Special article
REC Interv Cardiol. 2019;2:108-119
Requirements and sustainability of primary PCI programs in Spain for the management of patients with STEMI. SEC, AEEC, and SEMES consensus document
Requisitos y sostenibilidad de los programas de ICP primaria en España en el IAMCEST. Documento de consenso de SEC, AEEC y SEMES
a Área de Enfermedades del Corazón, Hospital Universitario de Bellvitge, IDIBELL, Universidad de Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain b Servicio de Cardiología, Hospital Universitario de León, León, Spain c Servicio de Cardiología, Hospital Clínico Universitario de Santiago, Santiago de Compostela, A Coruña, Spain d Servicio de Cardiología, Hospital Universitario de Salamanca, Salamanca, Spain e Servicio de Cardiología, Hospital Germans Trias i Pujol, Badalona, Barcelona, Spain f Servicio de Cardiología, Hospital Galdakao-Usansolo, Galdakao, Vizcaya, Spain g Servicio de Cardiología, Hospital Universitario La Paz, IDIPAZ, Madrid, Spain h Servicio de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain i Servicio de Cardiología, Hospital Álvaro Cunqueiro, Vigo, Pontevedra, Spain j Servicio de Cardiología, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain k Servicio de Cardiología, Hospital Universitario Virgen de la Victoria, Málaga, Spain l SUMMA 112, Madrid, Universidad Alfonso X el Sabio, Villanueva de la Cañada, Madrid, Spain m Servicio de Cardiología, Hospital do Salnés, Vilagarcía de Arousa, Pontevedra, Spain n Urgencias Sanitarias de Galicia 061, Santiago de Compostela, A Coruña, Spain o Servicio de Cardiología, Hospital Universitario 12 de Octubre, Madrid, Spain p Servicio de Cardiología, Hospital Universitario Reina Sofía, Córdoba, Spain
Abstract
In Mexico, the number of confirmed and estimated cases of COVID-19 has been going up gradually from the second week of March 2020. This directly and indirectly altered the normal care of patients with ischemic heart disease. This is a consensus document achieved by different societies (the Mexican Society of Interventional Cardiology [SOCIME], the Mexican Society of Cardiology [SMC], the Mexican National Association of Cardiologists [ANCAM], the National Association of Cardiologists at the Service of State Workers [ANCISSSTE]), and the Coordinating Commission of National Institutes of Health and High Specialty Hospitals [CCINSHAE]). Its main objective is to guide the decision-making process on coronary revascularization procedures for the management of patients with acute coronary syndrome in catheterization laboratories during the current health emergency generated by the SARS-CoV-2 pandemic.
Keywords: Myocardial infarction. Acute coronary syndrome. Percutaneous coronary intervention. COVID-19. SARS-CoV-2. Pandemic.
RESUMEN
En México, el número de casos confirmados y estimados de COVID-19 inició su ascenso progresivo a partir de la segunda semana de marzo de 2020, lo que alteró de forma directa e indirecta la atención habitual de los pacientes con cardiopatía isquémica. Este documento es un consenso de la Sociedad de Cardiología Intervencionista de México (SOCIME), la Sociedad Mexicana de Cardiología (SMC), la Asociación Nacional de Cardiólogos de México (ANCAM), la Asociación Nacional de Cardiólogos al Servicio de los Trabajadores del Estado (ANCISSSTE) y la Comisión Coordinadora de Institutos Nacionales de Salud y Hospitales de Alta Especialidad (CCINSHAE) que tiene como objetivo prioritario orientar las decisiones de revascularización coronaria, en particular en los pacientes con síndrome coronario agudo, en las salas de cateterismo, durante el tiempo que dure la urgencia sanitaria por la pandemia de SARS-CoV-2.
Palabras clave: Infarto de miocardio. Síndrome coronario agudo. Intervención coronaria percutánea. COVID-19. SARS-CoV-2. Pandemia.
INTRODUCTION
Ischemic heart disease is the leading cause of death in Mexico. Consequently, the number of patients with acute coronary syndrome (ACS) or stable chronic coronary syndrome who will require medical services is not expected to go down during the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The ongoing need to manage this disease during the COVID-19 pandemic compels us to recognise the exceptional situation and to search for an adequate response.
Several places across the world have suspended the management of ischemic patients or decided to use less invasive—also less effective—treatments. The idea behind this is to reduce the risk of contagion for patients, relatives, and healthcare workers. However, this decision has created huge controversy in the medical establishment: is it safe? Is it ethical? Is the safety of the medical team more important than the patients’ health? For how long should this measure be effective?
The Mexican Interventional Cardiology Society (SOCIME) backed by the Mexican Society of Cardiology (SMC), the National Association of Cardiologists of Mexico (ANCAM), and the National Association of Cardiologists at the Service of State Workers (ANCISSSTE) have published this document to guide the medical personnel in the decision-making process during the current COVID-19 pandemic.
PATIENT CLASSIFICATION
Healthcare workers looking after patients with ischemic heart disease should classify them into 2 groups based on the information available during the healthcare process: 1) patients with suspected SARS-CoV-2 infection or patients with symptoms of concurrent myocardial ischemia or other heart-related complications; 2) other patients, without a diagnosis or suspicion of SARS-CoV-2 infection, who require medical attention due to symptoms of myocardial ischemia. The reason for making this subdivision is to minimise the contagion of other patients, relatives, medical and hospital personnel by avoiding contact between uninfected patients and patients with SARS-CoV-2. Before being transferred to the cath lab all patients will be actively screened for the presence of fever, cough, and respiratory distress. The patients’ temperature and arterial oxygen saturation will need to be checked as well.
STABLE CHRONIC CORONARY SYNDROMES
There is general consensus that invasive procedures—whether percutaneous or surgical—should be differentiated in patients with stable chronic coronary syndrome during the current health emergery.1,2 The undersigned working group that participated in the writing of this document agrees on the adoption of this measure. However, it also admits that the number of patients who will not receive proper care will end up accumulating and a proportion of these patients will turn into new cases of acute ischemia. In conclusion, patients with stable chronic coronary syndrome during the current pandemic should receive optimal therapy and be warned of the importance of seeking urgent medical attention in the presence of symptoms of ischemic instability.
ACUTE CORONARY SYNDROMES
In the current context of the COVID-19 pandemic, there is no unanimous agreement to define the optimal treatment strategy for patients with ACS. We will be reviewing the information available accumulated over a very short period of time on the different treatment proposals reported followed by the position and recommendations from this group.
Classification
ACSs can be divided into: a) ST-segment elevation ACS including ST-segment elevation myocardial infarction (STEMI) and b) non-ST-segment elevation ACS including non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina.
Diagnosis
Two recommendations should be followed to minimize the risk of contagion. The first one should be social distancing and limited contact with patients during the physical examination or while performing the transthoracic echocardiogram to obtain the maximum amount of information while minimizing physical contact with the patient; the second recommendation is the use of coronary computed tomography angiography as a fast non-invasive imaging modality to confirm or discard the presence of coronary heart disease as the cause for the symptoms when appropriate.3 The main elements for diagnosis are:
-
- Suggestive symptoms.
-
- Twelve-lead electrocardiogram.
-
- Myocardial biomarkers.
-
- Coronary computed tomography angiography: when available, it should be considered in the case of reasonable doubt on the coronary origin of the symptoms. It speeds up the confirmation/discarding process, reduces hospital stay and use of hospital resources and minimizes stay in the cath lab and exposure to contagion for the heart team who work in the cath lab.
Management of STEMI
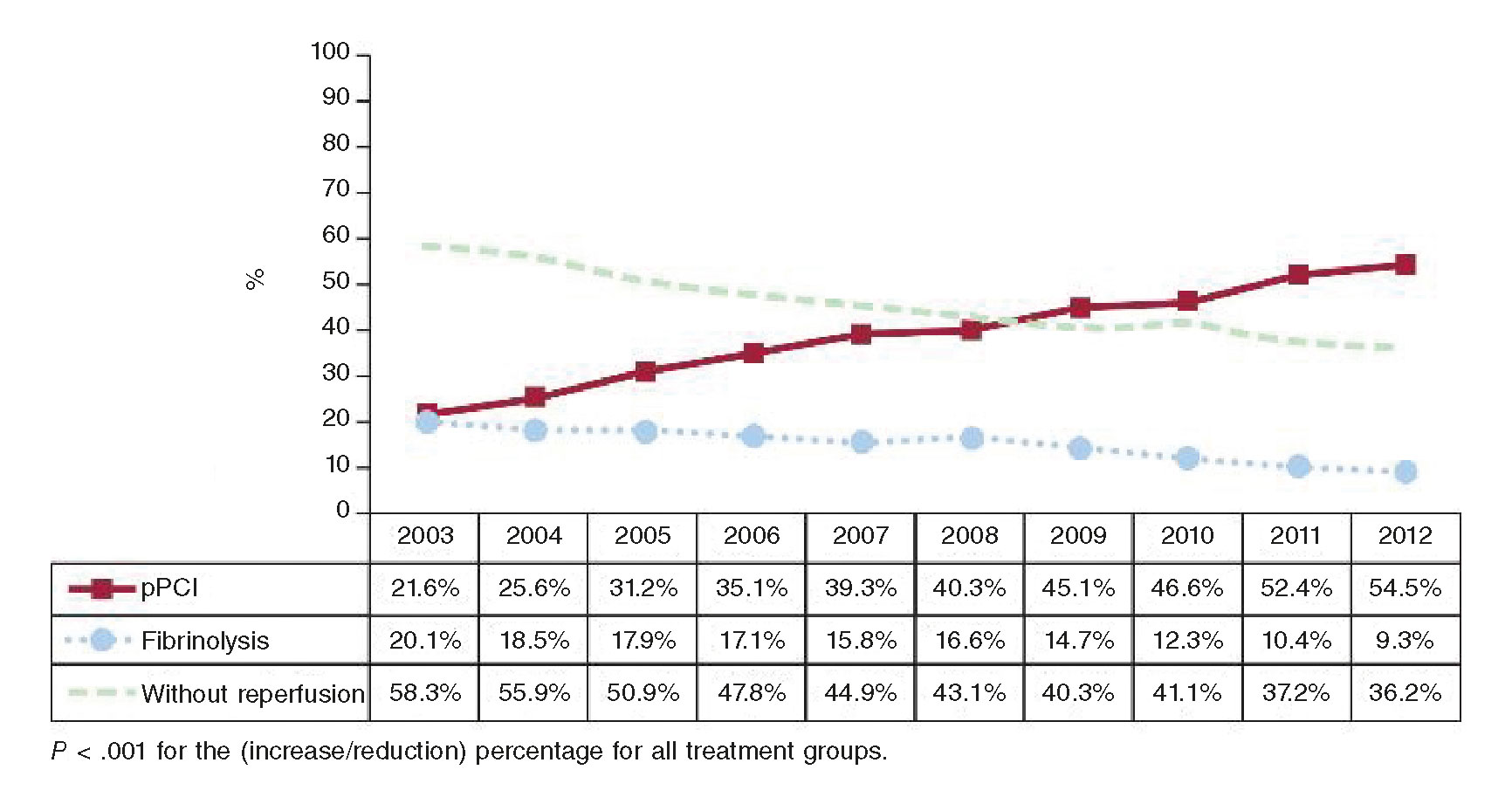
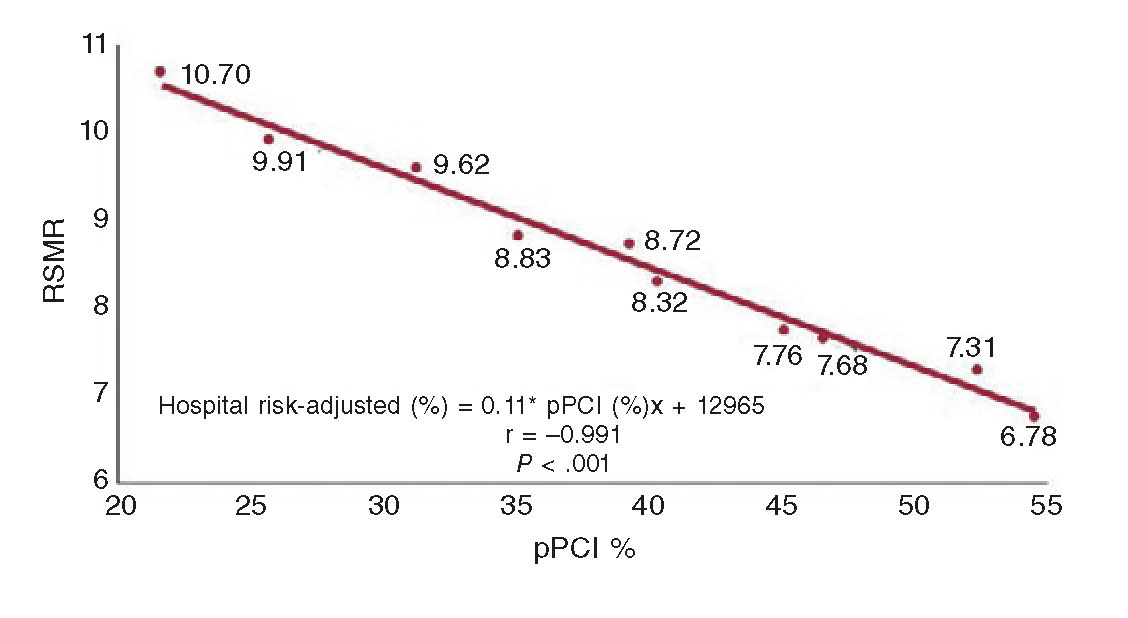
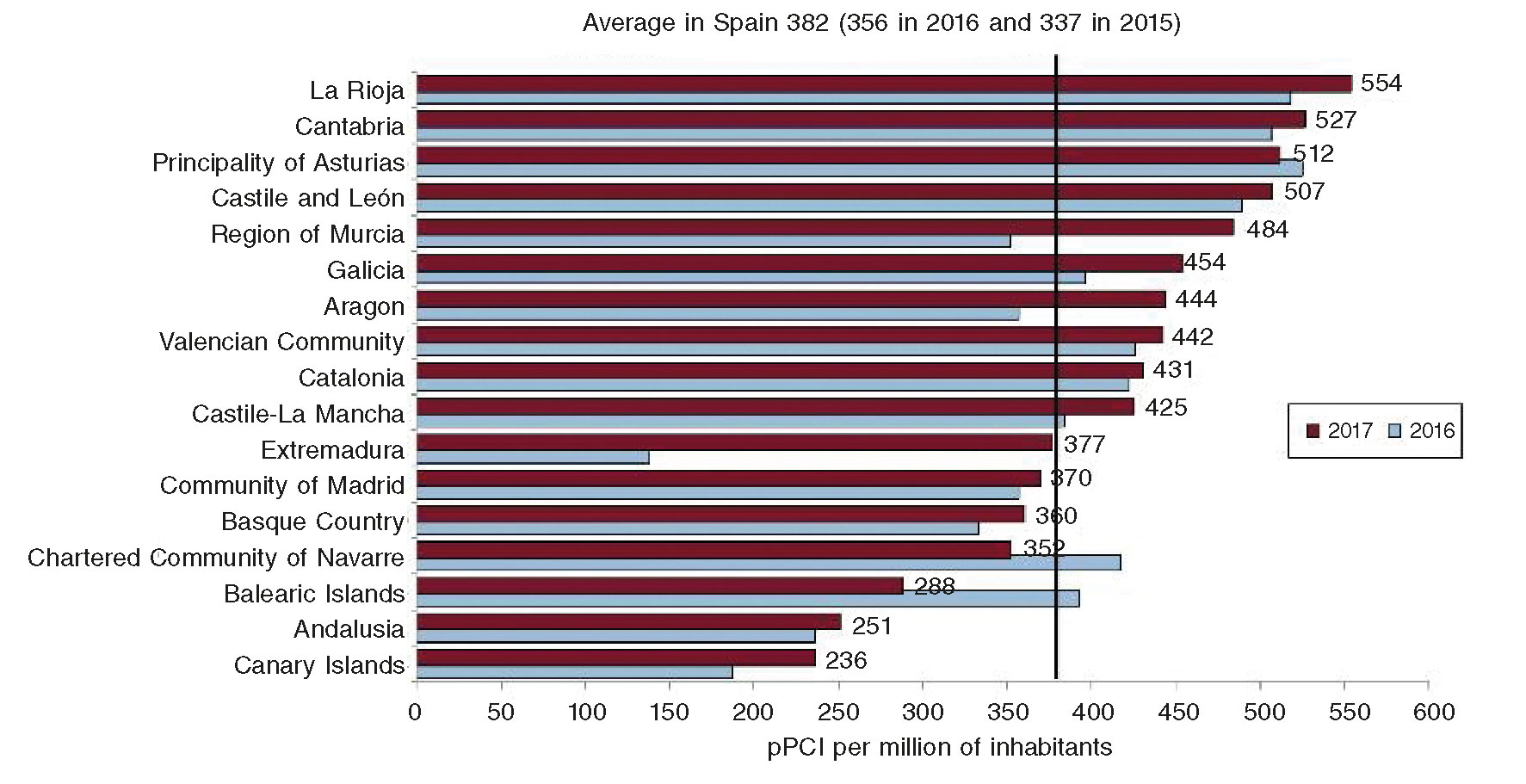
Primary percutaneous coronary intervention (pPCI) is the treatment of choice for coronary reperfusion. Fibrinolysis is spared for cases where percutaneous coronary intervention (PCI) is not available or when the patient transfer to a PCI capable hospital involves significant time delays.4 The COVID-19 pandemic has produced different points of view for the management of patients with STEMI; these different positions are indicative of the particular situations that medical societies are facing to design their recommendations.
Fibrinolysis as the strategy of choice
A report and the position adopted by opinion leaders recommend the use of fibrinolysis as the first reperfusion strategy in both uninfected patients and patients with suspicion or diagnosis of COVID-19. The PCI would be spared for cases of failed fibrinolysis and only if benefits exceed the possible risks.5 They recommend that patients with serious pneumonia due to SARS-CoV-2, unstable vital signs, and concurrent STEMI should receive support medical treatment only—no fibrinolysis or pPCI—until they have recovered from pneumonia. These recommendations are highly restrictive, do not anticipate the pPCI option and limit the possibility of bailout PCIs in selected cases.5
pPCI as the strategy of choice
Several medical societies are keeping to their usual recommendations during the current pandemic. Patients with STEMI (with or without suspicion of infection, with or without COVID-19) inescapably need medical attention and should receive pPCI as the reperfusion therapy, especially those with persistent angina or hemodynamic compromise; they also keep the bailout PCI option alive. They accept that fibrinolysis can be an alternative in patients with pneumonia due to SARS-CoV-2 who develop STEMI but remain stable.1,2,6 The Spanish Society of Cardiology (SEC) ranks pPCI as the preferred reperfusion strategy in most patients with severe pneumonia and difficulties moving or being transferred.7 At the present time, Spain is one of the countries with the highest contagion and mortality rates and its healthcare system has collapsed due to the excessive volume of patients with COVID-19; in the middle of this health crisis, consensus still favors the reperfusion of STEMI through pPCI.
Management of non-ST-segment elevation ACS
In general, the specific risk of complications of patients with NSTEMI or unstable angina should be identified to decide the best time to perform cardiac catheterization. Regardless of their risk, it is essential to know the patients’ coronary anatomy and based on the findings assess the revascularization method.8 As with STEMI, this routine common practice has changed during the current pandemic and several of the position papers on the management of ST-segment elevation ACS have been replicated.
Medical therapy as the strategy of choice
The general recommendation is to know the state of contagion before referring patients to the cath lab Patients without a diagnosis or suspicion of infection need to be tested to confirm or discard the presence of SARS-CoV-2. It is suggested that patients with suspicion of COVID-19 who have not been tested yet and those who have already tested positive should receive medical therapy. The interventional procedure should be deferred until they have recovered or the risk of contagion has gone down.7 It is recommended that patients without suspicion of infection or without COVID-19 (common patients) undergo PCI only if they are high-risk patients or remain unstable during the course of conservative treatment. Urgent PCIs will be spared only for patients with hemodynamic compromise or malignant arrhythmias regardless of their state of infection.6,7
PCI as the strategy of choice
Here the usual recommendations still stands, which is why high-and-intermediate risk patients should undergo PCI, regardless of their state of contagion.8 In low-risk patients with COVID-19 or with severe pneumonia and who remain unstable it is advised to consider medical therapy and defer the procedure. Early PCIs facilitate early hospital discharges. In patients with multivessel disease the PCI should be favored over coronary revascularization surgery. Patients admitted to hospitals without cath lab capabilities should not be transferred. In these cases, conservative treatment is advised followed by early hospital discharges.1,2
CONSENSUS ELEMENTS
The following variables were taken into consideration while the recommendations of this consensus document were being designed:
-
Contagion. When examined, patients who seek medical attention should be classified according to their current state of SARS-CoV-2 infection regardless of the presence of symptoms. A special subgroup are unstable patients with severe pneumonia who develop ACS.
-
Timeframe. At different times and different speeds peaks and troughs in the number of patients with COVID-19 are expected to happen.
-
Geographic location. More cases are expected in more heavily populated cities in particular the Mexico City metropolitan area, Guadalajara, Monterrey, and Puebla.
-
Healthcare system. There are different healthcare models available in Mexico. In general, in private hospitals cath labs are opened around the clock. In the public system several secondary care centers have cath lab capabilities with different care plans for the management of ACS. Most tertiary care centers have pPCI programs available around the clock too.
-
Therapeutic effectiveness. In the full spectrum of ACS, PCI is the treatment of choice. In patients with STEMI, fibrinolysis is a less effective alternative associated with a higher risk of bleeding complications. In patients with non-ST-segment elevation ACS, medical therapy in the acute phase is only intended to stabilize the atheromatous plaque and alleviate myocardial ischemia before the cath. lab. referral.
-
Risk profile. In the full spectrum of ACS, it is essential to examine patients at increased risk of adverse cardiovascular events early. This group of patients benefits the most from invasive treatment.
-
Hospital stay. Anticipating larger volumes of patients in a prolonged emergency situation, it is advised to shorten hospital stays to alleviate the work load, reduce the use of hospital resources, reduce the exposure of patients, relatives, medical and paramedic personnel to a potentially contaminated environment, empty beds, and facilitate the ongoing rotation of patients.
-
Rooms and special catheterization laboratories. According to the type, size, and resources of each hospital, whether public or private, a specific physical space should be spared to examine patients without COVID-19 or clinical suspicion away from patients with confirmed infections or a justified suspicion of infection. In public and private hospitals with at least 2 cath labs it is possible to use 1 cath lab for the specific care of patients with confirmed or suspected CODIV-19 and the other one for the management of common patients. In public or private hospitals with only 1 cath lab available, the heart team should look for alternatives to perform the procedures while minimizing the risk of contagion. This can be done by splitting the care schedule depending on the patients’ state of infection and only if the clinical indication for cardiac catheterization allows it (managing infected or suspicious patients in the morning, and uninfected or unsuspicious patients in the afternoon). Reducing the number of healthcare workers in the cath lab and organizing groups and shifts during specific timeframes helps too. Regardless of the number of cath labs available, it is a priority to observe the recommendations established for adequate protection of the heart team, which should be the smallest possible. Also, the cath lab should be disinfected before taking on the next patient.
-
Personal protective equipment. The medical personnel looking after patients, whether infected or suspicious, should all have the necessary personal protective equipment and observe the protection measures recommended like those from the Interventional Cardiology Association and Heart Rhythm Association of the Spanish Society of Cardiology.9
RECOMMENDATIONS
The objective of this document is to guide the decision-making process on the coronary revascularization of patients with ACS during the health emergency declared due to the current SARS-CoV-2 pandemic. Considering the elements described above and anticipating an epidemiological model similar to the one seen in countries like ours, we recommend making slight modifications to the management of patients with ACS while continuing to use the pharmacoinvasive reperfusion strategy prevalent in Mexico.
STEMI
Cardiovascular risk
We recommend that pharmacoinvasive strategy should start by identifying patients with higher risk of complications. The presence of 1 or more of the following characteristics is indicative of high risk:
– Age > 75 years.
