To the Editor,
The advances made in surgical techniques followed by the best candidate selection process possible and optimal preparation of patients eligible for surgery have contributed to reducing early postoperative mortality in patients treated with the Fontan procedure.1 However, the balance provided by the Fontan procedure is precarious and can fail for multiple reasons that can be resolved through interventional catheterizations (IC).2-4
This study describes the experience of our center performing IC and focuses on the type of interventional procedures performed and predictors of these.
We conducted a retrospective study of all patients < 18 years-old who underwent a Fontan procedure from January 2000 through December 2021 and were treated with IC due to suspected anatomical injury detected by echocardiography (annually) or magnetic resonance imaging (at 10 years and then every 3 years) or complications like protein-losing enteropathy, plastic bronchitis or hepatopathy. Since 2017, scheduled catheterizations are performed 10 years after the Fontan procedure.
All patients gave their prior written informed consent, and the study was approved by the hospital ethics committee. Possible sex and gender variables have been considered in accordance with the SAGER guidelines.
Descriptive statistics of the demographic, anatomical, hemodynamic, and surgical variables was used. Normally distributed variables were expressed as mean and standard deviation while those without a normal distribution were expressed as median and interquartile range [IQR]. Kaplan-Meier curves were drawn to estimate the IC-free survival rate. To identify predictors of the need for IC, univariate Cox logistics regression analysis was conducted. Variables with significance levels < .2 were included in the multivariate analysis. Also, hazard ratios (HR) with a 95% confidence interval (95%CI) were estimated.
A total of 74 patients treated with a Fontan procedure were identified. Their demographic, anatomical, and pre-catheterization characteristics are shown on table 1. After a median follow-up of 10.3 years [IQR, 5.3-13.3], a total of 59 IC were performed on 35 patients (47%) for a total 79 interventional procedures. The most common ones were embolization of collaterals between systemic and pulmonary veins (26.6%), stent implantation or dilation into the pulmonary branches (20.3%), closure of fenestration (19%), and embolization of aortopulmonary collaterals (16.5%). Other interventional procedures included fenestration dilation (5.1%), Fontan stent implantation or dilation (5.1%), aortic stent implantation or dilation (3.8%), endocavitary pacemaker implantation (1.3%), embolectomy (1.3%), and embolization of antegrade flow (1.3%). A total of 20, 9, 4, 1, and 1 patients were treated with 1, 2, 3, 4, and 5 IC, respectively.
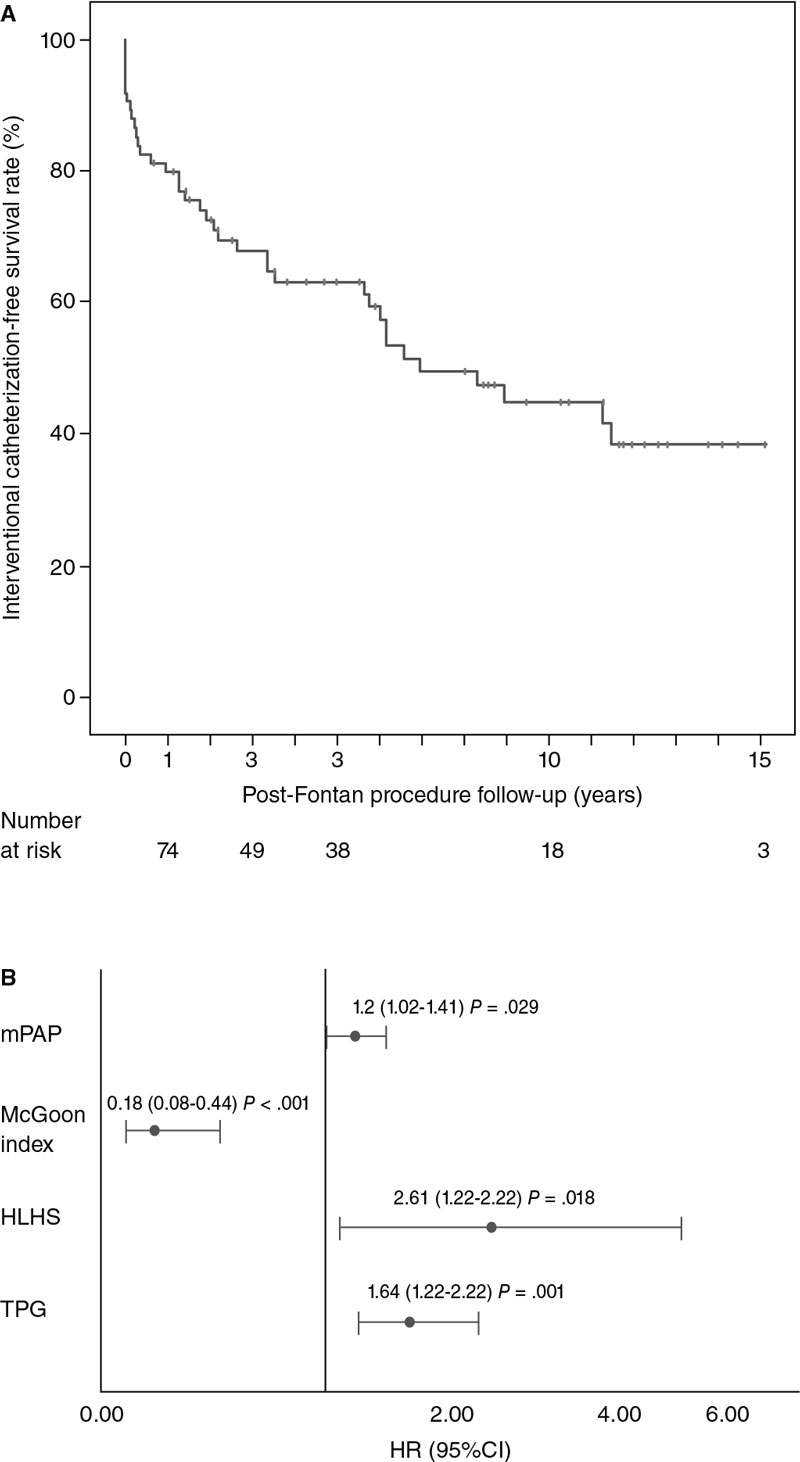
