Special article
REC Interv Cardiol. 2019;2:108-119
Requirements and sustainability of primary PCI programs in Spain for the management of patients with STEMI. SEC, AEEC, and SEMES consensus document
Requisitos y sostenibilidad de los programas de ICP primaria en España en el IAMCEST. Documento de consenso de SEC, AEEC y SEMES
a Área de Enfermedades del Corazón, Hospital Universitario de Bellvitge, IDIBELL, Universidad de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain b Servicio de Cardiología, Hospital Universitario de León, León, Spain c Servicio de Cardiología, Hospital Clínico Universitario de Santiago, Santiago de Compostela, A Coruña, Spain d Servicio de Cardiología, Hospital Universitario de Salamanca, Salamanca, Spain e Servicio de Cardiología, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain f Servicio de Cardiología, Hospital Galdakao-Usansolo, Galdakao, Vizcaya, Spain g Servicio de Cardiología, Hospital Universitario La Paz, IDIPAZ, Madrid, Spain h Servicio de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain i Servicio de Cardiología, Hospital Álvaro Cunqueiro, Vigo, Pontevedra, Spain j Servicio de Cardiología, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain k Servicio de Cardiología, Hospital Universitario Virgen de la Victoria, Málaga, Spain l SUMMA 112, Madrid, Universidad Alfonso X el Sabio, Villanueva de la Cañada, Madrid, Spain m Servicio de Cardiología, Hospital do Salnés, Vilagarcía de Arousa, Pontevedra, Spain n Urgencias Sanitarias de Galicia 061, Santiago de Compostela, A Coruña, Spain o Servicio de Cardiología, Hospital Universitario 12 de Octubre, Madrid, Spain p Servicio de Cardiología, Hospital Universitario Reina Sofía, Córdoba, Spain
ABSTRACT
Bayesian statistics assesses probabilistically all sources of uncertainty involved in a statistical study and uses Bayes’ theorem to sequentially update the information generated in the different phases of the study. The characteristics of Bayesian inference make it particularly useful for the treatment of cardiological data from experimental or observational studies including different sources of variability, and complexity. This paper presents the basic concepts of Bayesian statistics associated with the estimation of parameters and derived quantities, new data prediction, and hypothesis testing. The latter in the context of model or theory selection.
Keywords: Posterior distribution. Prior distribution. Predictive distribution. Bayesian probability. Bayes’ theorem.
RESUMEN
La estadística bayesiana valora de forma probabilística cualquier fuente de incertidumbre asociada a un estudio estadístico y utiliza el teorema de Bayes para actualizar, de manera secuencial, la información generada en las diferentes fases del estudio. Las características de la inferencia bayesiana la hacen especialmente útil para el tratamiento de datos cardiológicos procedentes de estudios experimentales u observacionales que contienen diferentes fuentes de variabilidad y complejidad. En este trabajo se presentan los conceptos básicos de la estadística bayesiana relativos a la estimación de parámetros y cantidades derivadas, predicción de nuevos datos y contrastes de hipótesis; estos últimos en el contexto de la selección de modelos o teorías.
Palabras clave: Distribución a posteriori. Distribución previa. Distribución predictiva. Probabilidad bayesiana. Teorema de Bayes.
INTRODUCTION: MATHETMATICS, PROBABILITY, AND STATISTICS
In the words of world famous astrophysicist Stephen Hawking, the goal of science is «nothing less than a complete description of the universe we live in».1 Male and female scientists alike pursue this objective by building theories and assessing their predictions. It is the very essence of the scientific method.
Statistics is a scientific discipline that designs experiments and learns from data. It formalizes the process of learning through observations and guides the use the knowledge accumulated in decision-making processes. Concepts like chance, uncertainty, and luck are almost as old as mankind, and reducing uncertainty has always been a common goal for most human civilizations. Probability is the mathematical language used to quantify uncertainty and is at the core of statistical learning that represents—in probabilistic terms—both the study populations and the random samples that come from such populations.
There is not such a thing as a single statistical methodology. The most widely known and used ones are, by far, frequentist statistics, and Bayesian statistics. Both share common goals and use probability as the language of statistical learning. However, both understand the concept probability different. As a matter of fact, it is the element on which they largely disagree. According to the frequentist concept, it is only legitimate to assign probabilities to random phenomena that can be defined through experiments that can be repeated multiple times, and only under identical and independent conditions.
The Bayesian concept of probability is a much wider idea because it allows us to assign probabilities to all elements with uncertainty regardless of their nature. Bayesian probability applies to the occurrence of random events, both those that can be repeated under the conditions required by frequentist probability and those that don’t (chances that Arnau, a 60-year-old male who lives alone will recover from a heart attack). The differences between both methodologies grow even larger because Bayesian probability assigns probabilities to different parameters (like the prevalence of people between the ages of 45 and 65 who have suffered a heart attack), statistical hypotheses (the efficacy of a new treatment for diabetic patients with heart failure is greater compared to conventional treatment), probabilistic models or even to missing data generated by non-randomized losses to follow-up (eg, ignoring the information of patients with losses to follow-up in a survival study on a given end-stage process would introduce biased information to the study).
The second distinctive element between both statistical methodologies is the use of Bayes’ theorem. For Bayesian statistics it is an essential tool to sequentially update the relevant information that comes from a study. Therefore, after an initial analytical phase, the knowledge generated will be used to start a new process of learning that will be providing new information on the problem at stake.
Both the frequentist and Bayesian concepts of probability share the same axiomatic system, and the same probabilistic properties. This common niche makes them share a common mathematical language too.
The map of basic Bayesian concepts and their different associations is not easy to explain without falling into a plethora of technicalities. And this is even more evident in real-world studies in the cardiovascular research setting. Therefore, in this article we will be working on very clear cases we believe are powerful examples regarding conceptual terms that are, nonetheless, simple, and devoid of technical complexities.
This article includes 6 different sections. The first one, this introduction, refers to the general wisdom regarding Bayesian statistics and its association with mathematics, probability, and statistics. The second section includes brief historical references on Bayesian methodology. The next section is about Bayes’ theorem in its most innocent version regarding the occurrence of random events. Afterwards, we’ll be dealing with the concepts and basic protocol of Bayesian statistics: previous distribution, function of verisimilitude, posterior distribution, and predictive distribution to predict experimental results. Also, we’ll include a brief explanation on the computational problems associated with the practical application of Bayesian methods and their power to generate inferences on relevant derived quantities. Hypothesis testing—the P value in particular—will also be dealt with later on as well as the Bayesian hypothesis testing proposal. The article will end with a small comment on the use of prior distributions.
IT ALL STARTED WITH BAYES, PRICE, AND LAPLACE
Knowing a little bit of Bayesian history is important because it allows us to put it into a temporal and social perspective that illuminates and boosts its learning. We’ll give a few relevant hints on this history now. McGrayne2 gives us an easy-to-understand and rigorous big picture on Bayesian history.
The very first time anybody heard of Bayes’ theorem was in Great Britain halfway into the 18th century through Reverend Thomas Bayes while trying to prove the existence of God through mathematics. He would never dare to publish his findings. Prior to his death, he bequeathed all his savings to his friend Richard Price who—if okay with it—was supposed to spend this money to publish the findings, which is something he eventually did. However, these results went totally unnoticed.
We’re still in the 18th century, but now we’ll have to travel to France to meet Pierre-Simon Laplace, one of the most prominent mathematicians in history. He discovered, independently of Bayes and Price, Bayes’ theorem in the format that we know today. Also, he developed the Bayesian concept of probability. After his death, his work fell into oblivion, under attack too because it was not in tune with the ruling idea of objectivity so embedded in the scientific world at the time.
Back to Great Britain now. Bletchley Park was a 19th century mansion in Northern London turned into a working center to break the secret messages of the German army during the Second World War (1939-1945). Here Alan Turing and his team—that included the Bayesian statistician Jack Godd—played a key role in the history of Bayesian statistics: Bayes’ theorem was tremendously useful to decipher the code of the Enigma machines the Germans were using to code and decode messages. After the war, the British government classified all the information that had anything to do with Turing, mathematics, statistics, and decoding as top secret. Bayes’ theorem became a useful tool for just a handful of scientists, and an anathema (or worse) for most of them. As a truly revealing anecdote, McGrayne2 tells the story when Good presented the details of the method that Turing and his team had used to decipher the Nazi codes to members of Britain’s Royal Statistical Society. This is what the next speaker had to say about the whole thing: «After that nonsense […]».
During the second half of the 20th century, the future of Bayesian statistics looked grim: support from the English-speaking academic world grew thin, and the rest of the scientific community knew very little about Bayesian statistics. Also, there were many computational difficulties to implement it to real-world studies with data. But what seemed to be destined to happened never did. We’re now back to the Second World War to Los Alamos National Laboratory in the state of New Mexico, United States. This center was created with absolute secrecy during Second World War to investigate the construction of nuclear weapons under the umbrella of the so-called Manhattan Project, led by the United States with the participation of Great Britain and Canada. It is in this context where the early Monte Carlo simulation methods were discovered back in 1946 by Polish mathematician Stanislaw Ulam while playing solitaire. Also, at that time, Metropolis et al.3 publish the first Markov chain Monte Carlo (MCMC) simulation algorithm while conducting his investigations on the H-bomb.
Several years go by without any direct links whatsoever between Bayesian statistics and MCMC methods. However, some studies are published—especially on image recognition—combining both elements.4 Encouraged by the technological advances made, especially in computing, Alan Gelfand, an American, and Adrian Smith, a British, collect former studies on MCMC methods and make a direct connection with Bayesian statistics.5 This will mark the beginning of the great Bayesian revolution that starts in the field of applications to, little by little, move on to the academic world. Bayesian inference is now recognized, accepted, and validated by the scientific community as a useful statistical methodology for scientific and social development.
BAYES’ THEOREM
The most widely known format of Bayes’ theorem is presented for the occurrence of random events. If A and B are random events, then
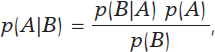
being p(A) the probability of event A, p(A|B) the associated probability A conditioned by the information that B occurred, and comparably p(B)> 0 and p(B|A). It is important to distinguish between probabilities p(A) and p(A|B). Both quantify the occurrence of A, but p(A) does so in absolute terms while p(B|A) does so in relative terms and conditioned by the information picked up in B. For example, nobody would question that the chances that a person may suffer from angina pectoris are higher if this person is hypertensive as opposed to not having that information available, p (Angina pectoris|Hypertension) > p (Angina pectoris).
Example I: Infections and tests
The prevalence that a certain infection affects any given population is .004. There is a test to detect its presence with a 94% sensitivity and a 97% specificity. We want to assess the chances that a person from such population is really infected if he tested positive for an infection.
We will use V and Vc to define a success that describes whether a person is infected or not, respectively. Therefore, p(V) = .004 and p(Vc) = .996. We’ll use (+) and (–) to describe a positive and negative test result for infection, respectively. In probabilistic terms, if a person is infected, he will test positive with a .94 probability, and negative with a .06 probability, p(+|V) = .94 and p(–|V) = .06 (false negative). If not infected, the person will test negative with a .97 probability and positive with a .03 probability, p(–|Vc) = .97 and p(+|Vc) = .03 (false positive).
According to Bayes’ theorem, the chances that a person is infected with a positive test result are
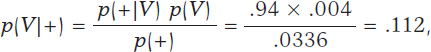
being p(+) = p(+|V) p(V) + p(+|Vc) p(Vc) = .0336 after implementing the total probability theorem (figure 1).
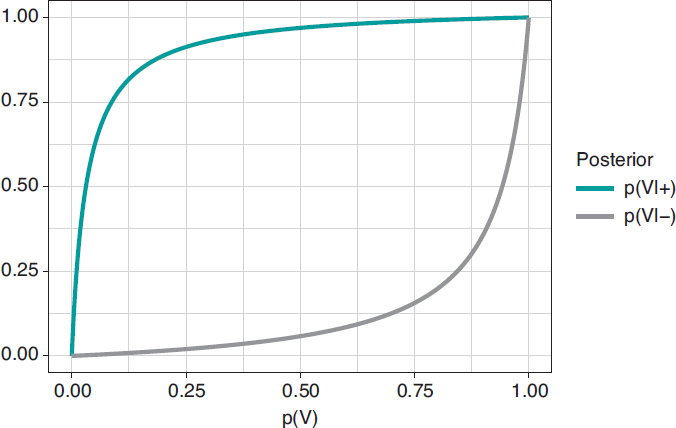
Figure 1. Posterior probability of infection with a positive test result p(V|+) (upper chart) and a negative test result p(V|–) (upper chart) in relation to the prior probability, p(V), of infection.
In principle, it is disconcerting that such a reliable test and with a positive test result for infection generates a small posterior probability, .112 favorable to the infection. However, if we take into consideration that the initial probability of being infected is p(V) = .004 and that, after the positive test result, probability is p(V|+) = .112, we’ll see that it has gone up from 4 to 112 by a thousand—has multiplied by a factor of 28—and we believe that the influence of the test result in such posterior probability is more relevant. Anyways, we would, at least, need a second test to increase the evidence for or against the infection.
Figure 2 shows 2 charts. The upper curve is the posterior probability of infection when the test is positive, p(V|+). The lower curve is the probability of infection too, but with a negative test result, p(V|–). In both cases, such posterior probabilities are represented in terms of prior probability, p(V), of being infected. When p(V) is close to 0, as it is the case with this example, the probability p(V|+) goes up a lot although, in absolute terms, it remains very low. On the contrary, when p(V) is close to 1, the probability p(V|–) will still be high despite evidence against a very reliable negative test result. The main element to understand this situation is that posterior probability, p(V|+) = .112, combines a very small probability of having an infection with a very high probability of testing positive when infected.
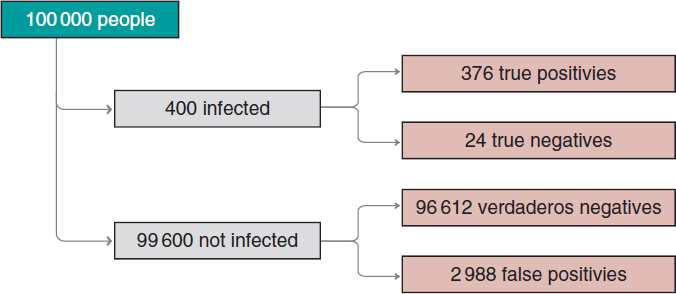
Figure 2. Approximate number of people infected, not infected, true positives, true negatives, false positives, and false negatives in a population of 100 000 inhabitants with an early prevalence for infection of .004 and a 94% sensitivity test and a 97% specificity test.
We’ll move on now to assess our results. The prevalence of infection, p(V) = .004 indicates that in a population of 100 000 people we should be expecting around 400 people infected and, approximately, 99 600 people not infected (figure 2). If the entire population were tested, we would expect to see that around 376 of the 400 people infected would test positive (true positives), as opposed to 24 (false negatives). In the group of healthy people, around 96 612 people would test negative (true negatives), but nearly 2988 people would test positive (false positives). If we looked at the number of people who tested positive, we’d have around 376 true positives, and 2988 false positives. Therefore, most people with a positive test (nearly 89%) would not actually be infected.
A second test with a positive result too would provide further evidence favorable to the infection. Its probability should be updated including the positive result of the second test as new information. If now (+1) and (+2) represent a positive test result for the first and second tests, the relevant probability would be p(V|+1,+2). The sequential use of Bayes’ theorem allows us to estimate such probability considering p(V|+1) = .112 as prior probability. The result obtained, p(V|+1,+2) = .798, is meaningful evidence favorable to an infection after 2 positive test results.
PARAMETER ESTIMATE
The true protagonists of basic Bayesian studies are probabilistic models governed by unknown parameters. These are at the core of Bayesian inferential machinery. We should mention that a parameter is a characteristic of a statistical population under study. Examples of parameters are the percentage of effectiveness of any given drug, the 5-year survival rate of soft tissue sarcoma, the basic reproduction factor R0 of an infection, etc. Parameters are estimated using partial information of the study population from data samples obtained through random procedures that guarantee their representativity and small population condition.
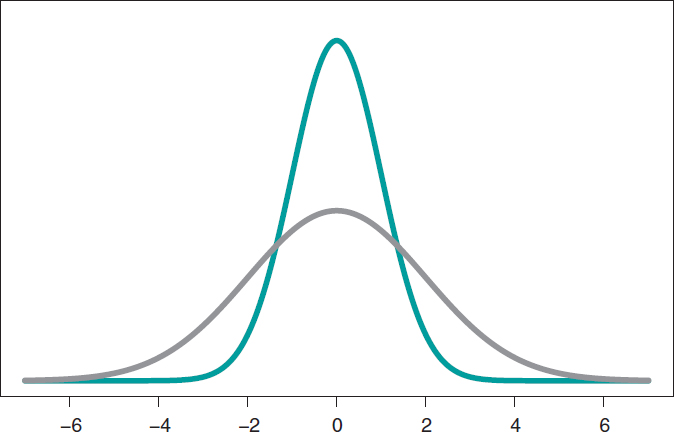
The most widely known probabilistic model is normal distribution with a dome-shaped symmetrical density function and defined by 2 different parameters, mean, µ, and standard deviation (σ). Mean is the center of gravity of distribution and corresponds to the peak of the dome. Standard deviation is a dispersion measurement that determines the width of the dome: in all normal distributions, the interval (µ − 3σ, µ + 3σ) includes 99.7% of the values of distribution. Therefore, the interval-related probability (−3, 3) in a normal distribution with mean = 0 and standard deviation = 1 would be the same as the one associated with the interval (−6, +6) of a normal distribution with mean = 0 and standard deviation = 2 (figure 3).
Figure 3. Chart of normal density with mean of 0, and standard deviation of 1 (green color), and normal density with mean of 0, and standard deviation of 2 (gray color).
Mean and standard deviation are unknown parameters in most studies based on normal data. In our case, and to avoid any technical complications, we’ll assume that the standard deviation is known. Therefore, the statistical process will only have eyes from the mean µ. Bayes’ theorem adapts itself to the territory of probability distributions with focus on the population mean symbol µ as parameter of interest according to the following formula
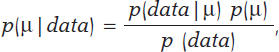
being p(μ) the previous distribution (or prior distribution) of μ that quantifies, in probabilistic terms, the initial information available on μ and p(μ | data), the posterior distribution of µ that contains the information on µ available when the initial information is added to data. The term p(data | μ) is the verisimilitude function of μ, a measurement that assesses the compatibility of data with the possible μ values. The element p(data) is the previous predictive distribution (also evidence in the automatic and machine learning setting), and assesses the plausibility of the data obtained.
Example II: the heart of boys and girls with spinal muscular atrophy
Falsaperla et al.6 present the results of an observational study on the heart electrical conduction system disorder that causes bradycardia or electrocardiographic abnormalities in boys and girls with spinal muscular atrophy type 1 and 2 (SMA1, and SMA2, respectively). We gained our inspiration from this study to build a simulated database. Therefore, the results from this example do not come from Falsaperla et al.6 and should not be compared to those from the original study.
We simulated the data on the PR interval length that extends from the origin of atrial depolarization until the origin of ventricular depolarization from 14 children with SMA2. We assumed a normal model with unknown measurement and known standard deviation. Out statistical goal was to estimate the mean.
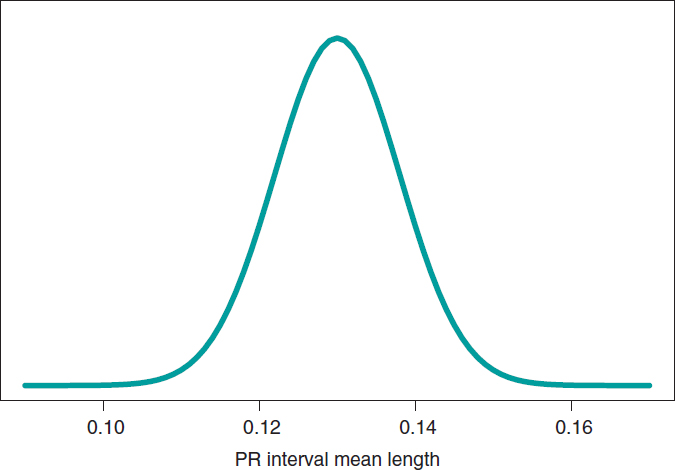
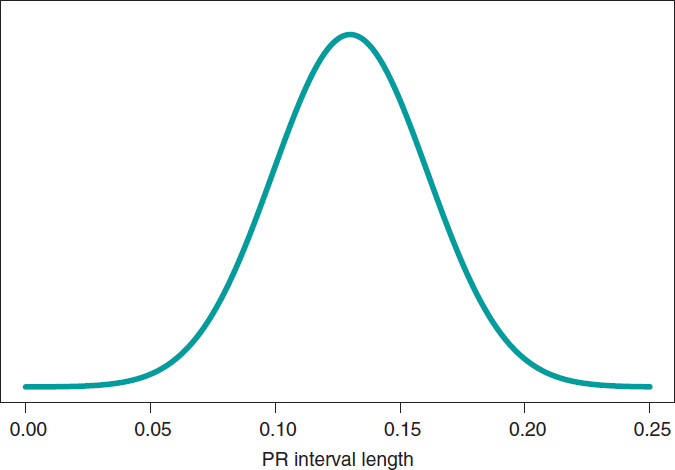
We’ll go on now with the Bayesian protocol. First, we need a previous distribution p(μ) to express our information of such parameter. Afterwards, we consider a scenario without new information on μ except for the information provided by data and use Jeffreys Prior to treat all possible μ values the same way.7 The posterior distribution of μ, p(μ | data), is a normal distribution with a mean of .13, and a standard deviation of .03/√∙14 seconds that we can graphically see on figure 4. We estimate that μ is .13 seconds, and directly assess the accuracy of such estimate through a credibility interval that tells us that the posterior probability of μ will be taking values between .114 and .146 seconds is .95. We give it a very low probability of .05 that μ will be > .146 or < .114.
Figure 4. Posterior distribution of the PR interval mean length in children with spinal muscular atrophy type 2.
A frequentist analysis of this data would never allow direct probabilistic assessments of μ. A frequentist 95% confidence interval for μ would provide the same numerical results compared to the Bayesian interval. However, it should be interpreted in a completely different way. The frequentist 95% confidence interval is on the capacity of the interval to include μ true value, and not on the possible μ values. The interval built, (.114, .146), has a .95 probability of capturing μ true value, but also a .05 probability of not doing so. We should remember that the frequentist concept of probability prevents allocating probabilities to parameters and establishing direct probabilistic assessments of μ.
PREDICTING NEW OBSERVATIONS
Prediction and estimation are fundamental statistical concepts. We estimate parameters, but we predict data and experimental results always through distributions of probability.
The posterior predictive distribution of the results of a future experiment is built by combining the probabilistic model that correlates future data and parameters with posterior distribution. A significant aspect of predictive process regarding estimation is its greater uncertainty. Overall, the precision of estimations improves with larger samples, which means that in the hypothetical case of having all the data available, our estimation would be accurate. The process of prediction does not have such a feature. Although the precision of predictions increases parallel to the size of the sample, in the hypothetical case of having access to all the information from a population, error-free predictions would still be impossible.
Example II: the heart of boys and girls with muscular atrophy (continues)
In the estimation stage we studied the PR interval mean length in girls and boys with SMA2, learning based on a sample of 14 simulated data. Now we’ll be dealing with totally different situation. We have a kid with SMA2 who did not participate in the study. Our objective is to predict his PR interval length. The goal now is not to estimate population means with SMA2, but to predict the value of the PR interval length in a particular kid.
Figure 5 shows the posterior predictive distribution of the PR interval length of a new boy with SMA2. Although this prediction is based on information from 14 children from the sample, it is about a new boy with SMA2. The anticipated value of this new child’s PR interval length is .13 seconds. The accuracy of prediction is quantified through prediction intervals. In this case with a .95 probability, the anticipated value will be between .069 and .191 seconds.
Figure 5. Posterior predictive distribution of the PR interval length of a new child with spinal muscular atrophy type 2.