– Cardiogenic shock (whether associated or not to mechanical complications).
– Refractory angina.
– Ventricular tachyarrhythmias.
– Bradyarrhythmia requiring temporary pacemaker implantation.
– Electrocardiographic pattern of diffuse ischemia.
pPCI
We recommend public and private hospitals with cath lab capabilities and, in particular, tertiary care or reference centers to keep offering pPCI over fibrinolysis. This is completely justified by its higher rate of success, lower risk of complications, and shorter hospital stays (hospital discharges within 36 h - 48 h). The fibrinolysis proposal as the primary reperfusion method even in hospitals with cath lab is questionable.
Fibrinolysis
We still recommend its indication as the reperfusion method in non-PCI capable centers. Unlike conventional pharmacoinvasive strategy, the transfer of stable patients with successful fibrinolysis for early elective cardiac catheterization is ill-advised; these patients should be followed and monitored later in time. Only patients with failed fibrinolysis should be transferred and only in the presence of hemodynamic or electric instability. In patients with severe pneumonia due to COVID-19 it is a valid option even for PCI-capable centers in the presence of high and growing volumes of suspicious or confirmed cases. Also, in the absence of high-risk markers, especially in patients admitted within the first 3 hours after symptom onset and without contraindications for fibrinolysis.
Periodic adjustment of reperfusion method
We recommend that in cities, regions, and hospitals with large volumes of patients with COVID-19 or suspected cases, whether critical, growing or rapidly spiking, the hospital management should decide on the most adequate reperfusion method with the heart team and the resources available. The primary reperfusion strategy should adjust periodically based on the temporal and geographic pattern of the infection.
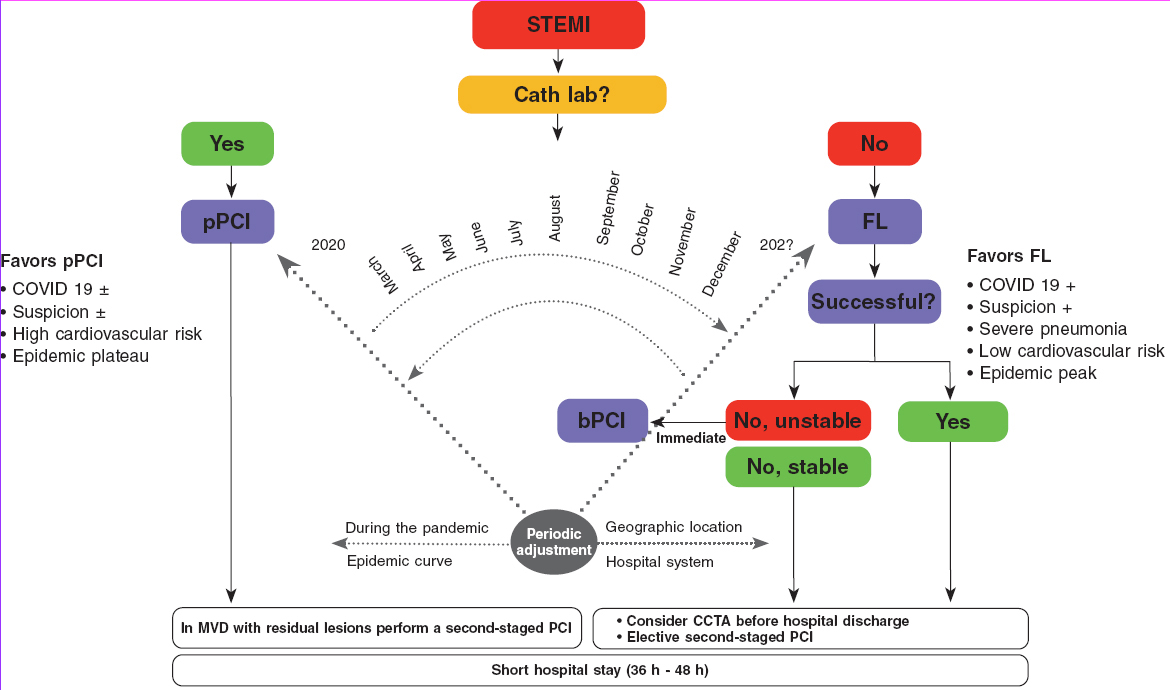
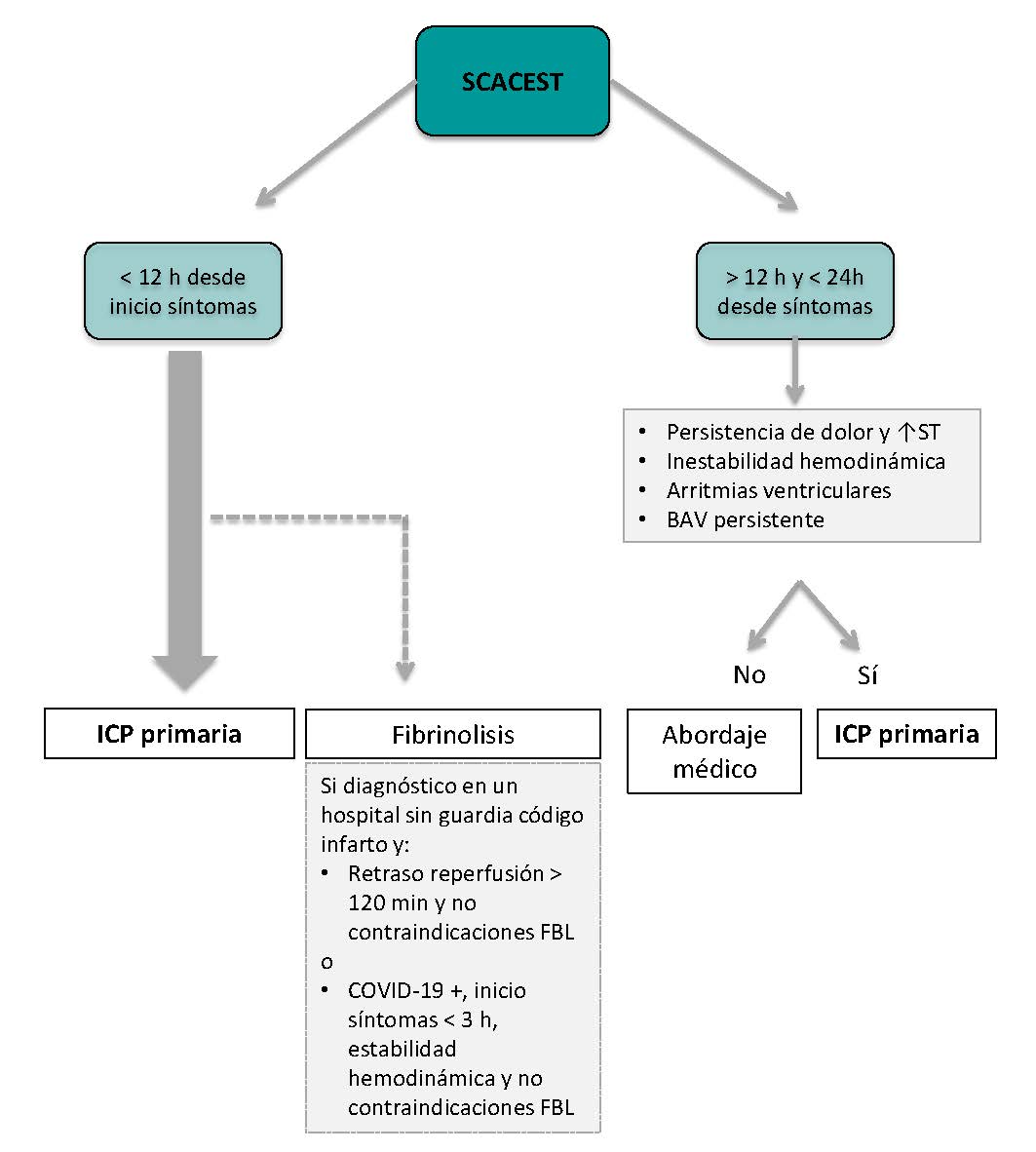
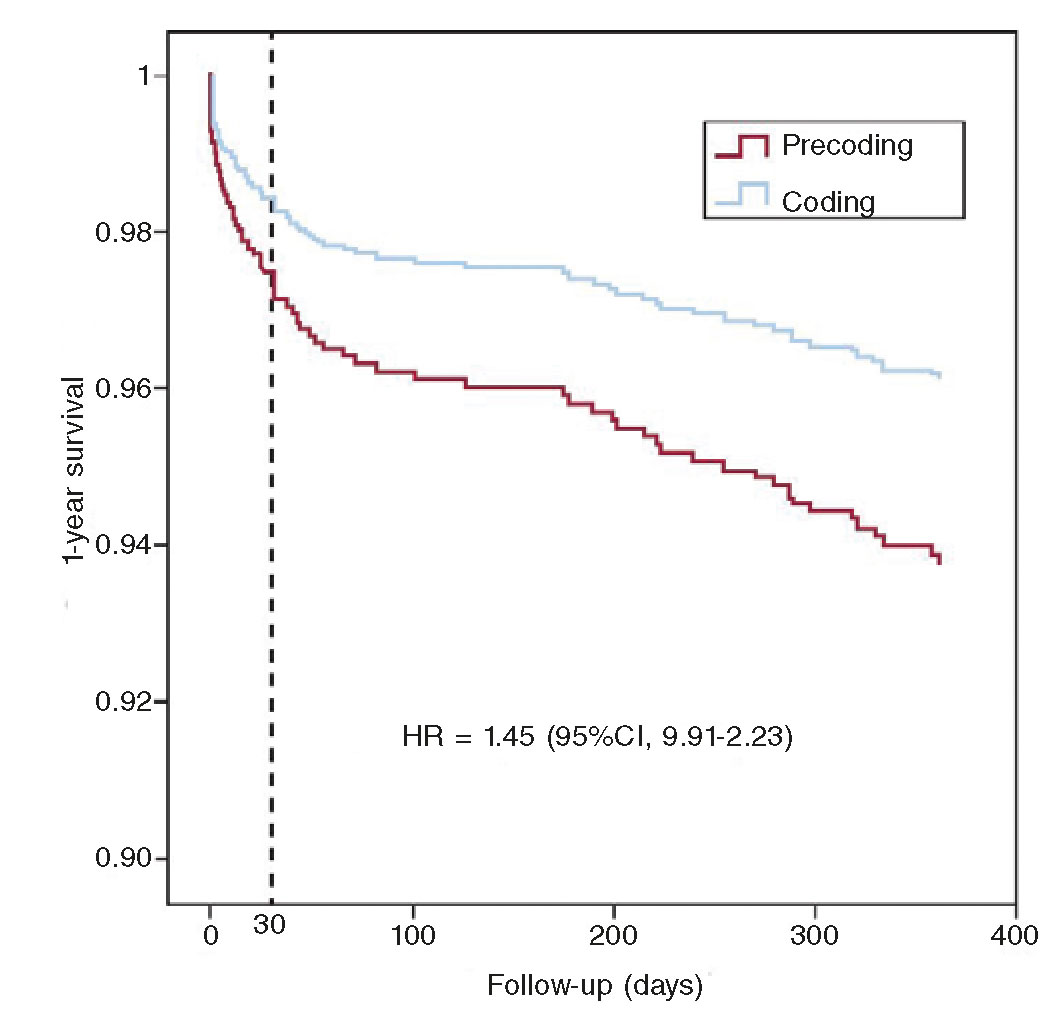
The algorithm shown on figure 1 for the management of patients with STEMI during the current COVID-19 pandemic recommends pPCI as the method of choice for coronary reperfusion. The reperfusion strategy should change as the pandemic evolves with the necessary, periodic adjustments based on the modification criteria already described.
Figure 1. Therapeutic algorithm for the management of patients with STEMI during the current SARS-CoV-2 pandemic. The arrow thickness is indicative of the preferred therapy. +, present; ±, present or absent; bPCI: bailout percutaneous coronary intervention; CCTA, coronary computed tomography angiography; FL, fibrinolysis; MVD, multivessel coronary artery disease; pPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
NSTEACS
Regardless of the presence of SARS-CoV-2, low-risk patients who respond to medical therapy can be discharged from the hospital to be studied later in time if the risk of contagion is lower or if they have recovered from COVID-19. If the patient’s coronary anatomy needs to be documented prior to hospital discharge, an option here is to perform a coronary computed tomography angiography and, based on the findings, plan the PCI or discharge the patient. Moderate-or-high risk patients (according to the criteria described above) and those who become unstable during the course of conservative treatment should be transferred to the cath lab regardless of their state of contagion.
Most concepts described for the management of STEMI are valid in these patients. PCI-capable public and private hospitals with experienced personnel and the necessary resources should keep the PCI option open in light of its high rate of success and short hospital stays. In patients with significant multivessel disease the hospital stay should be short and PCI revascularizations should be prioritized over coronary revascularization surgeries.
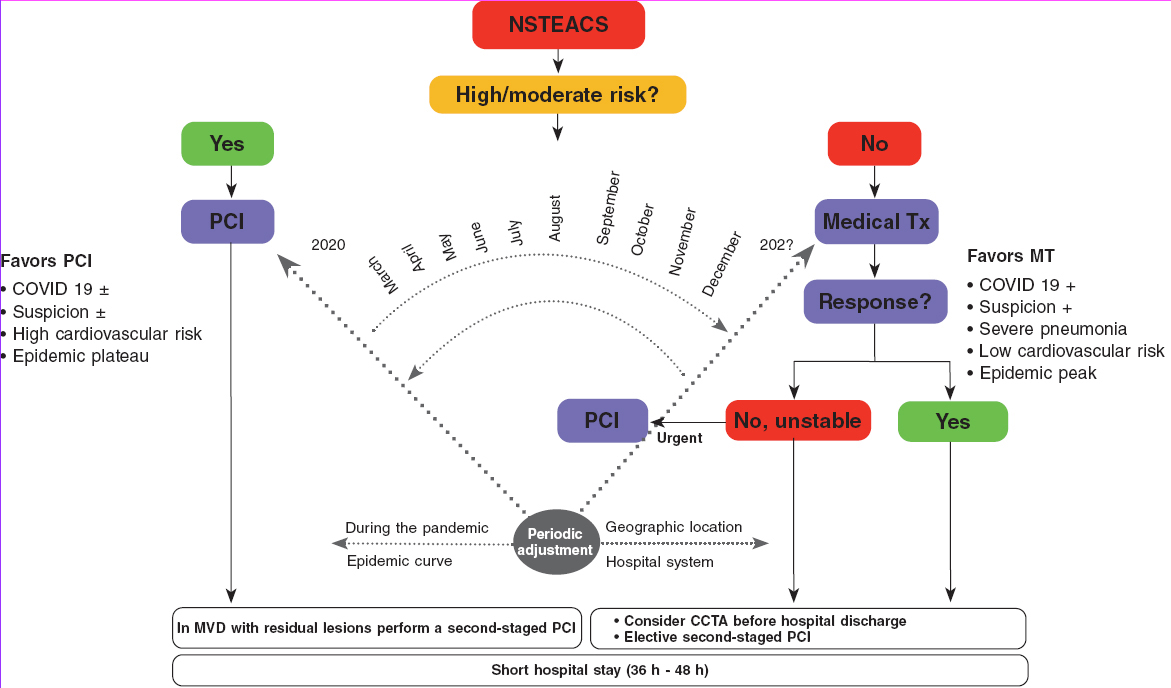
In the algorithm shown on figure 2 for the management of patients with NSTEACS during the current COVID-19 pandemic the use of PCI is recommended in high-risk patients. However, the reperfusion strategy should be dynamic as the pandemic evolves with periodic adjustments based on the modification criteria already described.
Figure 2. Algorithm for the management of patients with NSTEACS during the current SARS-CoV-2 pandemic. The arrow thickness is indicative of the preferred therapy. +, present; ±, present or absent; CCTA, coronary computed tomography angiography; MT, medical therapy; MVD, multivessel coronary artery disease; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; Tx, treatment.
Cardiovascular complications in patients with COVID-19
Patients with COVID-19 can have clinical signs of myocardial damage and secondary complications. Myocardial damage with elevated high-sensitivity cardiac troponin levels (7.2%), cardiogenic shock (8.7%), and arrhythmias (16.7%) has been reported in some patients. In the absence of coronary heart disease, the gradual increase of high-sensitivity cardiac troponin levels is a predictor of mortality.10 Cases of type I STEMI and fulminant myocarditis have also been reported.11
Most of the patients who develop complications are eligible for intensive therapy, and a high percentage of these may end up needing mechanical support of the ventricular function. The etiology of patients with cardiovascular complications can be coronary heart disease; this consideration will not be rare because the characteristics of patients more often affected are old age, overweight, high blood pressure, and diabetes mellitus. If the hypothesis of concomitant coronary heart disease is accepted there are two possible avenues: treat the case as that of a patient with NSTEMI and follow the corresponding algorithm or discard the presence of significant coronary heart disease using coronary computed tomography angiography.
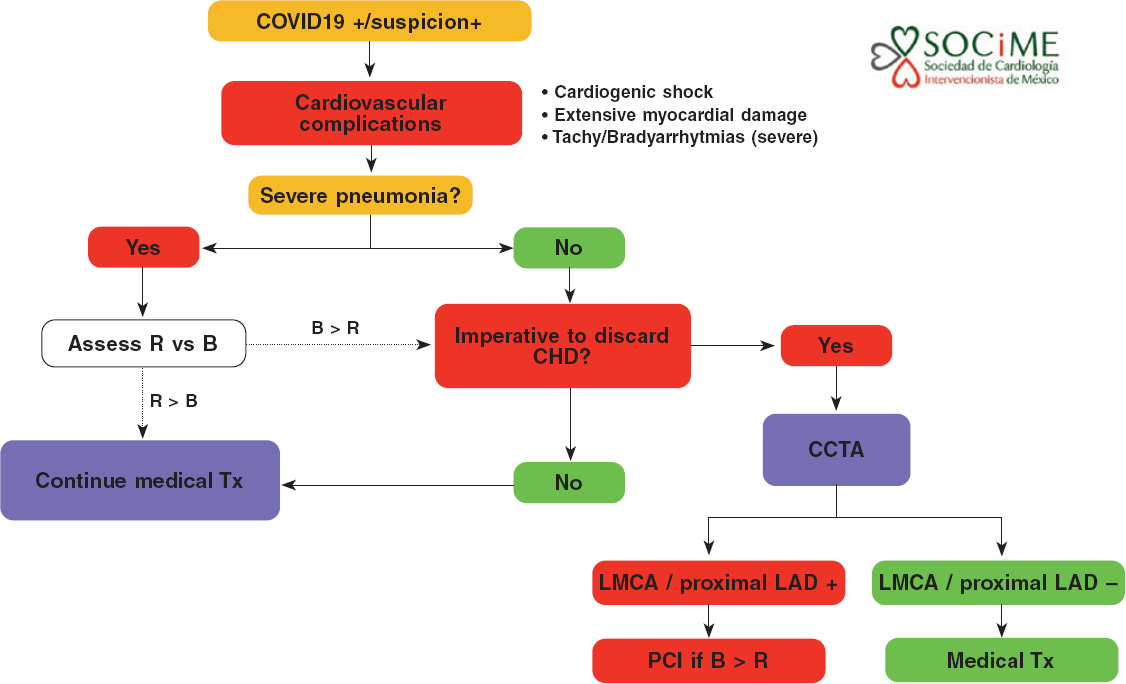
In patients with severe pneumonia, myocardial damage, hemodynamic or electric instability, and suspicion of coronary heart disease, the medical team should decide on the adequacy (benefits exceeding risks, patients who can be saved) of transferring the patient to the cath lab or performing a coronary computed tomography angiography. In particular, the presence of left main coronary artery disease or disease of the proximal left anterior descending artery in a patient who can be saved supports the need to perform an urgent PCI (figure 3).
Figure 3. Diagnostic-therapeutic algorithm for the management of patients with COVID-19 or suspicion of COVID-19 infection with cardiovascular complications. B, benefits; CHD, coronary heart disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending coronary artery; LMCA, left main coronary artery disease; R, risk; Tx, treatment.
VALIDITY OF RECOMMENDATIONS
The epidemiological model is totally unpredictable under the current circumstances; the rate of contagion, number of confirmed cases, and mortality rates are different across cities, regions, countries, and continents. However, estimates from the Mexican Ministry of Public Health suggest a peak of cases and more hospitalizations in the metropolitan area of Mexico City and main cities of the nation from May through August 2020. The flattening of the curve of infection will still take several months. No definitive projection on this regard can be given at the present time.
These recommendations will remain effective as necessary. They will be re-evaluated periodically together with the Mexican Ministry of Public Health to discuss the right time to go back to normal.
CONFLICTS OF INTEREST
None reported.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Jorge Gaspar Hernández, General Manager of the Ignacio Chávez National Institute of Cardiology for his remarks on this manuscript.
EDITOR’S NOTE
This document is subject to further iterations based on the evolution of the current COVID-19 pandemic. This manuscript has undergone a process of internal review of exceptional priority by the editorial staff due to the special interest of disclosing the information contained herein to the scientific community. The editors wish to thank Permanyer Publications for its collaboration and commitment for the quick publication of this document.
REFERENCES
1. Welt FGP, Shah PB, Aronow HD, et al. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic:From ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020. https://doi.org/10.1016/j.jacc.2020.03.021.
2. National Health Service. Specialty guides for patient management during the coronavirus pandemic. Clinical guide for the management of cardiology patients during the coronavirus pandemic. 2020. Disponible en:https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-cardiolgy-coronavirus-v1-20-march.pdf. Consultado 20 Mar 2020.
3. Yang S, Manjunath L, Willemink MJ, Nieman K. The role of coronary CT angiography for acute chest pain in the era of high-sensitivity troponins. J Cardiovasc Comput Tomogr. 2019;13:267-273.
4. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
5. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19:the protocols from Sichuan Provincial People's Hospital. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05993-9.
6. Society of Cardiovascular Angiography and Interventions. Mahmud E. The Evolving Pandemic of COVID-19 and Interventional Cardiology. Disponible en: https://www.scai.org/media-center/news-and-articles/evolving-pandemic-covid-19-and-interventional-cardiology. Consultado 18 Mar 2020.
7. Romaguera R, Cruz-Gonza?lez I, Jurado-Roma?n A, et al. Considerations on the invasive management of ischemic and structural heart disease during the COVID-19 coronavirus outbreak. Consensus statement of the Interventional Cardiology Association and the Ischemic Heart Disease and Acute Cardiac Care Association of the Spanish Society of Cardiology. REC Interv Cardiol.2020. https://doi.org/10.24875/RECICE.M20000121.
8. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation:Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
9. Romaguera R, Cruz-González I, Ojeda S, et al. Consensus document of the Interventional Cardiology and Heart Rhythm Associations of the Spanish Society of Cardiology on the management of invasive cardiac procedure rooms during the COVID-19 coronavirus outbreak. REC Interv Cardiol. 2020. https://doi.org/10.24875/RECICE.M20000116.
10. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system:acute and long-term implications. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa231.
11. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
ABSTRACT
The current COVID-19 outbreak is forcing healthcare workers to continuously reconsider the proper indications for cardiac catheterization. Human and material resources optimization, infection prevention for patients and healthcare workers, and transfer times force a rethink of the previously established protocols. This article is a consensus statement of the Interventional Cardiology Association and the Ischemic Heart Disease Association of the Spanish Society of Cardiology, and aims to provide information to healthcare workers on the indications of diagnostic or therapeutic cardiac catheterization during the current COVID-19 pandemic.
Myocardial infarction. Interventional cardiology. Angioplasty. Infection Prevention. COVID-19. Coronavirus. Pandemic.
