mariani MA Ebels T. Pulmonary versus aortic pressure behavior of a bovine pericardial valve. J Thorac Cardiovasc Surg. 2020;159:1051-1059.e1.
15. Stotut KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease:executive summary:a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e637-697.
16. Boshoff DE, Cools BL, Heying R, et al. Off label use of percutaneous pulmonary valved stents in the right ventricular outflow tract:time to rewrite the label?Catheter Cardiovasc Interv. 2013;81:987-995.
17. Kenny D, Rhodes J, Fleming G, et al. 3-Year outcomes of the Edwards SAPIEN Transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. J Am Coll Cardiol Interv. 2018;11:1920-1929.
18. Fleming GA, Hill KD, Green AS, et al. Percutaneous pulmonary valve replacement. Prog Ped Cardiol. 2012;33:143-150.
19. Cao QL, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014:84:1131-1137.
20. Kim GB, Song MK, Bae EJ, et al. Successful feasibility human trial of a new self-expandable percutaneous pulmonary valve (Pulsta valve) implantation using knitted nitinol wire backbone and trileaflet α-gal-free porcine pericardial valve in the native right ventricular outflow tract. Circulation Cardiovasc Interv. 2018;11:e006494.
21. Virk SA, Liou K, Chandrakumar D, Gupta S, Cao C. Percutaneous pulmonary valve implantation:a systematic review of clinical outcomes. Int J Cardiol. 2015;201:487-489.
22. Ansari MM, Cardoso R, Garcia D, et al. Percutaneous pulmonary valve implantation:present status and evolving future. J Am Coll Cardiol. 2015;66:2246-2255.
23. Sharma A, Cote AT, Hosking MCK, et al. A systematic review of infective endocarditis in patients with bovine jugular vein valves compared with other valve types. J Am Coll Cardiol Interv. 2017;10:1449-1458.
24. Nordmeyer J, Ewert P, Gewillig M, et al. Acute and midterm outcomes of the postapproval MELODY registry:a multicenter registry of transcatheter pulmonary valve implantation. Eur Heart J. 2019;40:2255-2264.
25. Shahanavaz S, Berger F, Jones TJ, et al. Outcomes of transcatheter reintervention for dysfunction of a previously implanted transcatheter pulmonary valve. J Am Coll Cardiol. 2020;13:1529-1540.
26. Feltes TF, Bacha E, Beekman RH, et al. Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease:A scientific Statement from the American Heart Association. Circulation. 2011;123:2607-2652.
27. Patel AB, Ratnayaka K, Bergersen L. A review:Percutaneous pulmonary artery stenosis therapy:state-of-the-art and look to the future. Cardiol Young. 2019;29:93-99.
28. Hiremath G, Qureshi AM, Prieto L, et al. Balloon Angioplasty and Stenting for Unilateral Branch Pulmonary Artery Stenosis Improve Exertional Performance. JACC Cardiovasc Interv. 2019;12:289-297.
29. Zablah JE, Morgan GJ. Pulmonary Artery Stenting. Interv Cardiol Clin. 2019;8:33-46.
30. Cohen JL, Glickstein JS, Crystal MA. Drug-Coated Balloon Angioplasty:A novel treatment for pulmonary artery in-stent stenosis in a patient with Williams syndrome. Pediatr Cardiol. 2017;38:1716-1721.
31. Giglia TM, Massicotte MP, Tweddell JS, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease:a scientific statement from the American Heart Association. Circulation. 2013;128:2622-2703.
32. Franco Díez E, Balbacid Domingo E, Arreo del Val V, et al. Percutaneous Interventions in Fontan Circulation. Int J Cardiol Heart Vasc. 2015;8:138-146.
33. Nakanishi T, Matsumoto Y, Seguchi M, et al. Balloon Angioplasty for Postoperative Pulmonary Artery Stenosis in Transposition of the Great Arteries. J Am Coll Cardiol. 1993;22:859-866.