SIMULATION AND GROUP COMPARISON
The Bayesian protocol with the 3 basic elements, previous distribution, verisimilitude function, and posterior distribution is common to almost all kinds of settings, both the basic ones including some parameters, as well as the complex ones with many sources of uncertainty with complex hierarchical structures. It is a robust and easy-to-use protocol, and a powerful and appealing idea.
Difficulties appear if we want to extrapolate this protocol to real studies of certain complexity. It is in just a couple of these cases that an analytical expression for the posterior distribution of parameters can be achieved. Impossible, nonetheless, for posterior distributions associated with derived quantities of interest. In most studies, mathematics becomes complicated, and posterior distributions are difficult to obtain. In these cases, the MCMC methods come to the rescue of Bayesian analysis. They can simulate our estimates of the relevant posterior distribution and from them generate the inferences or predictions required by the study.
In the following example we’ll be showing the most basic situation we have discussed: starting with a target posterior (analytical) distribution we’ll be simulating posterior distributions of relevant, non-analytical quantities of interest.
Example III: acute myocardial infarction and stents
The following example has been inspired by Iglesias et al.8. This is a study of 1300 patients with acute myocardial infarction treated with percutaneous coronary intervention. Each patient was randomized to sirolimus-eluting stent implantation with degradable polymer (group S) or everolimus-eluting stent implantation with durable polymer (group E).
We compared both treatments in relation to the rate of deaths 12 months after treatment. A total of 35 out of the 649 patients from group S stopped treatment or were lost within first year of follow-up, and 24 died. In group E, initially with 651 patients, 25 were lost or stopped treatment, and 22 died. The presence of missing data due to losses to follow-up is an important issue that should be dealt with carefully. In this case we’ll omit it because our goal is to illustrate Bayesian procedures using the least possible technicalities.
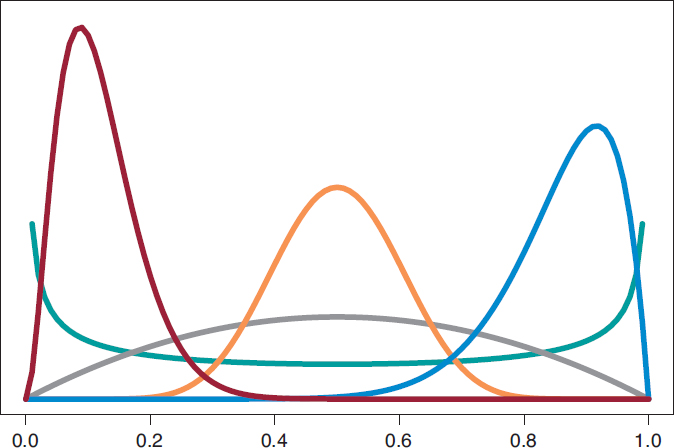
We’ll start by analyzing the risk of death θs and θE in groups S and E, respectively 1 year into treatment. Since anybody from either one of the 2 groups can die, or not, within the first year of treatment, in each group, the probabilistic model is binomial distribution that will be describing the number of deaths reported. The risk of death from each group is a rate with values that range between 0 and 1. For each rate we’ll be selecting beta distribution because it’s the proper probabilistic model to use with rates and doesn’t pose any estimate difficulties. β distribution (that we’ll representi as Be(α, β)) has 2 different parameters, α > 0 and β > 0, that determine the way of the distribution as well as its mean and variance. It is a flexible distribution that can be symmetrical or asymmetrical, positive or negative (figure 6).
Figure 6. Chart of β densities: Be(.5, .5) in green color, Be(2, 2) in gray color, Be(3, 22) in maroon color, Be(12, 2) in blue color, and Be(12, 12) in pumpkin color.
The prior β distribution that best describes the lack of information is Be(.5, .5). Its justification only responds to theoretical criteria. In each group, the posterior distribution of the risk of death will also be β whose updated parameters can be obtained by adding the number of deaths and the number of people alive in the study to the 2 values 0.5 and 0.5 of the prior beta distribution:
p(θs) = Be(.5, .5); p(θs | data) = Be(24.5, 590.5),
p(θE) = Be(.5, .5); p(θE | data) = Be(22.5, 604.5).
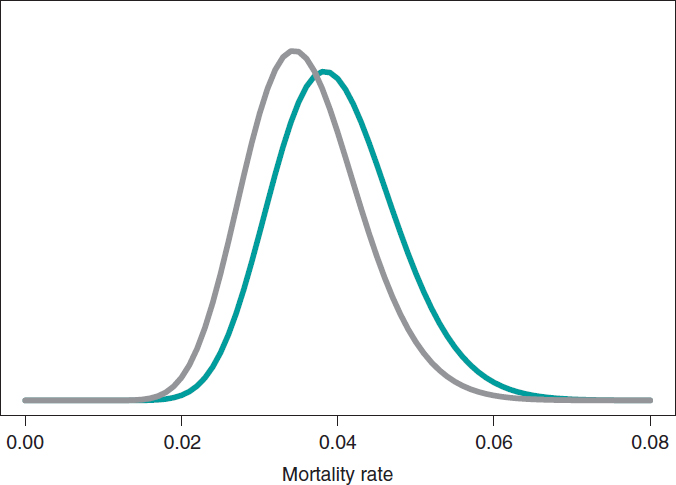
The estimation of the risk of death in patients from groups S and E is the mean of its posterior distribution, .040 and .036, respectively. Also, with a .95 probability the risk of death of group S is between .026 and .057, and between .023 and .052 in group E. These results indicate that the rate of death from both groups is small, although slightly higher in group S. The 95% credibility interval—both of θs and θEis very informative (figure 7).
Figure 7. Posterior distribution of the mortality rate reported in patients from group S (green color) and group E (gray color).
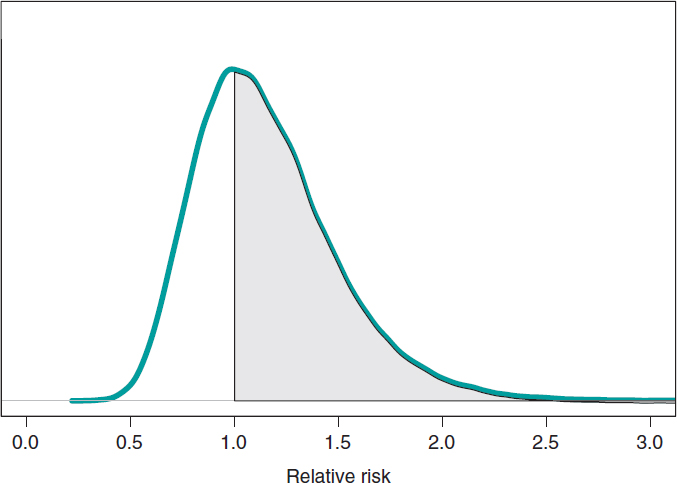
We assume that our goal is to compare the 1-year mortality risk in both groups. Although the tool we could think of first is hypothesis testing (that we’ll introduce later on), the epidemiological and statistical literature on this regard is abundant, and 2 groups are often compared through relative risk (RR) or absolute risk (AR).9 The 1-year RR of mortality in patients with type S stents vs patients with type E stents is RR = θs/θE, a ratio between 2 rates. RR values < 1 are indicative that the mortality rate from group S is lower compared to group E while values > 1 are indicative of precisely the opposite. Since RR is defined through θs and θE, the information from both rates, expressed through its posterior distribution, can propagate to RR as a posterior probability distribution, p(RR | data) (figure 8). This distribution is not analytical, but it can come close to it through a Monte Carlo simulation from the 2 posterior distributions p(θs | data) and p(θE | data). Using this approximation, the posterior RR mean is 1.160, its standard deviation, .346, and the posterior probability of RR being >1 is .641. An analogue frequentist analysis would be more complicated from the mathematical standpoint and would not provide a direct probabilistic assessment of RR.
Figure 8. Posterior distribution of the relative risk of mortality at 1 year in patients with S-type stents vs patients with E-type stents. The shadow region is the probability, .641, that the mortality rate of group S is higher compared to group E.
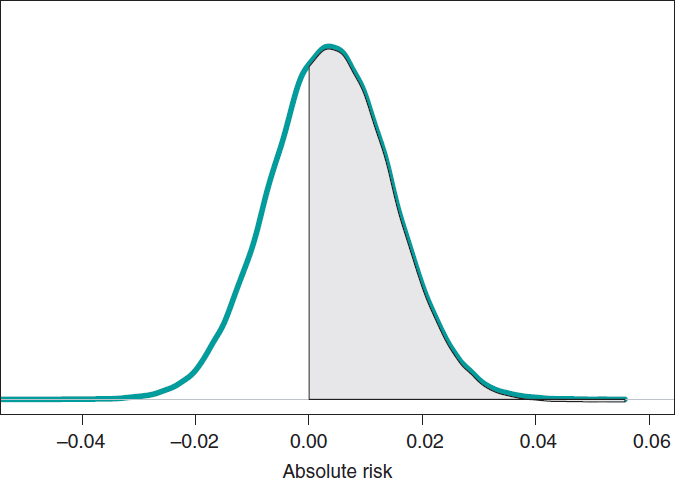
If we compare both groups through the AR of mortality at 1 year, our goal would be AR = θs-θE. This is a difference between 2 rates that could take values between −1 and 1. Negative values will be indicative that the mortality rate of group E is higher compared to groups S while positive values will indicate just the opposite. figure 9 shows the AR posterior distribution.
Figure 9. Posterior distribution of the absolute risk of mortality at 1 year in patients with S-type stents vs patients with E-type stents. The shadow region is the probability, .641, that the mortality rate of group S is higher compared to group E.
The posterior AR mean is .004, its standard deviation, .011, and with a .641 probability, the AR will be > 0.
HYPOTHESIS TESTING: FREQUENTIST P VALUES
Hypothesis testing is the topic that generates the most irreconcilable differences between the Bayesian and the frequentist scientific communities because it is here where the consequences of their different concepts of probability become more evident. Testing hypothesis means testing new theories. Most of the latest theories have a quiet appearance among the scientific community, but little by little they start accumulating evidence in their favor until the sitting evidence is debunked.
The most widely known and used concept in frequentist statistics is the P value, as well as its .05 value that in some studies appears as the magical number used to reject or accept hypotheses or scientific theories. P value is another tool in the frequentist inference armamentarium that simply does not exist in the Bayesian one regarding the testing of 2 different hypotheses: the null hypothesis H0 (that often represents the sitting scientific theory), and the alternative hypothesis H1 (the new theory). P values are always associated with data because without the latter there are no P values. Under these conditions, P value is the probability that a certain theoretical summary of data will be equal to the one observed or more incompatible with the null hypothesis supposing that such hypothesis holds true. Such compatibility is often represented by the P = .05 threshold. P values ≥ .05 keep confidence in the null hypothesis; P values < .05, however, are favorable to the alternative one.
The excessive, and sometimes, inappropriate use of P values in scientific studies is still under discussion in the statistical community. It started in small scientific circles, but the use of the P value soon became a somehow «magical» element rather than a scientific tool. Back in 2014, the American Statistical Association, one of the world’s leading statistical societies, approached this issue and drafted a document that has become the go-to guideline on this topic.10 The following ones are some of the conclusions on significant P values for the management of biometrical data:
- They are a probabilistic measurement of the compatibility of data with the null hypothesis. Smaller P values are associated with more data incompatibility with such hypothesis.
- Do not assess the probability that a hypothesis will hold true or not.
- The conclusions of a study should not only be based on whether a given P value exceeds this or that threshold. The use of the expression «statistically significant» (P < .05) to establish conclusions distorts all scientific procedures.
- Do not measure the size of an effect or the significance of a given result. All small effects can produce small P values when the size of the sample or the accuracy of measurements is big, and all big effects can generate big P values with small samples or imprecise observations.
The P value has been given an unfair treatment because it has been attributed fantastical and surreal properties that have turned against it. Controversy has shattered the scientific debate and encouraged criticism in scientific disciplines that use data to generate knowledge. The huge interest in today’s scientific reproducibility topics owes volumes to this debate.11-15
ACCUMULATING EVIDENCE FOR THE PROBABILISTIC ASSESSMENT OF NEW THEORIES
The Bayesian concept of probability is the key element to put hypotheses and theories to the test because it allows us to assign direct probabilities to both hypotheses and theories, both prior, p(theory holds true) and posterior, p(theory holds true|data ).16
Frequentist statistics is based on hypothesis testing using p-type probabilities (data|theory holds true) while Bayesian statistics is based on p-type probabilities (theory holds true|data). The p-type(data|theory holds true) frequentist probability assumes that the theory tested holds true, and based on that assumption assesses the concordance of data with such hypothesis. The p-type(theory holds true|data) Bayesian probability probabilistically assesses the certainty of the theory being tested in association with the data obtained.
The fundamental tool of Bayesian statistics to choose between hypotheses
H0: theory #1 holds true,
H1: theory #2 holds true,
based on a dataset is Bayes factor,17 the ratio between the probabilities associated with data according to both theories. It can also be expressed as the ratio between posterior odds (p(theory #1 holds true|data )/p(theory #2 holds true|data)) favorable to the certainty of theory #1 compared to theory #2 and the corresponding prior odds (p(theory #1 holds true)/p(theory #2 holds true). Like this:
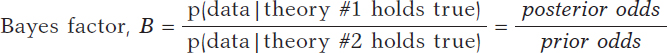
Bayes factor (B) holds evidence favorable to the certainty of theory #1 (compared to theory #2) provided by data: it turns prior probabilities into posterior probabilities. In logarithmic scale, log (B), the Bayes factor is also known as «weight of evidence», a term coined by Turing back in Bletchley Park during Second World War. Small Bayes factor values give little support to H0 vs H1; however, big Bayes factor values provide extensive support to H0.
Example I: Infections and tests (continues)
Let’s go back to the data from example I: Vallivana needs to be diagnosed on an infection with 2 positive test results. This problem can be faced as 2 hypotheses being tested:
H0: Vallibana has an infection
H1: Vallibana doesn’t have an infection
We assume that Vallibana does not have any particular characteristics that give her a probability of infection different from the remainder of the population. Therefore, we know that p(Vallibana has an infection) = .004, and p(Vallibana doesn’t have an infection) = .996. Vallibana’s prior odds favorrable to the infection compared to non-infection are:
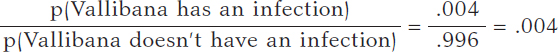
Vallibana takes the test, and it turns out positive (+1). She decides to retake it and tests positive again (+2.). Vallibana’s posterior odds favorable to the infection compared to non-infection are:
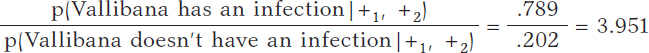
The Bayes factor in favor of Vallibana being infected, that is, the ratio between the posterior odds and the prior odds is 987.75. Indeed, this value provides strong evidence in favor of Vallibana being infected (+).
Example II: the heart of boys and girls with spinal muscular atrophy (continues)
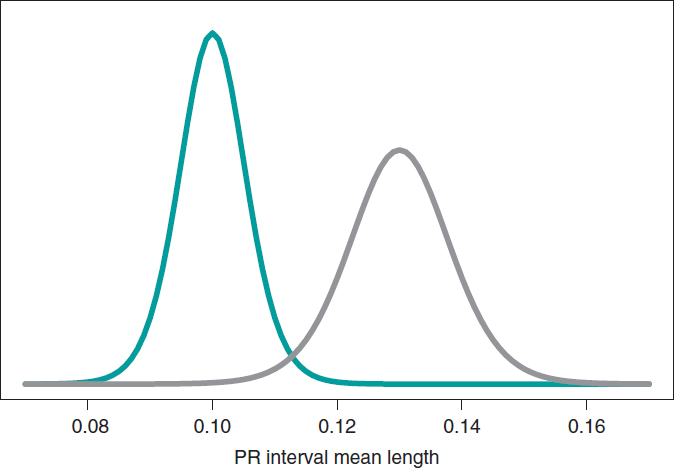
Now let’s go back to the study conducted by Falsaperla et al.6 from example II that aimed to compare the PR interval mean length in girls and boys with SMA1 and SMA2—that we’ll refer to as μ1 and μ2, respectively—through hypothesis testing:
H0: μ2 ≤ μ1
H1: μ2 > μ1
where the null hypothesis, H0, claims that the PR interval mean length in girls and boys with SMA2 is shorter or equal to that reported in children with SMA1. The alternative hypothesis H1 says otherwise. Here we’ll be working with simulated normal data in both groups: n1 = 14 observations in the SMA1 group with sample mean and standard deviation values of .10 and .02 seconds, respectively, and n2 = 14 observations in the SMA2 group with sample mean and standard deviation values of.13 and .03 seconds, respectively.
What we´ll do is build an inferential process for the mean of each group separately. In both cases, we’ll be considering a neutral previous distribution that gives all prominence to data. figure 10 shows the posterior distribution of the mean of each group. Both distributions are rather separate from one another, which means that the posterior probabilities associated with each hypothesis will be very different as well: .002 for H0, and .998 for H1.
Figure 10. Posterior distribution of the PR interval mean length in girls and boys with spinal muscular atrophy type 1 (green color), and type 2 (gray color).
p(H0|data) = p(μ2 ≤ μ1|data) = .002
p(H1|data) = p(μ2 > μ1|data) = .998
On a roughly basis, it is 500 times more likely that H1 will hold true than not. In light of such an overwhelming piece of evidence the wise decision would be to choose H1. The frequentist treatment of this testing is based on the P value. In our case, we’d obtain a P value of .002, which would imply rejecting the null hypothesis in favor of the alternative one. Both methodologies propose the same decision and provide the same numerical results: probabilities of .002. However, both probabilities are conceptually different. Bayesian probability tests the null hypothesis based on the data reported. Frequentist probability assesses the data observed with the assumption that the null hypothesis will hold true.
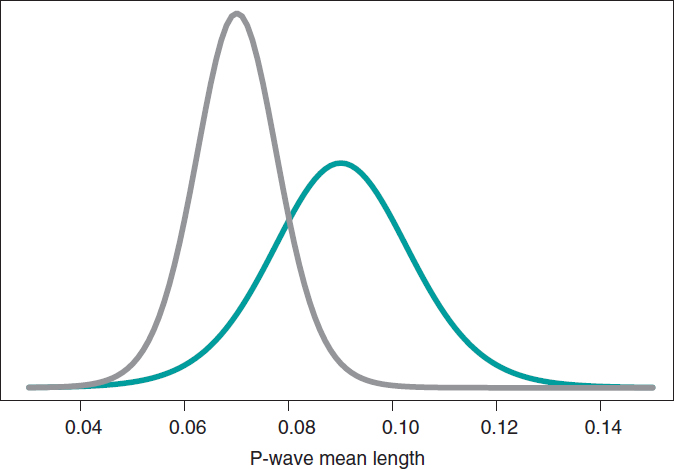
Still following in the footsteps of Falsaperla et al.6 we wish to mention that our examples are not based on original cases. They are merely illustrative of Bayesian procedures. We’ll now be working with the P-wave on the electrocardiogram. We wish to compare the P-wave mean length in children with SMA1 and SMA2. We’ll be simulating 14 observations of the P-wave length in the group of children with SMA1 and compared it to children with SMAs. The sample mean and standard deviation is .09 and .05 seconds in group SMA1, respectively, and .07 and .03 seconds in group SMA2. We’ll be comparing the means of both groups through hypothesis testing:
H0: mean P-wave in SMA1 = mean P-wave in SMA2
H1: mean P-wave in SMA1 > mean P-wave in SMA2
based on Bayesian inferential process like the one from the previous example. figure 11 shows the posterior distribution of the P-wave mean length in both groups. There are fewer data from the group of girls and boys with SMA2 compared to the group with SMA1. The posterior probability associated with each hypothesis is:
p(H0 | data) = p(mean P-wave in SMA1 ≤ mean P-wave in SMA2 | data)
p(H1 | data) = p(mean P-wave in SMA1 > mean P-wave in SMA2 | data)
These results provide a significant piece of evidence favorable to the alternative hypothesis that is almost 8 times more likely than H0. From the frequentist standpoint, the P value associated with data would be .107 (>.05), which is why we would conclude that data does not provide enough evidence to reject H0. Bayesian decision could perfectly be the same. However, the Bayesian analysis provides a direct assessment on the certainty of both hypotheses. The findings from the Bayesian analysis could be used as previous information in future studies with more data. Therefore, the posterior distributions obtained (figure 11) would be prior distributions in this new study. Bayes’ theorem allows us to generate knowledge sequentially searching for evidence for or against different hypothesis.
Figure 11. Posterior distribution of the P-wave mean length in girls and boys with spinal muscular atrophy type 1 (green color), and type 2 (gray color).
CARDIOLOGY POSES SOME OF DOUBTS ON THE IMPLEMENTATION OF METHODOLOGY IN CLINICAL TRIALS
One of the most controversial topics in Bayesian methodology is the selection of previous distributions. A Bayesian analysis will always allow us to avoid using any information on the amount of interest not provided by data. In this case, it works with previous distributions that play a neutral role in the learning process and that are useful only as the starting point of the Bayesian inferential protocol.
Previous informative distributions contain information that adds to the one provided by data like expert knowledge18-20 or results from previous studies.21,22 It is a highly valuable Bayesian characteristic in studies on which data is scarce like studies on rare diseases and orphan drugs. We should mention that inferential processes based on previous informative distributions should include sensitivity analyses of the results obtained regarding previously used distribution or distributions. Similarly, it has become popular to consider communities of previous distributions with diverse previous distributions—and a certain degree of skepticism or enthusiasm—with the effect under test because they provide a scientific framework of reference.
On many occasions, in clinical trials, the use of previous informative distributions reduces the sizes of frequentist samples based on preassigned values of the test power, and previous parameter estimates.22 An example of this situation is the BIOSTEMI trial.8 The 1300 patient-sample was estimated using Bayesian methods through a previous robust distribution as a mixture that included—in equal proportion—historical information of 407 patients from the BIOSCIENCE trial,23 and a practically non-informative distribution. The flexibility of Bayesian sequential learning is a key element of the so-called adaptative Bayesian designs24 that allow us to include additional information in different phases of the trial without damaging the consistency and reliability of results.
FUNDING
This study was partially funded by Biotronik Spain S. A, and by project PID2019-106341GB-I00 from the Spanish Ministry of Science and Innovation, and Universities of the Spanish Government.
AUTHORS’ CONTRIBUTIONS
C. Armero was involved in the structure, content, and drafting of this manuscript; P. Rodríguez, and J.M. de la Torre Hernández were actively involved in the review process of the manuscript final version.
CONFLICTS OF INTEREST
C. Armero, P. Rodríguez, and José M. de la Torre Hernández declared no conflicts of interest regarding the content, authorship, and publication of this manuscript. J.M. de la Torre Hernández is the editor-in-chief of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
REFERENCES
1. Hawking S. A Brief History of Time: The Origin and Fate of the Universe. New York: Bantam; 1988.
2. McGrayne SB. La teoría que nunca murió: De cómo la regla de Bayes permitió descifrar el código Enigma, perseguir los submarinos rusos y emerger triunfante de dos siglos de controversia. Barcelona: Crítica; 2012.
3. Metropolis N, Rosenbluth A, Rosenbluth M, Teller A, Teller E. Equations of state calculations by fast computing machines. J Chem Phys. 1953;21:
1087-1092.
4. Robert CP, Casella G. A Short History of Markov Chain Monte Carlo: Subjective Recollections from Incomplete Data. Stat Sci. 2011;26:102-115.
5. Gelfand AE, Smith AFM. Sampling-based approaches to calculating marginal densities. J Am Stat Assoc. 1990;85:398-409.
6. Falsaperla R, Vitaliti G, Collotta AD, et al. Electrocardiographic Evaluation in Patients With Spinal Muscular Atrophy: A Case-Control Study. J Child Neurol. 2018;33:487-492.
7. Robert CP, Chopin N, Rousseau J. Harold Jeffreys’s Theory of Probability Revisited. Stat Sci. 2009;24:141-172.
8. Iglesias JF, Muller O, Heg D, et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet. 2019;394:1243-1253.
9. Christensen R, Johnson W, Branscum A, Hanson TE. Bayesian Ideas and Data Analysis: An Introduction for Scientists and Statisticians. Boca Raton: CRC Press; 2011.