RESUMEN
El brote actual de COVID-19 está obligando a los profesionales sanitarios a replantear de forma continua las indicaciones de cateterismo cardiaco. La optimización de recursos materiales y humanos, la prevención de contagios a profesionales y pacientes, así como la gestión de los tiempos de traslado, hace totalmente necesario reformular los protocolos previamente establecidos. El presente texto es un documento de consenso de la Asociación de Cardiología Intervencionista y la Asociación de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares de la Sociedad Española de Cardiología que pretende dar información al personal sanitario sobre las indicaciones de cateterismo diagnóstico o terapéutico durante la pandemia actual de COVID-19.
Infarto. Cardiología intervencionista. Angioplastia. Infección. Prevención. COVID-19. Pandemia.
INTRODUCTION
The current outbreak of COVID-19 has caused all healthcare providers have to rethink the indications for cardiac catheterization on an ongoing basis. The optimization of material and human resources, the prevention of contagions to healthcare providers and patients, and management during patient transfers makes it absolutely necessary to reformulate the protocols previously established. This consensus document has been agreed by the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (ACI-SEC) and the Spanish Society of Cardiology Working Group on Ischemic Heart Disease and Acute Cardiovascular Care. It includes indications to perform cardiac catheterizations under the current situation. It is difficult to foresee the evolution of this pandemic and its health impact, which will probably force us to readjust this document based on the particular situation and dynamic of each center. It is important to emphasize that when the actual situation is over, we recommend going back to the indications included in the clinical practice guidelines published by the European Society of Cardiology.1
In order to perform cardiac catheterizations our advice is to follow the recommendations on prevention and management included in the consensus document published by the ACI-SEC and the Heart Rhythm Association of the Spanish Society of Cardiology.2
ELECTIVE PROCEDURES
Indication for elective procedures in the catheterization laboratory should be based on individual assessments of the risk of contagion/benefit from the intervention ratio. In the current situation postponing all elective procedures seems the most reasonable thing to do to minimize the possibility of contagion of disease-free patients (and people they may have been in contact with) in a setting of high prevalence of COVID-19 infection such as hospitals. Similarly, performing right catheterizations during this pandemic is ill-advised.
If the cath lab has enough material and human resources, the non-emergent catheterizations of already hospitalized patients without suspicion of COVID-19 or COVID-19-negative patients may be an option to promote early discharges (ie, a study to characterize dilated cardiomyopathy in a patient admitted with an index episode of heart failure).
NON-ST-SEGMENT ELEVATION ACUTE CORONARY SYNDROME
Differential diagnosis
A key element to be taken into consideration when indicating a cardiac catheterization in a patient with acute coronary syndrome (ACS) is the high prevalence of heart disease in patients admitted due to COVID-19,3 the significantly high troponin levels (8% to 12% higher) seen in confirmed cases of COVID-19 but without ACS yet,4 and the possibility that myocarditis complicates the COVID-19 infection.5 This highlights the importance of clinical judgement before establishing a diagnosis of ACS/acute myocardial infarction. In general, in patients hospitalized due to COVID-19 infection who experience high cardiac enzyme levels of coronary origin and remain asymptomatic we recommend following a conservative approach. Coronary angiography should be spared for cases of high suspicion of high-risk ACS, medical treatment-resistant recurrent ischemia, and when the patient’s vital prognosis following the infection anticipates good prognosis.
NON-ST-SEGMENT ELEVATION ACUTE CORONARY SYNDROME
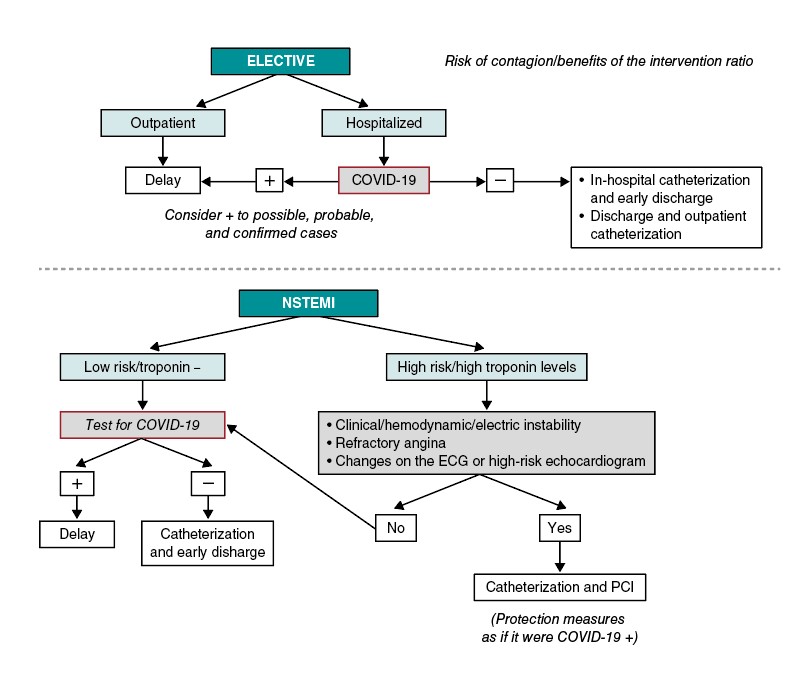
Figure 1 1 shows the approach suggested for patients with non-ST-segment elevation acute coronary syndrome (NSTEACS). In patients hospitalized due to NSTEACS with suspicion of COVID-19 we recommend running the diagnostic test before performing the cardiac catheterization to assess the risk/benefit ratio of the procedure. The current clinical guidelines on revascularization recommend an early invasive strategy (less than 24 h) in patients with at least 1 criterion of high risk and in less than 72 h in patients with at least 1 criterion of intermediate risk.6. In most of the patients with NSTEACS this interval should be enough to confirm or discard the infection. The procedure should be postponed if the patient’s clinical situation allows it in cases where the diagnostic test is not available yet. On the contrary, in patients with NSTEACS but with persistent ischemia or high-risk criteria like recurrent angina, diffuse ST-segment changes suggestive of left main coronary artery or ventricular dysfunction performing the catheterization within the first 2 hours may be an option, taking the necessary protective measures to avoid transmitting the infection.
Figure 1. Algorithm for the management of elective patients and non-ST-segment elevation myocardial infarction (NSTEMI). ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Patients negative for COVID-19 undergoing the procedure should be discharged early from the hospital.
In patients admitted to centers without a cath. lab. who need to be transferred, it is advisable, if possible, to follow a conservative approach and early discharge except for high-risk criteria or poor disease progression.
In selected patients with acute myocardial infarction—especially type 2 7— the conservative approach is the one recommended initially.
Revascularization in NSTEACS and multivessel disease
In patients with NSTEACS and multivessel disease and an indication for complete revascularization who remain hospitalized in centers where surgeries have been postponed, we recommend performing it within the same procedure to reduce hospital stay and avoid having to perform another procedure in the cath. lab.
ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION
The reperfusion treatment during the management of ST-segment elevation myocardial infarction (STEMI) of < 12 hour-duration since symptom onset should be primary percutaneous coronary intervention (pPCI) because it reduces the rates of mortality, reinfarction, stroke,1 and mechanical complications compared to fibrinolysis. Also, a significant percentage of patients undergoing pPCI can be discharged early and don’t require further invasive examinations which simplifies the management of these patients. This reduces the hospital stay and avoids collapsing the entire healthcare system. Nevertheless, during the COVID-19 pandemic the following key points should be taken into consideration:
- Due to the current care overload sustained by the ERs of our healthcare system, transfer times can take longer than usual in many cases.
- The transfer of patients with suspicion or confirmation of COVID-19 should take place safely and with infection under control. Also, after the transfer the ambulance should be properly disinfected. Therefore, the logistics required to guarantee safe transfers can also delay the entire process.
- Despite the preventive measures implemented to avoid transmitting the infection, the transfer of patients with an active infection for COVID-19 to a different center can infect the healthcare providers and, most important of all, other patients hospitalized and who are especially vulnerable to the disease.
- In patients already diagnosed with COVID-19 and in poor clinical state (especially patients hospitalized in intensive care units) with STEACS, repefusion treatment may yield no clinical benefits.
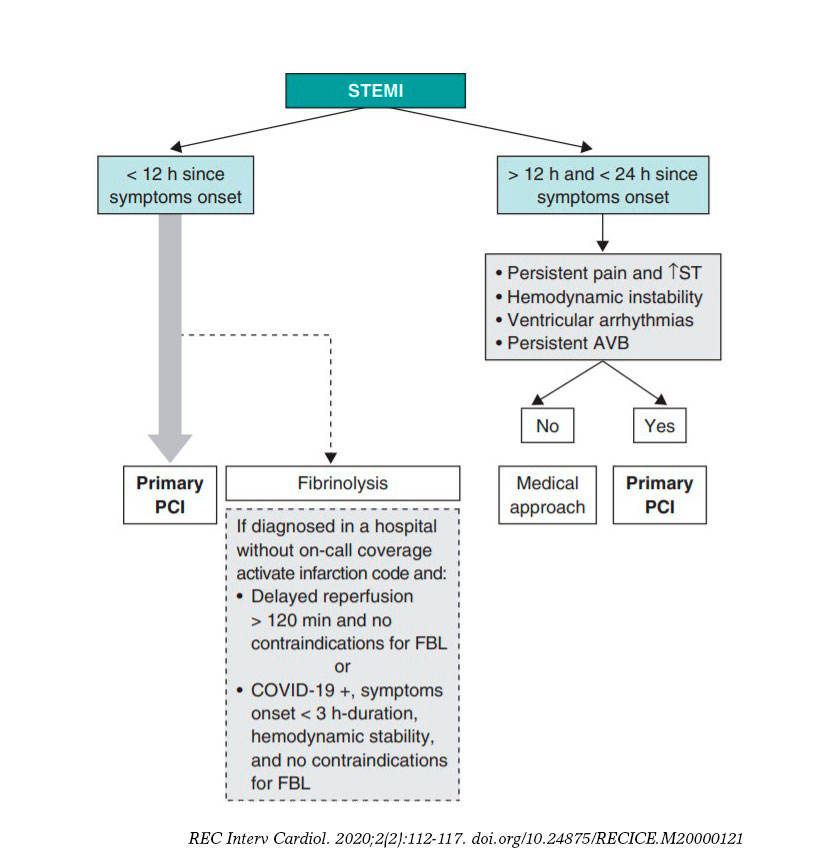
Reperfusion strategy
Figure 2 shows the management of STEMI. The ACI-SEC and the Spanish Society of Cardiology Working Group on Ischemic Heart Disease and Acute Cardiovascular Care recommend that the percutaneous coronary intervention should be the reperfusion strategy of choice in most cases. Fibrinolysis should be spared for cases diagnosed in non-PCI capable centers that meet one of the following requirements:
Figure 2. Algorithm for the management of patients with ST-segment elevation myocardial infarction (STEMI). AVB, atrioventricular block; FBL, fibrinolysis; PCI, percutaneous coronary intervention.
- Estimated time to the pPCI > 120 min.
- Patients who have tested positive to COVID-19 with poor clinical state that makes transfer difficult.
- Patients who have tested positive to COVID-19 with low hemorrhagic risk and symptoms of less than 3 hour-duration.
In cases where fibrinolysis may be an option, the lack of contraindications and the administration of the drug in less than 10 min from diagnosis should be guaranteed.1 Then, depending on the patient’s clinical state and the availability of beds in the ICU of the destination hospital, transfer to a center with cath. lab. capabilities may be considered. The rule of thumb here is to avoid transferring patients with confirmed reperfusion and good disease progression.
After the percutaneous coronary intervention, it is recommended that each patient be taken to their referring centers. However, the patients’ clinical situation and bed availability of each center should be individualized in each case.
Other considerations
As a general rule we recommend leaving the number of centers that are part of the infarction code program untouched. Although demand from out-of-hospital emergency medical services may change significantly during the COVID-19 pandemic, a hospitalized patient may have an indication for an urgent cardiac catheterization. In the current situation, transferring this patient to a different center may be more problematic than performing the procedure at the center where the patient is already hospitalized. Our recommendation here is that no PCI capable center should avoid treating infarctions.
Other clinical considerations:
- The diagnosis of STEMI in patients with complete left bundle-branch block is still complex to this day despite the use of different electrocardiographic criteria.8 Therefore, in patients with suspicion or confirmation of COVID-19 who require transfer for reperfusion we recommend agreeing on the diagnosis as much as possible to avoid unnecessary transfers.
- The management of patients with recovered sudden death without overt electrocardiographic criteria of STEMI is still controversial. Although a recent randomized clinical trial showed that these patients don’t benefit from an immediate coronary angiography,9 it is still being performed in most centers. However, due to their clinical situation—if infected—these patients are extremely prone to secreting microdroplets and infecting the healthcare providers. At the same time they are very vulnerable to infection if they are not already infected. For this reason, immediate PCIs are ill-advised in these patients.
- In patients with STEMI without cardiogenic shock and multivessel disease the overall recommendation is to perform a complete revascularization.10 However, in the current situation, we believe that the management of STEMI should be as simple as possible. In this sense, we believe that in most of these patients the management of non-culprit lesions should be postponed until the outbreak of COVID-19 is over. On the other hand, in patients with a clear need for complete revascularization at admission, we recommend the study of all lesions within the same procedure during the acute phase.
CARDIOGENIC SHOCK
In situations of acute coronary syndrome related cardiogenic shock, cardiac catheterization is indicated. The management of critically ill patients is especially complex because intubation, aspiration, and CPR maneuvers can prompt respiratory secretions in the form of aerosols and increase the exposure of the healthcare providers. All critically ill patients should be treated as patients with COVID-19.
The following key points should be taken into oconsideration:
- As in the rest of patients with cardiogenic shock, only the culprit vessel should be revascularized.11
- If intubation is necessary and possible, it should be performed before entering the cath. lab. and in the best conditions possible to comply with all COVID-19 prevention recommendations.
- Connection to a ventilator —a closed system— is recommended prior to manual ventilation with an ambu-bag. If ventilation with manual resuscitation is necessary, the use of high-efficiency particulate air (HEPA) filters between the tube and the bag is recommended.
- The extracorporeal membrane oxygenation (ECMO) machine used by the heart team should be purged at all times before the arrival of patients to reduce the possibility of infection and speed up the whole process. In patients with COVID-19 with cardiogenic shock, ECMO can be used as the first-line therapy compared to other devices like the Impella ventricular support system or the intra-aortic balloon pump counterpulsation.
STRUCTURAL HEART INTERVENTIONS
In general, all structural heart interventions should be postponed until the pandemic is under control. We should remember that most of these procedures require several days at the hospital, but at the same time the ICU/coronary unit beds may become necessary for patients with COVID-19. Also, in some cases structural heart interventions are performed under general anesthesia and intubation or on transesophageal echocardiography monitoring. All of them high-risk situations for the infection of patients and healthcare providers. On the other hand, patients undergoing structural heart interventions are often old and therefore especially susceptible to nosocomial infection due to COVID-19.
Other urgent procedures like aortic valvuloplasty or transcatheter aortic valve implantation in patients with angina at rest, recurrent syncope or refractory heart failure can be performed.
DRUGS
Regarding the drugs commonly used at the catheterization laboratory such as antithrombotic therapies, the recommendations are basically the same regardless of whether the patient has been infected with COVID-19 or not.
The medication to treat patients with COVID-19 may interact with the most common drugs currently used at the cath lab.12
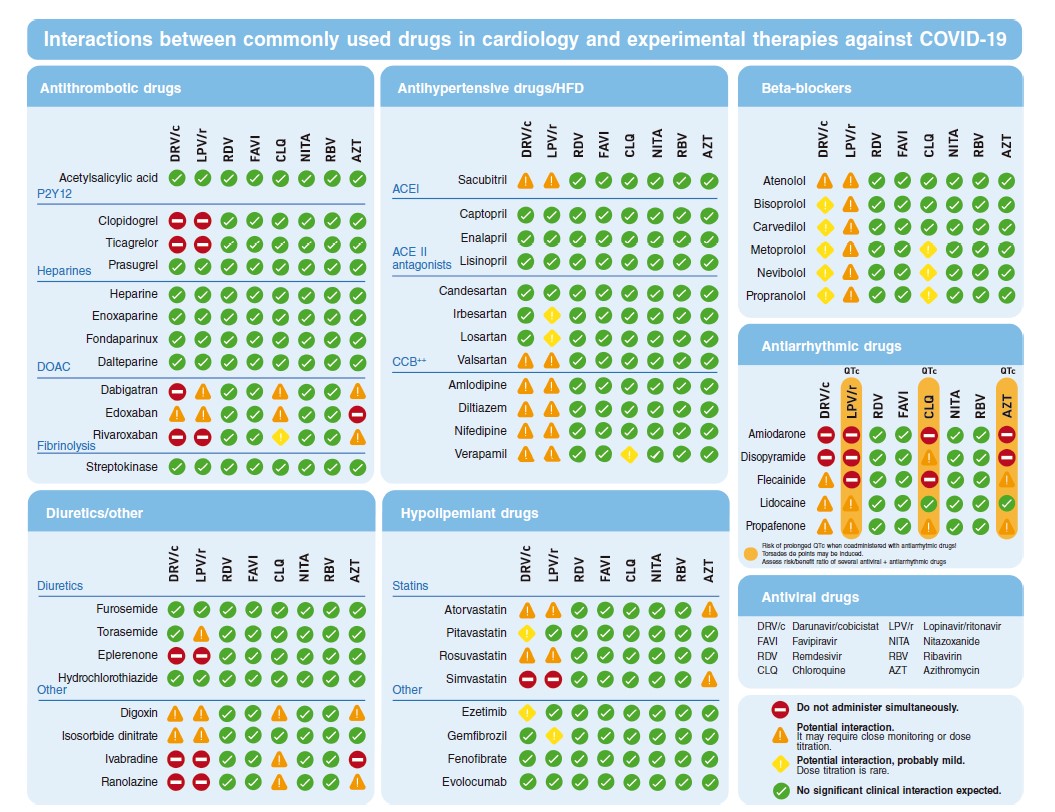
Figure 3 shows the most commonly used drugs in cardiology both in the context of ACS and stable coronary artery disease.
Figure 3. Possible interactions between the most commonly used drugs in cardiology and the possible treatments used against COVID-19. ACE II, angiotensin-converting enzyme antagonists; ACEI, angiotensin-converting enzyme inhibitors; CCB, calcium channel blockers; DOAC, direct-acting oral anticoagulants; HFD, heart failure drugs.
Antiplatelet therapy
- Adiro: no evidence of significant interactions.
- Oral P2Y12 platelet receptor antagonits: prioritize prasugrel. Concomitant treatment with lopinavir/ritonavir or darunavir/cobicistat increases the effect of ticagrelor and can reduce the effect of clopidogrel.
- Cangrelor: no evidence of significant interactions.
- Tirofiban: no evidence of significant interactions.
Anticoagulant drugs
- Unfractionated heparin: no evidence of significant interactions.
- Bivalirudin: no evidence of significant interactions.
- Enoxaparin: no evidence of significant interactions.
- Fondaparinux: no evidence of significant interactions.
Analgesics/sedatives
- Fentanyl/morphine: potential interactions; prioritize morphine.
- Midazolam: it should not be administered orally. It can be IV administered with special caution.
Inotropes and vasopressors
- Adrenaline: no evidence of significant interactions.
- Dobutamine: no evidence of significant interactions.
- Noradrenaline: no evidence of significant interactions.
- Dopamine: no evidence of significant interactions.
Other
- Nitroglycerin: no evidence of significant interactions.
- Verapamil: potential interactions. Administer and use with special caution and close monitoring.
- Furosemide: no evidence of significant interactions.
CONCLUSIONS
The current outbreak of COVID-19 has made us rethink the invasive approach to ischemic and structural heart disease. The recommendation of the Spanish Society of Cardiology Working Groups on Cardiac Catheterization and Interventional Cardiology and Ischemic Heart Disease and Acute Cardiovascular Care is to postpone all non-emergent procedures to avoid the infection of patients and healthcare providers and minimize the collapse of the healthcare system. However, the percutaneous coronary intervention should still be used for the management of ST-segment elevation myocardial infarction unless prescribed otherwise.
CONFLICTS OF INTEREST
The authors have declared no conflicts of interest regarding this manuscript. R. Moreno is associate editor of REC: Interventional Cardiology. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
EDITOR'S NOTE
This manuscript has undergone an especially rapid internal review by the editorial team due to the strong interest indisseminating the information among the scientific community. The editors thank Permanyer Publications for their collaboration and commitment to the prompt publication of this document.
REFERENCES
1. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
2. Romaguera R, Gonzalez-Cruz I, Ojeda S, et al. Consensus document of the Interventional Cardiology and Heart Rhythm Associations of the Spanish Society of Cardiology on the management of invasive cardiac procedure rooms during the COVID-19 coronavirus outbreak. REC Interv Cardiol. 2020.https://doi.org/10.24875/RECICE.M20000116.
3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513.
4. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020. https://doi.org/10.1016/j.pcad.2020.03.001.
5. Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa190.
6. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315.
7. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269.
8. Di Marco A, Anguera I, Rodriguez M, et al. Assessment of Smith Algorithms for the Diagnosis of Acute Myocardial Infarction in the Presence of Left Bundle Branch Block. Rev Esp Cardiol. 2017;70:559-566.
9. Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N Engl J Med. 2019;380:1397-1407.
10. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381:1411-1421.
11. Thiele H, Akin I, Sandri M, et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N Engl J Med. 2018;379:1699-1710.
12. University of Liverpool. Interactions with Experimental COVID-19 Therapies. Disponible en: https://www.Covid19-druginteractions.org. Consultado 17 Mar 2020.
ABSTRACT
During March 2020, the SARS-CoV-2 virus spread throughout Europe, with the spread being especially intense in Italy and Spain. Given the emergency created by the COVID-19 outbreak, routine activity has been altered in most cardiac catheterization and electrophysiology labs. Health staff working in these areas are faced with performing procedures in patients with a confirmed diagnosis of COVID-19 or with uncertainty in unconfirmed cases. This article is a consensus document of the Interventional Cardiology Association and Heart Rhythm Association of the Spanish Society of Cardiology and aims to provide information to health care professionals working in these invasive cardiology facilities (cardiac catheterization and electrophysiology labs, pacemaker implantation) in order to guarantee quality patient care and adequate levels of infection prevention.
Keywords: Interventional cardiology. Electrophysiology. Infection. Prevention. Prevention. COVID-19. Coronavirus. Pandemic.
RESUMEN
Durante marzo de 2020, el virus SARS-CoV-2 se ha extendido por toda Europa, con especial intensidad en Italia y España. Ante la emergencia creada por el brote de COVID-19, la inmensa mayoría de las salas de hemodinámica y electrofisiología han visto alterada su actividad habitual. Además se enfrentan a la realización de procedimientos en pacientes con diagnóstico confirmado de COVID-19 o con la incertidumbre en casos no confirmados. El presente texto es un documento de consenso de la Asociación de Cardiología Intervencionista y la Asociación del Ritmo Cardiaco de la Sociedad Española de Cardiología que pretende dar información al personal sanitario de estas instalaciones de cardiología invasiva (hemodinámica y electrofisiología y marcapasos) para garantizar una atención de calidad a los pacientes así como unos niveles los niveles adecuados de prevención de la infección.
Palabras clave: Cardiología intervencionista. Electrofisiología. Infección. Prevención. COVID-19. Coronavirus. Pandemia.
INTRODUCTION
On 31 December 2019, the authorities of the People’s Republic of China informed the World Health Organization of several cases of pneumonia of unknown cause in Wuhan, a city located in the Chinese province of Hubei. One week later, they confirmed that the cases were due to a new coronavirus, named SARS-CoV-2. During February, the virus spread through northern Italy and subsequently throughout the rest of Europe, including Spain, where measures to contain the spread were initiated on 10 March, 2020. On 13 March, 2020, the Spanish Government issued a decree (article 116.2 of the Spanish Constitution) declaring a state of alarm with immediate effect. The decree involves changes to the organization of health care installations, staff, and services. In line with the new legal situation, the respective health departments of the autonomous communities modified the regulations generally affecting the availability of health staff (eg, working hours, granting of leave, holidays or days off, exemptions), clinical care, and procedures, often restricting activity to emergency care. The Interventional Cardiology Association and the Heart Rhythm Association of the Spanish Society of Cardiology understand the need to make our commitment public and to adapt our practice to the best practices in the current regulatory context.