The IC-free survival rate was 63% and 45% at 5 and 10 years, respectively (figure 1A). No deaths were reported associated with cardiac catheterizations. A total of 4 patients (5%) experienced complications associated with the catheterizations (pulmonary thromboembolism, brachial neuropraxia, vasoactive drug administration during the procedure, and pulmonary atelectasis).
The diagnosis of hypoplastic left heart syndrome (HR, 2.62; 95%CI, 1.18-5.78), and the values of mean pulmonary artery pressure (HR, 1.2; 95%CI, 1.02-1.41), the transpulmonary gradient (HR, 1.64; 95%CI, 1.21-2.22), and the McGoon index (HR, 0.18; 95%CI, 0.07-0.44) prior to the Fontan procedure behaved as independent predictors of the need for IC after this surgery (figure 1B).
Table 1. Characteristics of patients, types of interventional procedures, and time elapsed since the Fontan procedure
| Characteristics | Age at the Fontan procedure |
|---|---|
| Type of heart disease | |
| Hypoplastic left heart syndrome | 19 (25.6) |
| Tricuspid atresia | 15 (20.3) |
| Complex heart disease with functionally univentricular heart | 13 (17.5) |
| Double-inlet left ventricle | 12 (16.2) |
| Pulmonary atresia with intact septum | 9 (12.2) |
| Ebstein anomaly | 3 (4.1) |
| Heterotaxy | 3 (4.1) |
| Dominant right ventricle | 25 (33.8) |
| Masculine sex | 40 (54.1) |
| Norwood surgery | 20 (27) |
| Age at the Fontan procedure (months) | 62.8 ± 27.6 |
| Weight at the Fontan procedure (kg) | 17.3 ± 5.4 |
| Type of Fontan procedure | |
| Extracardiac | 67 (90.5) |
| Lateral tunnel | 7 (9.5) |
| Fenestrated | 26 (35.1) |
| Pre-Fontan catheterization data | |
| mPAP (mmHg) | 12.2 ± 2.3 |
| TPG (mmHg) | 3.6 ± 1.6 |
| iPVR (WU·m2) | 1.1 ± 0.5 |
| EDVP (mmHg) | 10.4 ± 2.7 |
| Qp/Qs | 0.5 ± 0.1 |
| Nakata index (mm2/m2) | 243.8 ± 85.2 |
| McGoon index | 2 ± 0.5 |
| Type of interventional procedure | Time elapsed since the Fontan procedure, months |
| Embolization of SV-PV collaterals | 72.3 [35-90.5] |
| Stent implantation/dilation into the pulmonary branches | 46.3 [3-81.6] |
| Fenestration closure | 15.6 [9.3-23.3] |
| Embolization of aortopulmonary collaterals | 25.4 [6.7-93.1] |
| Fenestration dilation | 3.9 [0.2-63.3] |
| Stent implantation/dilation into Fontan. SVC or IVC | 138.4 [34.3-152.4] |
| Stent implantation/dilation into the aorta | 85.4 [4.4-122.2] |
| Endocavitary pacemaker implantation | 82.4* |
| Embolectomy | 0.26* |
| Embolization of antegrade flow | 1.43* |
|
EDVP, end-diastolic ventricular pressure; iPVR, indexed pulmonary vascular resistances; IVC, inferior vena cava; mPAP, mean pulmonary artery pressure; Qp/Qs, pulmonary to systemic flow ratio; SV-PV, systemic vein-pulmonary vein; SVC, superior vena cava; TPG: transpulmonary pressure gradient; WU, Wood units. |
|
Figure 1. A: Kaplan-Meier curve of interventional catheterization-free survival after Fontan procedure. B: Independent predictors of the risk of interventional catheterization. 95%CI, 95% confidence interval; HLHS: hypoplastic left heart syndrome; HR, hazard ratio; mPAP, mean pulmonary artery pressure; TPG: transpulmonary pressure gradient.