10. Wasserstein RL, Lazar NA. The ASA Statement on p-Values: Context, Process, and Purpose. Am Stat. 2016;70:129-133.
11. Goodman SN. Toward Evidence-Based Medical Statistics. 1: The P Value Fallacy. Ann Intern Med. 1999;130:995-1004.
12. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337-350.
13. Halsey LG, Curran-Everett D, Vowler SL, Drummond GB. The fickle P value generates irreproducible results. Nat Methods. 2015;12:179-185.
14. Ioannidis JPA. Why Most Published Research Findings Are False. PLoS Med. 2005;2:e124.
15. Ioannidis JPA. The Proposal to Lower P Value Thresholds to .005. J Am Med Assoc. 2018;319:1429-1430.
16. Kruschke JK. Doing Bayesian data analysis: A Tutorial with R, JAGS, and Stan. 2nd ed. Amsterdam: Academic Press/Elsevier; 2015.
17. Kass RE, Raftery AE. Bayes Factors. J Am Stat Assoc. 1995;90:773-795.
18. Hampson LV, Whitehead J, Eleftheriou D, et al. Elicitation of Expert Prior Opinion: Application to the MYPAN Trial in Childhood Polyarteritis Nodosa. PLoS One. 2015;10:e0120981.
19. Mason AJ, Gomes M, Grieve R, Ulug P, Powell JT, Carpenter J. Development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes: Application to the IMPROVE trial. Clin Trials. 2017;14:357-367.
20. Jansen JO, Wang H, Holcomb JB, et al. Elicitation of prior probability distributions for a proposed Bayesian randomized clinical trial of whole blood for trauma resuscitation. Transfusion. 2020;60:498-506.
21. Grant RL. The uptake of Bayesian methods in biomedical meta-analyses: A scoping review (2005–2016). J Evidence-Based Med. 2019;12:69-75.
22. Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Chichester: Wiley; 2004.
23. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non- inferiority trial. Lancet. 2014;384:2111-2122.
24. Schmidli H, Gsteiger S, Roychoudhury A, O’Hagan A, Spiegelhalter D, Neuenschwander B. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics. 2014;70:1023-1032.
ABSTRACT
Hypertension is the most prevalent cardiovascular risk factor. Despite pharmacological treatment, a high percentage of patients do not achieve an adequate blood pressure control. Renal sympathetic denervation is a minimally invasive intervention for the management of hypertension involving the interruption of the renal artery sympathetic nervous system using a catheter-based approach. The early studies showed promising results, but the controversial results coming from the SYMPLICITY HTN-3 trial sent this technique into oblivion. Over the last 3 years, new clinical trials have appeared including new devices used in different populations, which definitively proves the effectiveness of renal sympathetic denervation.
This joint position statement from the Spanish Society of Hypertension-Spanish League for Combating High Blood Pressure (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) reviews the evidence available on the efficacy and safety profile of renal sympathetic denervation for the management of hypertension. Based on the results of clinical trials, recommendations have been established on what patients may be eligible for renal sympathetic denervation and under what circumstances.
Keywords: Hypertension. Renal sympathetic denervation. Blood pressure.
RESUMEN
La hipertensión arterial es el factor de riesgo cardiovascular más prevalente. A pesar del tratamiento farmacológico, un alto porcentaje de pacientes no consiguen un adecuado control. La denervación renal es una intervención mínimamente invasiva para el tratamiento de la hipertensión que implica la interrupción de los nervios simpáticos renales mediante un abordaje con catéter. Los estudios iniciales mostraron resultados prometedores, pero los controvertidos resultados del ensayo SYMPLICITY HTN-3 llevaron al abandono de la técnica. En los últimos 3 años han aparecido los resultados de nuevos ensayos clínicos, con nuevos dispositivos y en diferentes poblaciones, que demuestran definitivamente la eficacia de la denervación renal.
En este documento de posicionamiento conjunto de la Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) y la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) se revisa la evidencia disponible sobre la eficacia y la seguridad de la denervación renal en el tratamiento de la hipertensión. A partir de los resultados de los ensayos clínicos, se generan recomendaciones sobre qué pacientes y en qué condiciones podrían ser candidatos a una denervación renal.
Palabras clave: Hipertension arterial. Denervacion renal. Presion arterial.
Abbreviations
ABPM: ambulatory blood pressure monitoring. BP: blood pressure. DBP: diastolic blood pressure. HMOD: hypertension-mediated organ damage. HTN: hypertension. RSD: renal sympathetic denervation. R-HTN: resistant hypertension. SBP: systolic blood pressure.
INTRODUCTION
The role of the sympathetic nervous system in the pathophysiology of hypertension (HTN) is well known. In 2007, to address the unmet need of patients with resistant HTN (R-HTN), the first percutaneous renal sympathetic denervation (RSD) procedures were performed. First observational studies showed positive results, and the use of RSD started in select centers around the world.1,2 However, in 2014, the publication of a study which did not demonstrate a greater efficacy of RSD vs a sham-control group to control blood pressure (BP)3 dramatically reduced the interest of the scientific community in this procedure, as well as in its clinical application. An increased knowledge of renal anatomy combined with the development of second-generation devices has led to new studies, in which the efficacy of RSD vs a sham-control group has been demonstrated.4-7 Although the road ahead is long, the new evidence provides a clear role for RSD in the management of patients with HTN.
The clinical practice guidelines on the management of hypertension published by the European Society of Cardiology and European Society of Hypertension (ESC/ESH) back in 2018 outlined the role of device-based approaches for the management of HTN in the context of clinical trials only.8 The practical effect of this is that it discouraged the use of RSD. Despite the short time that has gone by since the publication of these guidelines, the data provided by the new clinical trials would justify treating selected patients with RSD.
This document reviews the evidence available on RSD for the management of HTN, analyzes possible indications, and suggests strategies to identify potentially eligible patients, formulated from the opinion of a panel of experts selected by the Spanish Society of Arterial Hypertension-Spanish League for the fight against Arterial Hypertension (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC). The writing of the document was carried out by professionals proposed by these scientific societies undersigning this work following their experience in the management of patients treated with RSD. After the first draft, other experts with and without previous experience in RSD carried out a critical review of the document, and agreed on the changes that were deemed appropriate.
CLINICAL EVIDENCE ON THE ROLE OF RENAL SYMPATHIC DENERVATION IN THE MANAGEMENT OF HYPERTENSION
The supplementary data shows the epidemiological characteristics of HTN (section 1), as well as the role of sympathetic nervous system in the management of HTN (section 2), thus improving our understanding of clinical trials. Table 1 of the supplementary data shows the main studies in which the efficacy of RSD has been assessed.
Table 1. Studies prior to renal sympathetic denervation in patients with uncontrolled hypertension
| Evaluation of pharmacological treatment |
| Type and number of drugs |
| Drug adequate dosage |
| Assess use of aldosterone antagonist |
| Assess lack of therapy compliance |
| Assess intolerance to drug therapy |
| 24-hour ABPM study |
| Rule out pseudo-resistant hypertension or white coat effect |
| Confirm uncontrolled hypertension (SBP > 130 mmHg/DBP > 80 mmHg at the 24-hour levels or SBP > 135/DBP > 85 in the day’s levels) |
| Rule out secondary causes of hypertension (table 2) |
| Cardiovascular risk assessment |
| Coexistence of other cardiovascular risk factors such as dyslipidemia, diabetes or smoking |
| Presence of HMOD |
| Presence of established cardiovascular or kidney disease |
| Imaging of the renal anatomy by computerized tomography or nuclear magnetic resonance imaging (assessment of occlusive stenosis, accessory branches, arterial diameter) |
| Complementary tests recommended: |
| Hemogram, renal function parameters, liver and lipid profiles, and urine sediment tests to detect the presence of microalbuminuria |
| Specific analytical determinations: |
| Baseline plasma aldosterone-to-renin ratio |
| Thyroid hormones |
| Calcium-phosphorus metabolism with parathyroid hormone levels |
| Cortisol (basal and 24-hour urine ratios) |
| Catecholamines with 24-hour urinary metanephrines ratio |
| Polysomnography |
|
ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure, HMOD, hypertension-mediated organ damage; SBP, systolic blood pressure. |
Back in 2009, the first study on RSD in patients with R-HTN, the SYMPLICITY HTN-1 trial, was published. This study suggested a high efficacy profile of RSD, and a lower office systolic blood pressure (SBP) down to 27 mmHg at 12 months, without any significant complications being reported.1
The SYMPLICITY HTN-3 trial was the first one to include a control group with a sham procedure and a 24-hour BP endpoint. No differences between the 2 treatment groups in terms of the efficacy profile of BP control in patients with R-HTN were reported at the 6-month follow-up.3 The disagreement between the results of this study and the previous ones, as well as the identification of several confounding factors9 brings up the need for designing new studies specifically aimed at solving these questions.
Definitive evidence on the efficacy of RSD has come from the SPYRAL HTN and RADIANCE-HTN studies. The SPYRAL HTN-ON MED trial enrolled patients with uncontrolled HTN treated with 1 to 3 antihypertensive drugs, randomized to receive RSD or a sham procedure. The 24-hour ambulatory SBP and diastolic BP (DBP) levels, as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 6-month follow-up.4 With a similar design, the SPYRAL HTN-OFF MED Pivotal trial enrolled uncontrolled hypertensive patients with office SBP levels between 150 mmHg and 180 mmHg in the absence of antihypertensive treatment. The 24-hour SBP and office SBP levels were reported at the 3-month follow-up.5 The RADIANCE-HTN SOLO trial enrolled patients with HTN and ambulatory blood pressure monitoring (ABPM) levels ≥ 135/85 mmHg and ≤ 170/105 mmHg without pharmacological treatment. The 24-hour ambulatory SBP and DBP levels as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.6 The RADIANCE-HTN TRIO trial enrolled patients with R-HTN on a fixed-dose, single-pill combination of a calcium channel blocker, an angiotensin receptor blocker, plus a thiazide diuretic, randomized to receive ultrasound catheter-based RSD or a sham procedure. The 24-hour ambulatory SBP and DBP levels, as well as office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.7
Real-life registries have enrolled more than 3500 patients treated with RSD showing lower office BP and ABPM levels. Some registries have demonstrated that the reduction of BP is not associated with the medication burden or with an increased number of antihypertensive drugs. RSD has proven to be safe and has a low rate of complications associated with the procedure.10 The GLOBAL SYMPLICITY registry, with over 2900 patients is the largest and longest duration analysis to this date of renal sympathetic denervation to show the efficacy and safety profile of RSD in a real-life scenario.10 Table 2 of the supplementary data shows a summary of different registries on RSD.
Table 2. Causes of secondary hypertension
| Renal parenchymal diseases | Glomerulopathies Polycystic disease Renal tumors Obstructive uropathy |
| Renovascular diseases | Fibrodysplasia Atherosclerosis |
| Suprarrenal diseases | Primary hyperaldosteronism Cushing’s syndrome 17-alpha-hydroxylase deficiency Pheochromocytoma Apparent excess of mineralocorticoids |
| Vasculares diseases | Aortic coarctation Large vessel vasculitis |
| Endocrine-metabolic | Thyroid dysfunction Hyperparathyroidism Acromegaly |
| Neurological diseases | Dysautonomiay Intracranial hypertension Psychogenic |
| Toxic-pharmacological diseases | Corticosteroids Non-steroidal anti-inflammatory drugs Cyclosporines Tricyclic antidepressants Anovulatory drugs Erythropoietin Licorice Cocaine High doses of caffeine |
| Genetic diseases | Monogenic forms Liddle syndrome |
RSD has been confirmed as a safe intervention. The incidence rate of both immediate complications associated with the procedure and renal and vascular complications in the short- and mid-term (6-12 months) is very low and is mainly associated with local problems at the puncture site; serious renal complications (renal artery dissection or stenosis) are anecdotal. Table 3 of the supplementary data summarizes the safety data from the main randomized clinical trials that often have a short-term clinical follow-up.
Table 3. Precautions and contraindications to renal sympathetic denervation
| • Renal sympathetic denervation has not been evaluated in patients who are pregnant, nursing, intend to become pregnant or in patients with type I diabetes mellitus, previous renal angioplasty, indwelling ureteral stents, aortic grafts or abnormal renal anatomy |
| • Subjects in whom a reduction of blood pressure would be considered hazardous (such as those with hemodynamically significant valvular heart disease) |
| • Implantable pacemakers and implantable cardioverter/defibrillators may be adversely affected by radiofrequency ablation. Consider deactivating implantable cardioverter/defibrillators during ablation, have temporary external sources of pacing and defibrillation available during ablation, and perform a complete analysis of the functionality of the device implanted after ablation |
| • Avoid treating arteries with diameters < 3 mm or > 8 mm |
| • Avoid treating arteries with significant disease or flow-limiting obstructions |
POSSIBLE INDICATIONS FOR RENAL SYMPATHETIC DENERVATION WITH DATA FROM THE LATEST CLINICAL TRIALS
Data from both randomized clinical trials and registries prove that the RSD procedure is safe and effective reducing BP, which is consistent across different populations including high-risk subgroups, and with different devices. Section 3 of the supplementary data reviews various consensus documents and recommendations previously published by different scientific societies prior to the publication of the SPYRAL HTN and RANDIANCE-HTN clinical trials.
RSD can be considered in patients with resistant HTN (BP > 140/90 mmHg despite lifestyle changes treated with ≥ 3 antihypertensive drugs at optimal doses, one of them being a diuretic or HTN controlled with ≥ 4 drugs),8 and also in patients with uncontrolled HTN (BP > 140/90 mmHg in patients with poor therapeutic compliance), and high cardiovascular risk.
Renal sympathetic denervation in patients with resistant hypertension
Patients with R-HTN were the first group in whom the role of RSD was assessed. The SYMPLICITY HTN-3 trial failed to demonstrate the increased efficacy of RSD vs sham control in patients with R-HTN.3 However, subsequent analysis revealed design and execution limitations that cast doubts on the reliability of the results.9 In the recently published RADIANCE-HTN TRIO trial, patients with R-HTN treated with a standardized triple combination pill experienced a drop in their BP levels 2 months after RSD compared to a sham procedure.7 If the BP lowering effect and the safety of RSD are maintained in the long term, RSD might be an alternative to the addition of more antihypertensive medications in patients with R-HTN.
Renal sympathetic denervation in patients with uncontrolled hypertension
The new evidence available introduces a paradigm shift for a technique that was initially conceived for the management of R-HTN when all other therapeutic options fail, and is currently an option that should be taken into consideration in patients with persistent BP > 140/90 mmHg despite drug treatment.
The concept of uncontrolled HTN includes a high percentage of hypertensive patients (maybe even > 60%) with highly heterogenous clinical characteristics and cardiovascular risk. Given the invasive nature of the RSD procedure, and until more information becomes available on the reduction of cardiovascular events in more specific subgroups of patients, there are some high-risk situations in which BP control is essential to reduce the risk of cardiovascular events:
a) Patients with frequent hypertensive crises. Hypertensive crises with SBP levels > 180 mmHg and/or DBP levels > 110 mmHg can cause brain, cardiac or microvascular damage. Emergency visits for hypertensive crises exceed 4% of all visits to the emergency room.11 Even in the absence of hypertension-mediated organ damage (HMOD), episodes of hypertensive crisis can have long-term implications to the extent that these patients may have a 50% higher risk of suffering cardiovascular events compared to controlled hypertensive patients. Nonetheless, outside the crisis setting they show similar BP levels.12
b) Patients with low compliance to pharmacological treatment. Pharmacological treatment of HTN is generally a long-term option and in most cases, for life. Poor compliance is a common problem to the extent that almost one third of all hypertensive patients do not start a new prescription of antihypertensive drugs,13 and around 50% become non-compliant within the first year after starting treatment.13 In the SPYRAL HTN trials, the 24-hour ABPM levels showed decreased BP levels throughout the entire 24-hour period in patients treated with RSD compared to no changes in the control group in the absence of drugs or incomplete control in the presence of drugs.4,5 Furthermore, in the SPYRAL HTN OFF-MED trial, the treatment group experienced an average reduction of 9.2 mmHg in office SBP levels.5 A meta-analysis of 123 studies including 613 815 patients showed that a drop of office SBP levels of 10 mmHg was associated with a significantly lower risk of cardiovascular events.14 Poor compliance is a serious problem of public health since these patients in whom an adequate BP control is not achieved, even due to poor therapeutic compliance, have a high cardiovascular risk.15 However, we should stress that RSD alone cannot bring BP levels down enough to achieve BP control in most patients. In the RADIANCE-HTN SOLO trial, the 24-h ABPM only 25% of the patients treated with RSD reached values < 130/80 mmHg.6 In these non-compliant patients, the main strength of RSD is the “always on” effect regardless of pharmacokinetics and compliance to drugs.
c) Patients with hypertension-mediated organ damage. The presence of HMOD identifies a group of patients with high cardiovascular risk in whom conventional treatment has failed to prevent the progression of the disease.16 Achieving the BP levels recommended is especially important in these patients because, in the early stages of the disease, some types of HMOD can be reversed; in more advanced stages, HMOD is irreversible despite adequate BP control. But this is important since it slows its progression while reducing the cardiovascular risk of these high-risk patients.17 A meta-analysis including 698 patients treated with RSD revealed an independent effect of RSD on HMOD, which advocates for the use of RSD in this group of high-risk patients.18
d) Patients at high cardiovascular risk. The European guidelines on the management of HTN establish the factors that influence cardiovascular risk in hypertensive patients including clinical characteristics, analytical characteristics, presence of HMOD or established cardiovascular or kidney disease. All these factors establish a 10-year cardiovascular risk that is categorized into 4 groups: low, moderate, high or very high risk to the extent that, for example, in high-risk patients the estimated cardiovascular mortality is 5% and in very high-risk patients > 10%.8 The assessment of cardiovascular risk should play an important role in the decision-making process to the extent that the higher the risk, the greater the benefits expected with better BP control. Therefore patients at high or very high-risk would be eligible for RSD whenever BP control is not adequate.
Empowering the hypertensive patient in the setting of a shared decision-making process
Over the last few years, shared decision-making process has emerged as the go-to model in the management of different conditions. In the field of RSD, a recent survey revealed that 38% of hypertensive patients who still don’t take antihypertensive medication would prefer RSD to lifelong drug therapy even knowing that it would probably not replace medication in many cases. Just this already reduces BP significantly.19 With the evidence provided in recent trials, RSD could be a valid treatment option in patients with uncontrolled HTN and high to very-high cardiovascular risk in whom, in a shared decision-making process context, consensus with the patient can be reached. In any case, we should mention that the treatment of HTN always requires the adoption of healthy lifestyle habits, and the recommendation to patients should include drug treatment as the first option.
STUDY PRIOR TO RENAL SYMPATHETIC DENERVATION
Patients should be examined in a unit specialized in HTN and vascular risk 3 months prior to the procedure in a center with proven experience.20 Table 1 summarizes the studies to be conducted in patients eligible for RSD.
Uncontrolled HTN should be confirmed through 24-hour ABPM.21 After confirming the presence of uncontrolled HTN, the clinical situations that increase BP levels such as obesity or obstructive sleep apnea should be identified and corrected. Also, substances such as salt or certain drugs that may also lead to HTN should be suspended or minimized. Non-compliance to treatment, which is very common and not always identified by the patient, if not rigorously investigated, should be ruled out.22 It is essential to rule out secondary HTN (table 2) or, if diagnosed, treat it effectively. Still, it is not an absolute contraindication to RSD.23
RENAL SYMPATHETIC DENERVATION PROCEDURE WITH RADIOFREQUENCY DEVICES
Section 4 of the supplementary data shows more in-depth technical aspects of RSD. Figure 1 of the supplementary data summarizes the RSD procedure.
A better knowledge of the anatomy of renal nerves24 and the development of new ablation devices have optimized the treatment technique,5,6 which is based on 3 main objectives:
Figure 1. Detail of renal sympathetic innervation. Renal nerves are usually arranged in large bundles and only form a true plexus when they are close to entering the kidney. Some nerves bypass the main renal artery and join distally to the different arterial divisions of the main renal artery (late arrival nerves). In this case, a late arrival nerve is seen joining the proximal third of the anterior division of the main renal artery (blue asterisk). It can also be seen how the proximal main renal artery is occupied by fused ganglia of the solar plexus (GM), and by the lumbar sympathetic chain (LSC). Both provide innervation to the kidneys, but also to other abdominal and pelvic organs, which can be accidentally denervated if the proximal third of the main renal artery is treated. The image also shows that the maximum proximity of nerve fibers to the arterial wall mainly occurs at branch level, but also at main trunk level. This is the target area of treatment, always avoiding the application of radiofrequency at renal pelvis level. AG, adrenal gland; CT, celiac trunk; GM, ganglionic mass made up of the aorticorenal and celiac ganglia; LSC, lumbar sympathetic chain; RK, right kidney; SMA, superior mesenteric artery; blue asterisk, late arrival nerve. In red, arterial structures. In yellow, nervous tissue.
Management of the renal artery main trunk and branches
It is common for the renal nerves to reach the kidney after bypassing the main renal artery.24 In animal models, it has also been confirmed that the application of combined radiofrequency in the renal artery main trunk and branches reduced the content of norepinephrine in the renal tissue even more, and in the cortical axonal density, both associated with the response to RSD.25
In patients treated with RSD, the presence of untreated accessory arteries leads to a lower hypotensive response.26 Their identification and treatment is essential and, if they are amenable to treatment thanks to their diameter (minimum diameters of 3 mm), the treatment of accessory arteries is advised.
Last but not least, the perivascular space around the ostium and the proximal third of the main renal artery is often occupied by ganglia of solar plexus and by the lumbar sympathetic chain (figure 1). Both carry innervation to the kidneys, but also to other abdominal and pelvic organs, and they could be accidentally denervated if the treatment is applied to the ostium and proximal third of the main renal artery. Therefore, until more information becomes available, it seems reasonable to be cautious when treating the most ostial portion of renal arteries.24
Treatment of the 4 quadrants of the renal artery
The distribution of nerve fibers around the renal artery follows a variable pattern across different individuals.27 Preclinical studies in a porcine model have shown that the application of radiofrequency in one point produces effects on approximately 25% of the arterial circumference,27 and procedures that use multiple helically staggered ablations in the 4 quadrants are more effective reducing the norepinephrine content into the renal tissue.28
Application of the maximum possible number of ablation points
A post-hoc analysis of the SYMPLICITY HTN-3 trial confirmed that patients with a greater number of radiofrequency applications reduced their BP levels even more without any associated adverse events.9 We recommend applying the maximum number of ablation points possible, always respecting a distance of 5 mm among them with a 4-quadrant distribution.
Section 4 of the supplementary data shows how to perform a RSD procedure using a tetrapolar radiofrequency catheter. Table 3 shows the precautions and contraindications regarding RSD.
Care after renal sympathetic denervation procedure
Once the procedure is finished, it is important to ensure adequate hemostasis in the femoral puncture. Usually, in the absence of complications, patients can be discharged after 24 to 48 hours with the same antihypertensive treatment they had before the procedure or with treatment adjustments in cases that show an immediate response, but still with adjustment appointments within 5 to 7 days. Of note, the effects of the intervention can take weeks to materialize.29
CLINICAL MANAGEMENT AFTER RENAL SYMPATHETIC DENERVATION
The main objective of the follow-up should be to confirm the safety of the intervention and the absence of complications in the short-, mid-, and long-term follow-up, as well as to monitor the evolution of BP levels and the adjustment of drug treatment.
At the clinical follow-up, it is important to maintain a multidisciplinary team same as during the selection of candidates. Table 4 shows the clinical management after RSD.