Like other viruses in the coronavirus family, this pathogen causes various clinical manifestations encompassed within the term COVID-19, which include respiratory illness ranging from the common cold to severe pneumonia with respiratory distress syndrome, septic shock, and multiorgan failure.1 Moreover, prognosis is poor in patients with prior cardiovascular disease and COVID-19 infection.2 Most cases of COVID-19 notified to data have been mild, but the virus is highly contagious, mandating measures to be taken in all health care and nonhealth care settings.
Faced with the emergency created by the COVID-19 outbreak, the vast majority of cardiac catheterization and electrophysiology labs have experienced changes in their day-to-day running. Health care staff working in these areas are faced with performing procedures in patients with a confirmed diagnosis of COVID-19 and with uncertainty in those with unconfirmed infection. In addition, interventional cardiology units are generally closed units with the same team members working closely together in these areas, representing a risk for health care delivery if quarantines are declared in entire units.
The present article is a consensus document aiming to provide information to health staff in these invasive cardiology installations (cardiac catheterization, electrophysiology, and pacemakers) to guarantee delivery of quality patient care. The document also aims to provide information on how to ensure adequate levels of protection against infection among family members, persons living with infected individuals, workers in health care centers, health care workers attending infected individuals, and the remainder of the population in general.
STAFF MANAGEMENT AND INDICATIONS FOR PROCEDURES
We recommend that each unit take the appropriate measures to separate workers into groups so that possible quarantines can be applied to groups within each unit rather than the unit as a whole.
In elective patients, we recommend considering delaying procedures whenever possible.
APPROACH TO THE PATIENT BEFORE ENTRY TO THE LAB
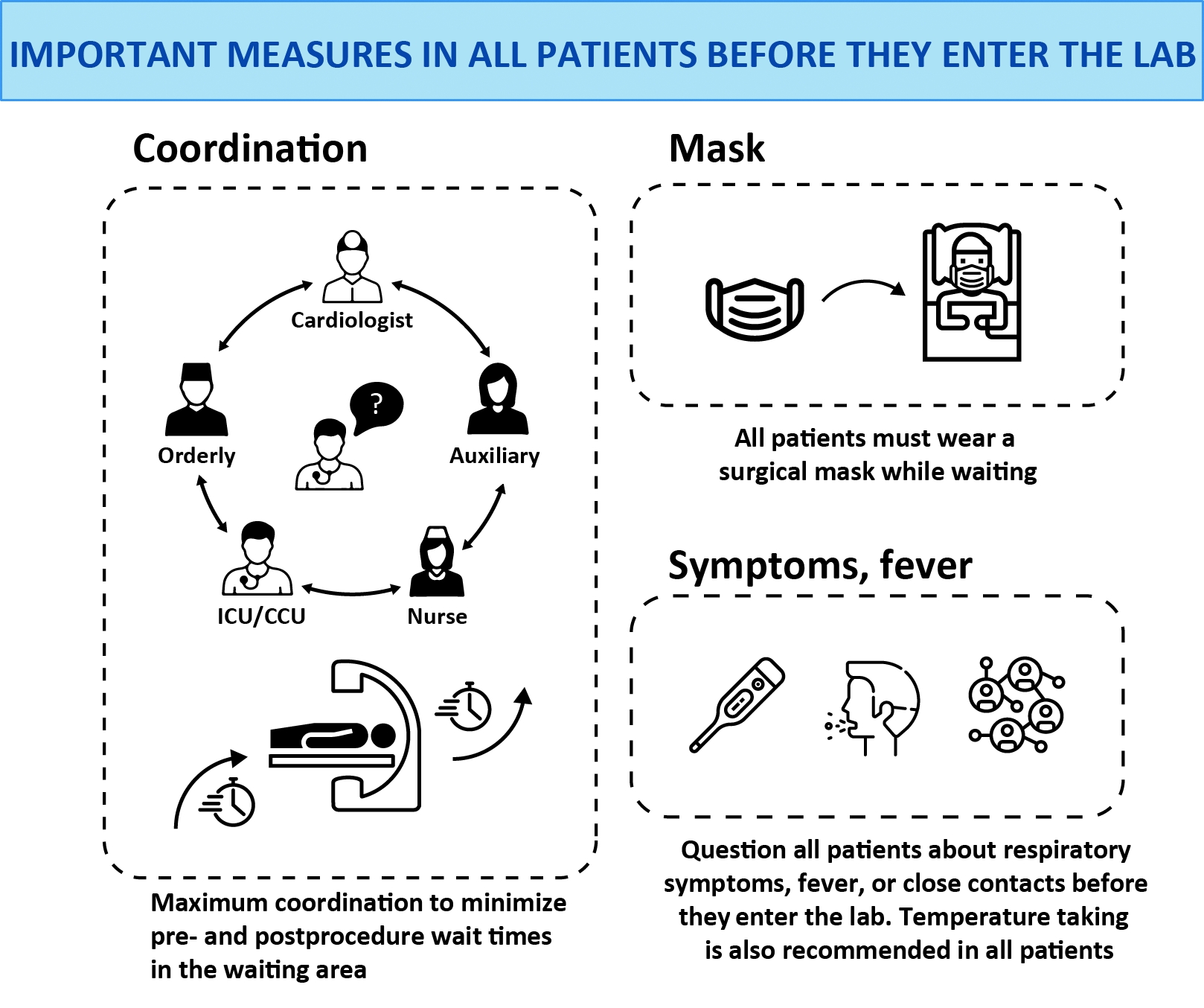
The following steps are recommended before patients enter cardiac catheterization and electrophysiology labs (figure 1):
- Maximal coordination to minimize pre- and postprocedure waiting times in waiting areas.
- Use of surgical masks in all patients while they wait.
- Questioning of all patients about respiratory symptoms, fever, and close contacts before entry to the lab; we also recommend temperature-taking in all patients.
Figure 1. Important measures in all patients before they enter the lab.
CCU, Cardiac/Coronary Care Unit; ICU, Intensive Care Unit.
APPROACH TO PATIENTS WITHOUT CONFIRMATION OF COVID-19 INFECTION
Given the current panorama and the possibility of having to treat asymptomatic patients or those with undiagnosed infection, we recommend taking maximal protection measures,3 especially in patients referred from the emergency department. Procedures involving manipulation of the airway and/or esophagus should also be considered high risk. The following measures are recommended:
- Patients: surgical mask before entry to the lab.
- Physicians and nurses: hand-washing, sterile fluid-impermeable gowns, sterile gloves, splash goggles, cap covering hair, and surgical mask.
- Cardiologists or circulating nurses: splash goggles, gloves, cap, and surgical mask.
In patients with respiratory symptoms in areas of community transmission, those with confirmed contacts and those who may require transesophageal echocardiography, manual ventilation, intubation, or any other type of airway manipulation, we recommend that the approach to infection prevention be the same as that used in patients being tested for COVID-19 infection or with confirmed infection (see next section). The approach to unstable patients, especially those with ST-segment elevation, should also be the same as that in patients with confirmed COVID-19 infection.
APPROACH TO PATIENTS WITH SUSPECTED OR CONFIRMED COVID-19 INFECTION
In patients with suspected or confirmed COVID-19 infection, we recommend the following measures:
- Consider procedures involving airway and/or esophageal manipulation as very high risk.
- Allow only essential staff to enter the lab.
- Keep doors shut at all times.
- Prepare drugs before patient entry to the lab.
- Avoid leaving the lab with contaminated equipment (eg, gown, gloves, mask, etc.) to collect material (eg, stents, catheters, etc) and consequently try to predict the necessary material as much as possible.
Material
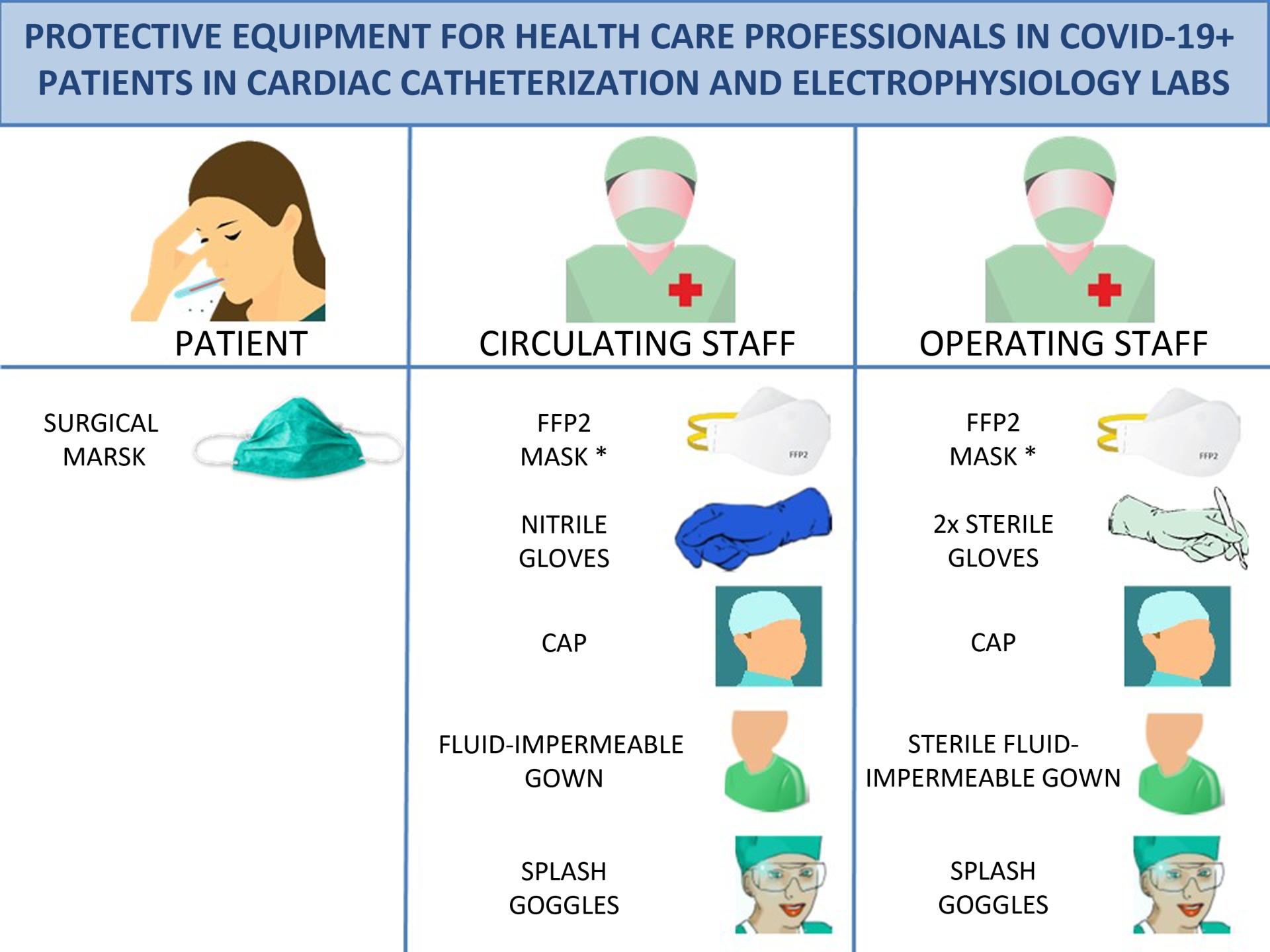
The recommended material is shown figure 2 and is described below:
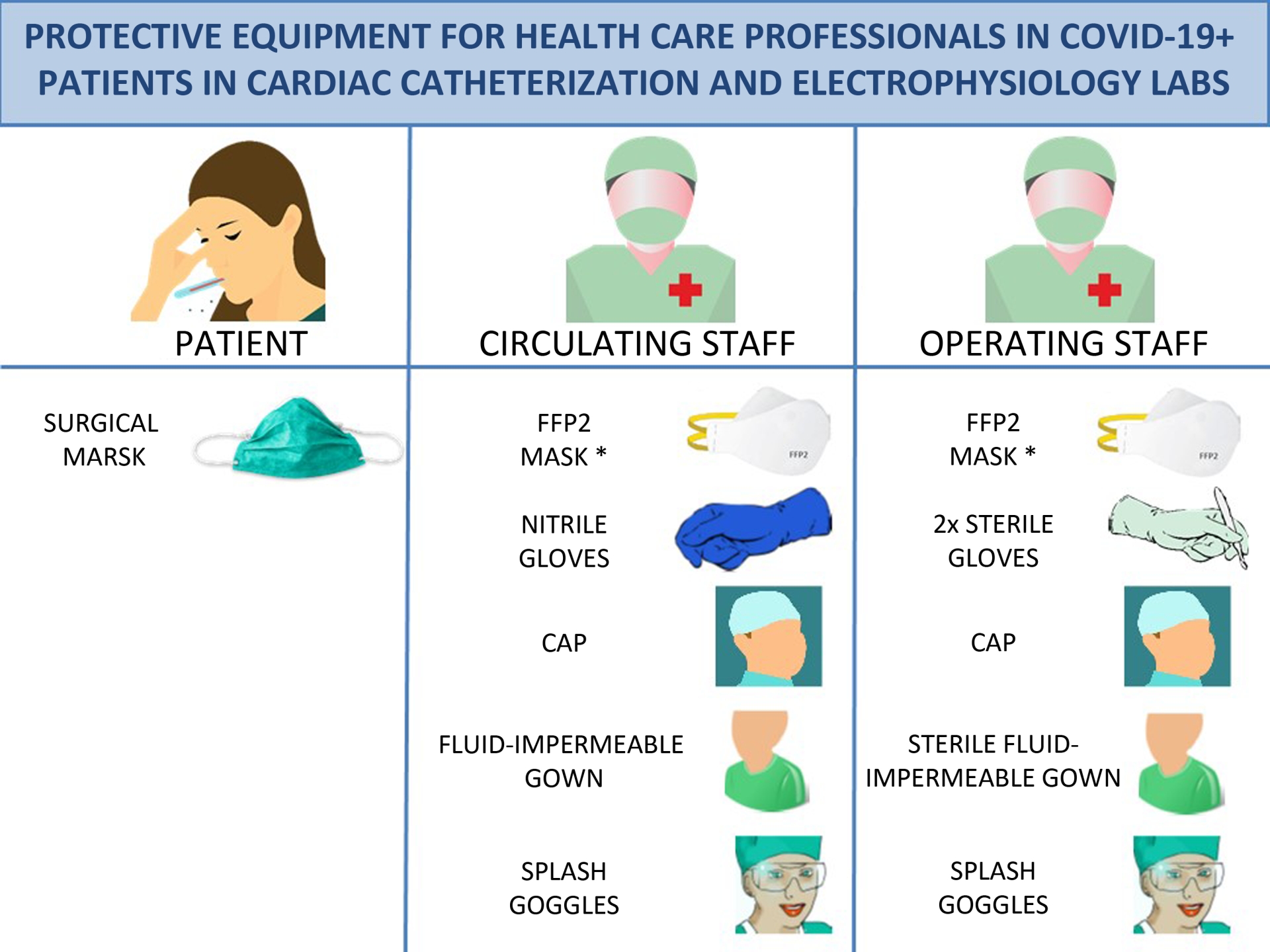
- Patients: surgical mask. It is important to stress that FFP2 masks are personal protection masks and not barrier masks. The air expelled by these masks is contaminated and so they should not be worn by infected patients. Patients should wear a face mask that acts as a barrier to secretions.
- Physicians and nurses: hand-washing, coated fluid-impermeable gown with cuff (if the gown is not fluid-impermeable, a plastic apron should be added), 2 pairs of gloves (whose use is recommended by some local authorities), splash goggles or conventional goggles and face shield, cap, and high filtration efficiency FFP2 mask if available4 (for procedures such as placement of implantable cardioverter-defibrillators, pacemakers and transcatheter prostheses, a surgical mask should be placed over the FFP2 mask). Closed work shoes are recommended or, if unavailable, boots.
- Cardiologists or circulating nurses: gloves, cap, fluid-impermeable gown and FFP2 face mask (if available).
Figure 2. Protective equipment for health care professionals in COVID-19+ patients in cardiac catheterization and electrophysiology labs. * For implantation of pacemaketers, implantable cardioverter-defibrillators and transcatheter prostheses, place a surgical mask over the FFP2 mask. FFP2, filtering face piece type 2.
Recommended steps for moving patients from the gurney to the operating table
Staff responsible for transferring patients with COVID-19 infection from the gurney to the operating table must wear previously placed individual personal protection equipment, including fluid-impermeable gown, cap, cuff-covering gloves, goggles and FFP2 mask (if available). On finishing the transfer, staff must undress as follows, remembering not to remove the mask under any circumstances while inside the lab.
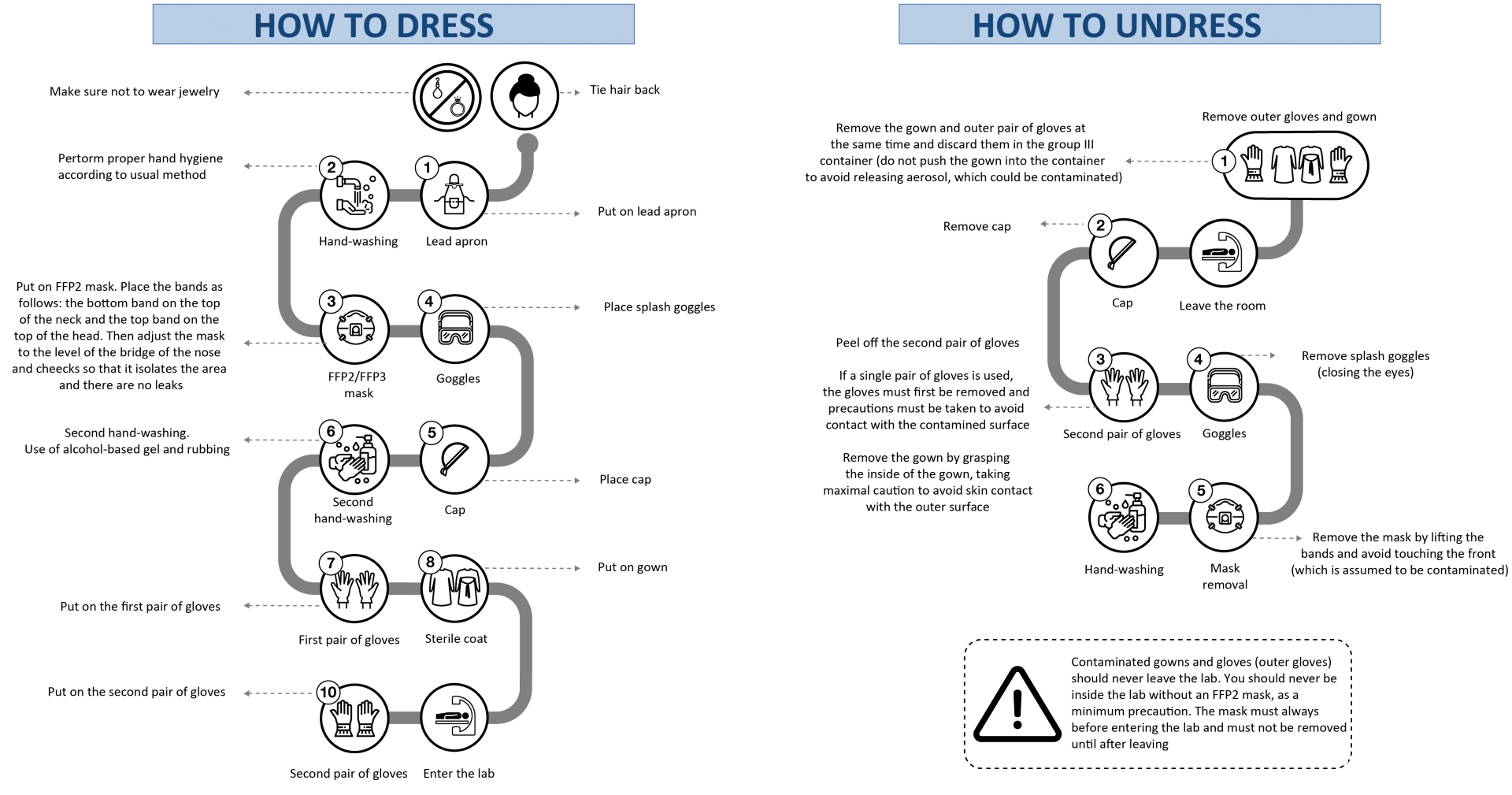
How to dress
The steps for dressing are described below (figure 3).
Outside the lab
- Do not wear jewelry.
- Tie hair back (if necessary).
- Put on lead apron.
- Perform correct hand hygiene using routine method.
- • Place FFP2 mask. The rubber bands should be placed in the following way: the bottom bands in the upper part of the neck and the top bands on the top of the head. Then adjust the height of the mask at the bridge of the nose and cheeks to isolate them and prevent leaks.5
- Place splash goggles.
- Place the cap.
- Perform second hand-washing, with use of alcohol-based hand sanitizer and rubbing.
- Put on the first pair of gloves.
- Put on the gown.
Inside the lab
- Roll the second pair of gloves over your fist.
How to undress
- We recommend that staff undress as shown in figure 3 and described below.
Inside the lab
- As you remove the gown, peel off the outer pair of gloves at the same time6 and discard into a group III container (do not push the gown down into the container to avoid releasing aerosol, which could be contaminated).
Outside the lab
- Remove cap.
- Peel off the second pair of gloves.
- Wash hands.
- Remove splash google with eyes closed.
- Remove the mask by lifting the elastic bands. Under no circumstances touch the front of the mask (which should be assumed to be contaminated).
- Wash hands
If only 1 pair of gloves are worn, first remove them, taking extreme care to avoid contact with the contaminated surface. Then remove the gown by grasping the inside of the gown, taking maximal caution to avoid skin contact with the outer surface.7
Important: Contaminated gowns and gloves (outer ones) should never leave the lab. Staff should never be inside the lab without an FFP2 mask, as a minimum precaution. Masks must always be placed before entering the lab and must not be removed until after staff have left the lab.
After completion of the procedure
After the end of the procedure:
- • We recommend disinfecting goggles with wipes impregnated with a wide spectrum biocidal agent to disinfect surfaces. Leave them wet and air dry. Use gloves to disinfect, due to the toxicity of the wipes and potential contamination of surfaces.
- Discard all material used in the procedure in a group III container for biomedical waste and then seal the container.
- Consider changing scrubs.
- Patients must wear a surgical mask during transfer to the ward or referral center and the orderly or physician (if required) must wear an FFP2 mask.
Lab cleaning
We recommend the following cleaning measures:
- Labs should be cleaned by following specific procedures for contact and drip isolation in each center. For example, by using sodium hypochlorite at a concentration of 1000 parts per million, leaving it in contact with the surface for 5 minutes.
- Cleaning cloths should be discarded (disposable).
- Cleaners should be equipped with personal protection equipment.
- After lab cleaning, consider cleaning all areas where the infected patient has been in contact with an ultraviolet-disinfectant robot.
- Labs should be cleaned at least 1 hour after the procedure, rather than immediately, to allow aerosol deposition.
Figure 3. Guidelines on how health staff in cardiac catheterization and electrophysiology labs should dress and undress. FFP, filtering face piece.
SPECIAL SITUATIONS: SEVERELY ILL PATIENTS
If oxygen is required, a mask should be placed over the nasal cannula or oxygen mask.
We discourage the use of nebulizers in patients with COVID-19 and also advise against the use of noninvasive positive pressure ventilation (continuous positive airway pressure [CPAP] and bilevel positive airway pressure [BiPAP]).
In patients requiring intubation and mechanical ventilation or cardiopulmonary resuscitation, extreme care should be taken to apply preventive measures due to the high risk of droplet release.