In our patients, the rate of interventional procedures performed (47%) is similar to that reported in the series by Downing et al.3 with an IC-free survival rate of 53% at 15 years. Although the number of procedures performed is quite similar, in their case, the closure of fenestration was the most common procedure of all due to their high rate of fenestrated Fontan (90%) compared to ours (35%).
Nonetheless, when our series was compared to others with older patients, significant differences were found. A total of 49% of the patients from the series of Van Dorn et al.4 (1978 through 2002) were treated with a traditional atriopulmonary connection. Most interventional procedures were pacemaker implantation or replacement (26%) or arrhythmia ablation (20%).
Our clinical practice attempts the closure of the fenestration 6 months after the Fontan procedure if the patient’s disease progression is favorable, pressure remains < 16 mmHg during the occlusion test, and proper cardiac output is preserved (> 2 L/min/m2 with a decrease of < 20% compared to baseline levels).
The presence of aortopulmonary collaterals has proven to have a negative effect on Fontan circulation, thus extending the duration of pleural effusions and causing ventricular volume overload. Therefore, we delve into an aggressive search and embolization of these collaterals in the catheterizations performed before and after the Fontan procedure.
The lack of stenosis in the Fontan conduit and pulmonary branches is essential to keep proper hemodynamics in Fontan circulation. Therefore, it seems logical to treat stenoses even in asymptomatic patients.
Regarding the risk factors associated with performing IC, the diagnosis of hypoplastic left heart syndrome was seen as an independent predictor of this event in both the series of Downing
et al.3 and our own. Elevated pulmonary pressures and resistances, and smaller pulmonary arteries are known factors of poor prognosis in this population.
FUNDING
None reported.
ETHICAL CONSIDERATIONS
All patients signed the informed consent and the study was approved by the hospital’s ethics committee. Possible sex and gender variables have been considered in accordance with the SAGER guidelines.
DECLARATION OF USE OF ARTIFICIAL INTELLIGENCE
Artificial intelligence has not been used during the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
A. Mendoza, and L. Albert: study idea, and data mining and analysis. M. Flores, D. Herrera, B. Toral, and A. Caro: manuscript review and edition.
CONFLICTS OF INTEREST
None whatsoever.
REFERENCES
1. Mendoza A, Albert L, Ruiz E, et al. Fontan operation. Hemodynamic factors associated with postoperative outcomes. Rev Esp Cardiol. 2012;65:356-362.
2. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19:37-43.
3. Downing TE, Allen KY, Goldberg DJ, et al. Surgical and catheter-based reinterventions are common in long-term survivors of the Fontan operation. Cir Cardiovasc Interv. 2017;10:e004924.
4. Van Dorn CS, Menon SC, Johnson JT, Day RW, Hoffman JL, Yetman AT. Lifetime cardiac reinterventions following the Fontan procedure. Pediatr Cardiol. 2015;36:329-334.