Table 4. Clinical management after renal sympathetic denervation*
| Blood pressure control |
| Home self-measurement of blood pressure is recommended to assess blood pressure decrease |
| Patient education to detect symptoms of hypotension |
| Pharmacological de-escalation, when appropriate |
| 24-hour ambulatory blood pressure monitoring levels at 3-6 months to assess response to RSD |
| 24-hour ambulatory blood pressure monitoring levels to assess long-term durability of renal sympathetic denervation |
| Renal function: in patients at risk of contrast nephropathy, control should follow after 7-10 days (individualize based on the clinical criteria) |
| Routine renal imaging modalities (echocardiography, computed tomography scan, magnetic resonance imaging) are ill-advised |
|
* Control after renal sympathetic denervation should be performed in a hypertension- specific unit as part of a regulated renal sympathetic denervation program. |
REQUIREMENTS OF A RENAL SYMPATHETIC DENERVATION PROGRAM
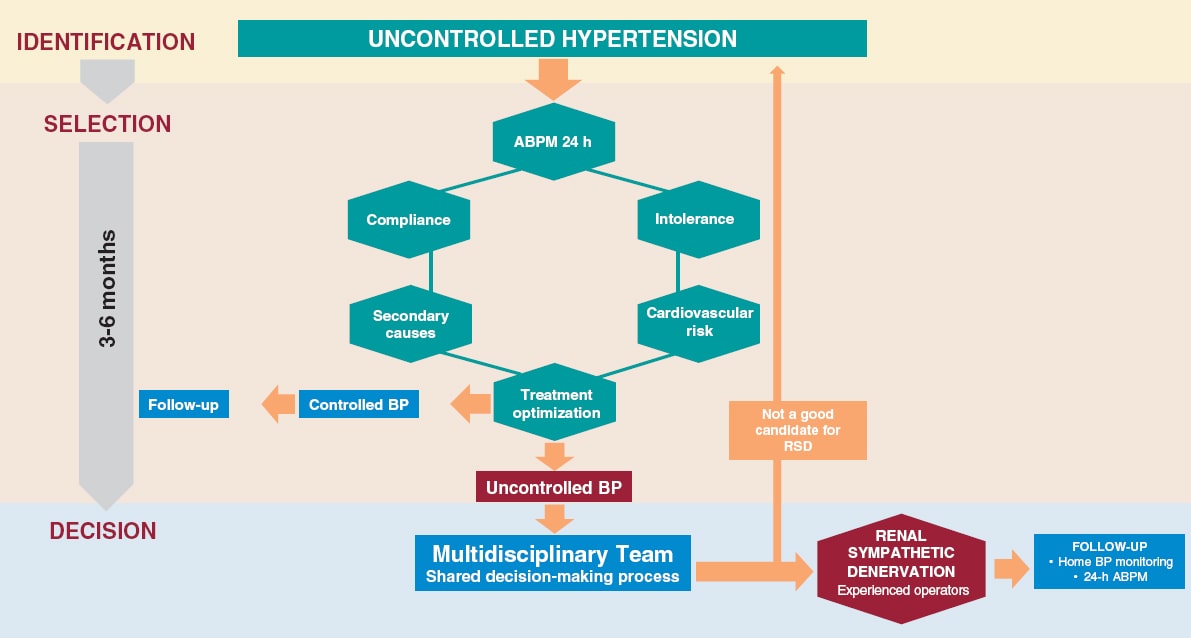
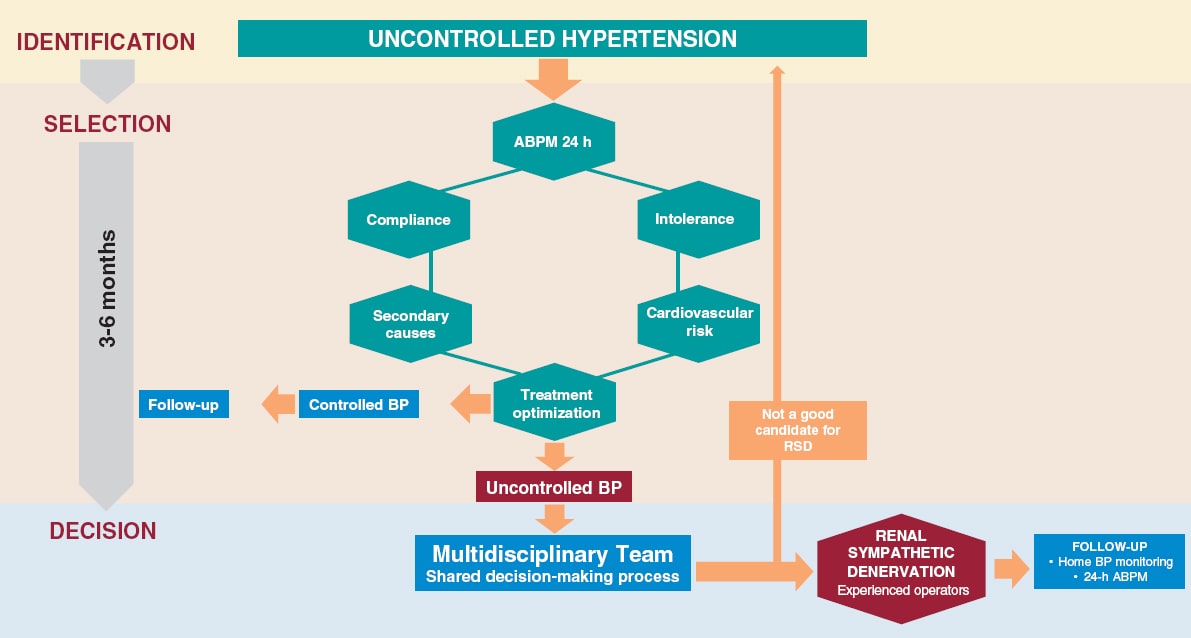
The success of a RSD program is based on the existence of a multidisciplinary team that performs a comprehensive assessment of the patient from the selection of candidates through their assessment prior to the intervention, the RSD procedure, and subsequent follow-up. This process should be carried out at specific units specialized in the management of HTN in collaboration with interventional cardiology units. Figure 2 shows the selection process of eligible patients.
Figure 2. Identification process, patient selection and decision on RDN. Patients with uncontrolled HTN (BP > 140/90 mmHg despite treatment) should be evaluated in an HTN unit. The lack of control should be confirmed by ABPM, assess adherence/intolerance to drugs, rule out secondary causes and cardiovascular risk. If, after optimizing the treatment, the lack of control persists, in patients at high or very high risk, and in a shared-decision process with the patient, RDN may be indicated. Adherence is defined as the extent to which a person’s behavior —taking medication, following a diet, and/or executing lifestyle changes— corresponds with the agreed recommendations from a healthcare provider. Drug intolerance refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Treatment optimization refers to lifestyle changes and pharmacological recommendations, including target doses, recommended by clinical practice guidelines.8 ABPM, ambulatory blood pressure monitoring; BP, blood pressure; RSD: renal sympathetic denervation.
We strongly discourage isolated procedures outside this controlled environment. RSD should not be performed in centers with volumes < 10 cases/year. Centers without a structured RSD program, but with eligible patients, should refer them to an experienced center rather than performing isolated procedures.
RSD procedures should be performed by operators experienced in the management of endovascular treatment. The SYMPLICITY HTN-3 trial post-hoc analysis showed the importance of an experienced interventional specialist given one of the factors influencing the results of the study was the operator’s lack of experience.9 Therefore, we recommend that procedures should be performed at centers with proven experience only and that, in centers that lack this experience, the possibility of monitoring should be available including assistance during the patient selection process and supervision of the procedure until enough experience is gained to ensure optimal results.
CONCLUSIONS
This expert consensus document has reviewed the information available regarding RSD in the management of patients with HTN. Also, it has established, for the first time, the indication for RSD in cases of uncontrolled HTN, especially in patients at high cardiovascular risk with HMOD or cardiovascular disease while taking the patient’s opinion into consideration as part of a shared decision-making process, and as long as it is evaluated by a multidisciplinary team and performed by experienced operators.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Study concept and design: O. Rodríguez-Leor, and J.A. García-Donaire; manuscript writing: O. Rodríguez-Leor, F. Jaén-Águila, J. Segura, I. J. Nuñez-Gil, A. García-Touchard, E. Rubio, M. Troya, J. Diego-Mediavilla, and J.A. García-Donaire; critical review: O. Rodríguez-Leor, Á. Cequier, R. Moreno, N. Martell, P. Beltrán, and E. Molina.
CONFLICTS OF INTEREST
O. Rodríguez-Leor, and J. A. García-Donaire have received personal fees from Medtronic, outside the submitted work. A. García-Touchard reports having received grants from Medtronic, and personal fees from Medtronic, also outside the submitted work. Á. Cequier reports having received grants and personal fees from Abbott Vascular, grants, and personal fees from Biosensors, grants from Boston Scientific, grants, and personal fees from Medtronic, grants from Biomenco, Cordis, Orbus Neich, and from the Spain Society of Cardiology, personal fees from Ferrer International, Terumo, Astra Zeneca, and from Biotronik outside the submitted work. R. Moreno is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. F. Jaén-Águila, J. Segura, I. J. Núñez-Gil, E. Rubio, M. Troya, J. Diego-Mediavilla, R. Moreno, N. Martell, P. Beltrán, and E. Molina declared no relationship whatsoever relevant to the contents of this paper worthy of disclosure.
REFERENCES
1. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension:a multicentre safety and proof-of-concept cohort study. Lancet 2009;373:1275-1281.
2. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial):a randomised controlled trial. Lancet. 2010;376:1903-1909.
3. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401.
4. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs:6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346-2355.
5. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal):a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444-1451.
6. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE HTN-SOLO):a multicentre, international, single-blind, randomised, sham controlled trial. Lancet. 2018;391:2335-2345.
7. Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO):a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476-2486.
8. Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology:ESH/ESC Task Force for the Management of Arterial Hypertension. Eur Heart J. 2018;39:3021-3104.
9. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the Symplicity HTN-3 trial. Eur Heart J. 2015;36:219-227.
10. Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes:3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–3482.
11. Janke AT, McNaughton CD, Brody AM, Welch RD, Levy PD. Trends in the incidence of hypertensive emergencies in US emergency departments from 2006 to 2013. J Am Heart Assoc. 2016;5:e004511.
12. Vleck M, Bur A, Woisetschläger C, Herkner H, Laggner AN, Hirschl MM. Association between hypertensive urgencies and subsequent cardiovascular events in patients with hypertension. J Hypertens. 2008;26:657-662.
13. Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence:analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284-290.
14. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death:a systematic review and meta-analysis. Lancet. 2016;387:957-967.
15. Gupta P, Patel P, Strauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113-1120.
16. Cordero A, Morillas P, Bertomeu-Gonzalez V, et al. Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome Investigators. Clustering of target organ damage increases mortality after acute coronary syndromes in patients with arterial hypertension. J Hum Hypertens. 2011;25:600-607.
17. Lonnebakken MT, Izzo R, Mancusi C, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6:e004152.
18. Kordalis A, Tsiachris D, Pietri P, et al. Regression of organ damage following renal denervation in resistant hypertension:a meta-analysis. J Hypertens. 2018;36:1614-1621.
19. Schmeider RE, Hogerl K, Jung S, Bramlage P, Veelken R, Ott C. Patient preference for therapies in hypertension:a cross-sectional survey of German patients. Clin Res Cardiol. 2019;108:1331-1342.
20. Schmieder RE, Redon J, Grassi G, et al. ESH Position Paper:Renal denervation - an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837-841.
21. Banegas JR, Messerli FH, Waeber B, et al. Discrepancies between office and ambulatory blood pressure:clinical implications. Am J Med. 2009;122:1136-1141.
22. Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218-225.
23. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension:when, who, and how to screen?Eur Heart J. 2014;35:1245-1254.
24. García-Touchard A, Maranillo E, Mompeo B, Sañudo JR. Microdissection of the Human Renal Nervous System:Implications for Performing Renal Denervation Procedures. Hypertension. 2020;76:1240-1246.
25. Mahfoud F, Tunev S, Ewen S, et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766-1775.
26. Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation?JACC Cardiovasc Interv. 2013;6:1085-1091.
27. Tzafriri AR, Mahfoud F, Keating JH, et al. Procedural and anatomical determinants of multi-electrode renal denervation efficacy –Insights from preclinical models. Hypertension. 2019;74:546-554.
28. Tzafriri AR, Mahfoud F, Keating JH, et al Innervations patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079-1087.
29. Fink G, Phelps JT. Can we predict the blood pressure response to renal denervation?Auton Neurosci. 2017;204:112-118.
ABSTRACT
Coronary vasoreactivity testing is a key diagnostic procedure in patients with suspected coronary spasm and research procedures intended to assess the coronary endothelial function. We should mention that coronary spasm has been observed in > 40% of the patients with angina and non-obstructive coronary stenosis. Also, that its dedicated treatment has proven to reduce ischemic symptoms and improve these patients’ quality of life. This technical report elaborated by the Working Group on Intracoronary Diagnostic Techniques of the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) summarizes the indications, preparation, performance, and interpretation of the vasoreactivity testing performed by intracoronary infusion of acetylcholine.
Keywords: Spasm provocation test. Coronary endothelial function.
RESUMEN
Las pruebas de vasorreactividad coronaria con infusión de acetilcolina son una prueba diagnóstica fundamental para pacientes con sospecha de enfermedad cardiaca secundaria a vasoespasmo y en procedimientos de investigación en los que se valora la función endotelial coronaria. Se calcula que más del 40% de los pacientes con angina y ausencia de lesiones coronarias presentan vasoespasmo como causa fundamental de los síntomas, y su tratamiento específico ha demostrado mejorar la calidad de vida en estos pacientes. El Grupo de Trabajo de Técnicas de Diagnóstico Intracoronario de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) ha elaborado el presente documento técnico que expone de manera práctica las indicaciones, la preparación, la realización y la interpretación de dichas pruebas.
Palabras clave: Prueba de provocación de vasoespasmo. Función endotelial coronaria.
Abbreviations:
DS: diameter stenosis. INOCA: ischemia with no obstructive coronary arteries. MINOCA: myocardial infarction with non-obstructive coronary arteries.
INTRODUCTION
Coronary vasoreactivity testing performed by intracoronary infusion of acetylcholine is basically used with 2 goals in mind: for endothelial function assessment and as a vasospasm provocation test in clinically suspicious cases. Although these tests have been known and used for decades, its use is not yet fully consolidated in our setting. This is mainly due to a scarce suspicion of myocardial ischemia due to micro or vasomotor disorders that has eventually lowered the demand for these tests. In addition, the lack of test standardization and training, the off-label use of acetylcholine, and the doubts surrounding these tests safety profile have not encouraged their widespread use in the routine clinical practice.
Over the last few years, this scenario has changed dramatically thanks to the growing evidence on the importance of diagnosing the causes of myocardial ischemia not directly related to fixed stenoses. Currently, invasive coronary spasm provocation tests are formally recommended by the European Society of Cardiology in its clinical practice guidelines on the management of chronic coronary syndromes, non-ST-segment elevation acute coronary syndromes, and ST-segment elevation acute coronary syndromes.1-3 The most common indications are to treat patients with angina or ischemia but without non-obstructive coronary lesions (ANOCA, INOCA—in this document, both coined under the term INOCA—), myocardial infarction with non-obstructive coronary arteries (MINOCA), persistent angina after coronary revascularization, obstructive coronary artery disease with clinical suspicion of associated angina of microvascular origin, and finally, patients with recovered sudden death of undetermined causes.1-3 Table 1 summarizes all clinical indications and level of recommendation to perform vasospasm provocation testing. Although this paper focuses on coronary vasoreactivity testing, we should remember that its use is often recommended simultaneously with other coronary functional testing performed using pressure guidewires like coronary flow reserve and microcirculation resistance measurements.1-5 The specific diagnosis of functional damage to coronary arteries and its targeted therapies have both improved the quality of life of patients with INOCA.6 The treatment recommended for coronary vasospasm is calcium channel blockers, nitrates, and nicorandil.6,7
Table 1. Clinical indications for the coronary vasospasm provocation test performed by intracoronary infusion of acetylcholine
| Class | Indication | Clinical specifications |
|---|---|---|
| Class I (highly recommended) | Clinical suspicion of vasospastic angina without objective documentation of ischemia or obstructive coronary artery disease in patient with chronic symptoms | – Vasospastic angina can occur predominantly at rest (35%), during exertion (30%), as a mixed pattern (30%) or dyspnea (5%)4 – The epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Acute coronary syndrome without presence of culprit lesions on the coronary angiography | – Carefully review the angiography to discard embolisms and radiolucent images consistent with thrombus or coronary dissections – The use of intravascular imaging (intracoronary ultrasound or optical coherence tomography) for this type of lesions is advised – Exclude other causes for high troponin levels (like myocarditis) through segmental assessments (ventriculography or echocardiography) and magnetic resonance imaging |
|
| Recovered inexplicable sudden death | – After excluding structural and/or arrhythmic heart disease | |
| Study of syncope preceded by thoracic pain | – After excluding structural and/or arrhythmic heart disease | |
| Recurrent angina despite revascularization | – First assess the pressure guidewire to exclude epicardial functional disease and microcirculation disorders in maximum hyperemia | |
| Class IIa (recommended) | Clinically documented vasospastic angina in a spontaneous event or on the non-invasive provocation test that is unresponsive to medical therapy | – Patients unresponsive to therapy with calcium channel blockers and nitrates or nicorandil – Epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Class IIb (debatable) | Clinically documented vasospastic angina or on the non-invasive provocation test that responds to medical therapy to know the type and degree of vasospasm | – The specification of the macro/microvascular spasm and whether it causes the occlusion of the artery can be relevant for the patient’s prognosis – Epicardial and microvascular function assessment in maximum hyperemia with a pressure guidewire is advised |
| Class III (ill-advised) | Asymptomatic patients | Asymptomatic patients |
| Patients with ejection fractions < 35% | Patients with ejection fractions < 35% | |
| Significant epicardial coronary artery disease (left main coronary artery and/or 3 vessels) | Significant epicardial coronary artery disease (left main coronary artery and/or 3 vessels) | |
|
Adapted with permission from the consensus document elaborated by the COVADIS Working Group (Coronary Vasomotion Disorders International Study).5 |
||
Based on the current clinical practice guidelines, the objective of the Working Group on Intracoronary Diagnostic Techniques of the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) is to facilitate and standardize the use of coronary vasoreactivity testing. Thus, this paper has been drafted to expose all technical steps in a practical way to encourage the performance and interpretation of these tests in our setting.
NORMAL ENDOTHELIAL FUNCTION OF CORONARY ARTERIES
The modulatory function of vascular endothelium in the blood flow towards the myocardium is intrinsically associated with its metabolic characteristics. Compared to the skeletal muscle, the heart has pretty high oxygen needs (some 20 times higher). The way this oxygen supply is achieved is through very high tissue extraction at baseline: at rest the myocardium extracts approximately 70% to 80% of the oxygen transported by hemoglobin compared to 30% of the skeletal muscle. This explains why, unlike other organs, the mechanism through which the heart regulates oxygen supply to the myocardium changing metabolic needs is fast regulation and constant blood flow into the coronary system.8
Microcirculation (arteries and arterioles < 400 µm) is basically responsible for regulating coronary blood flow. Although regulation is complex and includes metabolites, hormones, neurotransmitters, and other factors, the main protagonist is the vascular endothelium that produces nitric oxide—a powerful vasodilator—in response to different stimuli. Also, other vasodilator factors—like the hyperpolarizing endothelial factor—and vasoconstrictor factors like endothelin. Endothelium-dependent vasodilation can be stimulated through different ways, but the most commonly used one is the infusion of acetylcholine.
In normal conditions, an artery with a healthy endothelium responds to acetylcholine by releasing nitric oxide that translates into vasodilation. In the presence of artery denudation from the endothelium or if the action of the nitric-oxide synthase enzyme is blocked, the artery responds to acetylcholine with vasoconstriction due to the stimulation of smooth muscle muscarinic receptors not counteracted by the nitric oxide of endothelial origin. Therefore, the infusion of acetylcholine can be used to assess endothelial function: if normal, vasodilation becomes evident. If not, vasoconstriction kicks in. The macrovascular compartment endothelial function (epicardial) can be assessed on an angiography. However, to assess the endothelium-dependent response in microcirculation, blood flow should be measured using a Doppler guidewire or thermodilution. From the macrovascular point of view, visually evident epicardial vessel vasoconstriction in response to acetylcholine is considered endothelial dysfunction. Figure 1 shows examples of vasodilation (physiological) and vasoconstriction responses (suggestive of endothelial dysfunction) to the administration of acetylcholine. From the microvascular point of view, flow reductions or increases < 50% in response to the administration of acetylcholine are considered anomalous.9 Figure 2 shows examples of microvascular function assessment using the Doppler technique or intracoronary thermodilution.
Figure 1. Possible results of the vasospasm provocation test with acetylcholine. Case 1: physiological response (vasodilator response to acetylcholine). Case 2: endothelial dysfunction with vasoconstriction with respect to baseline that does not meet the criteria for micro or macrovascular spasm. Case 3: macrovascular spasm with significant vasoconstriction of the left coronary tree. Case 4: microvascular spasm due to moderate vasoconstriction of the left tree meeting the clinical and ECG criteria for ischemia. ACh, acetylcholine; DS, diameter stenosis; NTG, nitroglycerin.
Figure 2. Combined assessment of macro and microvascular function. A: pressure-Doppler guidewire study (Combowire, Philips, The Netherlands). After the angiography and measurement of baseline flow velocity, growing doses of acetylcholine are injected. After the second dose, moderate vasoconstriction of the left anterior descending coronary artery occurs followed by an occlusive spasm at circumflex artery level plus a slower flow velocity in the left anterior descending coronary artery indicative of microvascular vasoconstriction. The spasm is solved with intracoronary nitroglycerin followed by the adenosine non-endothelium-dependent assessment of microvascular function. The patient shows macro and microvascular endothelial dysfunction and a normal non-endothelium-dependent microvascular function. B: macrovascular endothelial function assessment appears normal; adenosine non-endothelium-dependent assessment with thermodilution guidewire (Pressurewire X, Abbott, United States). Coronary flow reserve is 5.9, and the index of microcirculatory resistance is 11, which is suggestive of a normal microvascular function. In conclusion, physiological examination without any relevant findings.
CORONARY VASOSPASM PROVOCATION TESTING
Different stimuli can be used to provoque epicardial or microvascular coronary spasm. Non-pharmacological stimuli like hyperventilation or coming into contact with cold are associated with an excessive number of false negatives for clinical use. Non-invasive coronary vasospasm assessment (based on changes on the ECG or the echocardiography through the IV administration of ergonovine) is associated with a risk of causing nitrate-resistant flow-limiting coronary spasm.4 For this reason, to this date, invasive studies based on the intracoronary administration of drugs are considered the single most sensitive and safe method. Actually, to this date, it is the method recommended by European guidelines and consensus docments.2,7 The direct administration of drugs allows us to use lower doses and establish a time correlation between the development of coronary spasm followed by symptom onset and changes on the ECG. Also, it facilitates immediate treatment through the direct administration of nitrates.4,10 The use of acetylcholine vs ergonovine is advised too since the former acts on a specific pathway (by stimulating cholinergic receptors only), and its safety profile is good because its half-life is shorter. Also, because it responds faster to nitrates in case of vasoconstriction.11 Also, acetylcholine allows us to assess the vascular endothelial response specifically, which is an additional advantage. The studies that compared the results of vasospasm provocation testing with acetylcholine vs ergonovine found similar sensitivity and high matching (94%) between the two. Therefore, in the presence of a negative acetylcholine test no additional studies with different drugs are advised.12
ACETYLCHOLINE
Acetylcholine is a neurotransmitter largely found in the nervous system (central, autonomous, and peripheral). It is used in the neuromuscular junction, in all synapses of the parasympathetic autonomous system, and in the first synapsis of the sympathetic nervous system. The muscarinic receptor of acetylcholine has 5 different subtypes; among them, subtype M2 is largely found in the myocardium where it reduces the heart rate and the cardiac conduction system; subtype M3 is found in the coronary arteries both in the endothelium and the smooth muscle. In the coronary arteries, the M3 receptor stimulates the contraction of the vascular smooth muscle (vasoconstriction). Also, it stimulates the endothelial production of nitric oxide that spreads into the smooth muscle reducing the concentration of calcium, and causing relaxation (vasodilation).13,14 Acetylcholine is rapidly hydrolyzed in both the neuromuscular junction and blood by the action of cholinesterases. When infused intracoronary in the doses described here, no systemic effects occur, and its cardiac effects only last a few minutes.