ROUTINE CARDIOLOGY DRUGS IN PATIENTS WITH COVID-19
Angiotensin converting-enzyme inhibitors/angiotensin II receptor antagonists
There is no evidence to support the hypothesis that these drugs may cause deleterious effects during COVID-19 infection. In contrast, there is much evidence of their cardiovascular benefits in specific populations. Therefore, unless there is a change in current evidence, we advise against their withdrawal unless there is hemodynamic instability.8
Antithrombotic agents
Any inflammatory process increases platelet reactivity. However, there is no current evidence to support any use other than routine use during COVID-19 infection. Therefore, the use of antithrombotic and antiplatelet agents should continue to be considered according to the patient’s clinical situation and bleeding risk.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors thank Agustín Fernández Cisnal (Hospital Clínico de Valencia, Valencia, Spain) for his help in creating the figures for this article.
EDITOR'S NOTE
This manuscript has undergone an especially rapid internal review by the editorial team due to the strong interest in disseminating the information among the scientific community. The editors thank Permanyer Publications for their collaboration and commitment to the prompt publication of this document.
REFERENCES
1. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the dianosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (strandard versión). Military Med Res. 2020. https://doi.org/10.1186/s40779-020-0233-6.
2. Zeng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020. https://doi.org/10.1038/s41569-020-0360-5.
3. Società Italiana di Cardiologia Interventistica. Gestione sale di emodinamica e cardiologia interventistica per emergenza covid-19. Available at: https://gise.it/Uploads/EasyCms/GM%20CF%20per%20PD%20gestione%20covid-19%20-_14892.pdf. Accessed 14 Mar 2020.
4. Procedimiento de actuación par los servicios de prevención de riesgos laborales frente a la exposición al SARS-CoV-2 . Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/PrevencionRRLL_COVID-19.pdf. Accessed 6 May 2020.
5. Generalitat de Catalunya. Procedimiento de actuación frente a casos de infección por el nuevo coronavirus SARS-CoV-2. Available at: http://canalsalut.gencat.cat/ca/salut-a-z/c/coronavirus-2019-ncov/material-professionals/index.html#googtrans(ca|es). Accessed 14 Mar 2020.
6. World Health Organization. Coronavirus disease (COVID-19) outbreak: rights, roles and responsibilities of health workers, including key considerations for occupational safety and health. Available at: https://www.who.int/docs/default-source/coronaviruse/who-rights-roles-respon-hw-covid-19.pdf?sfvrsn=bcabd401_0. Accessed 14 Mar 2020.
7. World Health Organization. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). Interim guidance 27 February 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf. Accessed 14 Mar 2020.
8. Sociedad Española de Cardiología. No hay evidencia clínica ni científica para suspender tratamientos de IECA y ARA debido a la infección por COVID-19. Available at: https://secardiologia.es/institucional/socios/comunicados/comunicados-oficiales/11446-no-hay-evidencia-clinica-ni-cientifica-para-suspender-tratamientos-de-ieca-y-ara-debido-a-la-infeccion-por-covid-19. Accessed 14 Mar 2020.
ABSTRACT
Severe tricuspid regurgitation is independently associated with an increased mortality. Recent studies show that surgery for isolated tricuspid valve (TV) is still associated with the highest surgical risk of all valve procedures. Percutaneous treatments for the management of tricuspid regurgitation have become an alternative to surgery in select patients of high surgical risk. To this day, the most widely used device is MitraClip in the tricuspid position. We reviewed the different devices used today for transcatheter treatments paying special attention to MitraClip. We describe the basic standpoints on transesophageal echocardiography screening and step-by-step procedure for the implantation of the clip. This document stands as a guide to systematize tricuspid valve assessments and the steps involved in device implantation to achieve successful procedures.
Keywords: Tricuspid regurgitation. Transesophageal echocardiography. Transcatheter tricuspid valve repair.
RESUMEN
La insuficiencia tricuspídea se asocia de manera independiente con un aumento de la mortalidad. Investigaciones recientes demuestran que la cirugía aislada de la válvula tricúspide presenta la mortalidad quirúrgica más alta dentro de los procedimientos valvulares. Las terapias percutáneas han surgido como una alternativa a la cirugía en pacientes seleccionados de alto riesgo quirúrgico. El dispositivo MitraClip en posición tricuspídea ha sido el más empleado. El propósito de este artículo es revisar los diferentes dispositivos transcatéter para el tratamiento de la insuficiencia tricuspídea, con especial énfasis en el MitraClip. Se describirán las vistas básicas de la exploración por imagen y el procedimiento paso a paso para la inserción del clip. Este documento pretende ser una guía para la sistematización de la evaluación de la válvula tricúspide y los pasos del implante del dispositivo para asegurar el éxito del procedimiento.
Palabras clave: Insuficiencia tricuspídea. Ecocardiograma transesofágico. Reparación tricuspídea transcatéter.
Abbreviations RV: right ventricle. TTE: transthoracic echocardiogram. TEE: transesophageal echocardiogram. TR: tricuspid regurgitation. TV: tricuspid valve.
INTRODUCTION
Although traditionally tricuspid valve disease has been considered a less serious condition compared to left-sided valvular heart disease, nearly 2 million patients in the United States suffer from moderate tricuspid regurgitation (TR). However, less than 10 000 patients are treated with surgery each year. Functional TR amounts to 90% of all cases of TR and is often due to tricuspid valve (TV) annular dilation (mainly in the anteroposterior diameter) and right ventricle (RV) dilation due to progressive left heart disease.1 TV has been considered “the forgotten valve” for years. This problem may be explained because it was believed that TR was well-tolerated and decreased after treatment of the left-sided valvular heart disease. However, patients with significant TR and heart failure often remain very symptomatic.2 The presence of moderate or severe TR is associated with a higher mortality rate regardless of other variables like the ejection fraction or pulmonary pressures. Mortality rate is > 25% per year.3-5
Current data confirm that repairing the TV while performing left heart cavity surgery is safe. However, reinterventions due to persistent TR are associated with high morbidity and mortality rates.6 Recent studies show that isolated TV surgery is still the valve surgery that is most associated with higher surgical risk and mortality rates between 8.8% and 9.7%.7 Therefore, the percutaneous therapies for the management of TR are an alternative to conventional surgery for patients who, until recently, were eligible for conservative medical treatment only for being high surgical risk patients.
Depending on the repair anatomical target, devices can be divided into coaptation, valve annuloplasty, and replacement devices (whether in the orthotopic or heterotopic position). The most widely used technique is the edge-to-edge repair with the MitraClip device (Abbott Vascular, Santa Clara, United States) in the tricuspid position. The TriValve registry8 included over 650 procedures with the MitraClip device (66% of all percutaneous procedures performed on the TV). This article has 2 objectives: first, to propose a protocol for the echocardiographic assessment of TV in patients with TR to decide on the suitability and feasibility of performing tricuspid repair with this system and guiding the procedure on a step-by-step basis; and second, to review briefly other transcatheter percutaneous devices available today that have already become clinically relevant.
TRICUSPID VALVE IMAGING FOR INTERVENTIONAL PROCEDURES
All patients eligible for tricuspid percutaneous procedures should initially be treated with a transthoracic echocardiogram (TTE). If the valve can be repaired using the edge-to-edge technique, a more advanced assessment using the transesophageal echocardiogram (TEE) should be performed.
TVs that clearly interfere with pacemaker or implantable defibrillator leads, leaflet perforation, severe restriction or significant thickening should not be considered favorable on the TTE. The traditional views for TV assessment through TTE are the parasternal view on the TV modified long axis, the short axis, the modified apical 4-chamber view, and the subcostal view in the short axis.
TEE assessments are crucial to decide whether a procedure using this device is feasible or not. Anatomy should be adequate, yet images should have enough quality to guide the procedure; at times, anatomical variants or the presence of intracardiac prosthetic material can make the procedure unfeasible. The current TEE guidelines established by the American Society of Echocardiography include additional images for TV assessment purposes.9 Multiplane rotations and multiple views are important for correct leaflet identification including adjacent structures used as reference.10
TRICUSPID VALVE ASSESSMENT THROUGH TRANSESOPHAGEAL ECHOCARDIOGRAM
The essential views for a detailed assessment of the TV regarding transcatheter procedures are:
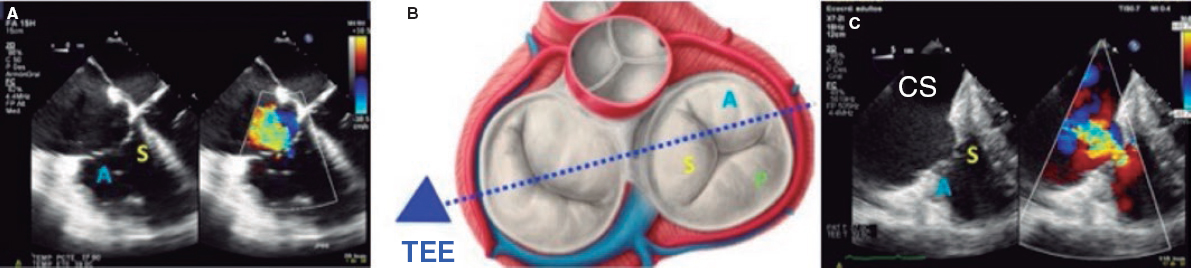
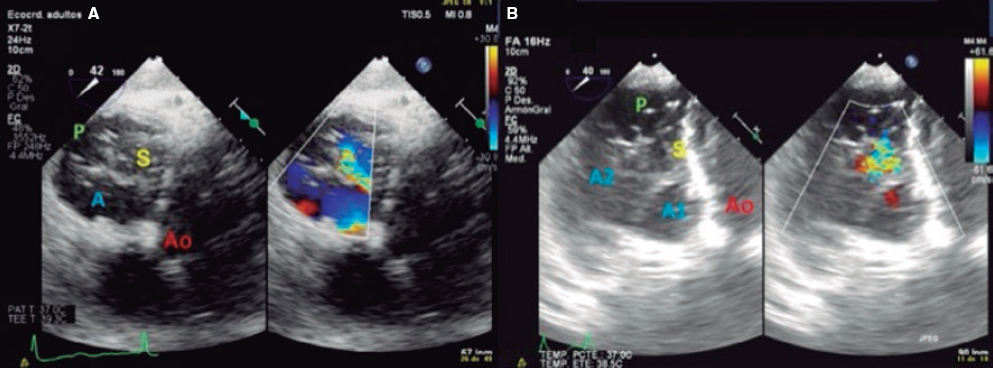
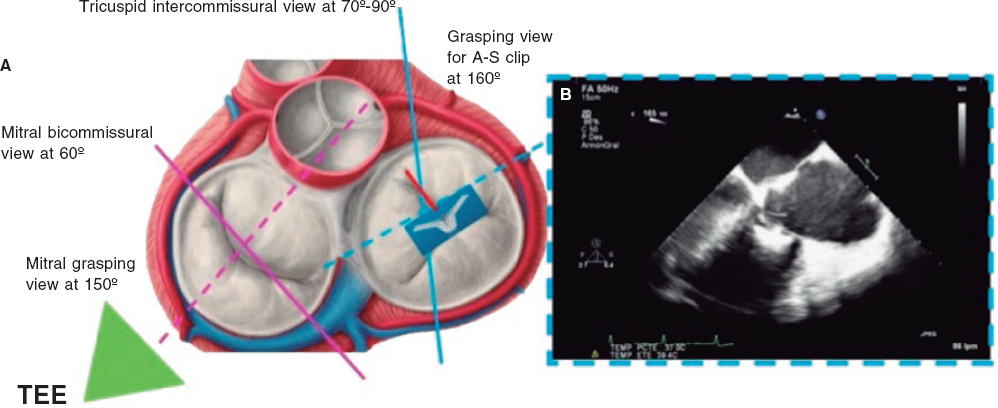
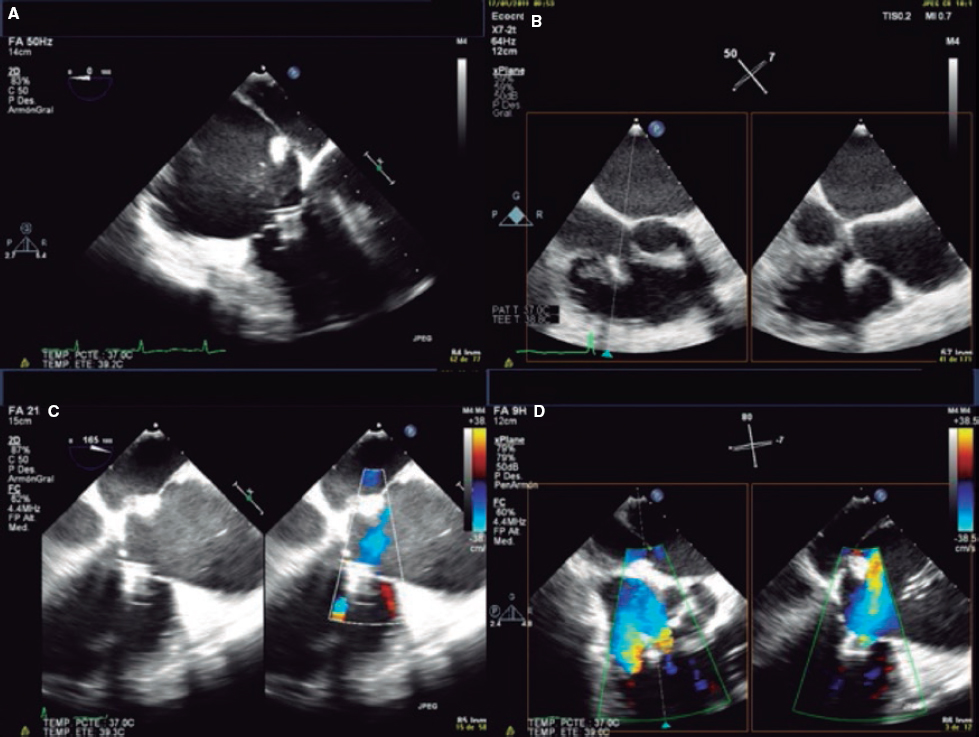
1. Mid-esophageal 4-chamber view at 0º. The septal leaflet (adjacent to the aorta) and the anterior leaflet (adjacent to the RV free wall) can be seen when the transducer is in anteflexion position (figure 1). The posterior leaflet can be seen when it is in the retroflexion position.
Figure 1. A: mid-esophageal 4-chamber view showing the septal leaflet and the anterior leaflet. B: imaginary view in the 4-chamber view. C: deep transesophageal view. A, anterior; TEE, transesophageal echocardiogram; S, septal; CS, coronary sinus.
To optimize the images of the right heart structures, the transducer should be turned clockwise. The artifacts of the septum and aortic or mitral valves can block the view of the septal leaflet. Tricuspid annulus is measured in this view with open flap-like cusps at the end of the diastole.
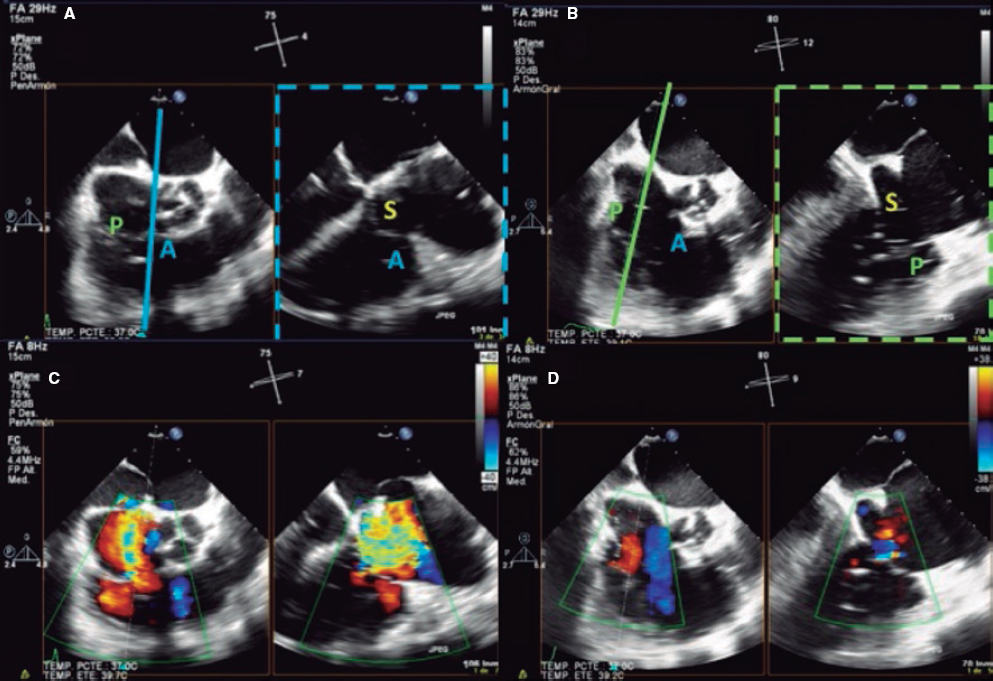
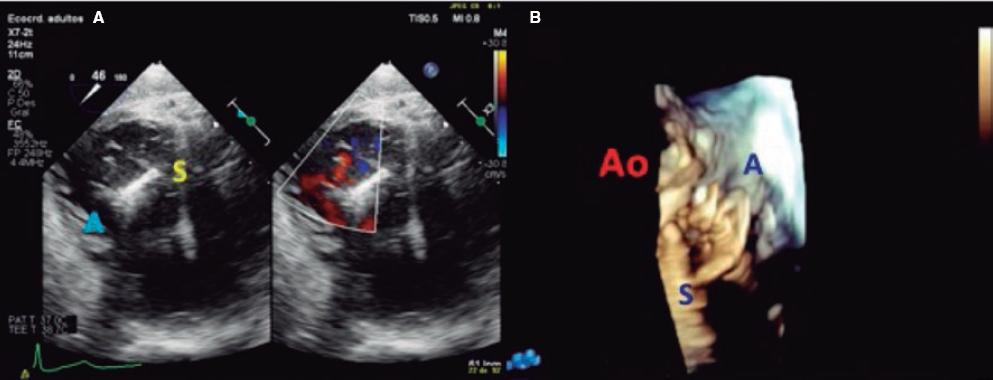
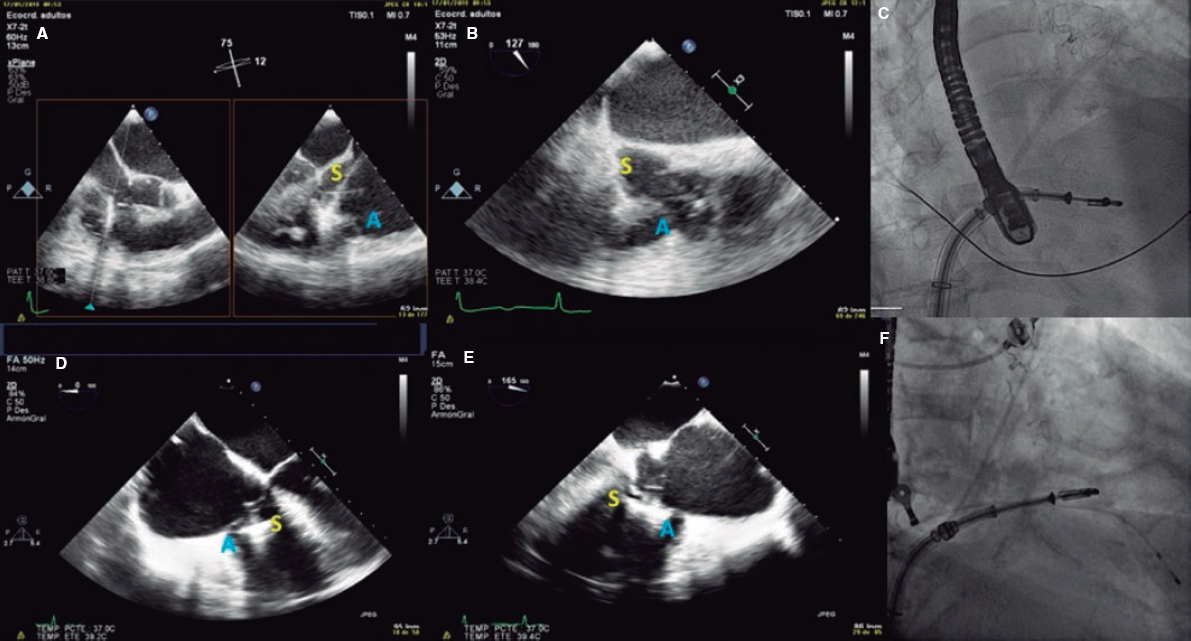
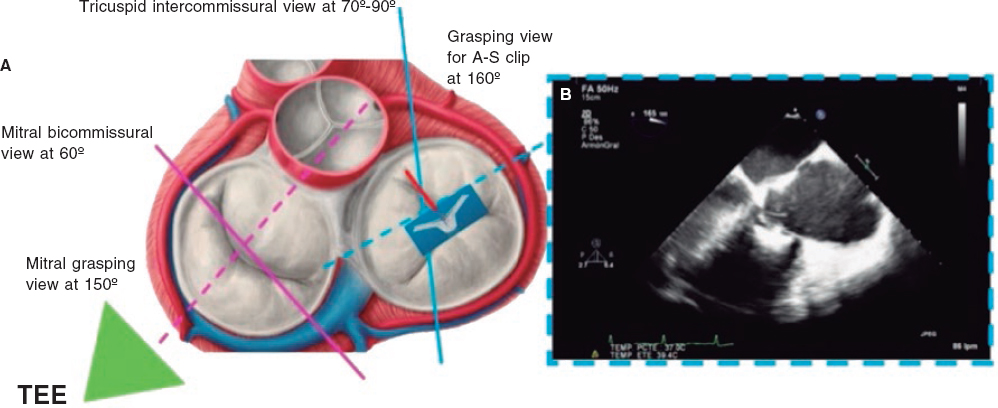
2. Mid-esophageal view of the RV inflow and outflow tract (intercommissural tricuspid view) between 60° and 90°. It shows the imaginary line from the anteroseptal (A-S) commissure up to the posteroseptal (P-S) commissure. The anterior leaflet close to the aorta and the posterior leaflet on the lateral side can be seen (video 1 of the supplementary data). This is the reference view to obtain biplanar images for the assessment of the septal leaflet. By bringing the cursor close to the aorta (X-plane tool), the orthogonal view shows the A-S coaptation and the anterior and septal leaflets. By moving it away from the aorta towards the lateral position, the view shows the P-S coaptation and the posterior and septal leaflets. The same views should be acquired using the Doppler color technique (figure 2) to see whether the origin of the regurgitant jet is of anterolateral or P-S predominance.
Figure 2. Intercommissural mid-esophageal view. A: when placing the cursor next to the aorta, the orthogonal view shows the anteroseptal coaptation line. B: by moving the cursor towards the most lateral region, the orthogonal view is acquired showing the posteroseptal coaptation line. C and D: color biplanar imaging showing the origin of regurgitation jet. A, anterior; P, posterior; S, septal.
Also, these views are useful to measure the length of the leaflets, see coaptation defects, assess the movement of the leaflets, and see the presence of strings that can make the procedure difficult. The presence of serious restrictions in the movement of the septal leaflet limits the performance of the procedure with the current device.
3. Deep esophageal 4-chamber view at 0°. Since the right inferior border of the heart is close to the diaphragm, the deepest insertion of the TEE transducer reaches the distal esophagus, close to the gastroesophageal junction; it may be that this view will only show the right atrium and coronary sinus and no images of the left atrium (figure 1). This prevents left heart structure-related artifacts from happening like the acoustic shadowing that the mitral prosthetic material can cause on the septal leaflet. This is the optimal view to acquire 3D volumes.