ACETYLCHOLINE-INDUCED ENDOTHELIAL DYSFUNCTION AND CORONARY VASOSPASM
Endothelial dysfunction is associated with the number of cardiovascular risk factors and is a well-known precursor of atherosclerosis.15 Also, the presence of endothelial dysfunction has been associated with the appearance of ischemia in the ischemia exercise test, heavier calcification and presence of necrotic and lipidic content in the vascular wall, and more cardiovascular adverse events in the long run.16-18 The prevalence of a vasoconstrictor response to the intracoronary infusion of acetylcholine, therefore, similar to an epicardial endothelial dysfunction, is variable depending on the characteristics of the patients being more common among males.19 In the studies conducted in patients with INOCA, the prevalence of endothelial dysfunction is somewhere between 45% and 75%.19,20
Although, to this point, no cut-off value has been universally accepted, it has been confirmed that moderate degrees of vasoconstriction (20% to 50%) with respect to the artery baseline diameter after the intracoronary infusion of acetylcholine have an important prognostic impact.18,21,22 Quantitative coronary angiography studies consider the variability of the technique when measuring changes in the mean luminal diameter of a segment with respect to the different doses of acetylcholine (usually the dose with the highest vasoconstriction with respect to the baseline one). Small imaging variations due to respiratory movements in every cine coronary arteriography, different limits of the study segment in the different measures taken, the analysis of diameters at different times of the cardiac cycle between the baseline image and maximum vasoconstriction, and the operator’s variability are the reasons why vasoconstriction can only be confirmed after variability is excluded from this measuring process. Several studies have established this variability (2 times the standard deviation of the percent difference) somewhere between 3% and 6%. Therfore, endothelial dysfunction is defined as a vasoconstrictor response that is greater than this variability.23,24
The pathophysiological factors of vasospastic angina, both in their macro and microvascular manifestations are less known and, also, probably multifactorial. Vasospastic angina has been associated with the presence of coronary plaques, vascular smooth muscle cell hyperreactivity, a high baseline vagal tone, hyperreactivity to sympathetic stimulation, and finally, to a significant degree of endothelial dysfunction.10 Vasospastic angina, both macro and microvascular, is more common among women.4 The traditional criteria to define vasospastic angina of macrovascular origin have been described by the Coronary Vasomotion Disorders International Study Group (COVADIS).5 In their document they describe the diagnostic criteria of this disease that go beyond the traditional definition of variant angina described by Prinzmetal et al.25. We should mention that, unlike the definition of endothelial function where baseline angiography is used as the reference, to define macrovascular spasm the COVADIS group recommends assessing the coronary spasp in the segment with the greatest constriction of all after the administration of acetylcholine and then compare it with the diameter of the same segment after the infusion of nitroglycerin.4 Also, this group recommends the use of drug provocation testing performed by intracoronary infusion of acetylcholine given its high sensitivity and specificity values (90% and 99%, respectively).26 Based on former studies and the traditional definition, the prevalence of epicardial coronary artery vasospasm, whether associated with microvascular spasm or not, occurs in 30% to 40% of the patients with INOCA.6,27
Fewer consensus documents have been published on the definition and diagnosis of microvascular spasm.28,29 Over the last few years, the appearance of thoracic pain and changes on the ECG suggestive of ischemia in response to acetylcholine and in the absence of macrovascular spasm have been accepted for the diagnosis of microvascular spasm (located in the arterioles). By this definition, 25% of the patients with INOCA meet the microvascular spasm criteria.27
TEST PERFORMED BY INTRACORONARY INFUSION OF ACETYLCHOLINE
Preparing the patient
The best way to prepare patients eligible for the coronary vasoreactivity test with acetylcholine is still under discussion. Historically, these procedures used to be performed in a dedicated procedure while avoiding and withdrawing all kinds of vasodilator drugs (like calcium channel blockers and nitrates) for, at least, 18 hours before the infusion of acetylcholine.27,30,31 However, after the publication of the randomized clinical trial CorMicA and the consensus document of the European Association of Percutaneous Coronary Interventions (EAPCI) on the study of patients with INOCA, conventional wisdom has changed.6,7 Currently, the use of intracoronary functional testing is recommended including the vasoreactivity test to acetylcholine within the same diagnostic procedure where the coronary angiography is performed.
This brings greater comfort to the patient, uses cath lab resources more efficiently, and alleviates the pressure of the hospital agenda. In any case, this procedure should be fully adapted to the needs and possibilities of every cath lab; in polymedicated patients with vasodilators or in inexperienced centers using this test the scheduled procedure should be used.
If radial access is used in patients eligible for a coronary vasoreactivity test the administration of calcium channel blockers to prevent radial spasm is ill-advised. In these cases, the administration of low doses of nitroglycerin through the introducer sheath (100 µg to 200 µg) can be considered. However, its effect will probably mostly be gone by the time acetylcholine is infused. Also, the coronary vasoreactivity test can be performed after studying microvascular function with a pressure guidewire (with the corresponding administration of intracoronary nitroglycerin before advancing the guidewire).6,7 In this case, a 2- to 3-min washout period should be observed before the infusion of acetylcholine.6,7
Finally, a specific informed consent should be obtained before running any vasoreactivity tests. Also, 12-lead ECG monitoring is required to assess the results. The use of radiotransparent wiring and electrodes is advised here to avoid interfering with the cine-fluoroscopy images obtained for each of the doses infused during coronary angiography. Figure 3 shows a schematic sample of how to prepare a patient before running a coronary vasoreactivity test with intracoronary acetylcholine.
Figure 3. Preparation of the patient before running any vasoreactivity tests. ACh, acetylcholine; Ca, calcium; Cine, cine coronary arteriography; IC, informed consent; NTG, nitroglycerin.
Regarding the use of beta-blockers, certain groups also recommend their cessation before running the test to avoid any possible vasoconstrictor effects. Until more scientific data become available, the opinion of this group is that beta-blockers do not affect the results of the test significantly and in no way give false negative results.
Preparing the acetylcholine
The acetylcholine available in Spain is a preparation for the intraocular injection of 20 mg of acetylcholine chloride (powder) for its dilution in a 2 mL vial of physiological saline solution. After the solution has been prepared, the drug is still unstable, which is why the best thing to do is to prepare it right before the test; if several consecutive tests are going to be performed, the same preparation can be used. Figure 4 summarizes the way to prepare the acetylcholine solutions suggested for the test. An important safety tip is to identify correctly every solution of acetylcholine we will be using; saline solution systems and color syringes for every dose can be useful.
Figure 4. Preparation of growing doses of acetylcholine. ACh, acetylcholine; DS, diameter stenosis; PSS, physiological saline solution.
Intracoronary infusion protocol
Over the last 30 years, different protocols on the administration and doses of intracoronary acetylcholine have been used. Table 2 shows the different protocols used in landmark studies.6,10,19,29,30,32-34 There are differences regarding the routes of administration (manual infusion through guide catheter or controlled selective infusion into an artery through an infusion pump and microcatheter), the number of doses infused (from 2 to 4), the amount of acetylcholine used (from 0.3 µg to 200 µg), and the infusion time (from 20 seconds to 3 minutes). Below we will be seeing the most widely accepted protocols based on the objective pursued (endothelial function assessment or vasospasm provocation) followed by a proposal according to the last consensus documents published to this date.
Table 2. Comparison of the different protocols of coronary vasoreactivity to acetylcholine
| Group | Infusion method | Doses used | Infusion time per dose | Comments |
|---|---|---|---|---|
| Harvard Working Group30 | Infusion through microcatheter and infusion pump | 4 dilutions of 10–7, 10–6, 10–5, and 10–4 per liter (infusion at a rate of 0.8 mL/min) into the LCA | 2 minutes | – Designed for endothelial function assessment – A final concentration of 10–9, 10–8, 10–7, and 10–6 is estimated (equivalent to a selective total dose per artery of 0.03 µg, 0.3 µg, 3 µg, and 30 µg) – It is performed on the LCA |
| Mayo Clinic32 | Infusion through microcatheter and infusion pump | 3 dilutions of 10–6, 10–5, and 10–4 per liter (infusion at a rate of 1 mL/min) followed by a bolus of 100 µg (through the same catheter) | 3 minutes (final bolus for 20 to 30 seconds) | – Mixed protocol for endothelial function assessment (equivalent to a selective total dose per artery of 0.5 µg, 5 µg, and 50 µg) and vasospasm assessment with a bolus of 100 µg – It includes the functional assessment of microcirculation with Doppler guidewire during the infusion of acetylcholine – It is performed on the LCA |
| Korea Working Group33 | Manual infusion through guide catheter | 3 doses of 20 µg, 50 µg, and 100 µg into the LCA | 1 minute | – It is performed on the LCA |
| Japanese Circulation Society10 | Manual infusion through guide catheter | 3 doses of 20 µg, 50 µg, and 100 µg into the LCA In the absence of vasospasm 2 doses of 20 µg and 50 µg into the RCA are advised |
20 seconds | – Vasospasm provocation test on the LCA and RCA – The implantation of an electrode catheter to perform it is advised |
| Standford Working Group19 | Manual infusion through guide catheter | 4 doses of 20 µg, 50 µg, 100 µg, and 200 µg into the LCA | 1 minute | – It is performed on the LCA |
| Stuttgard Working Group34 | Manual infusion through guide catheter | 4 doses of 2 µg, 20 µg, 100 µg, and 200 µg into the LCA In the absence of vasospasm into the LCA an 80 µg dose into the RCA is advised |
20 seconds | – It studies both the LCA and the RCA |
| The CorMicA trial and the COVADIS Working Group6,29 | Mixed pump and manual infusion | 3 growing doses of 0.18 µg/mL, 1.82 µg/mL, and 18.2 µg/mL administered using an infusion pump through the guide catheter The procedure is completed with a manual bolus of 100 µg (50 µg into the RCA) |
2 minutes for every growing dose, and 20 seconds for the final bolus | – It is performed on the LCA after microcirculation assessment with adenosine through a pressure guidewire – It assesses the endothelial function and the vasospasm provocation test in the same procedure |
| Protocol of the ACI-SEC (present document) | Manual infusion through guide catheter | 3 doses of 2 µg, 20 µg, and 100 µg into the LCA In case of suspected vasospasm into the RCA the test should be started in this artery with doses of 2 µg, 20 µg, and 50 µg |
20 seconds | – For endothelial function assessment purposes, the doses should be infused more slowly for 2 to 3 minutes – It is performed on the LCA |
|
ACI-SEC, Interventional Cardiology Association of the Spanish Society of Cardiology; RCA, right coronary artery; LCA, left coronary artery. |
||||
Endothelial function assessment
Growing doses of acetylcholine are used for endothelial function assessment. If this procedure is performed by selective drug infusion into 1 of the main coronary vessels with a microcatheter (usually the left anterior descending coronary artery), the concentrations used are 10 moL/L to 6 moL/L, 10 moL/L to 5 moL/L, and 10 moL/L to 4 moL/L. Considering the left anterior descending coronary artery flow (some 80 mL/min), it is estimated that the drug reaches concentrations that are 100 lower in coronary microcirculation. Using the microcatheter these dilutions are injected the into the proximal left anterior descending coronary artery or into the artery to be interrogated at a rate of 1 mL/min for 3 minutes or 2 mL/min for 2 minutes through an infusion pump.24,35 Infusion starts with the least concentrated dilution and, if no complications or overt vasospasm are reported the next infusion should start 2 to 3 minutes later. In practice, this method injects 0.5 µg, 5 µg, and 50 µg of acetylcholine in each of the doses. As already mentioned, in the presence of a non-dysfunctional vascular endothelium, the physiological response is the vasodilation of major epicardial vessels.
The procedure described, although widely used in clinical trials, is somehow complicated and expensive, which is why easier and more practical alternatives have been developed for macrovascular endothelial function assessment. The most important one that has already become the standard may be the one used in the ENCORE trials (Evaluation of nifedipine and cerivastatin on recovery of coronary endothelial function)36,37 consisting of the infusion of growing doses of 2 µg, 20 µg, and 100 µg directly into the left main coronary artery for 3 minutes each followed by the performance of an angiography after every dose. Also, it consists of the assessment of the arterial diameter compared to the one measured on the baseline angiography. If macrovascular endothelial function needs to be assessed, the recommendation is to follow this infusion pattern. As we will be seeing, this protocol has already been widely adopted in recent publications and, with minor changes, has become the go-to protocol for the diagnosis of coronary vasospasm although with a faster infusion of the doses.
Microvascular endothelial function can also be assessed using dedicated guidewires for the simultaneous measurement of coronary flow. In general, this procedure is performed using a Doppler guidewire (Combowire, Philips, The Netherlands),9 although the assessment can also be performed through thermodilution with thermistor-based temperature measuring guidewires (Pressurewire, Abbott, United States).38,39 Figure 2 shows 2 examples of this procedure.
Coronary spasm provocation testing
Although there are different protocols on doses and infusion times, the protocol recommended here for vasospasm provocation has been widely accepted by the most experienced groups. In addition, there are data available on its safety profile in many patients and is the protocol backed by the EAPCI in its recent consensus document.7
Three doses of 2 µg, 20 µg, and 100 µg are used in the left coronary artery, and 3 doses of 2 µg, 20 µg, and 50 µg in the right coronary artery. If the test is negative or inconclusive and the previous doses are well-tolerated, a 200 µg or a 80 µg dose can be used in the left or right coronary artery, respectively, if suspicion runs high.
Regarding the infusion time, a slow 20 second-bolus can be administered, although this is based on clinical tolerability. The highest doses, especially in the right coronary artery, often require infusions at a slower rate to avoid sinus arrest-induced bradycardia or atrioventricular block. It is important to carefully and slowly wash the guide catheter with a saline solution to avoid the sudden injection of the remaining drug into the catheter by the time the cine-fluoroscopy imaging is acquired. After every dose both the symptoms and the repolarization and angiographic changes should be assessed while paying special attention to the appearance of epicardial spasms or significant reductions of coronary flow velocity. At the end of the test, intracoronary nitroglycerin is infused (200 µg to 300 µg) and spasm is solved within a few seconds.
Safety and complications
Before indicating the test, the presence of factors that may be correlated with a risk of complications associated with the intracoronary infusion of acetylcholine should be discarded. The test should be carefully performed in patients with a past medical history of asthma or bronchospasm and serious disorders of automaticity and cardiac conduction.
Although safe in experienced hands, coronary vasoreactivity tests to acetylcholine are not stranger to potentially serious complications. These tests should always be performed paying extra care by trained personnel and ready to face the possible complications that may arise. In a metanalysis of different studies with over 6000 procedures, the rates of major (eg, ventricular arrhythmias, need for cardiopulmonary resuscitation or infarction), and minor complications (symptomatic bradycardia, transient atrioventricular block, appearance of ventricular arrhythmias or air embolism) were 1% and 6%, respectively.40 We should mention that no death was reported in this metanalysis.40 Table 3 shows the most common complications and their corresponding treatments.
Table 3. Complications associated with the intracoronary infusion of acetylcholine
| Complication | Percentage | Comment | Treatment |
|---|---|---|---|
| Bradycardia and/or transient atrioventricular block | 3.23% | More common in high doses and when infused fast, especially into the RCA | Stop the infusion for a few seconds until going back to rhythm. Study the possibility of going on with the test at a slower infusion rate |
| Appearance of atrial fibrillation | 2.38%* | It is often self-limiting, but also fast, and its clinical tolerance is poor. It is a reason to stop the test whose outcome will be undetermined | If hemodynamic tolerance is good, use antiarrhythmic drugs; if poor, study the possibility of electrical cardioversion |
| Ventricular fibrillation, ventricular tachycardia or need for resuscitation | 1.00% | Due to acute ischemia following flow-limiting vasospasm | Nitroglycerin and defibrillation |
| Shock and/or myocardial infarction | 0.07% | Due to flow-limiting spasm at multivessel or left main coronary artery level | Nitroglycerin plus inotropic support +/– ventricular support |
| Transient hypotension | 0.05% | It is often unsignificant | Stop the infusion for a few seconds until going back to rhythm. Study the possibility of going on with the test at a slower infusion rate |
| Coronary artery dissection | 0.02% | Catheter-induced coronary artery dissection | Stenting |
| Air embolism | 0.02% | Operator-dependent complication; it is more common when infusion is performed through a microcatheter. It can be serious if not treated fast | Administer oxygen at 100% and wash the artery with saline serum multiple times (after making sure there is no more air). Inotropic and/or ventricular support (or both) may be needed |
| Catheter-induced spasm | 0.02% | More common in the RCA | Try to avoid nitroglycerin in the absence of flow lost. It is often a transient phenomenon |
|
The percentages disclosed were estimated based on 6183 procedures reported in 9 different studies. * According to the CorMicA trial, the rate of atrial fibrillation with the fastest doses infused was 6%.6 RCA, right coronary artery. Adapted with permission from Ciliberti et al.40 |
|||
During the infusion of acetylcholine, sinus bradycardia, sinus arrests or episodes of atrioventricular block are common. This is often associated with too fast infusions, especially in the right coronary artery. If these complications occur, infusion should stop for a few seconds and restarted at a slower velocity. Atrial fibrillation can sometimes occur, but it often solves spontaneously; the most persistent cases often solve after the administration of amiodarone or other antiarrhythmic drugs. If bradyarrhythmias make a comeback, the test should stop immediately or be performed with a transient pacemaker in very selected cases where the test is considered indispensable.
An unwanted effect of the test is flow-limiting vasospasm that is not well-tolerated. In general, the consequences depend on the time elapsed between the occurrence of the vasospasm and the infusion of intracoronary nitrates to reverse it. The ischemia originated can cause hypotension and ventricular fibrillation that should be treated with nitroglycerin and immediate defibrillation. To stop this from going unnoticed, the patient’s blood pressure should be checked halfway into the infusion of acetylcholine, especially after the highest doses have been infused and when injected into a dominant left coronary branch. Under no circumstance a growing dose of acetylcholine should be infused if a significant or flow-limiting spasm or any other important complication have been spotted after the infusion of lower doses. Also, we should remember that, at the end of the infusion, the guide catheter still contains 2 mL of acetylcholine dilution that should be slowly pushed with a saline solution to stop it from entering the bolus with the injection of contrast. Same as it happens with any other invasive coronary procedures, and especially in this test, preloaded nitroglycerin should be available and ready to be infused. In most of the cases its infusion causes vasodilation, and fast flow recovery without needing further doses. On the other hand, atropine is a cholinergic receptor antagonist that can be used as an antidote when necessary.
Some operators perform the vasoreactivity test using the pressure guidewire inside the coronary artery as a safety measure. This brings more stability to the catheter, provides better selective infusion of dilutions, and allows us to monitor distal pressure (that can decrease in the case of flow-limiting spasm). Also, it controls the velocity of manual infusion in case of a long infusion without a pump (temperature or velocity changes are indicative that infusion is happening too fast). However, we should remember that the passage of the guidewire itself can cause vasospasm. Actually, it can simulate pseudo-spasms in tortuous arteries due to curve rectification.
INTERPRETING THE CORONARY VASOSPASM PROVOCATION TESTING
General concepts
Interpreting this test rests on 3 basic pillars:
-
The reproduction of the patient’s common symptoms that motivated the test. With the last dose of acetylcholine patients often experience changes of rhythm (eg, P-wave block or bradycardia) that can cause symptoms. These disorders should be distinguished from the patient’s usual angina symptoms.
-
The presence of changes on the ECG suggestive of ischemia, especially if accompanied by the angina symptoms that motivate the study. This assessment is often performed a few seconds after the infusion of each dose of acetylcholine. We should remember that in the presence of epicardial spasm with decreased blood flow in some of the epicardial arteries, the ST-segment does not need to be elevated or more changes on the ECG need to be present. That is so because patient’s safety is a priority at all time. Also, we should remember that, sometimes, the same injection of contrast or saline solution causes changes on the ECG. That is why serial ECGs (or collections of registries) should be performed a few seconds after the infusion of acetylcholine and before the cine coronary arteriography required (with the corresponding infusion of contrast).
-
The presence of angiographic coronary spasm (macrovascular) as seen on the serial registries (with cine-fluoroscopy) after every dose of acetylcholine. Spasm is defined as an obstruction with "65; 90% stenosis with respect to the diameter of the artery in this segment after the infusion of nitroglycerin. Diameter stenosis (DS) can be determined visually or using a quantitative coronary angiography. The DS is measured by obtaining the minimal luminal diameter after the dose of acetylcholine with greater vasoconstriction (MLD_ACh) with respect to the reference vessel diameter calculated after the infusion of nitroglycerin (RVD_NTG) with the following formula:
DS = 100 – [(MLD_Ach / RVD_NTG) × 100]
In practice, it is better to use the quantitative coronary angiography on the proximal segments of major arteries than in more distal segments where the reference diameter is often small and, according to the formula described above, could underestimate the DS.
Possible test results
Figure 1 shows the 4 results that can be obtained from a vasospasm provocation test performed by the infusion of intracoronary acetylcholine:
-
Negative test with vasodilator response (with respect to baseline). The presence of vasodilator response without symptom onset or changes on the ECG is suggestive of a normal endothelial function at epicardial level.
-
Negative test with vasoconstrictor response (with respect to baseline). The presence of epicardial vasoconstriction after acetylcholine without criteria of epicardial or microvascular vasospasm (defined by the lack of symptoms, changes on the ECG or significant vasoconstriction) is indicative of endothelial dysfunction, especially if vasoconstriction is confirmed after the infusion of the first few doses. Since the vasospasm provocation test has not been designed to assess the endothelial function (that requires a slower infusion rate) a certain degree of vasoconstriction is often seen with the highest dose due to the acetylcholine-induced direct stimulation of the vascular smooth muscle, which is not necessarily suggestive of epicardial endothelial dysfunction.
-
Positive test for epicardial spasm. The diagnosis of epicardial vasospasm requires the 3 following simultaneous findings:
-
Reproduction of symptoms after the infusion of acetylcholine.
-
Changes on the ECG suggestive of ischemia, usually in the ST-segment (whether depression or elevation > 0.1 mV). The appearance of negative U-waves has been described too.
-
Spasm with a "65; 90% diameter stenosis with respect to the same segment after the infusion of nitroglycerin that can be flow-limiting, focal, multisegmental or diffuse.
-
-
Positive test for microvascular spasm. Microvascular spasm has been defined as the reproduction of common angina symptoms plus the finding of changes on the ECG indicative of ischemia (basically, ST-segment depression or elevation > 0.1 mV) in the absence of coronary spasm with a "65; 90% diameter stenosis (with respect to nitroglycerin).
LEGAL ASPECTS PERTAINING TO THE USE OF INTRACORONARY ACETYLCHOLINE
The use of drugs in off-label indications different from those approved in their instructions for use and outside the clinical trial setting like intracoronary acetylcholine for diagnostic purposes requires the approval of local pharmaceutical committees. Because the Spanish Agency of Medicines and Medical Devices accepts the use of these drugs under very particular circumstances no general consensus has been achieved and local approvals are still required.
Also, in observance of Royal Decree 1015/2009 of 19 June,41 the use of a drug through a route of administration different from the one described in the drug labeling requires the provision of information as well as the patient’s written informed consent prior to its administration. For that reason, some centers also require the signing of a specific informed consent to be able to use intracoronary acetylcholine. These documents are available on the center intranet or the hospital pharmacy.
CONCLUSIONS
Figure 5 summarizes the key takeaways of this document. In conclusion, vasoreactivity testing with acetylcholine is an essential part of the assessment of patients with non-obstructive coronary artery disease and symptoms or ischemia. The result of this assessment allows us to target specific therapies and has proven effective in the routine clinical practice. Cath labs should be prepared to perform this kind of tests, and computers should be ready to use and interpret them.
Figure 5. Key takeaways of this document. ECG, electrocardiogram; INOCA, ischemia with non-obstructive coronary arteries; MINOCA, myocardial infarction with non-obstructive coronary arteries.
FUNDING
This document had no funding whatsoever.
AUTHORS’ CONTRIBUTIONS
E. Gutiérrez and J. Gómez-Lara equally contributed to the manuscript first draft, and to the figures, and tables. The remaining authors performed a thorough revision of the paper and made comments and changes to its content and form.
CONFLICTS OF INTEREST
None reported.
REFERENCES
1. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
2. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
3. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367.
4. Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349-2358.
5. Beltrame JF, Crea F, Kaski JC, et al.;Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565-2568.
6. Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina:The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855.
7. Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology &Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520.
8. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009-1086.
9. Lerman A, Burnett JC, Jr., Higano ST, McKinley LJ, Holmes DR, Jr. Longterm L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123-2128.
10. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801.
11. Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Safety and optimal protocol of provocation test for diagnosis of multivessel coronary spasm. Heart Vessels. 2016;31:137-142.
12. Sueda S, Kohno H, Fukuda H, et al. Induction of coronary artery spasm by two pharmacological agents:comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003;14:451-457.
13. Saternos HC, Almarghalani DA, Gibson HM, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018;50:1-9.
14. Lamping KG, Wess J, Cui Y, Nuno DW, Faraci FM. Muscarinic (M) receptors in coronary circulation:gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler Thromb Vasc Biol. 2004;24:1253-1258.
15. Gutierrez E, Flammer AJ, Lerman LO, Elizaga J, Lerman A, Fernandez-Aviles F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175-3181.
16. Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345-2352.
17. Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525-1530.
18. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-1906.
19. Pargaonkar VS, Lee JH, Chow EKH, et al. Dose-Response Relationship Between Intracoronary Acetylcholine and Minimal Lumen Diameter in Coronary Endothelial Function Testing of Women and Men With Angina and No Obstructive Coronary Artery Disease. Circ Cardiovasc Interv. 2020;13:e008587.
20. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary Vasomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655-662.
21. Hasdai D, Gibbons RJ, Holmes DR, Jr., Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390-3395.
22. Hoshino M, Yonetsu T, Mizukami A, et al. Moderate vasomotor response to acetylcholine provocation test as an indicator of long-term prognosis. Heart Vessels. 2016;31:1943-1949.
23. Davis SF, Yeung AC, Meredith IT, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457-462.
24. Gomez-Lara J, Oyarzabal L, Brugaletta S, et al. Coronary endothelial and microvascular function distal to polymer-free and endothelial cell-capturing drug-eluting stents. The randomized FUNCOMBO trial. Rev Esp Cardiol. 2021. https://doi.org/10.1016/j.rec.2021.01.007.
25. Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. A variant form of angina pectoris;preliminary report. Am J Med. 1959;27:375-388.
26. Okumura K, Yasue H, Matsuyama K, et al. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883-888.
27. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723-1730.
28. Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20.
29. Ford TJ, Ong P, Sechtem U, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease:Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864.
30. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-1051.
31. Takagi Y, Yasuda S, Takahashi J, et al. Clinical implications of provocation tests for coronary artery spasm:safety, arrhythmic complications, and prognostic impact:multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258-267.
32. Widmer RJ, Samuels B, Samady H, et al. The functional assessment of patients with non-obstructive coronary artery disease:expert review from an international microcirculation working group. EuroIntervention. 2019;14:1694-1702.
33. Kim JW, Park CG, Suh SY, et al. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol. 2007;100:1083-1086.
34. Ong P, Athanasiadis A, Sechtem U. Intracoronary Acetylcholine Provocation Testing for Assessment of Coronary Vasomotor Disorders. J Vis Exp. 2016;114:54295.
35. Hasdai D, Cannan CR, Mathew V, Holmes DR, Jr., Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203-208.
36. ENCORE Investigators. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease:the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function). Circulation. 2003;107:422-428.
37. Luscher TF, Pieper M, Tendera M, et al. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease:the ENCORE II study. Eur Heart J. 2009;30:1590-1597.
38. Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium-dependent microvascular function in humans:validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188-2194.
39. Diez-Delhoyo F, Gutierrez-Ibanes E, Sanz-Ruiz R, et al. Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients With Acute Myocardial Infarction. Circ Cardiovasc Interv. 2019;12:e007257.
40. Ciliberti G, Seshasai SRK, Ambrosio G, Kaski JC. Safety of intracoronary provocative testing for the diagnosis of coronary artery spasm. Int J Cardiol. 2017;244:77-83.
41. Real Decreto 1015/2009, de 19 de junio, por el que se regula la disponibilidad de medicamentos en situaciones especiales. BOE núm. 174, de 20/07/2009. Available online: https://www.boe.es/eli/es/rd/2009/06/19/1015. Accessed 25 Apr 2021.
ABSTRACT
Over the last two decades, several key advances have been made in the field of interventional cardiology including new techniques and treatments, organizational changes such as the management of acute myocardial infarction, and the arrival of satellite catheterization laboratories. All these advances require the updating of the requirements and equipment that are needed in an interventional cardiology unit. This consensus document by the Interventional Cardiology Association of the Spanish Society of Cardiology, the Ischemic Heart Disease and Acute Cardiac Care Association of the Spanish Society of Cardiology and the Spanish Association of Nursing in Cardiology which describes the recommendations that should be followed by percutaneous coronary intervention capable hospitals or centers intend to build interventional cardiology units. It also describes the requirements for provision, qualification of professionals, technological and material resource allocation, and aspects related to supervised catheterization laboratories and structural heart disease programs.
Keywords: Catheterization laboratory. Interventional cardiology. Acute myocardial infarction. Structural heart disease.
RESUMEN
En las últimas dos décadas han tenido lugar grandes avances en el campo de la cardiología intervencionista. Estos incluyen no solo nuevas técnicas y tratamientos, sino también cambios en la organización, como la atención continuada al infarto agudo de miocardio y la aparición de salas tuteladas. Todos estos avances hacen necesaria una actualización de los requisitos y del equipamiento necesarios en una unidad de hemodinámica y cardiología intervencionista. En este documento de consenso de la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología, la Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares de la Sociedad Española de Cardiología, y la Asociación Española de Enfermería en Cardiología, se establecen las recomendaciones que deberían cumplir los centros hospitalarios donde esté instalada o se pretenda instalar una unidad de hemodinámica y cardiología intervencionista, los requisitos de dotación y cualificación de profesionales, las dotaciones tecnológicas y los materiales necesarios, y aspectos relacionados con las salas tuteladas y los programas de tratamiento de la cardiopatía estructural.
Palabras clave: Sala de cateterismo. Cardiología intervencionista. Infarto agudo de miocardio. Cardiopatía estructural.
Abbreviations:
ACI-SEC: Interventional Cardiology Association of the Spanish Society of Cardiology. AEEC: Spanish Association of Nursing in Cardiology. PCI: percutaneous coronary intervention. PCT: patient care technician. TAVI: transcatheter aortic valve implantation. UIC: unit of interventional cardiology.
INTRODUCTION
Over the last 2 decades, interventional cardiology has been one of the fastest growing medical specialties. The invasive management of acute coronary syndrome, the optimization of short and long-term results of percutaneous coronary intervention (PCI) techniques, and the development of percutaneous techniques to treat a large number of structural heart disease cases have become very popular. This has facilitated the management of patients with cardiovascular diseases who require diagnostic or therapeutic invasive techniques at the unit of interventional cardiology (UIC) at one time or another.
Parallel to this increasing healthcare demand, we have seen another very significant increase in the number of UIC-capable centers, the need for material and human resources, and development of the technology used. Some rules and regulations have changed over the last few years. The latest clinical practice guidelines were published by the Spanish Society of Cardiology (SEC) 20 years ago,1 and the very latest recommendations from the Spanish Ministry of Health were established almost 10 years ago.2 That is why it seems necessary to update these recommendations, in our field, on the hemodynamic and interventional cardiology requirements and adapt them to the current situation where most UICs already have continuous infarction care plans and structural heart programs implemented. For this reason, the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC), the Spanish Society of Cardiology Working Group on Ischemic Heart Disease and Acute Cardiovascular Care, and the Spanish Association of Nursing in Cardiology (AEEC) have published this document.
HOSPITAL REQUIREMENTS
This document divides cath labs into autonomous cath labs (run with medical personnel from the center) and satellite or supervised cath labs (run with personnel from other centers to secure the provision of healthcare).
These are the requirements for hospitals to qualify as PCI-capable centers (figure 1):
- Presence of a coronary care unit or cardiac surgery intensive care unit.
- Presence of a cardiology unit and on-call cardiologists are highly recommended as well.
- Ability to treat vascular complications surgically at the center or at a partner center with patient transfer times under 60 minutes.
- Access to a nephrology and dialysis unit.
- Access to a hematology unit and blood bank.
- Access to a radiological protection unit in or out of the hospital setting.
Figure 1. Summary of hospital requirements regarding human and material resources that should become available at the interventional cardiology unit. PCI, percutaneous coronary intervention.
These requirements are applicable both to autonomous and satellite units. Regarding cardiac surgery, its existence in situ should not be considered a requirement per se for the center to qualify as a PCI-capable UIC.3 Also, a protocol needs to be agreed upon with a cardiac surgery unit to facilitate the transfer of patients for emergency surgeries in less than 60 min. Traditionally, structural heart procedures have not been deemed necessary to perform procedures such as valvuloplasties or percutaneous closures of interatrial defects. However, with the rise of structural heart procedures of higher risk and complexity like transcatheter aortic valve implantation (TAVI) or the management of mitral regurgitation using the MitraClip system, this question has gained popularity and the current clinical practice guidelines consider it necessary.
HUMAN RESOURCES
Chief, Director or Head of the UIC
The chief of the UIC should be a cardiologist accredited in the practice of interventional cardiology by the ACI-SEC. Although back in 2011, the Spanish Ministry of Health recommended, at least, more than 5 years of professional practice and over 500 procedures performed,2 we believe the time has come to update these numbers. Therefore, we recommend over 1000 diagnostic procedures and over 500 therapeutic procedures performed.
The basic functions and responsibilities of the head of the UIC are:
- Coordinate healthcare, training and research activities at the UIC.
- Develop and establish procedural protocols, checklists, and outcome assessments.
- Plan the annual objectives of the activities that will be performed and manage the provision of healthcare, education, training, and research at the UIC. Also, elaborate plans with the annual needs of the UIC.
- Implement policies to provide the office material, devices or technologies needed to run the UIC properly.
- Promote the electronic registry of procedures and results and be in charge of sending it to the ACI-SEC annual registry.
- Facilitate the communication and coordination of actions with other cardiology and hospital units.
- See that the rules, regulations, and general policy of both the cardiology unit and the hospital are observed in line with both the cardiology unit and hospital board of directors.
- Draw up an annual report of the activities developed at the UIC.
- Design internal sessions for the training of medical staff and non-professional healthcare workers.
- Participate in the general sessions held at the UIC, especially in the medical-surgical sessions held by the heart team.
- Make sure that the rules and regulations on radiation protection are implemented and being observed. Also, make sure that the UIC personnel has completed the radiation protection courses required by law.
- Be an active leader in and out of the UIC.
- Periodic assessments of:
- The quality of the clinical practice developed at the UIC by creating, reviewing, and updating the protocols of processes as well as diagnostic and therapeutic procedures.
- The activity, productivity, cost, efficiency, and safety of all the activities developed at the UIC.
- The degree of adherence to the goals set by the UIC with periodic follow-ups and problem solving approaches.
Medical staff
As explained in the “Training and competences” section below, the UIC medical personnel should hold a valid degree as cardiology specialists issued in Spain, follow the recommendations established by the ACI-SEC regarding specific training in interventional cardiology,4 and be in possession of the radiation protection course level 2.5
The functions and responsibilities of the interventional cardiologists who work at the UIC are:
- Perform the invasive procedures often performed in interventional cardiology.
- Perform evaluations of patients prior to performing any diagnostic or therapeutic invasive procedures including possible contraindications and individual risk assessments and confirm that the patients’ legally required informed consent is duly signed.
- Make diagnostic and therapeutic decisions based on validated protocols and clinical pathways established by both the hospital and the cardiology unit.
- Know the different procedures included in the UIC repertoire as well as their indications, risks, and methodologies.
- Collaborate with the remaining UIC team and coordinators to achieve the goals set.
- Know how the equipment works, its indications, and functioning.
- Know which structural heart diseases are eligible for percutaneous treatment and its indications.
- When performing structural heart procedures, it is mandatory to know about techniques and procedures, indications, risks, and contraindications. Also, to be competent in the management of all potential complications that may occur.
The recommended number of cardiologists with full dedication to interventional cardiology to provide scheduled care at an UIC with just 1 cath lab and be able to cover vacations is 3. At UICs with 2 cath labs the recommended number of cardiologists with full dedication to interventional cardiology is 5. For every additional cath lab over 2, 1 interventional cardiologist should be added. This is applicable to supervised cath labs too in such a way that in those centers where the UIC acts as the reference to 1 or more supervised cath labs, the number of interventional cardiologists needed should increase by a factor of 1 for every supervised cath lab available. In any case, if the UIC has continuous infarction care plans implemented (on a 24 hour/day basis 365 days/year), the minimum number of interventional cardiologists required for its correct functioning is 4.6 In order to guarantee the availability of continuous infarction care plans, in some centers (especially those with just 1 cath lab) it may be necessary to hire part-time interventional cardiologists who also work in other areas of cardiology.
The UIC accredited health professionals will facilitate and engage themselves in the development of training programs for both internal medicine residents (IMR) and internship recipients as long as the UIC is accredited to that effect. On the other hand, to perform special procedures, other health professionals who may not be part of the UIC such as cardiology specialists (echocardiography specialists or acute coronary care unit cardiologists) and anesthesiologists may be requested.
Nurse supervision
Nurse supervisors should have an adequate level of experience and training in interventional cardiology, as well as other specific functions and responsibilities. It is advisable that they should be accredited as experts in interventional cardiology by the AEEC.7 These are their responsibilities:
- Organize the UIC nursing staff.
- Supervise and coordinate the day’s work together with the head of the UIC.
- Organize the management of patients before and after performing the procedures.
- Prepare and keep the areas involved in observation and care totally operational.
- Implement the hospital protocols and clinical practice guidelines on monitorization, drug therapy, pre- and postoperative care while making sure that the patients’ safety is guaranteed at all times.
- Develop and establish procedural protocols, checklists, and outcome assessments.
- Organize continuous education and training programs for the nursing staff.
- Assess the competence of health professionals.
- Take good care of equipment and tools.
- Make sure that tools and drugs are always available.
Nursing staff
The minimum requirements for the nursing staff who work at the UIC are:
- Hold a bachelor’s degree in nursing.
- Have UIC training and be trained in the kind of procedures performed in this setting at least in the management of diagnostic procedures and PCI techniques.
- Have a radiological protection degree (preferably the level 2 radiological protection course).5
- At least 50% of the nursing team needs to be accredited in interventional cardiology by SEC/ACI-SEC/AEEC.7
Also, the UIC nursing team needs to be competent in cardiovascular care in general and in the early management of patients, their mental preparedness, and be able to handle postprocedural monitorization. Also, they need to be experienced in the management of cardiovascular disease, critical coronary care unit, management of cardiovascular drugs, be skilled starting IVs, experienced using cardiovascular tools, competent in the use of interventional cardiology tools and skilled using them.2,8
Regarding their competences at the UIC, the nursing team can be divided into: instrument nurses, circulating nurses, and polygraph nurse specialists; still, the entire nursing team needs to be competent in the 3 areas described above. The team should also be skilled in preparing the patients before the procedure and immediate monitorization are tasks that should be performed by expert UIC nurses.
The number of nurses required for the proper functioning of the UIC depends on the type of procedure performed. In order to perform diagnostic studies and PCIs, the minimum number of nurses is 2 (in this case, the same nurse does both the circulating and polygraph monitoring tasks) being 3 the ideal number. At least 3 nurses are required to perform structural heart procedures. The AEEC estimates that for quality care purposes, the proper number of nurses at a cath lab should be 3 in order to cover the aforementioned instrument, circulating, and polygraph monitoring tasks.8 In any case, at least 2 of these 3 nurses need to be experts in order to provide safe and quality care for the patients during and after cardiac catheterization.
Radiology technicians
Since radiation protection training is mandatory for both the UIC medical staff and the nursing team, radiology technicians are not considered essential workers at the UIC. Actually, most UICs simply don’t have them among their staff. However, if they are among the UIC staff, they need to take regular care and maintenance of the radiology equipment and know the different software applications and quantitative coronary angiography systems. Also, they need to understand how non-angiographic imaging modalities (intracoronary ultrasound and optical coherence tomography) and physiological systems (intracardiac pressures and intracoronary pressure guidewires) work. Also, they need to make sure that radiation emissions are safe both for the patients and the healthcare personnel.
Patient care technicians and non-professional healthcare workers
Patient care technicians (PCT) play an important role preparing the patients and helping the nursing team. At least 1 PCT needs to be present in UICs with 1 or 2 cath labs, and 2 PCTs in UICs with more than 2 cath labs.
The administrative personnel and auxiliary health workers are essential employees at the UIC. Administrative personnel handle medical appointments and all sorts of healthcare related documents. Auxiliary health workers are in charge of swiftly escorting the patients in and out of the hospital. At least 1 administrative clerk with full dedication to the UIC should be present in large volume centers with more than 1 cath lab. The cleaning personnel prepare the cath lab between cases while maintaining the proper hygienic conditions.
TRAINING AND COMPETENCES
The SEC, through the ACI-SEC, has been implementing an internal accreditation system for healthcare professionals and training centers since 1998.4 The requirements to access this accreditation system and the core curriculum update in interventional cardiology proposed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI)9 are the basis of the recommendations described below.
Interventional cardiology training
Training in interventional cardiology should guarantee an absolute knowledge of all diagnostic and therapeutic invasive techniques available. Over the last few decades, Spanish cardiologists have been treating not only coronary artery disease but also non-valvular10 and valvular11,12 structural heart diseases percutaneously. It is important to stress that interventional cardiologists are also involved in the comprehensive cardiac management of the patients including the indication for the procedure, evaluations using imaging modalities, the management of all possible complications, and clinical follow-up.
The prerequisites to obtain the professional accreditation in interventional cardiology are:
- Hold a medical degree as cardiology specialist issued by the Spanish Ministry of Education.
- Be well-trained in acute cardiac care to treat any possible complications and clinical impairment that may occur while procedures are being performed.
- Have a level 2 accreditation in radiological protection oriented towards the practice of interventional cardiology.5
- Be properly trained in both diagnostic and therapeutic interventional cardiology. According to the accreditation criteria established by ACI-SEC, training should take place over a 2-year period in a UIC licensed and registered to host this kind of training.4 Since certain highly complex procedures are performed in a limited number of large volume centers, training can be carried out in more than 1 national accredited traing centre or international center with a good professional reputation. Ideally, the UIC medical staff should be accredited and licensed for the practice of interventional cardiology. Also, they can be carrying out this training as specialists already hired by the UIC. At the ICU there can be 1 or more cardiologists at different levels of advanced training under the supervision of already accredited interventional cardiologists.
- To have performed, at least, 250 therapeutic coronary procedures, half of them as lead operator. These procedures should be properly documented and certified by the head of the UIC from the center where the training sessions are taking place.
- Master all manual and cognitive skills on the selection of patients, devices, equipment, tools, drugs, information, and writing of documents. In summary:
- Coronary procedures: master all arterial and venous vascular accesses, hemostasis systems, and devices; PCIs for the management of simple lesions and acute coronary syndromes; and be experienced using complex techniques while performing PCIs including intravascular imaging, functional studies, treatment of bifurcations, chronic occlusions, calcified lesions, and implantation of circulatory mechanical support systems.
- Direct participation in the primary angioplasty program for the management of infarction.
- Have theoretical experience and practical knowledge as an assistant operator in structural heart procedures including transcatheter approaches for the management of different heart valve diseases and closures of septal defects, left atrial appendages, and paravalvular dehiscences.
- Know the care provided, and therapy administered to every patient before and after the procedure. Also keep a proper follow-up after hospital discharge.
- Have the capacity to treat all the possible complications that may occur associated with coronary procedures and coadjuvant therapies.
Training centers
In order to guarantee proper training in interventional cardiology, the clinical practice guidelines on the management of myocardial revascularization recommend13 that training should be carried out in large volume centers with an independent UIC and a structured acute coronary syndrome care program on a 24/7 basis.
In Spain, the ACI-SEC has an accreditation program with training centers adapted to the reality of our context that establishes the following minimum requirements4:
- The UIC needs to be included in a cardiology unit accredited by the SEC and the National Specialty Commission for the training of cardiology specialists via IMR.
- The UIC needs to have at least 1 cath lab that should meet all the requirements anticipated by the current Spanish legislation and be certified by the center radiological protection unit including supervision by accredited personnel for manipulation and supervision purposes.
- The center should perform at least 500 PCIs every year. Centers that perform, on average, 500 PICs annually can train a specialist during the first year and a second specialist during the second year. However, centers that perform at least 1000 PCIs/year can train 2 specialists during the first year and 2 during the second year.
- The training program coordinator needs to be competent in all the cognitive and technical activities included in interventional cardiology with an overall historic volume of procedures performed non-inferior to 1000 PCIs and a mean annual activity of 200 PCIs.
- The UIC should offer satisfactory advanced training with enough cases with all the possible subgroups of risk and complexity including PCIs for the management of acute myocardial infarction.
- The presence of a heart team on-call (on a 24/7 basis) for the management of patients with acute coronary syndrome that may require emergency procedures is essential. Similarly, this service should be able to provide immediate care to patients who may experience complications resulting from interventional techniques and procedures.
- The UIC should observe a minimum level of activity and scientific curiosity in interventional cardiology.4
Keeping the accreditation
There is a direct correlation between results and the volume of PCIs performed per center and operator, both regarding PCIs in general and14 in PCIs to treat infarctions.15 In order to remain competent in performing PCIs to treat acute coronary syndrome, interventional cardiologists need to perform at least 75 PCIs every year. This represents a total of 400 PCIs performed each year by PCI-capable centers on a 24/7 basis). They also need to perform at least 75 PCIs each year to treat acute coronary syndromes (a total of at least 200 annual PCIs per center). Ideally, centers and operators with fewer PCIs performed should partner with larger volume centers.13
Under the current accreditation system, at the time this manuscript was being written, in order to maintain competency the ACI-SEC required that every 5 years, all accredited professionals will be compelled to justify the activity developed during this time. The applicants will need to prove that they performed at least a total of 75 annual PCIs documented and certified by the head of the UIC.4
MATERIAL RESOURCES
Physical space
The ideal location of the UIC is a place close or with easy access to the emergency unit and the cardiology unit hospitalization areas. The UIC should be built with the following physical spaces in mind:
- Total area: at least 200 m2.
- Cath lab: it should have at least 50 m2 of useful surface depending on the equipment included in it. The height of the ceiling should be at least 3 meters. Its design should be rectangular, and the walls need to be covered with lead. It should have an entry door for the patients separate from the door that gives access to the control zone. Both doors should also be covered with lead. The entry door to the cath lab needs to have a red light that should automatically turn on when x-rays are activated. The floor should be antielectrostatic.
- Control zone of the radiology equipment and polygraphy machine connected (ideally through a microphone amplifier circuit) to the examination room and separated from it by radiation shielding lead glass. Ideally, it should be located at the smaller side of the cath lab facing the patient table and opposite the radiology equipment. It can be individual for every cath lab or used with several cath labs.
- Technical room hosting the back-up equipment for the angiography system, polygraph, and transmission of images. The current equipment requires less space. Still, 10 m2 are needed based on the specific needs of every manufacturer. It should have its own independent cooling system and all electric wires should be insulated.
- Reception area for patient preparedness and care. It can be an area adjacent to the cath lab or work as a day hospital does. It should be close to the examination rooms and used for pre- and postprocedural care. In the presence of outpatient catheter and PCI programs it is advisable to adapt this space as a day hospital to fit individual chairs or beds for privacy purposes. Every space should have its own individual monitoring system (electrocardiogram, arterial blood pressure, and oxygen saturation), gas outlets, and power sockets. This area needs to be monitored at all times by nurses and doctors in charge of the operations and procedures performed in such areas.