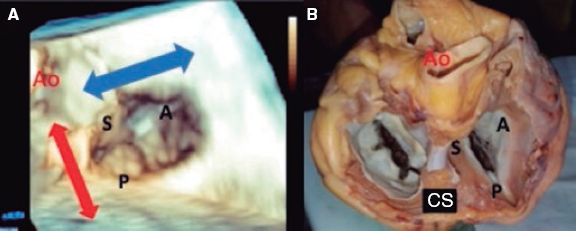
4. Transgastric view. These views are acquired by advancing the transducer towards the stomach. Starting at the baseline transgastric short axis and by rotating the transducer between 20° and 40° and making small clockwise rotations with anteversion, the optimal view to see the 3 tricuspid leaflets is acquired (TV short axis). This is the only 2D (bidimensional) view to see the 3 cusps and commissures simultaneously with the posterior leaflet in the nearby field, the anterior leaflet in the distant field, and the septal one adjacent to the septum (figure 3 and video 2 of the supplementary data). Images need to be optimized to make sure we are positioned on the tip of the leaflets and parallel to the valvular view. To do this we can start with a 2-chamber transgastric view of the RV at around 90º-110°. By using the X-plane tool to place the cursor on the tip of the leaflets, the orthogonal view will be acquired (that will be the TV short axis). This view provides a great deal of information on the TV:
Figure 3. Transgastric view. A: transgastric short axis view of a tricuspid valve with 3 cusps. B: transgastric short axis view of an anatomical variant with 2 scallops in the anterior leaflet. A: anterior; A1, scallop 1 of the anterior leaflet; A2, scallop 1 of the anterior leaflet; Ao, aorta; P, posterior; S, septal.
-
– Number of leaflets: there are usually 3, but we may find valves with several scallops and even 4 cusps in up to 40% of the patients.11
-
– Portion of the valve occupying each leaflet: it is very useful to make a graphic representation of what part of the circumference occupies each leaflet.
-
– Location of the regurgitant jet origin: the leaflets distal edge needs to be approached for a correct visualization of the coaptation defect in the short axis.
The impact of 3D images is not as powerful when performing procedures on the TV compared to the mitral valve. Lang et al.12 suggest a standard image visualization to see a frontal view of the TV by placing the septal leaflet in the inferior position (at the 6:00 hour position). These recommendations were established at the end of the last decade when there were no transcatheter procedures and the surgical view was trying to be replicated. Rotating the image 90° with the aortic valve in the 11:00 hour position is currently the preferred option. This should improve our understanding of the anatomy of the TV by creating a common language between cardiac imaging specialists and interventional cardiologists.
The quantitative analysis of leaflets and annulus is performed through multiplanar reconstructions of 3D views.13 By placing orthogonal views on the tricuspid annulus at the leaflet insertion site, the TV short axis is acquired. After precise adjustment the annular diameters and area dimensions can be obtained (figure 1 of the supplementary data).
The new classification of TR with new degrees of massive and torrential severity can be crucial for the adequate classification of patients and to assess the procedural final results.14
KEY TRANSESOPHAGEAL ECHOCARDIOGRAM VIEWS FOR TRICUSPID VALVE CLIP REPAIR
There are 4 key TEE views to guide the TV clip implantation: 4-chamber, bicommissural, transgastic, and grasping views (capture of tricuspid leaflets inside the device). The former views have already been described; we will now focus on the latter.
Grasping view
This is the view that better shows the leaflets that should be treated and the clip with wide open arms. Finding the best grasping view for clip implantation in the tricuspid position is one of the most complex and important steps during the procedure. The first limitation to find it is that, unlike what happens in the mitral valve, there are no clear anatomical references to guarantee the perpendicularity of the clip with respect to the leaflets. That is why a correct alignment at transgastric level is critical. After finding this alignment, the transducer is removed to look for the grasping view at mid-esophageal level.
The most common strategy is to place a clip between the A and S leaflets and towards the TV most central region. To acquire the grasping view and see the A and S leaflets we should start at the intercommissural view and the multiplanar view, and the grasping view will often be found at around 160° (figure 4). Another option is to perform a TEE transducer sweep from 0° to 180° until finding a direct grasping view to actually see the leaflets and the clip with wide open arms. In any case, every patient should be handled individually since the TV has more anatomical variations than the mitral valve. Also, the angle between the intercommissural view and the coaptation line of the A and S leaflets can be < 90º and mislead the multiplanar assumptions. The real-time 3D TEE can be useful to guide the clip, but not so much with mitral procedures.
Figure 4. Search for the grasping view for the clip in the anteroseptal (A-S) position with respect to the center of the tricuspid valve. A: grasping view for A-S clip (discontinuous blue line). B: transesophageal echocardiogram (TEE) at 160° showing the clip with wide open arms and the anterior and septal leaflets resting on them.
STEP-BY-STEP PROCEDURE
In most cases, the MitraClip implantation in the tricuspid position is performed through femoral access under general anesthesia. It is monitored through fluoroscopy and TEE; the combination of the 2 is essential to achieve optimal results. This is the step-by-step procedure with the key data that should be assessed through imaging modality:
1. Percutaneous access through the right femoral vein using the Seldinger technique. A high-support guidewire is inserted through a 7-Fr introducer sheath towards the superior vena cava (SVC) and after access pre-dilation, the guide catheter (GC) is advanced towards the right atrium.
2. The GC is inserted similar to the way it is done in the mitral valve procedure and the handle is ± rotated approximately 180º in the direction (−). The GC is also rotated 180º counterclockwise to face the anterior region. Once in the right atrium, the dilator and the guidewire are removed, and the catheter is softly aspirated to avoid air embolism. The rotation is then released (−).
3. At this point, the procedure can be performed in 2 different ways. The first is using the handle (+) to increase the GC cuve and point directly at the TV. In this case, the clip catheter is normally inserted into the GC (blue line with blue line) while the GC guarantees the correct positioning of the device to perform leaflet grasping. In the second way, the most widely used, the clip catheter is inserted misaligned (90° counterclockwise or 180°; the authors of this article prefer the 180° misalignment). This technique facilitates the straddling (of the clip radiopaque markers on the GC) of the clip catheter on the GC (this should be done towards the SVC to avoid damaging surrounding structures). After reaching the straddling position, the A or M wheel (depending on the patient’s anatomy) will deflect the tip of the clip towards the target TV region. This technique allows a more versatile range of motion with the device to avoid orientation or distance issues between the tip of the GC and the TV target area. Over the next few steps we will be describing the GC-clip catheter misalignment technique. From the echocardiographic standpoint, these steps are monitored in the 90º-110° bicaval view with biplane trying to not damage any anatomical structures.
4. Once the straddling position has been reached, the A or M wheel (or a combination of the 2) is activated to deflect the clip towards the TV. The wheel selected will depend on the trajectory of the clip. The objective is to achieve the most perpendicular trajectory possible to the TV and avoid, as much as possible, the catheter from coming too close to the interatrial septum (called septal hugging). This circumstance will make the clip point at the TV lateral region. Fluoroscopy in the 45º left, anterior, oblique view is used to see the tip of the clip move towards the left of the screen, away from the septum, pointing towards the operator (figure 2 of the supplementary data). Echocardiographic monitoring will then move from the bicaval to the intercommissural view.
5. When the clip remains perpendicular on the TV, the GC clockwise rotation will move the clip towards the septal position. The GC counterclockwise rotation will move it towards the lateral region. The stabilizer advancement will bring the clip closer to the A-S commissure. After retraction, it will pass to the P-S commissure. This will allow complete range of motion and guarantee the correct location of the grasping (figure 5).
Figure 5. A: transesophageal echocardiogram in 3D zoom mode; periprocedural frontal view. The guide catheter can move inside the right atrium towards the aortic valve by advancing the entire system (red arrow) or towards the posterior leaflet by retracting it; the movement towards the septum (blue arrow) is possible through clockwise rotation of the guide catheter, and towards the right ventricle free wall through counterclockwise rotation. B: explanted heart, anatomic view. A, anterior; Ao, aorta; P, posterior; S, septal; CS, coronary sinus.
6. When the desired position has been achieved, the clip opens and perpendicularity is assessed. This should be done by using a 2D TEE in the transgastric short axis or by 3D zooming in the atrial view. The clip should be rotated smoothly so that it remains completely perpendicular to the desired grasping site (figure 6 and video 3 of the supplementary data).
Figure 6. With the clip on the tricuspid annulus the transgastric short axis (A) or 3D zoom (B) should be used to steer the clip. A, anterior; Ao, aorta; S, septal.
7. Afterwards, the clip closes at 60º and is advanced slowly towards the RV just underneath the leaflets to avoid interfering with RV strings or structures. It is advisable to perform this maneuver through fluoroscopy guidance in a 30º right, anterior, oblique view (RV long axis). This fluoroscopic view will confirm that the clip describes a straight trajectory and maintains its perpendicularity. Fine tuning the rotation of the clip can be made while advancing towards the RV, but it should not be done underneath the leaflets (to avoid string interference). Then, the clip slowly opens at 120º to later retract and guarantee the capture of the leaflets (figure 3 of the supplementary data and video 4 of the supplementary data).
8. Back in the RV, the perpendicularity of the clip with respect to the target coaptation line should be confirmed on the transgastric short axis view. Then, the grasping view should be looked for, that is, the view that better shows the target leaflets and the clip with wide open arms. It is essential to have good ultrasound imaging during grasping to guarantee the correct insertion of the leaflets and the perpendicularity of the arms of the clip. Repeated suboptimal captures of the leaflets should be avoided to not cause excessive damage (TV leaflets are thinner and more fragile compared to the mitral valve leaflets) (figure 7).
Figure 7. Examples of grasping view. A and D: patient 1, grasping from the intercommissural view at 75° and from the direct grasping view at 125°. B and E: patient 2, direct grasping view acquired at 0° and 160°. C and F: right, anterior, oblique projection to steer the movement of the clip. A, anterior; S, septal.
9. Once the leaflets have been captured, their insertion should be confirmed through multiple 2D TEE views. Also, the presence of tissue bridges should be verified (though 3D TEE views). Multiplane is very useful for assessment purposes. A TTE or an intracardiac echocardiography should be used in cases of uncertain leaflet insertion. The TV mean gradient should be measured to discard stenosis; in general, mean gradients > 3 mmHg are not recommended (figure 8).
Figure 8. A-D: grasping assessment in multiple views for leaflet insertion assessment and reduction of tricuspid regurgitation.
10. Standard release of the clip. Once it has been completely released, the degree and location of residual TR and need for new clip implantation should be assessed. The position of the new clips will depend on the location and amount of residual TR jets.
Since MitraClip XTR was introduced for the first time, most procedures have been performed using this device. It allows easier captures even when there are coaptation defects among the leaflets. The step-by-step procedure described here refers to the standard MitraClip device used today in mitral procedures. A modification of the Mitraclip device (in the GC) will soon be available to facilitate the steering and positioning of the clip (with excellent results in the 30-day assessment in the TRILUMINATE trial.15) This new version will replace the device mitral version. The steps will change too. Similarly, we will have a new iteration of the system within the next few months (TriClip).
There are several technical possibilities for clip implantation in the TV; the strategy can vary depending on the surface occupied by each leaflet and the location of the regurgitation jet. For the management of A-S coaptation defects, implanting the clip as close as possible to the center of the valve maximizes the reduction of TR. In patients with large coaptation defects, the most common strategy is to treat the A-S coaptation line using several clips and avoid the bicuspidization of the TV (video 5 of the supplementary data). An alternative to this can be to implant a first clip between the anterior and septal leaflets plus a second clip between the posterior and septal leaflets to create a triple-orifice TV (figure 4 of the supplementary data). In this sense, the triple-orifice technique can be superior to the A-S bicuspidization one because it achieves a direct reduction of coaptation defects and counteracts annular dilation by exerting traction forces in the annular anterior and posterior regions. However, the analysis of both strategies did not find any differences between the 2 regarding TR or clinical improvement.16
The selection of patients for tricuspid valve repair should include a combination of clinical, hemodynamic, and anatomical characteristics. Table 1 shows the criteria that define the optimal candidates who are eligible for TR repair using the MitraClip device.17 It should be emphasized that all patients should have symptomatic TR despite receiving optimal medical treatment, high surgical risk, and severe TR.
Table 1. Criteria to identify the ideal candidates for tricuspid repair with the MitraClip device
| Optimal | Possible | Exceptional |
|---|---|---|
| Secondary TR with normal leaflets | Secondary TR with normal appearance of the leaflets or primary TR with valve prolapse | Significant leaflet thickening (rheumatic) or severe valve shortening or destruction or prolapse |
| Small coaptation defect (< 3-4 mm) and good leaflet mobility | Moderate coaptation defect (4-7.2 mm), reduced leaflet mobility | Large coaptation defect (> 7.2 mm) or severe leaflet restriction |
| Good TEE window | Enough echocardiographic window to see the leaflets | Insufficient echocardiographic window to see the leaflets |
| Without PM or ICD leads | Presence of PM or ICD leads without significant leaflet or clip interaction | PM or ICD lead-induced TR |
| Normal-moderately depressed RV function Normal size or moderately dilated RV | Moderately reduced RV function, moderate RV dilation | Severely reduced RV function or severe RV dilation |
| Normal pulmonary systolic pressure | < 60 mmHg-65 mmHg and pulmonary resistances < 4 WU | > 60 mmHg-65 mmHg or pulmonary resistances < 4 WU |
|
ICD, implantable cardioverter-defibrillator; PM, pacemaker; RV, right ventricle; TEE, transesophageal echocardiogram; TR, tricuspid regurgitation; WU, Wood units. Reproduced with permission from Hausleiter et al.17. |
||
The echocardiographic predictors of success during TR repair with the MitraClip device recently published are: the location of the jet in the A-S coaptation line or central region, and the coaptation defect between leaflets < 7.2 mm are the best predictors with the NTR device.17 Procedural success is also essential because it is a variable independently associated with better prognosis.8,18
OTHER TRANSCATHETER DEVICES TO TREAT TRICUSPID REGURGITATION
Table 2 and table 3 show other transcatheter devices for the management of TR.
Table 2. Transcatheter tricuspid percutaneous repair devices
Table 3. Transcatheter tricuspid valve replacement percutaneous devices
Edge-to-edge repair devices
Pascal
This device is used for edge-to-edge repair by capturing the leaflets of the TV. This device uses clasps (metal blades to capture the leaflets) and paddles (arms that go into the leaflets) to gently grasp the leaflets against a spacer. The clasps move independently for better leaflet capture in the device, while the spacer increases the coaptation surface reducing leaflet stress. The complete system is 22-Fr and the device can be fully elongated favoring its interaction with subvalvular apparatus. The procedure is TEE-guided through transfemoral access and under general anesthesia. Under TEE and fluoroscopy guidance, the device is steered towards the TV and implanted similar to the MitraClip. In a recent communication, the early experience with the Pascal repair system for TR repair was reported. Out of 12 patients treated, 92% reduced their TR in, at least, 1 degree, and all of them improved their functional class at the 30-day follow-up.19 This early experience is encouraging, but more data are needed before using this device in a larger community of patients.
Annuloplasty devices
Trialign
The Trialign device (Mitralign Inc., Tewksbury, Massachusetts) is a transjugular suture-based annuloplasty system to reduce the tricuspid annular diameter through plicated leaflet tissue. During the procedure a pair of polyester sutures are released into the tricuspid annulus next to the anteroposterior and P-S commissures. It is then cinched using a polyester suture that obliterates the tricuspid posterior leaflet and fixates to the atrial side. The results of the SCOUT trial20 have already been published on the successful implantation of a pair of sutures in 14 out of 16 patients (87.5%). The mean reduction after the procedure was 37% in the tricuspid annulus and 59% in the regurgitant orifice area. A pair of sutures can be implanted in the anterior annulus to optimize the final outcome. There was no perioperative mortality and quality of life tests improved at the 1-month follow-up.
Cardioband
The Cardioband system (Edwards LifeSciences, CA, United States) is a percutaneous direct annuloplasty device for TV repair. It is implanted into the tricuspid annulus by a series of anchors that pass through the annulus and into the baseline RV myocardium following the natural shape of the annulus and sparing the septal region where it meets the atrioventricular node. Afterwards, it is cinched using TEE-guidance and the annular septal-lateral diameter is reduced.
The 6-month data of the TRI-REPAIR trial21 have been published. It included 30 patients and procedure technical success was 100%. There was a 9% reduction of the annular septal-lateral diameter, and a 50% reduction of the PISA (proximal isovelocity surface area) at the 6-month follow-up. Eighty-eight percent of the patients had New York Heart Association (NYHA) functional class I-II and improved results in the quality of life tests.
TriCinch
The TriCinch (4Tech Cardio, Galway, Ireland) is an annuloplasty device with a deflectable catheter and a coil system in its distal end that anchors to the tricuspid annulus. This anchoring generates traction in the entire annulus through a band connected to it. The anchor is implanted in the pericardial space to avoid detachment at follow-up. Fixation site is in the middle region of the anterior tricuspid annulus under fluoroscopy and echocardiographic guidance. Then the catheter is tightened to cinch the tricuspid annulus, reduce its A-S dimension, and improve leaflet coaptation. Lastly, a self-expandable nitinol stent is released inside the inferior vena cava (IVC) to secure the system and keep the tension applied. The implantation of the TriCinch device preserves the native anatomy and facilitates other future treatment options. The experience with animals confirms the safety and efficacy profile of this device.22
Valve replacement devices
The transcatheter replacement of the TV can be orthotopic or heterotopic. The first experiences with this technique used aortic valves for percutaneous implantation in the tricuspid position. The “valve-in-valve” or “valve-in-ring” percutaneous implantation of an aortic valve in the tricuspid position is more common. Acceptable short-term results with these procedures have been reported.23 However, the development of transcatheter tricuspid valve replacement systems in native TV is much more interesting.
GATE
The GATE bioprosthesis (NaviGATE Cardiac Structures, Lake Forest, CA, United States) is the only device available today experienced clinically in humans as compassionate use. Also, it is the only device that allows fully orthotopic transcatheter tricuspid valve replacements. It is a xenopericardial leaflet bioprosthesis inserted into a self-expandable nitinol scaffold. The nitinol stent is wider in the ventricular region, giving it a tronco-conical morphology that reduces the transvalvular gradient and minimizes the outflow tract obstruction (since very little material protrudes towards the ventricle). It can be implanted using the jugular approach or the right atrium through minithoracotomy; the femoral access is still under development. The necessary size for the jugular access is ≥ 14 mm because a 42-Fr introducer sheath is used. In both cases, at least 7 cm are necessary from the annular entry site to steer the device (which should be angulated 70º) so that it is perfectly coaxial to the annular plane.
The prosthesis is available in 5 different sizes (36 mm, 40 mm, 44 mm, 48 mm, and 52 mm). A slight oversizing (from 5% to 10%) with respect to the annular dimensions is advisable. To select the device size, it is required to measure the annulus using TEE and computed tomography scan. On the CAT scan, the distance between the annulus to the right coronary artery is a very important feature to avoid damage and have guidance during the procedure. The x-ray projection imaging of the implant is also derived from the CAT scan.
To this day, very few implants have been performed worldwide and always with compassionate use. Recently, the experience of the University of Columbia with 5 consecutive procedures has been published.24 Procedural success was 100% and the route of access was minithoracotomy in all cases. Only 1 patient died within the first 30 days after the procedure. Successful implantation was associated with improved RV remodeling, increased cardiac output, and a better NYHA functional class in most patients.
CAVI
The objective of CAVI (caval valve implantation) is to avoid return flow from TR to the cava veins. This means that the devices per se do not eliminate TR. They improve systemic venous congestion instead. The procedure is performed by implanting valves (in the early experience, non-dedicated balloon-expandable valves) into the origin of the IVC and SVC. The implant can be simple (IVC only) or double depending on the anatomical characteristics of the venous system drainage into the right atrium. There a special self-expandable CAVI device available too, the TricValve (P&F Products Features Vertriebs, Vienna, Austria), that consists of a nitinol stent specifically designed for low-pressure circulation. The largest sizes available are 38 mm and 43 mm for the SVC and IVC, respectively. This means that the optimal diameters of the anchoring area should be ≤ 35 mm (from 28 mm to 43 mm).
Both valves are released using the transfemoral access in a 27-Fr dedicated catheter. The IVC device is released with the portion covered inside the right atrium and the waist inside the diaphragm hiatus to allow the drainage of venous hepatic system. Although it has been the first transcatheter device ever used, it poses some problems because it does not correct TR. The long-term impact of the ventricularization of the right atrium, the persistent overload of the right atrium and RV, the persistent low cardiac output state, and the impact on the function of the RV prevent a more widespread use of this technique. The consequences of thrombotic complications remain unknown too.
Tricento
The Tricento device (NTV AG, Muri, Switzerland) is a sophisticated CAVI concept. It consists of a bicaval, anchored, covered stent with an element of the lateral bicuspid valve made out of a porcine pericardium that only requires a low closing pressure. The nitinol stent has radiopaque markers to allow precise positioning and orientation during implantation. Using the transfemoral approach, the device is released through a 24-Fr catheter. The Tricento is fully repositionable and retrievable until it is completely released. Because of the patients’ great anatomical variability, the stent should be individualized. Once again, the idea is to avoid return flow from TR towards the cava veins. However, all the main setbacks of the CAVI concept are applicable here.
CONCLUSION
There are several transcatheter therapies under development for the management of TR. This article standardized the basic echocardiographic views available for the selection of patients. Also, it stands as a guide for the TV implantation of MitraClip, the most widely used device today. Given the non-stop advance of these devices and cardiac imaging, both imaging and interventional protocols will be jointly developed.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest whatsoever regarding this manuscript.
SUPPLEMENTARY DATA
Video 1. Moñivas Palomero V. DOI: 10.24875/RECICE.M19000091
Video 2. Moñivas Palomero V. DOI: 10.24875/RECICE.M19000091
Video 3. Moñivas Palomero V. DOI: 10.24875/RECICE.M19000091
Video 4. Moñivas Palomero V. DOI: 10.24875/RECICE.M19000091
Video 5. Moñivas Palomero V. DOI: 10.24875/RECICE.M19000091
BIBLIOGRAFÍA
1. Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation:a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331-2336.
2. Taramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol. 2012;59:703-710.
3. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405-409.
4. Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185-1194.
5. Topilsky Y, Inojosa JM, Benfari G, et al. Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur Heart J. 2018;39:3584-3592.
6. Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation:which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127-132.
7. Zack CJ, Fender EA, Chandrashekar P, et al. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol. 2017;70:2953-2960.
8. Taramasso M, Alessandrini H, Latib A, et al. Outcomes After Current Transcatheter Tricuspid Valve Intervention:Mid-Term Results From the International TriValve Registry. JACC Cardiovasc Interv. 2019;12:155-165.
9. Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination:recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921-964.
10. Hahn RT. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2016;9:e005332.
11. Holda MK, Zhingre Sanchez JD, Bateman MD, Iaizo PA. Right Atrioventricular Valve Leaflet Morphology Redefined:Implications for Transcatheter Repair Procedures. JACC Cardiovasc Interv. 2019;12:169-178.
12. Lang RM, Badano LP, Tsang W, et al. American Society of Echocardiography;European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1-46.
13. Muraru D, Hanh RT, Soliman OI, et al. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc Imaging. 2019;12:500-515.
14. Santoro C, Del Castillo AM, Gonzalez-Gomez A, et al. Mid-term outcome of severe tricuspid regurgitation:are there any differences according to mechanism and severity?Eur Heart J Cardiovasc Imaging. 2019;20:1-8.
15. Nickenig G, Hausleiter J, Hahn R, et al. Percutaneous Edge-to-Edge Repair for Tricuspid Regurgitation:Primary Outcomes from the TRILUMINATE clinical trial. En:EuroPCR;2019 May 21;Paris. Available online: https://abstractbook.pcronline.com/export/pdf/id/110069.
16. Braun D, Orban M, Orban M, et al. Transcatheter Edge-to-Edge Repair for Severe Tricuspid Regurgitation Using the Triple-Orifice Technique Versus the Bicuspidalization Technique. JACC Cardiov Interv. 2018;11:1790-1792.
17. Hausleiter J, Braun D, Orban M, et al. Patient selection, echocardiographic screening and treatment strategies for interventional tricuspid repair using the edge-to-edge repair technique. EuroIntervention. 2018;14:645-653.
18. Besler C, Orban M, Rommel KP, et al. Predictors of Procedural and Clinical Outcomes in Patients With Symptomatic Tricuspid Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. JACC Cardiov Interv. 2018;11:1119-1128.