- Storage unit: since it is advisable to leave the fewest possible pieces of equipment at the cath lab (and always stored in specific surgical furniture and appliances) in order to secure the proper conditions of sterility and asepsis, the storage spaces should be big enough to store the material and equipment used during the procedures. Computerized storage control systems integrated into information and replacement systems are advisable and often used in an increasing number of UICs.
- Other zones intended to be part of this area: administrative area, waiting room and briefing room for families, medical report office, waiting room for the medical personnel, locker room for patients and medical personnel, and separate bathrooms.
Conditions of sterility and air quality
UNE regulation 100713 dated September 2005 classified all UICs as high-risk areas16, class I, and categorized them traditionally as operating rooms type B (ISO class 7),17 indicative that the air diffusion system recommended is turbulent flow.
According to UNE regulation 17134018 which classifies hospital aeras based on risk and type of ventilation/filtration used, cath labs are ranked as high-risk areas. The UICs built from 2012 onwards need to meet the aforementioned regulation to guarantee a sterile environment to be able to perform all kinds of minimally invasive procedures like TAVI, left atrial appendage closures, and use of the MitraClip system. Also, the conditions of sterility and asepsis allow us to use these UICs as conventional operating rooms too (for example, for the management of vascular complications). However, UICs built before this regulation became effective are not bound to it unless remodeled (table 1).
Table 1. Conditions of sterility and quality of air that all current cath labs should meet
| • Minimum advisable flow recirculation with 25 movements/hour of which ≥ 1200 m3/h should be with fresh air from outside |
| • Single air-handling unit |
| • Recirculated air should be treated exactly the same as the outside air by the same air-conditioner |
| • Periodic microbiological tests |
| • Air velocity at the room occupancy area between 0.2 m/s and 0.3 m/s |
| • Have, at least, 3 levels of filtration including these types of filters: |
| - Pre-filter EU4 |
| - Air-conditioning EU9 air filter |
| - Final H13 filter in the surgical area |
UICs should provide enough surgical coverage to perform procedures under conditions of sterility. The use of disposable material is also advised.19 Under conditions of special risk of infection for healthcare personnel, personal protection equipment is advised.20 In any case, all measures related to sterility at the cath lab and infection avoidance for the healthcare personnel should be discussed with both the preventive medicine and risk management units.
Radiology equipment and clinical support systems
In general, the UIC radiological and additional equipment should include (figure 2):
Figure 2. Equipment present at the cath lab. 1: patient table. 2: x-ray tube. 3: radiation shielding lead glass with upper leaded glass section and articulated adjustable arm mounted on the ceiling. 4: leaded skirt mounted on the table. 5: automated contrast media injector. 6: on/off ceiling light with adjustable articulated arm. 7: monitors. 8: infusion pumps. 9: anesthetic and respiratory equipment. 10: crash cart or code cart with defibrillator. 11: smart cabinet for material storage with automatic replacement. 12: table with the equipment needed to perform the procedure. 13: console of the pressure wire.
- A 100 kW-standard X-ray generator.
- Flat-panel imaging digital detector with field sizes big enough to facilitate the use of coronary and structural techniques. Twenty inches is the recommended size.
- Collimation system.
- Anticollision systems are mandatory to avoid short range collisions with the patients. Also, a grid should be incorporated to the system.
- Ceiling or floor mounted motorized arm with iso centric tilt movement and possibility of cranial and caudal ≥ ± 40° and lateral and oblique ≥ ± 90° angulations without having to move table or patient.
- Examination table: low-attenuation carbon fiber board or equivalent with longitudinal and cross-sectional movement capabilities—automatic or manual—and electromagnetic system blocking system on the table. Vertical movement needs to be motorized. Also, it needs to include accessories and outlets to adapt additional components (injector pump, polygraph, consoles for coronary physiology monitoring, etc).
- Contrast injector: automatic injectors are advised but not required.
- Monitors: mounted on the ceiling and movable or adjustable for correct visualization. The monitor can be a single ≥ 55-inch flat screen or multiple ≥ 19-inch monitors. They need to show at least 3 different imaging sources such as real-time radiological images, the reference radiological image, polygraphy, echocardiogram, intracoronary ultrasound, optical coherence tomography, computerized tomography scan or fusion tools.
- Polygraph: it should display continuous ECG monitoring, invasive arterial pressure with at least 2 independent pressure transducers, oxygen saturation through pulse oximetry, cardiac output cable, hemodynamic wave data recording, and be capable of processing hemodynamic data (eg, valvular areas, vascular resistances, gradients, and cardiac output). It needs to be programmed with software that should allow the reception of the working list, the sending of information to a storage system, and the transmission of images with storage and post-processing editing capabilities. Ideally, it should be controlled from the examination table and the working station should be located in the control zone outside the cath lab.
- We should be able to generate final reports on the procedure performed including data from the Kerma-air product (KAP) and dose-area product (DAP) that will be included in the patient’s medical history.
- Radiological contrast: iso-osmolar contrast media are advised since they are associated with a lower risk of contrast-induced nephropathy.
- Radiological protection systems:
- Protective lead curtains for the examination table, at least where the table controls are located and on the side where the procedure will be performed. The minimum lead cover should be 0.5 mm thick.
- Suspended and articulated transparent protection shield to protect the exposed medical personnel who participate in the procedure and remain close to the patient table. At the same time, it facilitates the visualization of the patient and should adapt to the patient contour.
- Radiological protection equipment for the medical staff: lead aprons, lead thyroid collar cover, lead glasses, and dose meters.
- Cold light operating lamp: hanging from the ceiling from an articulated arm and highly movable to light up specific sections of the surgical field.
- Intercommunicator between the examination room and the control room.
- Uninterruptible power supply systems for monitorization and life support purposes. Also, if possible, radiological equipment with enough power (15 min) to perform a fluoroscopy in case of possible power cuts.
Image acquisition systems and storage
The image acquisition system should be digital with a proper dynamic range for routine clinical applications. It should cover the low doses of the different imaging modalities of x-rays and the higher doses of digital acquisition including the most demanding ones of the digital subtraction angiography. The frequency range in pulsed fluoroscopy or graphic representation should be of 30 or more images per second. It should allow processing, visualization, and digital storage.
This equipment needs to include coronary and ventricular quantification applications. Currently, there are applications available for quantitative computed tomography assessment to plan procedures, and fusion systems of transesophageal echocardiography plus digital angiography that can be useful when performing interventional procedures to treat structural heart diseases.
The images of every patient will be saved and stored permanently in a filing system compatible with multiple DICOM modality worklist services (digital imaging and communication on medicine) of cardiac images with integrated DICOM-3 services. These images need to be stored in the corresponding hospital or health service PACS (picture archiving and communication system) so that all studies can be seen and analyzed from the different working stations connected to this server. For that purpose, TCP/IP communications protocols (transmission control protocol/ internet protocol) are required. These protocols should be used in full compliance with data protection legislation. The capabilities of compact disc and digital versatile disc recording and reading are advisable in compliance with the DICOM standard anticipating the possibility of exporting images and angiographic series to other imaging formats.
For real-time image processing and simultaneous image acquisition purposes, a working station will be required for case review and analysis that will join the working station of the image acquisition system. It should be located in the same control zone as the x-ray and polygraph equipment.
Resuscitation and life support systems
UICs should have specific resuscitation and life support systems:
- Crash carts or code carts: the entire UIC staff should be trained in cardiopulmonary resuscitation techniques. The cart should be placed at the patient’s bedside and include the following components that should be reviewed periodically:
- Defibrillator/monitor equipped with transcutaneous pacing capabilities.
- Oxygen administration systems.
- Orotracheal intubation equipment (laryngoscope and tubes).
- Ventilation system.
- Aspiration system.
- Drugs required for hemodynamic drug support, sedation, and management of cardiorespiratory arrest.
- Ventilator.
- Infusion pumps.
- Temporary transvenous pacemaker insertion equipment (electrocatheter and generator).
- Pericardiocentesis kit.
Specific material to perform coronary interventions
Added to the conventional material used to achieve diagnosis and perform coronary interventions (diagnostic catheters, guide catheters, angioplasty guidewires, angioplasty balloons, and coronary stents), it is advisable to have specific coronary stents available to treat coronary perforations, and plaque modification devices to treat nondilatable coronary lesions with conventional balloon or heavily calcified coronary lesions.21
Intracoronary diagnostic tools
In a large number of patients, the use of pressure guidewires or intracoronary imaging modalities will be required as established by the latest clinical practice guidelines on the management of myocardial revascularization.13
Clinical practice guidelines consider pressure guidewires as the proper tool to identify hemodynamically relevant coronary lesions in stable patients (indication class I, level of evidence A) and guide revascularization in patients with multivessel disease (indication class IIa, level of evidence B).13
Also, clinical practice guidelines indicate the use of intracoronary imaging modalities (both intracoronary ultrasound and optical coherence tomography) to study the mechanisms of stent failure and for implant optimization purposes in selected patients (indication class IIa, level of evidence B). Also, intracoronary ultrasound is considered the imaging modality of choice to study the severity of left main coronary artery lesions and for result optimization purposes (indication class IIa, level of evidence B)13.
Therefore, we believe it is necessary for UICs to have functional assessment methods available (eg, pressure guidewires) and intracoronary imaging modalities as well.
Circulatory support systems
Circulatory support systems are required at the UIC to approach complex angioplasties in high-risk patients and for the management of hemodynamically unstable patients or cardiogenic shock. Actually, this is very important in centers with continuous infarction care programs running, especially large volume centers and satellite cath labs without surgical coverage in situ. These systems can be:
- Intra-aortic counterpulsation balloon: catheters should be available at the UIC. However, the console can be stationed at the coronary care unit or cardiac surgery intensive care unit. It should be adaptable to any type of balloon, portable, and have a minimum power supply of 3 hours.
- Percutaneous left ventricular assist devices: the most commonly used one is transaortic microaxial blood pump. It is used for the management of patients with cardiogenic shock and to approach very high-risk PCIs. Its use should follow the recommendations established by the clinical practice guidelines.
- Venoarterial extracorporeal membrane oxygenation: this system is advisable in large volume centers that treat patients with refractory cardiogenic shock, cardiac arrest that remains unresponsive to cardiopulmonary resuscitation maneuvers, and refractory malignant ventricular arrythmias.
SPECIFIC CONSIDERATIONS
Supervised cath labs
Over the last decade, the model of satellite or supervised cath labs in large volume center units has been widely implemented. The reason for this is to bring coronary intervention techniques to a larger number of centers so patients can have access to these services without detriment to the advantages that experienced tertiary levels bring. The characteristics of a satellite or supervised cath lab are:
- The interventional cardiology staff is stationed in another reference unit but still provides coverage to this center to perform the procedures required.
- The head of the satellite cath lab and the head of the reference UIC where the medical staff is stationed is the same person.
- In general, these satellite cath labs are implemented in level II hospitals without cardiac surgery in situ.
-
The requirements of these centers are:
- They should meet all remaining requirements and have the same support units as an autonomous cath lab.
- They require fewer medical staff and health professionals compared to the reference unit. Actually, 1 interventional cardiologist should be enough. At least 2 nurses should be available. However, 3 nurses are advisable per satellite cath lab and day of occupancy. The medical personnel should be stationed at the reference center. The nursing staff and auxiliary health workers can be stationed at the hospital where the satellite cath lab is located.
- A prior written informed consent model needs to be implemented including the fact that emergency surgeries will be performed in a different partner center.
- Patient transfer time to the reference center when emergency surgery is required will not exceed 60 minutes.
A written agreement will need to be signed between the management of both centers for the provision of these services including a budget with all the expenses derived from buying the material required. These centers can be included in healthcare networks.
According to the legislation that regulates the planning and arrangement of the healthcare services provided by each Spanish autonomous community, satellite cath labs can have extraordinary continuous activity programs available on a 24/7 basis 365 days/year to provide urgent care especially within the framework of institutional infarction code programs. The implementation of these programs will be the sole responsibility of the reference UIC.
Structural heart procedures or urgent procedures will not be performed in the supervised cath lab. but in the reference center. However, very complex coronary procedures or interventions requiring special devices in clinically stable patients are ill-advised and they should be performed at the reference center.
Optimization of the infarction care program (primary angioplasty program)
The requirements and needs of infarction care networks have already been described in detail.6 In summary, hospitals with primary angioplasty programs require:
- A coronary care unit or general intensive care unit with levels of care 2 and 3 according to the Acute Cardiovascular Care Association.22
- Cardiologists on call physically present.
- A cardiac surgery unit capable of treating infarction related mechanical complications, or at least partnerships with centers with other cardiac surgery units, capable of transfering patients in less than 60 minutes.
-
Added to the material required to sustain life support and perform resuscitation maneuvers, UICs with infarction code care programs should include ventricular assist devices too. These UICs require a 24/7 on-call service throughout the year. The personal requirements for training and accreditation purposes are:
- At least 4 ACI-SEC- accredited interventional cardiologists in the on-call medical staff.
- A total of over 400 PCIs performed at the UIC every year. Also, each operator should perform at least 75 PCIs and 30 primary angioplasties each year.
- At least 2 nurses on-call and 1 PCT are required with proper training performing procedures in situ with the operators and expertise in the equipment used. It is advisable that the whole nursing staff should be part of the UIC.
- The quality of the program should be assessed using some sort of control mechanism including reperfusion times and mortality results. Similarly, it is advisable to participate in a regional or national registry to guarantee that the quality control process is properly carried out.
Structural heart intervention programs
The specific recommendations to perform structural heart procedures are:
- To perform structural heart procedures, interventional cardiologists should have been accredited in hemodynamics and interventional cardiology by the ACI-SEC at an accredited center. This training qualifies the accreditee to be able to perform PCIs.
- At least 2 cath labs are required in centers that perform structural heart procedures, to be able to assist the infarction care network while long structural heart procedures are still underway.
- UICs capable of performing structural heart procedures should have enough room for the echocardiography specialist and anesthesiologist when required.
- Transesophageal echocardiography (ideally with a 3D probe) is required. The availability of intracardiac echocardiography at the UIC setting is not mandatory and its use is regulated by the recommendations established by the clinical practice guidelines.
- The availability of cardiac surgery in centers that perform structural heart procedures like such as valvuloplasties (mitral, aortic or pulmonary) or percutaneous closures of interatrial septal defects or other short circuits has traditionally not been considered a necessity. The general recommendations established for all UICs apply here too when these procedures need to be performed (possibility of patient transfer in less than 60 minutes to a center with cardiac surgery capabilities). These are also the recommendations established to approach the closure of left atrial appendage.23 Regarding TAVI, developed by cardiology almost 20 years ago,24 the current clinical practice guidelines require the availability of cardiac surgery at the treating center,25,26 although these requirements may change in the future.27 The recommendations established for the management of the MitraClip system regarding the need for surgery are similar to those established for TAVI.28
- Hybrid cath labs are not required, but if structural heart procedure is performed in an operating room, it should include all the necessary equipment for constant hemodynamic monitorization purposes, a kinescope, and high-quality fluoroscopy with possibility of a wide array of angles, projections, and image storage; a mobile C-arm would not be suitable here. In this case, the necessary equipment to perform PCIs, implant transvenous pacemakers, use different types of vascular introducers of different sizes and lengths, as well as bailout devices in cases of device migration, transseptal puncture equipment and pericardiocentesis kit, vascular closure devices, and devices to perform vascular interventions will also be required.
- High-resolution monitors are required for the simultaneous visualization of hemodynamic monitorization images (pressures, electrocardiogram, oxygen saturation). Also, they need to be able to show images acquired using other imaging modalities like echocardiography.
- Two operators and 3 nurses are needed to perform structural heart procedures. In some cases, 1 echocardiography specialist and 1 anesthesiologist may be required. The presence of a cardiac or vascular surgeon may be required too to perform certain procedures.
- The head of the UIC or the coordinators of the structural heart program should be cardiologists with at least 1 year of specific training in structural heart procedures in a large volume centers, experienced in this kind of procedure. Also, they should have at least 5-year experience performing interventional procedures (both PCIs and noncoronary interventional techniques) including transseptal punctures, valvular procedures, and intracardiac device implantation and retrieval. If experience is limited with some of the techniques described, these procedures should be supervised until the proper experience has been gained.
- The number of procedures recommended for both the center and the operator is well-defined for TAVI: at least 50 per year.29 This is the case with transfemoral TAVI, the only access of which there is evidence in randomized clinical trials as an alternative to aortic valve replacement surgery. Clinical practice guidelines are a little unclear regarding other techniques.30-32 However, our recommendation is that at least 15 left atrial appendage closures and percutaneous mitral valve repairs and 10 percutaneous closures of interatrial septal defects should be performed every year.
- Centers capable of performing structural heart procedures will need to send the data from the procedures performed to the official registries of the ACI-SEC and the SEC. Also, data will be subject to the consequences that may arise from supervising these data.
Latest programs: cardiac arrest code and management of acute pulmonary embolism
Over the last few years, the management of cardiac arrest and acute pulmonary embolism has improved significantly. Actually, interventional cardiology has progressively gained interest in these conditions.
Regarding cardiac arrest, performing emergency coronary angiographies to treat acute coronary syndrome can bring clinical benefits. Our recommendation is that patients with out-of-hospital cardiac arrests should be transferred to specific centers. Actually, this kind of care for this type of patient has become a customary practice for some hospitals and is now called “cardiac arrest code”. Requirements for these centers are:
- Inclusion in an acute myocardial infarction care network.
- Cardiologists on call.
- Coronary care unit or cardiac surgery intensive care unit with circulatory support system implantation capabilities.
- Capability of performing therapeutic hypothermia.
- Neurology/neurophysiology unit.
Maastricht types III and IV controlled asystole organ donation programs are not required but highly recommended.
Regarding acute pulmonary embolism, more and more UICs have included the management of this condition in their repertoire using embolectomy catheters in hemodynamically compromised patients contraindicated for thrombolysis.
Based on experience and efficiency, both the cardiac arrest code and the management of pulmonary embolism should be handled by personnel from the continuous infarction care program-according to the internal reality of every hospital and the healthcare regulations of the department of health of the Spanish autonomous community concerned.
CONCLUSION
Over the last few years, the wide invasive management of acute coronary syndrome, development of acute myocardial infarction care networks, creation of supervised cath labs, the arrival and development of new diagnostic and therapeutic coronary techniques and coronary interventions for the management of structural heart disease, as well as legislation changes have changed the equipment, and human resources associated with UICs. This document comes as a response to the need for adapting the current situation to the recommendations in our context regarding requirements in hemodynamics and interventional cardiology. In the future, the recommendations published in this document will need to be updated based on the future steps interventional cardiology may take.
FUNDING
No funding.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest regarding this manuscript. R. Moreno is associate editor of REC: Interventional Cardiology; the editorial protocol of the journal was observed to guarantee an impartial manuscript handling.
REFERENCES
1. Morís de la Tassa C, Cequier Fillat A, Moreu Burgos J, Pérez Hernández H, Aguirre Salcedo JM. Guías de práctica clínica de la Sociedad Española de Cardiología sobre requerimientos y equipamiento en hemodinámica y cardiología intervencionista. Rev Esp Cardiol. 2001;54:741-750.
2. Palanca Sanchéz I, Castro Beiras A, Macaya Miguel C, Elola Somoza J, Bernal Sobrino JL, Paniagua Caparrós JL;Grupo de Expertos. Unidades asistenciales del área del corazón:estándares y recomendaciones. Madrid:Ministerio de Sanidad, Política Social e Igualdad;2011.
3. Esplugas E, Hernández RA, López Bescós L, Moreu J, Pomar JL. The performance of coronary angioplasties at centers without cardiac surgery. The recommendations of the Sociedad Española de Cardiología. Rev Esp Cardiol. 1999;52:5-12.
4. Sistema de acreditación para el ejercicio y la enseñanza de Hemodinámica y Cardiología Intervencionista dirigido a Profesionales y Unidades de Formación. Asociación de Cardiología Intervencionista de la Sociedad Espa-ñola de Cardiología (ACI-SEC). Available online: https://www.hemodinamica.com/institucional/ acreditacion/. Accessed 23 Sep 2020.
5. Orden SCO/3276/2007, de 23 de octubre, por la que se publica el Acuerdo de la Comisión de Recursos Humanos del Sistema Nacional de Salud, mediante el que se articula el segundo nivel de formación en protección radiológica de los profesionales que llevan a cabo procedimientos de radiología intervencionista. Available online: https://www.boe.es/eli/es/o/2007/10/23/sco3276. Accessed 23 Sep 2020.
6. Cequier A, Pérez de Prado A, Cid AB, et al. Requisitos y sostenibilidad de los programas de ICP primaria en España en el IAMCEST. Documento de consenso de SEC, AEEC y SEMES. REC Interv Cardiol. 2019;2:108-119.
7. Gómez M, Rodríguez V, Pedrosa CP, et al. Perfil Profesional de Enfermería de Hemodinámica y Cardiología Intervencionista. Sistema de Acreditación Competencias Avanzadas. 2018. Available online:http://hemodinamica.enfermeriaencardiologia.com/wp-content/uploads/documento-de-perfil-normas-de-acreditacion.pdf Accessed 23 Sep 2020.
8. Fernández JM, García FJ, Gómez M, et al. Manual de Procedimientos de Enfermería en Hemodinámica y Cardiología Intervencionista. Madrid:Asociación Española de Enfermería en Cardiología;2014.
9. Van Belle E, Teles R, Pyxaras S, et al. Core curriculum EAPCI. EAPCI core curriculum for the interventional cardiologists. Committee for Education and Training. EuroIntervention. 2020. https://doi.org/10.4244/EIJ-D-18-00448.
10. Zabala Argüelles JI, García E, Zunzunegui Martínez JL, et al. Cierre percutáneo de la comunicación interauricular:resultados a medio plazo de esta nueva opción terapéutica. Rev Esp Cardiol. 2000;53:21-6.
11. Calvo OL, Sobrino N, Gamallo C, Oliver J, Dominguez F, Iglesias A. Balloon percutaneous valvuloplasty for stenotic bioprosthetic valves in the mitral position. Am J Cardiol. 1987;60:736-737.
12. Medina A, Bethencourt A, Coello I, Hernandez E, Goicolea J, Melián F, et al. A new type of adjustable vascular introducer for balloon valvuloplasty:technical note. Cardiovasc Intervent Radiol. 1989;12:169-171.
13. Neumann FJ, Sousa-Uva M, Ahlsson A, et al.;ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
14. Hannan EL, Wu C, Walford G, et al. Volume-outcome relationships for percutaneous coronary interventions in the stent era. Circulation. 2005;112:1171-1179.
15. Nallamothu BK, Wang Y, Magid DJ, et al. National Registry of Myocardial Infarction Investigators. Relation between hospital specialization with primary percutaneous coronary intervention and clinical outcomes in ST-segment elevation myocardial infarction:National Registry of Myocardial Infarction-4 analysis. Circulation. 2006;113:222-229.
16. UNE. UNE 100713:2005. Instalaciones de acondicionamiento de aire en hospitales. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0034264. Accessed 23 Sep 2020.
17. UNE. UNE 14644-1:2016. Salas limpias y locales anexos. Parte 1:Clasificación de la limpieza del aire mediante la concentración de partículas (ISO 14644-1:2015). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0057435. Accessed 23 Sep 2020.
18. UNE. UNE 171340:2020. Validación y cualificación de salas de ambiente controlado en hospitales. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0064465. Accessed 23 Sep 2020.
19. European Committee for Standardization. European Standard EN 137951:2019. Surgical clothing and drapes Requirements and test methods Part 1: Surgical drapes and gowns. Available online: https://www.sls.se/media/v3hf5pb1/pren-13795-1.pdf. Accessed 23 Sep 2020.
20. Romaguera R, Cruz-González I, Ojeda S, et al. Gestión de las salas de procedimientos invasivos cardiológicos durante el brote de coronavirus COVID-19. Documento de consenso de la Asociación de Cardiología Intervencionista y la Asociación del Ritmo Cardiaco de la Sociedad Española de Cardiología. REC Interv Cardiol. 2020;2:106-111.
21. Cubero-Gallego H, Tizón-Marcos H, Vaquerizo B. Opciones actuales para el tratamiento de las lesiones calcificadas. REC Interv Cardiol. 2020;2:129-139.
22. Bonnefoy-Cudraz E, Bueno H, Casella G, et al. Editor's Choice —Acute Cardiovascular Care Association Position Paper on Intensive Cardiovascular Care Units:An update on their definition, structure, organisation and function. Eur Heart J Acute Cardiovasc Care. 2018;7:80-95.
23. Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI Expert Consensus Statement on Catheter-Based Left Atrial Appendage Occlusion —An Update. EuroIntervention 2020;15:1133-1180.
24. Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis:first human case description. Circulation. 2002;106:3006-3008.
25. Baumgartner H, Falk V, Bax JJ, et al.;ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
26. Bavaria JE, Tommaso CL, Brindis RG, et al. 2018 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document:operator and institutional recommendations and requirements for transcatheter aortic valve replace ment. J Am Coll Cardiol. 2019;73:340-374.
27. Jiménez Quevedo P, Pan M, Moreno R, Pérez de Prado A. Scientific evidence versus expert opinion. Should we modify clinical practice guidelines?Rev Esp Cardiol. 2020;73:187-189.
28. Nishimura RA, O'Gara PT, Bavaria JE, et al. 2019 AATS/ACC/ASE/SCAI/ STS Expert Consensus Systems of Care Document:A Proposal to Optimize Care for Patients With Valvular Heart Disease:A Joint Report of the American Association for Thoracic Surgery, American College of Cardi ology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:2609-2635.
29. Wassef AWA, Rodes-Cabau J, Liu Y, et al. The learning curve and annual procedure volume standards for optimum outcomes of transcatheter aortic valve replacement:findings from an international registry. JACC Cardiovasc Interv. 2018;11:1669-1679.
30. Glikson M, Wolff R, Hindricks G, et al.;ESC Scientific Document Group. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion —an update. EuroIntervention. 2020;15:1133-1180.
31. Chhatriwalla AK, Vemulapalli S, Holmes DR Jr, et al. Institutional Experience With Transcatheter Mitral Valve Repair and Clinical Outcomes:Insights From the TVT Registry. JACC Cardiovasc Interv. 2019;22:1342-1352.
32. Horlick E, Kavinsky CJ, Amin Z, et al. SCAI expert consensus statement on operator and institutional requirements for PFO closure for secondary prevention of paradoxical embolic stroke. Catheter Cardiovasc Interv. 2019;93:859-874.
Abstract
In Mexico, the number of confirmed and estimated cases of COVID-19 has been going up gradually from the second week of March 2020. This directly and indirectly altered the normal care of patients with ischemic heart disease. This is a consensus document achieved by different societies (the Mexican Society of Interventional Cardiology [SOCIME], the Mexican Society of Cardiology [SMC], the Mexican National Association of Cardiologists [ANCAM], the National Association of Cardiologists at the Service of State Workers [ANCISSSTE]), and the Coordinating Commission of National Institutes of Health and High Specialty Hospitals [CCINSHAE]). Its main objective is to guide the decision-making process on coronary revascularization procedures for the management of patients with acute coronary syndrome in catheterization laboratories during the current health emergency generated by the SARS-CoV-2 pandemic.
Keywords: Myocardial infarction. Acute coronary syndrome. Percutaneous coronary intervention. COVID-19. SARS-CoV-2. Pandemic.
RESUMEN
En México, el número de casos confirmados y estimados de COVID-19 inició su ascenso progresivo a partir de la segunda semana de marzo de 2020, lo que alteró de forma directa e indirecta la atención habitual de los pacientes con cardiopatía isquémica. Este documento es un consenso de la Sociedad de Cardiología Intervencionista de México (SOCIME), la Sociedad Mexicana de Cardiología (SMC), la Asociación Nacional de Cardiólogos de México (ANCAM), la Asociación Nacional de Cardiólogos al Servicio de los Trabajadores del Estado (ANCISSSTE) y la Comisión Coordinadora de Institutos Nacionales de Salud y Hospitales de Alta Especialidad (CCINSHAE) que tiene como objetivo prioritario orientar las decisiones de revascularización coronaria, en particular en los pacientes con síndrome coronario agudo, en las salas de cateterismo, durante el tiempo que dure la urgencia sanitaria por la pandemia de SARS-CoV-2.
Palabras clave: Infarto de miocardio. Síndrome coronario agudo. Intervención coronaria percutánea. COVID-19. SARS-CoV-2. Pandemia.
INTRODUCTION
Ischemic heart disease is the leading cause of death in Mexico. Consequently, the number of patients with acute coronary syndrome (ACS) or stable chronic coronary syndrome who will require medical services is not expected to go down during the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The ongoing need to manage this disease during the COVID-19 pandemic compels us to recognise the exceptional situation and to search for an adequate response.
Several places across the world have suspended the management of ischemic patients or decided to use less invasive—also less effective—treatments. The idea behind this is to reduce the risk of contagion for patients, relatives, and healthcare workers. However, this decision has created huge controversy in the medical establishment: is it safe? Is it ethical? Is the safety of the medical team more important than the patients’ health? For how long should this measure be effective?
The Mexican Interventional Cardiology Society (SOCIME) backed by the Mexican Society of Cardiology (SMC), the National Association of Cardiologists of Mexico (ANCAM), and the National Association of Cardiologists at the Service of State Workers (ANCISSSTE) have published this document to guide the medical personnel in the decision-making process during the current COVID-19 pandemic.
PATIENT CLASSIFICATION
Healthcare workers looking after patients with ischemic heart disease should classify them into 2 groups based on the information available during the healthcare process: 1) patients with suspected SARS-CoV-2 infection or patients with symptoms of concurrent myocardial ischemia or other heart-related complications; 2) other patients, without a diagnosis or suspicion of SARS-CoV-2 infection, who require medical attention due to symptoms of myocardial ischemia. The reason for making this subdivision is to minimise the contagion of other patients, relatives, medical and hospital personnel by avoiding contact between uninfected patients and patients with SARS-CoV-2. Before being transferred to the cath lab all patients will be actively screened for the presence of fever, cough, and respiratory distress. The patients’ temperature and arterial oxygen saturation will need to be checked as well.
STABLE CHRONIC CORONARY SYNDROMES
There is general consensus that invasive procedures—whether percutaneous or surgical—should be differentiated in patients with stable chronic coronary syndrome during the current health emergery.1,2 The undersigned working group that participated in the writing of this document agrees on the adoption of this measure. However, it also admits that the number of patients who will not receive proper care will end up accumulating and a proportion of these patients will turn into new cases of acute ischemia. In conclusion, patients with stable chronic coronary syndrome during the current pandemic should receive optimal therapy and be warned of the importance of seeking urgent medical attention in the presence of symptoms of ischemic instability.
ACUTE CORONARY SYNDROMES
In the current context of the COVID-19 pandemic, there is no unanimous agreement to define the optimal treatment strategy for patients with ACS. We will be reviewing the information available accumulated over a very short period of time on the different treatment proposals reported followed by the position and recommendations from this group.
Classification
ACSs can be divided into: a) ST-segment elevation ACS including ST-segment elevation myocardial infarction (STEMI) and b) non-ST-segment elevation ACS including non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina.
Diagnosis
Two recommendations should be followed to minimize the risk of contagion. The first one should be social distancing and limited contact with patients during the physical examination or while performing the transthoracic echocardiogram to obtain the maximum amount of information while minimizing physical contact with the patient; the second recommendation is the use of coronary computed tomography angiography as a fast non-invasive imaging modality to confirm or discard the presence of coronary heart disease as the cause for the symptoms when appropriate.3 The main elements for diagnosis are:
-
- Suggestive symptoms.
-
- Twelve-lead electrocardiogram.
-
- Myocardial biomarkers.
-
- Coronary computed tomography angiography: when available, it should be considered in the case of reasonable doubt on the coronary origin of the symptoms. It speeds up the confirmation/discarding process, reduces hospital stay and use of hospital resources and minimizes stay in the cath lab and exposure to contagion for the heart team who work in the cath lab.
Management of STEMI
Primary percutaneous coronary intervention (pPCI) is the treatment of choice for coronary reperfusion. Fibrinolysis is spared for cases where percutaneous coronary intervention (PCI) is not available or when the patient transfer to a PCI capable hospital involves significant time delays.4 The COVID-19 pandemic has produced different points of view for the management of patients with STEMI; these different positions are indicative of the particular situations that medical societies are facing to design their recommendations.
Fibrinolysis as the strategy of choice
A report and the position adopted by opinion leaders recommend the use of fibrinolysis as the first reperfusion strategy in both uninfected patients and patients with suspicion or diagnosis of COVID-19. The PCI would be spared for cases of failed fibrinolysis and only if benefits exceed the possible risks.5 They recommend that patients with serious pneumonia due to SARS-CoV-2, unstable vital signs, and concurrent STEMI should receive support medical treatment only—no fibrinolysis or pPCI—until they have recovered from pneumonia. These recommendations are highly restrictive, do not anticipate the pPCI option and limit the possibility of bailout PCIs in selected cases.5
pPCI as the strategy of choice
Several medical societies are keeping to their usual recommendations during the current pandemic. Patients with STEMI (with or without suspicion of infection, with or without COVID-19) inescapably need medical attention and should receive pPCI as the reperfusion therapy, especially those with persistent angina or hemodynamic compromise; they also keep the bailout PCI option alive. They accept that fibrinolysis can be an alternative in patients with pneumonia due to SARS-CoV-2 who develop STEMI but remain stable.1,2,6 The Spanish Society of Cardiology (SEC) ranks pPCI as the preferred reperfusion strategy in most patients with severe pneumonia and difficulties moving or being transferred.7 At the present time, Spain is one of the countries with the highest contagion and mortality rates and its healthcare system has collapsed due to the excessive volume of patients with COVID-19; in the middle of this health crisis, consensus still favors the reperfusion of STEMI through pPCI.
Management of non-ST-segment elevation ACS
In general, the specific risk of complications of patients with NSTEMI or unstable angina should be identified to decide the best time to perform cardiac catheterization. Regardless of their risk, it is essential to know the patients’ coronary anatomy and based on the findings assess the revascularization method.8 As with STEMI, this routine common practice has changed during the current pandemic and several of the position papers on the management of ST-segment elevation ACS have been replicated.
Medical therapy as the strategy of choice
The general recommendation is to know the state of contagion before referring patients to the cath lab Patients without a diagnosis or suspicion of infection need to be tested to confirm or discard the presence of SARS-CoV-2. It is suggested that patients with suspicion of COVID-19 who have not been tested yet and those who have already tested positive should receive medical therapy. The interventional procedure should be deferred until they have recovered or the risk of contagion has gone down.7 It is recommended that patients without suspicion of infection or without COVID-19 (common patients) undergo PCI only if they are high-risk patients or remain unstable during the course of conservative treatment. Urgent PCIs will be spared only for patients with hemodynamic compromise or malignant arrhythmias regardless of their state of infection.6,7
PCI as the strategy of choice
Here the usual recommendations still stands, which is why high-and-intermediate risk patients should undergo PCI, regardless of their state of contagion.8 In low-risk patients with COVID-19 or with severe pneumonia and who remain unstable it is advised to consider medical therapy and defer the procedure. Early PCIs facilitate early hospital discharges. In patients with multivessel disease the PCI should be favored over coronary revascularization surgery. Patients admitted to hospitals without cath lab capabilities should not be transferred. In these cases, conservative treatment is advised followed by early hospital discharges.1,2
CONSENSUS ELEMENTS
The following variables were taken into consideration while the recommendations of this consensus document were being designed:
-
Contagion. When examined, patients who seek medical attention should be classified according to their current state of SARS-CoV-2 infection regardless of the presence of symptoms. A special subgroup are unstable patients with severe pneumonia who develop ACS.
-
Timeframe. At different times and different speeds peaks and troughs in the number of patients with COVID-19 are expected to happen.
-
Geographic location. More cases are expected in more heavily populated cities in particular the Mexico City metropolitan area, Guadalajara, Monterrey, and Puebla.
-
Healthcare system. There are different healthcare models available in Mexico. In general, in private hospitals cath labs are opened around the clock. In the public system several secondary care centers have cath lab capabilities with different care plans for the management of ACS. Most tertiary care centers have pPCI programs available around the clock too.
-
Therapeutic effectiveness. In the full spectrum of ACS, PCI is the treatment of choice. In patients with STEMI, fibrinolysis is a less effective alternative associated with a higher risk of bleeding complications. In patients with non-ST-segment elevation ACS, medical therapy in the acute phase is only intended to stabilize the atheromatous plaque and alleviate myocardial ischemia before the cath. lab. referral.
-
Risk profile. In the full spectrum of ACS, it is essential to examine patients at increased risk of adverse cardiovascular events early. This group of patients benefits the most from invasive treatment.
-
Hospital stay. Anticipating larger volumes of patients in a prolonged emergency situation, it is advised to shorten hospital stays to alleviate the work load, reduce the use of hospital resources, reduce the exposure of patients, relatives, medical and paramedic personnel to a potentially contaminated environment, empty beds, and facilitate the ongoing rotation of patients.
-
Rooms and special catheterization laboratories. According to the type, size, and resources of each hospital, whether public or private, a specific physical space should be spared to examine patients without COVID-19 or clinical suspicion away from patients with confirmed infections or a justified suspicion of infection. In public and private hospitals with at least 2 cath labs it is possible to use 1 cath lab for the specific care of patients with confirmed or suspected CODIV-19 and the other one for the management of common patients. In public or private hospitals with only 1 cath lab available, the heart team should look for alternatives to perform the procedures while minimizing the risk of contagion. This can be done by splitting the care schedule depending on the patients’ state of infection and only if the clinical indication for cardiac catheterization allows it (managing infected or suspicious patients in the morning, and uninfected or unsuspicious patients in the afternoon). Reducing the number of healthcare workers in the cath lab and organizing groups and shifts during specific timeframes helps too. Regardless of the number of cath labs available, it is a priority to observe the recommendations established for adequate protection of the heart team, which should be the smallest possible. Also, the cath lab should be disinfected before taking on the next patient.
-
Personal protective equipment. The medical personnel looking after patients, whether infected or suspicious, should all have the necessary personal protective equipment and observe the protection measures recommended like those from the Interventional Cardiology Association and Heart Rhythm Association of the Spanish Society of Cardiology.9
RECOMMENDATIONS
The objective of this document is to guide the decision-making process on the coronary revascularization of patients with ACS during the health emergency declared due to the current SARS-CoV-2 pandemic. Considering the elements described above and anticipating an epidemiological model similar to the one seen in countries like ours, we recommend making slight modifications to the management of patients with ACS while continuing to use the pharmacoinvasive reperfusion strategy prevalent in Mexico.
STEMI
Cardiovascular risk
We recommend that pharmacoinvasive strategy should start by identifying patients with higher risk of complications. The presence of 1 or more of the following characteristics is indicative of high risk:
– Age > 75 years.
– Cardiogenic shock (whether associated or not to mechanical complications).
– Refractory angina.
– Ventricular tachyarrhythmias.
– Bradyarrhythmia requiring temporary pacemaker implantation.
– Electrocardiographic pattern of diffuse ischemia.
pPCI
We recommend public and private hospitals with cath lab capabilities and, in particular, tertiary care or reference centers to keep offering pPCI over fibrinolysis. This is completely justified by its higher rate of success, lower risk of complications, and shorter hospital stays (hospital discharges within 36 h - 48 h). The fibrinolysis proposal as the primary reperfusion method even in hospitals with cath lab is questionable.
Fibrinolysis
We still recommend its indication as the reperfusion method in non-PCI capable centers. Unlike conventional pharmacoinvasive strategy, the transfer of stable patients with successful fibrinolysis for early elective cardiac catheterization is ill-advised; these patients should be followed and monitored later in time. Only patients with failed fibrinolysis should be transferred and only in the presence of hemodynamic or electric instability. In patients with severe pneumonia due to COVID-19 it is a valid option even for PCI-capable centers in the presence of high and growing volumes of suspicious or confirmed cases. Also, in the absence of high-risk markers, especially in patients admitted within the first 3 hours after symptom onset and without contraindications for fibrinolysis.
Periodic adjustment of reperfusion method
We recommend that in cities, regions, and hospitals with large volumes of patients with COVID-19 or suspected cases, whether critical, growing or rapidly spiking, the hospital management should decide on the most adequate reperfusion method with the heart team and the resources available. The primary reperfusion strategy should adjust periodically based on the temporal and geographic pattern of the infection.
The algorithm shown on figure 1 for the management of patients with STEMI during the current COVID-19 pandemic recommends pPCI as the method of choice for coronary reperfusion. The reperfusion strategy should change as the pandemic evolves with the necessary, periodic adjustments based on the modification criteria already described.
Figure 1. Therapeutic algorithm for the management of patients with STEMI during the current SARS-CoV-2 pandemic. The arrow thickness is indicative of the preferred therapy. +, present; ±, present or absent; bPCI: bailout percutaneous coronary intervention; CCTA, coronary computed tomography angiography; FL, fibrinolysis; MVD, multivessel coronary artery disease; pPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
NSTEACS
Regardless of the presence of SARS-CoV-2, low-risk patients who respond to medical therapy can be discharged from the hospital to be studied later in time if the risk of contagion is lower or if they have recovered from COVID-19. If the patient’s coronary anatomy needs to be documented prior to hospital discharge, an option here is to perform a coronary computed tomography angiography and, based on the findings, plan the PCI or discharge the patient. Moderate-or-high risk patients (according to the criteria described above) and those who become unstable during the course of conservative treatment should be transferred to the cath lab regardless of their state of contagion.
Most concepts described for the management of STEMI are valid in these patients. PCI-capable public and private hospitals with experienced personnel and the necessary resources should keep the PCI option open in light of its high rate of success and short hospital stays. In patients with significant multivessel disease the hospital stay should be short and PCI revascularizations should be prioritized over coronary revascularization surgeries.
In the algorithm shown on figure 2 for the management of patients with NSTEACS during the current COVID-19 pandemic the use of PCI is recommended in high-risk patients. However, the reperfusion strategy should be dynamic as the pandemic evolves with periodic adjustments based on the modification criteria already described.
Figure 2. Algorithm for the management of patients with NSTEACS during the current SARS-CoV-2 pandemic. The arrow thickness is indicative of the preferred therapy. +, present; ±, present or absent; CCTA, coronary computed tomography angiography; MT, medical therapy; MVD, multivessel coronary artery disease; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; Tx, treatment.
Cardiovascular complications in patients with COVID-19
Patients with COVID-19 can have clinical signs of myocardial damage and secondary complications. Myocardial damage with elevated high-sensitivity cardiac troponin levels (7.2%), cardiogenic shock (8.7%), and arrhythmias (16.7%) has been reported in some patients. In the absence of coronary heart disease, the gradual increase of high-sensitivity cardiac troponin levels is a predictor of mortality.10 Cases of type I STEMI and fulminant myocarditis have also been reported.11
Most of the patients who develop complications are eligible for intensive therapy, and a high percentage of these may end up needing mechanical support of the ventricular function. The etiology of patients with cardiovascular complications can be coronary heart disease; this consideration will not be rare because the characteristics of patients more often affected are old age, overweight, high blood pressure, and diabetes mellitus. If the hypothesis of concomitant coronary heart disease is accepted there are two possible avenues: treat the case as that of a patient with NSTEMI and follow the corresponding algorithm or discard the presence of significant coronary heart disease using coronary computed tomography angiography.
In patients with severe pneumonia, myocardial damage, hemodynamic or electric instability, and suspicion of coronary heart disease, the medical team should decide on the adequacy (benefits exceeding risks, patients who can be saved) of transferring the patient to the cath lab or performing a coronary computed tomography angiography. In particular, the presence of left main coronary artery disease or disease of the proximal left anterior descending artery in a patient who can be saved supports the need to perform an urgent PCI (figure 3).
Figure 3. Diagnostic-therapeutic algorithm for the management of patients with COVID-19 or suspicion of COVID-19 infection with cardiovascular complications. B, benefits; CHD, coronary heart disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending coronary artery; LMCA, left main coronary artery disease; R, risk; Tx, treatment.
VALIDITY OF RECOMMENDATIONS
The epidemiological model is totally unpredictable under the current circumstances; the rate of contagion, number of confirmed cases, and mortality rates are different across cities, regions, countries, and continents. However, estimates from the Mexican Ministry of Public Health suggest a peak of cases and more hospitalizations in the metropolitan area of Mexico City and main cities of the nation from May through August 2020. The flattening of the curve of infection will still take several months. No definitive projection on this regard can be given at the present time.
These recommendations will remain effective as necessary. They will be re-evaluated periodically together with the Mexican Ministry of Public Health to discuss the right time to go back to normal.
CONFLICTS OF INTEREST
None reported.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Jorge Gaspar Hernández, General Manager of the Ignacio Chávez National Institute of Cardiology for his remarks on this manuscript.
EDITOR’S NOTE
This document is subject to further iterations based on the evolution of the current COVID-19 pandemic. This manuscript has undergone a process of internal review of exceptional priority by the editorial staff due to the special interest of disclosing the information contained herein to the scientific community. The editors wish to thank Permanyer Publications for its collaboration and commitment for the quick publication of this document.
REFERENCES
1. Welt FGP, Shah PB, Aronow HD, et al. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic:From ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020. https://doi.org/10.1016/j.jacc.2020.03.021.
2. National Health Service. Specialty guides for patient management during the coronavirus pandemic. Clinical guide for the management of cardiology patients during the coronavirus pandemic. 2020. Disponible en:https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-cardiolgy-coronavirus-v1-20-march.pdf. Consultado 20 Mar 2020.
3. Yang S, Manjunath L, Willemink MJ, Nieman K. The role of coronary CT angiography for acute chest pain in the era of high-sensitivity troponins. J Cardiovasc Comput Tomogr. 2019;13:267-273.
4. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
5. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19:the protocols from Sichuan Provincial People's Hospital. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05993-9.
6. Society of Cardiovascular Angiography and Interventions. Mahmud E. The Evolving Pandemic of COVID-19 and Interventional Cardiology. Disponible en: https://www.scai.org/media-center/news-and-articles/evolving-pandemic-covid-19-and-interventional-cardiology. Consultado 18 Mar 2020.
7. Romaguera R, Cruz-Gonza?lez I, Jurado-Roma?n A, et al. Considerations on the invasive management of ischemic and structural heart disease during the COVID-19 coronavirus outbreak. Consensus statement of the Interventional Cardiology Association and the Ischemic Heart Disease and Acute Cardiac Care Association of the Spanish Society of Cardiology. REC Interv Cardiol.2020. https://doi.org/10.24875/RECICE.M20000121.
8. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation:Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
9. Romaguera R, Cruz-González I, Ojeda S, et al. Consensus document of the Interventional Cardiology and Heart Rhythm Associations of the Spanish Society of Cardiology on the management of invasive cardiac procedure rooms during the COVID-19 coronavirus outbreak. REC Interv Cardiol. 2020. https://doi.org/10.24875/RECICE.M20000116.
10. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system:acute and long-term implications. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa231.
11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Considerations on the invasive management of ischemic and structural heart disease during the COVID-19 coronavirus outbreak.
- Consensus document of the Interventional Cardiology and Heart Rhythm Associations of the Spanish Society of Cardiology on the management of invasive cardiac procedure rooms during the COVID-19 coronavirus outbreak
- Percutaneous management of tricuspid regurgitation. Image-guided step-by-step MitraClip procedure
- Requirements and sustainability of primary PCI programs in Spain for the management of patients with STEMI. SEC, AEEC, and SEMES consensus document
Special articles
Original articles
Editorials
Original articles
Editorials
Post-TAVI management of frail patients: outcomes beyond implantation
Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital General Universitario de Elche, Elche, Alicante, Spain
Original articles
Debate
Debate: Does the distal radial approach offer added value over the conventional radial approach?
Yes, it does
Servicio de Cardiología, Hospital Universitario Sant Joan d’Alacant, Alicante, Spain
No, it does not
Unidad de Cardiología Intervencionista, Servicio de Cardiología, Hospital Universitario Galdakao, Galdakao, Vizcaya, España