19. Fam NP. Pascal tricuspid:early experience technology and clinical updates. En:Transcatheter Cardiovascular Therapeutics 2018;2018 Sept 23;San Diego. Available online: https://www.tctmd.com/slide/pascal-tricuspidearly-experience-technology-and-clinical-updates.
20. Hahn RT, Meduri CU, Davidson CJ, et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty:SCOUT Trial 30-Day Results. J Am Coll Cardiol. 2017;69:1795-1806.
21. Nickenig G, Weber M, Schueler R, et al. 6-Month Outcomes of Tricuspid Valve Reconstruction for Patients With Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;73:1905-1915.
22. Taramasso M, Latib A, Denti P, et al. Percutaneous repair of the tricuspid valve using a novel cinching device:acute and chronic experience in a preclinical large animal model. EuroIntervention. 2016;12:918-925.
23. McElhinney DB, Cabalka AK, Aboulhosn JA, et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves:An International, Multicenter Registry Study. Circulation. 2016;133:1582-1593.
24. Hahn RT, George I, Kodali SK, et al. Early Single-Site Experience With Transcatheter Tricuspid Valve Replacement. JACC Cardiovasc Imaging. 2019;12:416-429.
ABSTRACT
Primary percutaneous coronary intervention (pPCI) is the best modality of reperfusion in ST segment elevation in acute myocardial infarction (STEMI). Its application requires networked assistance systems whose implementation requires significant organizational and logistical changes. In comparison with other scenarios of urgent action, a series of aspects are specific to the pPCI programs. They require immediate, clearly pre-established strategies, carried out by highly skilled professionals who attend to a high volume of highly complex patients. The lack of homogeneity in the creation and development of reperfusion programs in Spain has led to great differences in their implementation. If the pPCI programs are not carried out under adequate conditions, their sustainability can be complex. The present document, agreed between different scientific societies, aims to analyze the current situation in Spain of the network care programs for STEMI, identify their limitations and deficiencies, assess their vulnerability and establish a series of recommendations to ensure their sustainability.
Keywords: ST-segment elevation acute myocardial infarction. Primary percutaneous coronary intervention. Requirements. Medical emergency systems. Program sustainability.
RESUMEN
El intervencionismo coronario percutáneo primario (ICPp) es la mejor forma de reperfusión en el infarto agudo de miocardio con elevación del segmento ST (IAMCEST). Su aplicación requiere sistemas asistenciales en red cuya implementación exige cambios organizativos y logísticos importantes. En comparación con otros escenarios de actuación urgente, una serie de aspectos son específicos de los programas de ICPp. Requieren de estrategias inmediatas claramente preestablecidas, realizadas por profesionales muy expertos y que atienden a un elevado volumen de pacientes de alta complejidad. La falta de homogeneidad en la creación y desarrollo de los programas de reperfusión en España hallevado a grandes diferencias en su implantación. Si los programas de ICPp no se efectúan en condiciones adecuadas su sostenibilidad puede ser compleja. El presente documento, consensuado entre diferentes sociedades científicas, tiene por objetivos analizar la situación actual en España de los programas de atención en red al IAMCEST, identificar sus limitaciones y deficiencias, evaluar su vulnerabilidad y establecer una serie de recomendaciones para asegurar su sostenibilidad.
Palabras clave: Infarto agudo de miocardio con elevación del segmento ST. Intervencionismo coronario percutáneo primario. Requisitos. Sistemas de emergencias médicas. Sostenibilidad de los programas.
Abbreviations: AACC: autonomous communities. STEMI: ST-segment elevation acute myocardial infarction. PPCI: primary percutaneous coronary intervention. SEC: Spanish Society of Cardiology. MES: medical emergency system. CICU: cardiac intensive care unit.
INTRODUCTION
Ischemic heart disease is still the leading cause of mortality worldwide and it amounts to up to 20% of the overall deaths in Europe.1 The ST-segment elevation acute myocardial infarction (STEMI) is one of the most relevant forms of presentation. Early reperfusion is the most effective way to reduce morbimortality in STEMI. It was soon confirmed that fibrinolysis could not reestablish the patency of the coronary artery found in up to 20%-45% of patients2 with a 5%-10% acute coronary re-occlusion after successful fibrinolysis and a rate of late occlusion close to 30%.3 Primary angioplasty or more precisely, primary percutaneous coronary intervention (pPCI) did obtain significantly higher success rates in reperfusion above 90%,4 which is associated with a 30% relative reduction in mortality rate at 30 days and a lower risk of reinfarction and stroke.5 For this reason, pPCI became the gold standard treatment for the management of STEMI. However, the benefit of pPCI over fibrinolysis is only evident when performed at adequate centers by experienced teams (infarction centers) and within the first 120 minutes after the first medical contact.6
The pPCI not only is more effective and safer compared to fibrinolysis but recent studies show that it is cost-effective from the socioeconomic point of view, regardless of the initial high cost of its logistical and technological implementation.7,8 In a study conducted in the United States,9 reperfusion through pPCI was associated with lower hospital costs initially compensated with the higher personnel costs (on call 24/7); however, when used in large volumes of patients, the pPCI was much more cost-effective. This higher cost-effectiveness is the product of eliminating the cost of fibrinolysis, the lower incidence of ischemic and hemorrhagic complications, the lower need for coronary angiographies and ischemia detection imaging modalities, the shorter initial hospital stay due to readmissions, and patients’ early return to professional life.
In an attempt to offer the best reperfusion strategy to the highest number of patients and within the recommended timeframes, several scientific societies recommended the creation of STEMI care network systems in the community and regional settings to provide the fastest possible healthcare to these patients.6 However, the implementation of these reperfusion networks requires important organizational and logistical changes. And this is a complex situation in Spain since the allocation of resources from the different healthcare administrations of the 17 autonomous communities (AACC) is decentralized and, therefore, budgets are managed independently. The organization and implementation of the healthcare system is also decentralized in this system. This leads to the heterogeneous provision of services. The way pPCI networks work and the results obtained from these networks are highly influenced by several factors such as geography, the number of pPCI-capable centers, referral times, the availability of adequate resources, infrastructure, and the characteristics of healthcare systems per se.10 Similarly, the heterogeneity of the financial situation and the structures of the different healthcare systems have led to great inequalities in the ways these networks have been organized; differences noticeable not only among different countries, but also among different geographical areas within the same country.10-13 All these aspects translate into challenges and threats when it comes to the optimal implementation of STEMI care systems in Spain and they should be studied and analyzed in search of proposals and solutions that meet their needs.
This document has been agreed by different scientific societies (table 1) with the following goals in mind:
Table 1. Scientific societies, working groups, and representatives that have participated and signed the consensus document
| Representative | Society |
|---|---|
| Ángel Cequier | Spanish Society of Cardiology (SEC) |
| Armando Pérez de Prado | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Ana Belén Cid | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Javier Martín-Moreiras | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Oriol Rodríguez-Leor | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| José Ramón Rumoroso | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Ana Serrador | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Raúl Moreno | Working Group on Hemodynamics and Interventional Cardiology of the SEC |
| Sergio Raposeiras | Working Group on Ischemic Heart Disease and Acute Cardiac Care of the SEC |
| Albert Ariza | Working Group on Ischemic Heart Disease and Acute Cardiac Care of the SEC |
| Esteban López de Sá | Working Group on Ischemic Heart Disease and Acute Cardiac Care of the SEC |
| Andrés Íñiguez | SEC |
| José Luis López Sendón | SEC |
| Francisco Javier Delgado | Spanish Association of Nursing in Cardiology |
| Rocío Gil Pérez | Spanish Association of Nursing in Cardiology |
| José Julio Jiménez- Alegre | Spanish Society of Emergency Medicine |
| Manuel José Vázquez | Spanish Society of Emergency Medicine |
| José Manuel Flores | Spanish Society of Emergency Medicine |
| Héctor Bueno | SEC |
| Manuel Anguita | SEC |
-
Analyze the actual situation of reperfusion-based STEMI care programs in Spain.
-
Identify the limitations and deficiencies of such programs.
-
Assess their vulnerabilities and sustainability. Establish a series of recommendations on the needs for personnel, infrastructures and incentives required for their optimization.
-
Achieve agreed consensus from the different scientific societies involved in the management and treatment of patients with STEMI.
-
Establish a combined national registry of all the actual pPCI programs.
ORGANIZATION OF HEALTHCARE NETWORKS FOR THE MANAGEMENT OF REPERFUSION IN SPAIN
Initial experiences and progress made by healthcare networks
The first experiences with structured programs of reperfusion with pPCI for the management of patients with STEMI in Spain date back to 2000 to 2001 in the Community of Navarre and the Region of Murcia, respectively where all patients were referred to a single reference center.14 The first multicenter program started in Galicia back in 2005. A series of centralized pPCI programs were defined then where geographical location-based isochrones were categorized, the healthcare centers where patients should be referred to were identified, and the healthcare and transfer resources needed were described. From early on, it was evident that these networks determined an increase in the number of STEMI patients treated with the best reperfusion therapy available at the time.15
Back in the year 2008, the AACC of Murcia, Navarre, Galicia, and the Balearic Islands had already implemented network structures that, at that time, meant that 12.8% of the Spanish national territory was covered. That same year, the Stent for Life initiative was born, a European project implemented by the European Association of Percutaneous Cardiovascular Interventions, and designed by interventional cardiologists, health Administration officials, patients, and healthcare industry providers. The goal of this initiative was to stimulate and facilitate the implementation of healthcare networks that would allow any STEMI patient to have access, in the least amount of time possible, to reperfusion therapy through pPCI. Spain started collaborating in this program back in 2009 in an attempt to identify regions with implementation deficiencies and to promote the elimination of barriers responsible for delays in the healthcare process. The project was based on prior experiences in the development of new projects. Also, it was decided to create and maintain reliable activity registries with campaigns aimed at training the population and making health authorities more sensitive to this issue.16 Nowadays, this initiative is called Stent, Save a Life and it has been brought to other continents to promote the development of healthcare networks in developing counties.
In 2009, Catalonia established its own pPCI and 3 years later, in 2012 Asturias, Cantabria, and Castile-La Mancha followed. Then, it would be the turn for Madrid, Valencia, Aragon, La Rioja, and the Basque Country. All these AACC have active regional programs generically known as Infarction Code. These changes in the reperfusion strategies reduced significantly the rates of thrombolysis, while the percentage of patients treated with pPCI increased gradually with an increase of territory coverage and number of interventions performed accross the country10,17 which is consistent with what was already happening in other European countries.18
Numerous experiences have been reported that exemplify the benefits of implementing STEMI care networks, both in the European19 and domestic settings.20 One study10 analyzed the connection between the implementation of reperfusion networks for the management of STEMI in AACC, the regional rate of pPCI and hospital mortality. Throughout the entire period studied, there was a higher rate of pPCIs in all AACC (54.5% in 2012 vs 21.6% in 2003) (figure 1). It was confirmed that crude mortality rate was higher in non-reperfused patients (17.3%) compared to patients who had undergone pPCIs (4.8%) or fibrinolysis (8.6%; P < .001), with a lower risk-adjusted standardized mortality ratio (of 10.2% in 2003 and 6.8% in 2012; P < .001). In sum, the implementation of reperfusion networks was associated with a 50% increase in the rate of pPCIs and a 14% reduction in mortality rate (figure 2).
Figure 1. Changes in reperfusion strategies for the management of ST-segment elevation acute myocardial infarction in the Spanish health public system between 2003 and 2012. The evolution of reperfusion strategies can be seen over the entire study period. The percentage of patients treated with primary percutaneous coronary interventions (pPCI) increased gradually and simultaneously with moderate drops in the rate of thrombolysis. (Reproduced with permission from Cequier et al.10)
Figure 2. Correlation between the rates of primary percutaneous coronary interventions (pPCI) and mortality in the management of patients with ST-segment elevation acute myocardial infarction in the Spanish health public system between 2003 and 2012. There was a significant correlation between the rates of pPCI and mortality over the entire study period. RSMR, risk-standardized mortality rate. (Reproduced with permission from Cequier et al.10)
Reperfusion networks in Spain: current situation
Situation of the pPCI networks
Over the last few months, we have been able to provide coverage to the entire Spanish territory through regional healthcare net- works for the management of STEMI patients. In some cases, these pPCI programs do not have fully structured regional networks and depend on local circuits limited to a certain number of hospitals. Taking all these aspects into consideration, the number of per population-pPCIs has changed dramatically in our country, with a reduced number of patients treated with pPCI in AACC that did not have complete and structured programs before17 (figure 3). Consequently, the lack of homogeneity in the creation and development of the different reperfusion programs in the different AACC has brought many differences to these programs, some of them created exclusively by healthcare providers themselves with almost no institutional support. Also, other programs have seen the involvement of the Administration with detailed analyses of subdivisions, logistics, and infrastructure, and the provision of all necessary resources. This has originated a significant heterogeneity in the structure and organization of the different programs and in the assessment of different quality indicators and results. The information available shows that establishing a systematic and organized pPCI program makes a very favorable impact on prognosis compared to having unstructured pPCI programs without a preestablished transfer logistics20 (figure 4). Since there is not such a thing as a national pPCI registry, we do not know what the real volume of activity is in these programs or under what quality standards they have been implemented. Recent initiatives started by the Spanish Society of Cardiology (SEC) aim at promoting healthcare and evaluating the results of STEMI care (SEC-CALIDAD).21
Figure 3. Primary percutaneous coronary interventions (pPCI) per million of inhabitants over the years 2016 and 2017 in all Spanish autonomous communities. Those communities without fully structured network programs (Andalusia, Canary Islands, and Extremadura back in 2016) show the lowest number of patients with ST-segment elevation acute myocardial infarction treated with pPCI (Reproduced with permission from Cid Álvarez et al.17)
Figure 4. Prognostic impact of patients from a primary percutaneous coronary intervention (pPCI) program in a subdivided region before (precoding) and after (coding) the implementation of pre-established intervention and transfer logistics measures with regional hospitals and emergency medical services. There is a significant improvement of survival rate over the first year after implementation. (Reproduced with permission from Gómez- Hospital et al.20)
Situation of healthcare providers conducting pPCIs
In an effort to obtain information on how the actual pPCI programs work in Spain, in May 2018 the Working Group on Hemodynamics and Interventional Cardiology of the SEC conducted a survey among all its members called Needs and requirements of primary angioplasty program: a professional survey (O. Rodríguez-Leor, personal information). The questionnaire collected information on the activity of the programs already running in all the centers and on the degree of satisfaction of the personnel involved. Out of the 390 interventional cardiologists surveyed, 172 (44%) filled in the questionnaire. The mean age of respondents was 45 ± 8 years old (interquartile range: 39-50 years) and they all had over 9 ± 6 years of experienced managing primary angioplasty programs. The centers performed an average 292 procedures per year (200-410). All AACC were represented in this survey.
A very important aspect was that 45% of the respondents were not getting any breaks after night code activations. In almost half of the cases of those who actually got a break, they did not have access to their resting hours the next day because these hours had to be adjusted to the actual logistics needs or volume of activity of their department (figure 5). Another remarkable aspect is that over 50% of the respondents said their intention was to stop doing night calls whenever they were eligible due to their age (figure 6A). On the other hand, a high percentage of respondents (85%) said that the management of patients had room for improvement within their actual programs (figure 6B).
Figure 5. Hourly schedules and types of rest after being activated to perform primary percutaneous coronary interventions during night shift. Only 25% take a break of at least 8 hours after performing this procedure. Forty-five percent immediately move on to their routine clinical practice the next day with no downtime. (Data gathered from a survey conducted among members of the Working Group on Hemodynamics and Interventional Cardiology of the Spanish Society of Cardiology.)
Figure 6. A: Willingness to remain available or leave the on-call primary percutaneous coronary intervention (pPCI) programs after no longer being mandatory due to age. Fifty-four percent of respondents will leave these programs. B: Answers to the question of whether respondents thought that there was room for improvement for the management of patients with ST-segment elevation acute myocardial infarctions within pPCI programs. Eighty-five percent said that there was room for improvement. (Data gathered from a survey conducted among members of the Working Group on Hemodynamics and Interventional Cardiology of the Spanish Society of Cardiology.) DK/DA, did not know/did not answer.
Back in 2012-2013, another survey was conducted among the nurses of 52 centers that showed that in 64% of the cases, those who had been on call did not have any time to rest, and only 7% had access to some sort of rest over the next 12 hours (V. Rodríguez, personal information). Eighty-four percent of respondents did not get any time in lieu.
The results from these surveys should make us think of the future threats and risks of the actual models of human resources in certain pPCI programs.
SPECIFICITIES OF pPCI PROGRAMS
The particular characteristics and specific requirements of pPCI programs can put them in a scenario of vulnerability that will gradually make it very difficult to keep their sustainability if these programs are not implemented under the right conditions from the point of view of structure, organization, human resources, and equipment.
One of the key aspects of pPCI is the immediacy of the healthcare provided. In STEMI patients there is a direct correlation between the time the artery has remained occluded and early and long-term morbimortality. The occluded artery should be opened as soon as possible, which is why the time elapsed since the patient is first assisted until the artery is opened is a critical factor to determine the effectiveness of the procedure and results.22,23 Although time limit when it comes to selecting the optimal reperfusion strategy (pPCI vs fibrinolysis) is 120 minutes, the recommendations of the clinical practice guidelines established by the European Society of Cardiology have become more and more demanding. They indicate that the maximum amount of time that should elapse since the diagnosis of STEMI is established until angioplasty guidewire crossing should not exceed 60 minutes in patients admitted to pPCI-capable centers, and should not exceed 90 minutes in patients transferred from other centers.6 A pPCI program should guarantee achieving diagnoses immediately, conducting urgent transfers and interventional procedures in a short span of time. The implementation of a pPCI program requires close collaboration of all teams involved in the healthcare system (primary care, regional hospitals, emergency rooms, emergency systems and transfers, and infarction centers).
Compared to other urgent care settings, even high-risk settings, there is a series of aspects exclusive and specific to pPCI programs:
-
They require immediate healthcare that should be perfectly coordinated among the different levels of healthcare with clearly preestablished strategies and logistics and conducted by experienced teams. The teams involved should be on call 24/7, and ready to act as swiftly as possible when required, without a preestablished schedule, and in less than a 60-90-minute timeframe after activation.
-
The transfer of patients to the corresponding infarction centers should have top priority over all other types of emergencies or procedures.
-
Patients have a high mortality risk and they usually need complex healthcare actions, so an adequate infrastructure is required.
-
The volume of STEMI patients with an indication for pPCI is very high.
The following features define the landmarks for a healthcare network system to be successful in the management of STEMI patients:
-
Establish a clear-cut definition of the corresponding geographical regions.
-
Have a written, agreed, and common protocol available for all healthcare providers involved.
-
Make sure that the transfer of patients is conducted by accredited professionals with a well-established level of competence and on adequately equipped-means of transportation (ambulances or helicopters), that is, through the appropriate systems of medical emergencies.
-
Facilitate immediate transfers to pPCI-capable centers. In cases where diagnosis is achieved out of the infarction center setting, the transfer to the hospital should be direct, without stops or re-evaluations in non-pPCI capable hospitals.
-
Upon arrival at the infarction center, the patient should be immediately transferred to the cath. lab and without any prior stops at the ER.
-
If a patient is initially assisted at a non-pPCI capable center, he should be evaluated in a monitored area by experienced personnel. If the patient is to be transferred to an infarction center to undergo the pPCI, then the time elapsed from diagnosis until the patient leaves the center should not exceed 30 minutes.
REQUIREMENTS AND NEEDS FOR THE RIGHT DEVELOPMENT OF pPCI HEALTHCARE NETWORKS
Subdivisions. Geographical regions
Spain is the fourth largest country in Euope, yet its population density is lower than most Western European countries, with a very irregular territorial distribution. This circumstance, especially in healthcare network systems makes it difficult to provide similar health coverage in the entire territory. In order to solve this problem, it is important to subdivide healthcare into geographical regions where healthcare to patients can be provided effective and homogeneously. Although certain recommendations have already been established by the European Society of Cardiology on what the reference population should be per cath. lab, its implementation in the pPCI setting is much more complex. Initially, a subdivision based on time isochrones is required so access to pPCI is guaranteed from the different healthcare providers to the largest amount of patients possible with transfer times within the timeframes recommended by the clinical practice guidelines, but only towards adequately equippedinfarction centers capable of performing a minimal number of pPCIs each year.24 The geographical barriers among different AACC should not be an obstacle for transfer logistics, since patients with an indication for pPCI should be transferred to the closest infarction center. Prior administrative agreements signed by the AACC involved should be taken into consideration. Patients who live in remote areas or who due to logistics or climate issues cannot undergo pPCI within the timeframe recommended should be treated as soon as possible with fibrinolysis and then transferred to infarction centers so that the pharmaco-invasive strategy can be initiated.25,26
Logistics-related aspects. Communications systems
To achieve these goals, logistics requires a 100% committment from all healthcare providers involved, particlularly from the transfer systems. It is important to have agile communication systems from a centralized structure (emergency coordination centers) capable of promptly notifying the infarction centers on the arrival of a patient, the transfer estimate time, and the patient’s clinical state so that the receiving center can adapt its daily activity to the arrival of an unexpected urgent procedure.24 Some programs include patients’ clinical description and electrocardiogram results before activation, thereby reducing the amount of diagnostic mistakes. On rare occasions, when several patients need to be transferred to the same center at the same time, or when the receiving center is busy with uncancellable activities, patients should be transferred to other close-by infarction center capable of providing the healthcare required.
Medical emergency systems
Medical emergency systems (MES) with doctors aboard the ambulance are a reality in Spain; however, the decentralization and transfer of health competences to the different AACC has generated different healthcare models. In the pPCI programs, MESs should be easily accessed over the phone through emergency coordination centers capable of triaging the urgent care requested. This request for help can come from private households, the street, or primary hospital or care centers. Afterwards, MESs activate the units that provide initial care and, if required, the transfer of the patient to the infarction center. Emergency coordination centers should have STEMI categorized as a time-dependent condition that requires priority care to achieve better results.24 Within the pPCI program setting, emergency coordination centers should meet a series of prerequisites and have:
-
A system capable of taking calls that will not collapse.
-
A computer system capable of recording the time of call, preliminary medical examination, type of resources allocated, the time these resources were allocated, activation time of the healthcare unit, arrival time to the place help was requested from, and timeframes of initial transfer and final arrival at destination.
-
A multidisciplinary team including phone dispatchers, nurses, doctors, and technicians trained in the management of telephone calls.
-
A periodic quality re-assessment protocol of the different components of the care provided.
The care provided to STEMI patients should be initiated by the first medical team available with the aim to examine the patient and interpret the electrocardiogram in less than 10 minutes. If diagnosis is confirmed, the corresponding healthcare protocol should be activated, and the patient transferred to a pPCI-capable center. A complete transfer of information on the patient’s clinical situation among the healthcare unit, the emergency coordination center, and the destination hospital is crucial. This approach should also be used with patients initially assisted in non-pPCI capable centers.6,24
The initial transfer of these patients should happen in MES mobile units with teams including physician, nurse and paramedics trained and accredited according the level of competence established. The receiving hospital should know what the estimated time of arrival should be able to organize the care to be provided (on business hours) or to activate the treating hemodynamics team (off business hours). The patient should be transferred directly to the cath. lab without stopping at the ER.6,24
It is essential that the MES team writes down a complete medical report including the aforementioned timeframes, the initial assessment, the interpretation of electrocardiograms, the medication administered, the patient’s progression, and any other complications that may arise. These teams should conduct periodic quality assessments of the healthcare provided to detect possible ways to improve this process. A system for the periodic exchange of information between the MESs and the hospitals treating the patients transferred should be established to analyze the quality and observance of the plans of action previously agreed on. There should always be MES resources available based on the established population ratios.
Infrastructure and equipment
The pPCI-capable interventional cardiology units and the cardiology services and centers where these units are headquartered should meet structural, functional, and organizational requisites to guarantee safety, quality, and efficiency conditions to perform such procedures. The following quality standards and accreditation criteria for reference units on the performance of pPCIs procedures27 and the standards and criteria that regulate the entire STEMI process have been recently agreed upon and published by the SEC (SEC-EXCELENTE):21
-
The receiving pPCI unit should have a protocol agreed by the hospital board of directors.
-
This unit should design and implement a training program including continuous practical training for its staff.
-
The pPCI reference units should guarantee non-stop 24/7 all year round full medical coverage. Units with 12-hour-day programs are acceptable but not recommended and only if the MES coordination system incorporates clear-cut criteria on the transfer of STEMI patients based on hourly availability.
-
As well as the standard equipment including ventilators, the cath lab should also have circulatory support systems, intra- aortic balloon counter-pulsation systems, electro-catheters, external pulse generator units, and a fully equiped crash cart to be able to perform advanced resuscitation maneuvers in the context of a patient with STEMI and its possible complications.
-
The hospital where the pPCI unit is headquartered should have the following services available:
-
– It is advisable to have a cardiac intensive care unit (CICU) or general intensive care unit (ICU) available capable of providing level-2 and level-3-care according to the standards established by the Acute Cardiovascular Care Association28 and meeting the standards recommended for this type of units.29
-
– Cardiac surgical services. In units that do not offer this service within the same hospital, a formal agreement signed with a nearby cardiac surgical service should be available for the quick transfer (< 60 minutes) of patients who may require urgent heart surgery.30
-
– Hematology and blood bank service or unit.
-
– Diagnostic imaging service including computed tomography scan capabilities.
-
– Cardiologist on call on location.
Regarding CICUs, the care of patients after undergoing pPCI should be consistent with the structure and functioning of each hospital. It is advisable that the centers of pPCI receiving units have a cardiology unit-dependant CICU. The decision to hospitalize a patient should be based on the level of care required by each patient (table 2).
| Level | Description of the need for healthcare |
|---|---|
| 0 | Patients whose needs can be met through normal ward care in an acute hospital |
| 1 | Patients at risk of their condition deteriorating, or those recently relocated from higher levels of care, whose needs can be met on an acute ward with additional advice and support from the critical care team |
| 2 | Patients requiring closer observation or intervention including support for a single failing organ system or post-operative care and those ‘stepping down’ from higher levels of care |
| 3 | Patients requiring advanced respiratory support alone or monitoring and support for two or more organ systems. This level includes all complex patients requiring support for multi-organ failure |
CICUs with level-2 and level-3 care should:
-
Have a medical and nursing responsible.
-
Keep a physician in charge of the unit on call and on location 24 hours/day.
-
Maintain the ratio of 1-2 nurses per patients requiring level-3-care and 1-3 nurses per patients requiring level-2-care.
-
Be fully equipped with material that meet the standards established by the clinical guidelines designed by the SEC.
-
Have at least two beds for every 100 000 inhabitants within the area of influence the pPCI program specifies for receiving units, and no less than six beds.
Units with level-1-care should have a nurse/patient ratio > 1:6, have the necessary equipment and material available, and meet the standards designed by the European clinical practice guidelines.6 The amount of beds recommended is nine beds for every 100 000 inhabitants within the scope of influence of the pPCI program, but it can be smaller if there is a referral program to send the patients back to their reference hospital. The amount of beds required for level-1-care is and conventional hospitalization (level-0-care) is reduced accordingly.
Human resources
The interventional cardiology units included in the pPCI network should have a 24/7, all year round service on call.6 The team on call should include:
-
Interventional cardiologists: during the procedure, together with the interventional cardiologist, the presence of a second physician responsible for the patient’s clinical stability is required. It is advisable that the team of a pPCI program includes at least four cardiologists accredited by the Working Group on Hemodynamics and Interventional Cardiology of the SEC.6 To achieve and maintain the training required for the management of STEMI, 30 pPCIs need to be performed per year and per operator.31 Small-volume centers should be included into wider infarction care networks.32,33
-
Graduate nurses: two graduate nurses trained in direct procedural care with sufficient knowledge of the equipment and material at hand are required. It is advisable that nursing staff be part of the unit and accredited in the field of hemodynamics based on defined criteria. Although the presence of an additional specialist is advisable, he should not replace the nurses in any of their fields of expertise. The participation of an assistant nurse trained in pPCI procedures is also advisable.
After activation, the time of arrival of the hemodynamics team should not exceed 30 minutes. The necessary measures to work within this timeframe should be implemented.
Post-procedural transfers. Early discharge
The transfer of patients once the pPCI has been performed is not uniform and is influenced by several aspects. Depending on the infrastructures, the personnel available, the availability of beds, the initial location of the patient after the pPCI, his clinical stability, and the level of complexity of the receiving hospital, the strategies for the management of patients post-pPCI can vary significantly. There are centers that accept and hospitalize all patients after the pPCI; others, however, keep them under observation for 12-24 hours prior to transferring them, and there are centers that transfer all patients as long as they remain stable after the pPCI.
Taking into consideration the pPCI setting and its possible complications, it is advisable that patients remain under strict observation the 12-24 hours following the procedure, preferably at the infarction center. Depending on the degree of clinical stability and the characteristics of the receiving center, patients can be transferred to their reference hospitals or remain hospitalized under observation or complete the necessary additional treatments that may be required. The models established for the transfer of patients to other centers are determined by the patient’s clinical characteristic, the applicable regional protocols, and the resources available. Patients whose procedure has had optimal results, who have undergone an adequate clinical evaluation, have no signs or symptoms compatible with persistent ischemia, do not show any traces of arrhythmia, remain hemodynamically stable, do not require vasoactive or mechanical support, and without a new revascularization scheduled are eligible for early transfers. Although some MESs already implement them, the widespread use of risk stratification indices and scores to allocate the most adequate resource for the transfer of these patients, and the systematic assessment of these indices and scores is strongly advisable.
Several studies have shown that after a successful pPCI in low-risk patients who were completely revascularized, a significant percentage of patients can be discharged from hospital to their home two or three days after the procedure. Patients eligible for early discharge after STEMI can be identified using risk scores such as the PAMI-II criteria (Second Primary Angioplasty in Myocardial Infarction), the Zwolle risk score, etc.6
Additional prerequisites of pPCI programs
The units that are part of a pPCI network should meet a series of additional requisites:
-
Be able to perform over 400 conventional pPCIs per year and over 75 conventional pPCIs per interventional cardiologist per year.34
-
Have an organizational and functions manual available that includes the actual clinical practice guidelines for the management of acute myocardial infarctions approved by SEC and procedural guidelines adapted to the unit setting.
-
Make available specific informed consent forms to patients and their families, explaining the characteristics of the diagnostic and therapeutic process to be performed.
-
Implement a specific mechanism to assess the pPCI program with indicators of process (especially timeframes) and results including complications.
-
Carry out a formalized process to improve the safety and quality provided to patientS and also keep a record of adverse events.
-
Together with the cardiology service, these units should be incorporated into a system that compares quality optimal practices (benchmarking), process indicators (especially timeframes), severity scores, complexity, and results to eventually to make comparisons with the other pPCI units.
-
PPCI units and the cardiology services should hold periodic meetings to systematically analyse any safety incidents that may have occurred in the unit and, especially, to establish the corresponding prevention measures.
-
Both should provide detailed information of all patients in order to constitute the STEMI National Registry of the Working Group on Hemodynamics and Interventional Cardiology of the SEC, quantify the activity that goes on in the Registry and for code insertion purposes, in the Basic Minimum Data and the RECALCAR Registry.
VULNERABILITIES OF THE ACTUAL pPCI PROGRAMS
Some pPCI programs were initially developed with partial or incomplete prior analyses, limited official support, and poor resources. The progressive expansion of pPCI programs and their widespread use in most STEMI patients have allowed to identify a series of deficiencies and shortfalls that may make them vulnerable and eventually jeopardize their own sustainability.
Impact on daily activity and volume-based care
According to the 2017 registry run by the Working Group on Hemodynamics and Interventional Cardiology, pPCIs amount to almost 30% of all interventional procedures performed in the cath lab.17 Data from different studies35,37 show that between 55% and 70% of all pPCI procedures are performed during off business hours (nights and weekends); however, the remaining 30%-45% procedures on STEMI patients are performed during business hours. The arrival of this large and unexpected volume of urgent and non-scheduled patients to the cath lab disturbs the scheduled daily activity of the unit since the patients require the fastest possible care these cath lab can provide. Depending on the level of adequacy that each center can establish to anticipate this, the routine activity of these cath labs may change, and cases may be suspended (ie, in-patients, cath lab scheduled patients or patients from other centers) and the usual working hours extended to avoid cancellations. Thus, optimizing the STEMI treatment may deteriorate the care that should be provided to other patients with serious heart conditions.
Psychological, legal, and economic implications in healthcare providers
The lack of appropriate sizing of the personnel involved in pPCI programs may be the reason behind two important issues: the development of psychosocial issues and the emergence of situations with legal implications. Dealing with large volumes of pPCIs during night shift requires an adequate number of healthcare providers to take on the unit activity scheduled for the next day. Both the design and makeup of the personnel of infarction centers should take this matter into serious consideration. Without an appropriate sizing of the personnel involved, staff will often have to perform elective procedures deprived of sleep and without the adequate resting time. These procedures have been associated with a higher number of suboptimal results,38 which may have legal consequences for the healthcare providers due to civil liability insurance limitations. If the pPCI procedures are performed during the night shift, an 8-hour resting period after the procedure seems like a reasonable amount of time before the staff goes back to their daily routine.
Also due to the fast action required the peculiarities of pPCI for the management of STEMI often puts a lot of stress on the healthcare providers, because of the complexity of the procedure, and the risk of complications for the patients, all of it due to the procedure per se or due to morbimortality. The variability of schedules, the required urgent action, and the immediate availability of the personnel involved determine a very particular type of “availability”. This may contribute to the higher incidence of the so-called burnout syndrome, depression and anxiety among the staff under high healthcare and emotional pressure as experienced in the cath lab.39 Added to this, there is the high incidence of trauma problems interventional cardiologists suffer39 and, in the long run and with a stochastic component, the possibility of developping certain types of neoplasms.40
In this context it should be mentioned that many pPCI programs in Spain are not categorized as individual specialty programs. Therefore, the financial retributions to these healthcare providers are no different from the retributions of other providers dealing with urgent interventions of lower complexity less frequently, in low or no risk patients and that can often be scheduled several hours in advance. All these aspects are perceived by interventional cardiologists as a mismatch that goes against their best interest. In Spain this is specifically seen in transplant programs and is probably one of the reasons that explains the level of excellence that these programs have achieved in this country.
Ignoring all these aspects can be detrimental to the teams and could in fact lead to situations of discouragement among the staff working in some of these programs. This may have negative repercussions in the level of excellence required by pPCI programs, especially taking into consideration the special profile of the patients involved. Recent data indicate that because of this many highly-experienced healthcare providers (who also happen to be those who do better) will leave these programs as soon as they are entitled due to their age. Also, in order to legally request leaving these mandatory programs, they may claim physical or psychological issues, which would be totally justified and easy to accomplish. All of this puts pPCI programs in a situation of great vulnerability.
PROPOSALS TO ENSURE THE SUSTAINABILITY OF pPCI PROGRAMS
Perfectly established subdivisions to reduce any possible delays
The national territory should be subdivided based on the nearness of hospital centers in order to make sure that the management of STEMI patients happens with the lowest possible delays. This subdivision should entertain concepts such as distance and transfer times to infarction centers. Isochrones should identify distance-related delays to these centers and delays affecting the state of communications. Patients who live in distant neighborhoods or with logistics issues should not undergo pPCI procedures within the intervals recommended; instead, they should be treated immediately with fibrinolysis, and then transferred to the corresponding cardiac center.6
Consensus strategies to reduce transfer times
Regardless of the initial level of care when the diagnosis of STEMI is achieved and when the system is activated (whether from private households, the street, primary care center or regional hospital), protocols for the transfer of patients to pPCI centers should be established. Each pPCI center should agree with the MESs and their corresponding reference hospitals on the best possible strategy to reduce the intervals of treatment. The adequate sizing of these may also be necessary to guarantee the level of care required. Agile communication systems with a centralized structure (emergency coordination center), are key if we want to establish the logistics of the entire process.
The MES emergency coordination center should prioritize the transfer of STEMI patients. Communication with the tertiary hospital through internal phone lines should be established to inform on the estimated transfer timeframe and the patient’s clinical state so that the receiving center can adapt its scheduled daily activity to the arrival of this unexpected emergent procedure. All these timeframes should be recorded prospectively to make the corresponding analysis of any delays that may have occurred during the transfer and be able to identify potential room for improvement. These protocols should be reviewed periodically on a result assessment basis.
Adequacy of pPCI-capable infarction centers
The degree of adequacy will depend on the number of pPCIs performed. The scope and benefit of the pPCI to a significant number of STEMI patients requires specific measures that should take into consideration a series of aspects that make it a particularly complex procedure. PPCI centers should be structured, organized, and properly sized to be able to handle significant increases in the number of urgent procedures. These programs should also include the transfer of patients through the entire intrahospital pathway. Several additional resources are necessary with different factors in each and every one of them:
-
Ensure the presence of a second physician while the pPCI is being performed.
-
The nursing staff should have the experience required while the pPCI is being performed. Depending on the patient’s clinical situation, additional nurses trained in cardiology care may be needed so that the interventional cardiology team can be solely committed to achieving reperfusion.
-
Prepare specific areas with personnel trained on the initial care of STEMI patients while they are waiting for the procedure to be performed and once it has been performed, and also additional beds in the cardiac intensive care unit depending on the volume of pPCIs to be performed.
-
Have large enough teams available to take on additional unscheduled urgent tasks depending on the volume of tasks to be performed.
-
Establish strategies to return pPCI patients to their reference hospitals in the hours following the procedure. This scenario should be protocolized based on the procedural results obtained, the patient’s clinical situation, and the characteristics of the receiving center. This approach is mandatory in centers with high pPCI volumes.
Priority phone availability versus physical presence
Physical presence of hemodynamics teams to perform pPCIs at the very hospital is an option that has always been entertained, but it is an actual reality in very few hospitals worldwide. The timeframe between activation and the opening of the artery using the appropriate strategy of phone location should not exceed 60 minutes and different studies have found no differences at all when it comes to delays in the provision of adequate care and results regardless of whether the team that performs the intervention is physically stationed at the center or is available through the phone. Additionally, considering an adequate number of interventional cardiologists per center, accredited nurses, and the remaining personnel involved in the program, the activities scheduled for the next day could be compromised if the team on call is physically stationed at the hospital, not if it is available through the phone.
Recognition and the right incentives
It is advisable to give pPCI programs a special status by taking into consideration that we are dealing with professionals with a high level of training who neeed to be available at all time, have an immediate capacity of reaction, and be available on different schedules and all at stressful settings due to the complexity of the procedures performed and the risk involved when managing this type of patients. A similar example of this is the special consideration given today to heart transplant programs.
Mandatory audited registry with periodic comparative analyses
It is essential to keep a mandatory prospective registry that includes patients’ baseline characteristics, those of the infarct, the time elapsed between symptom onset and revascularization, the procedural characteristics and the results obtained, and the patient’s clinical progression until he is discharged from the hospital (including reference centers when transferring patients during the acute phase). Both intervention times and results should be monitored periodically in every center and compared to other centers in order to guarantee the quality of the services provided and, if necessary, make the necessary adjustments. Keeping track of all this through a nationwide, verifiable, and independent registry with public data available of all the existing pPCI programs with results by sectors and centers should be mandatory in our country.
There is this pressing need to know the results of the programs implemented in different AACC. Mortality in STEMI is highly dependent on the quality of care received and comparing results among different countries, AACC and hospitals is not only a need but also an obligation that the Administration and the scientific societies should observe to keep healthcare providers and the population duly informed.
CONFLICTS OF INTEREST
R. Moreno is an Associate Editor of REC: Interventional Cardiology.
REFERENCES
1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe:epidemiological update 2016. Eur Heart J. 2016;37:3232-3245.
2. Grines CL. Should thrombolysis or primary angioplasty be the treatment of choice for acute myocardial infarction?Primary angioplasty —the strategy of choice. N Engl J Med. 1996;335:1313-1316.
3. GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med. 1993;329:1615-1622.
4. GUSTO Angiographic Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673-682.
5. Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction:quantitative review of randomised trials. Lancet. 2006;367:579-588.
6. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
7. Aasa M, Henriksson M, Dellborg M, et al. Cost and health outcome of primary percutaneous coronary intervention versus thrombolysis in acute ST-segment elevation myocardial infarction —Results of the Swedish Early Decision reperfusion Study (SWEDES) trial. Am Heart J. 2010;160:322-328.
8. Wailoo A, Goodacre S, Sampson F, et al. Primary angioplasty versus thrombolysis for acute ST-elevation myocardial infarction:an economic analysis of the National Infarct Angioplasty project. Heart. 2010;96:668-672.
9. Stone GW, Grines CL, Rothbaum D, et al. Analysis of the relative costs and effectiveness of primary angioplasty versus tissue-type plasminogen activator:the Primary Angioplasty in Myocardial Infarction (PAMI) trial. The PAMI Trial Investigators. J Am Coll Cardiol. 1997;29:901-907.
10. Cequier A, Ariza-Sole A, Elola FJ, et al. Impact on Mortality of Different Network Systems in the Treatment of ST-segment Elevation Acute Myo-cardial Infarction. The Spanish Experience. Rev Esp Cardiol. 2017;70:155-161.
11. Alter DA, Austin PC, Tu JV, Canadian Cardiovascular Outcomes Research T. Community factors, hospital characteristics and inter-regional outcome variations following acute myocardial infarction in Canada. Can J Cardiol. 2005;21:247-255.
12. Bertomeu V, Cequier A, Bernal JL, et al. In-hospital mortality due to acute myocardial infarction. Relevance of type of hospital and care provided. RECALCAR study. Rev Esp Cardiol. 2013;66:935-942.
13. Laskey W, Spence N, Zhao X, et al. Regional differences in quality of care and outcomes for the treatment of acute coronary syndromes:an analysis from the get with the guidelines coronary artery disease program. Crit Pathw Cardiol. 2010;9:1-7.
14. Carrillo P, Lopez-Palop R, Pinar E, et al. Program of Coronary Angioplasty in Acute Myocardial Infarction in the Region of Murcia (Spain):APRIMUR Registry. Rev Esp Cardiol. 2002;55:587-596.
15. Widimsky P, Wijns W, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe:description of the current situation in 30 countries. Eur Heart J. 2010;31:943-957.
16. Feldman MF, Goicolea J, Macaya C. The Aims of the Stent-for-Life Initiative in Spain in 2010-2013. Rev Esp Cardiol. 2011;11(Supl):6-8.
17. Cid Álvarez AB, Rodríguez Leor O, Moreno R, Pérez de Prado A. Spanish Cardiac Catheterization and Coronary Intervention Registry. 27th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2017). Rev Esp Cardiol. 2018;71:1036-1046.
18. Kristensen SD, Laut KG, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011:current status in 37 ESC countries. Eur Heart J. 2014;35:1957-1970.
19. Schiele F, Hochadel M, Tubaro M, et al. Reperfusion strategy in Europe:temporal trends in performance measures for reperfusion therapy in ST-elevation myocardial infarction. Eur Heart J. 2010;31:2614-2624.
20. Gómez-Hospital JA, Dallaglio PD, Sanchez-Salado JC, et al. Impact on delay times and characteristics of patients undergoing primary percutaneous coronary intervention in the southern metropolitan area of Barcelona after implementation of the infarction code program. Rev Esp Cardiol. 2012;65:911-918.
21. Rodríguez-Padial L, Bertomeu V, Elola FJ, et al. Quality Improvement Strategy of the Spanish Society of Cardiology:The RECALCAR Registry. J Am Coll Cardiol. 2016;68:1140-1142.
22. Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA. 2010;303:2148-2155.
23. Tarantini G, Razzolini R, Napodano M, Bilato C, Ramondo A, Iliceto S. Acceptable reperfusion delay to prefer primary angioplasty over fibrin-specific thrombolytic therapy is affected (mainly) by the patient's mortality risk:1 h does not fit all. Eur Heart J. 2010;31:676-683.
24. Cequier A, Maristany J. Angioplàstia primària. Estrategies per a la seva aplicacióa diferents àmbits sanitaris. Rev Soc Catalana Cardiol. 2005;5:290-297.
25. Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-2718.
26. Sinnaeve PR, Armstrong PW, Gershlick AH, et al. ST-segment-elevation myocardial infarction patients randomized to a pharmaco-invasive strategy or primary percutaneous coronary intervention:Strategic Reperfusion Early After Myocardial Infarction (STREAM) 1-year mortality follow-up. Circulation. 2014;130:1139-1145.
27. López-Sendón J, González-Juanatey JR, Pinto F, et al. Quality Markers in Cardiology. Main Markers to Measure Quality of Results (Outcomes) and Quality Measures Related to Better Results in Clinical Practice (Performance Metrics). INCARDIO (Indicadores de Calidad en Unidades Asistenciales del Area del Corazon):A SEC/SECTCV Consensus Position Paper. Rev Esp Cardiol. 2015;68:976-995.
28. Bonnefoy-Cudraz E, Bueno H, Casella G, et al. Editor's Choice —Acute Cardiovascular Care Association Position Paper on Intensive Cardiovascular Care Units:An update on their definition, structure, organisation and function. Eur Heart J Acute Cardiovasc Care. 2018;7:80-95.
29. Palanca I, Elola FJ, Bernal JL, Paniagua JL. Unidad de cuidados intensivos. Estándares y recomendaciones. Agencia de Calidad del SNS. Madrid:Mi-nisterio de Sanidad y Política Social;2009.
30. Dehmer GJ, Blankenship JC, Cilingiroglu M, et al. SCAI/ACC/AHA Expert Consensus Document:2014 update on percutaneous coronary intervention without on-site surgical backup. J Am Coll Cardiol. 2014;63:2624-2641.
31. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-651.
32. Tubaro M, Danchin N, Goldstein P, et al. Pre-hospital treatment of STEMI patients. A scientific statement of the Working Group Acute Cardiac Care of the European Society of Cardiology. Rev Esp Cardiol. 2012;65:60-70.
33. Nuñez Gil IJ, Fernández-Ortiz A, Escaned J, et al. Long term experience with a novel interventional cardiology network model:Learned lessons. J Hos Administ. 2016;5:87-94.
34. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
35. Dharma S, Dakota I, Sukmawan R, Andriantoro H, Siswanto BB, Rao SV. Two-year mortality of primary angioplasty for acute myocardial infarction during regular working hours versus off-hours. Cardiovasc Revasc Med. 2018;19:826-830.
36. Graham MM, Ghali WA, Southern DA, Traboulsi M, Knudtson ML;APPROACH Investigators. Outcomes of after-hours versus regular working hours primary percutaneous coronary intervention for acute myocardial infarction. BMJ Qual Saf. 2011;20:60-67.
37. Siudak Z, Rakowski T, Dziewierz A, et al. Primary percutaneous coronary intervention during on- vs off-hours in patients with ST-elevation myocardial infarction. Results from EUROTRANSFER Registry. Kardiol Pol. 2011;69:1017-1022.
38. Sandoval Y, Lobo AS, Somers VK, et al. Sleep deprivation in interventional cardiology:Implications for patient care and physician-health. Catheter Cardiovasc Interv. 2018;91:905-910.
39. Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory:Time for a safer environment. Catheter Cardiovasc Interv. 2009;73:432-438.
40. Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111:1368-1372.
Editorials
Expanding the role of drug-coated balloons in native large coronary artery disease
aDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy
bCardio Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
cDivision of Cardiology, Montefiore Medical Center, Bronx, New York, United States
Original articles
Editorials
The role of angiography-derived physiological assessment techniques in the post-FAVOR III Europe era?
aServicio de Cardiología, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain
Interviews
An interview with Camino Bañuelos
aServicio de Cardiología, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Madrid, Spain
bServicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, A Coruña, Spain