Innovation in interventional cardiology
New on the market is a microcatheter bearing the Medtronic stamp (USA). Medtronic, in collaboration with Surmodics Inc. (USA), has released a microcatheter that aspires to become the standard for multiuse microcatheters in percutaneous coronary intervention. Surmodics is a company specialized in the production of hydrophilic coatings that has been collaborating with Medronic for many years on a mission to improve the performance of their devices.
The Telemark microcatheter has three key features:
- It is made of a mixed (flat and round) braided mesh with Xtreme technology, which provides improved push force transmission.
- It has a Pristyne hydrophilic coating, which improves deliverability and lesion crossing.
- It has a very low-profile, tapered design, from 2.6 to 1.4 French.
The crossability, deliverability, and lubricity of this microcatheter are significantly superior to that of other standard microcatheters on the market. Use of this Telemark device will soon become established in everyday clinical practice.
Palabras clave: microcatéter, oclusión coronaria crónica total. Keywords: microcatheter, chronic total occlusion.
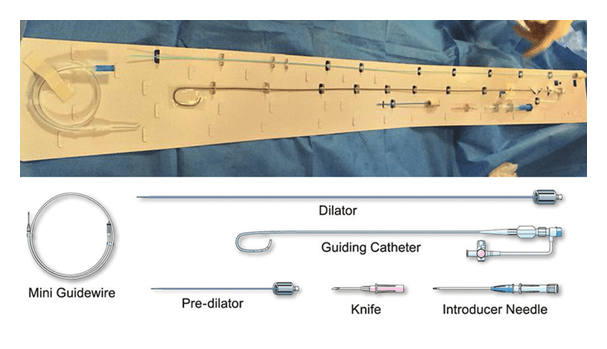
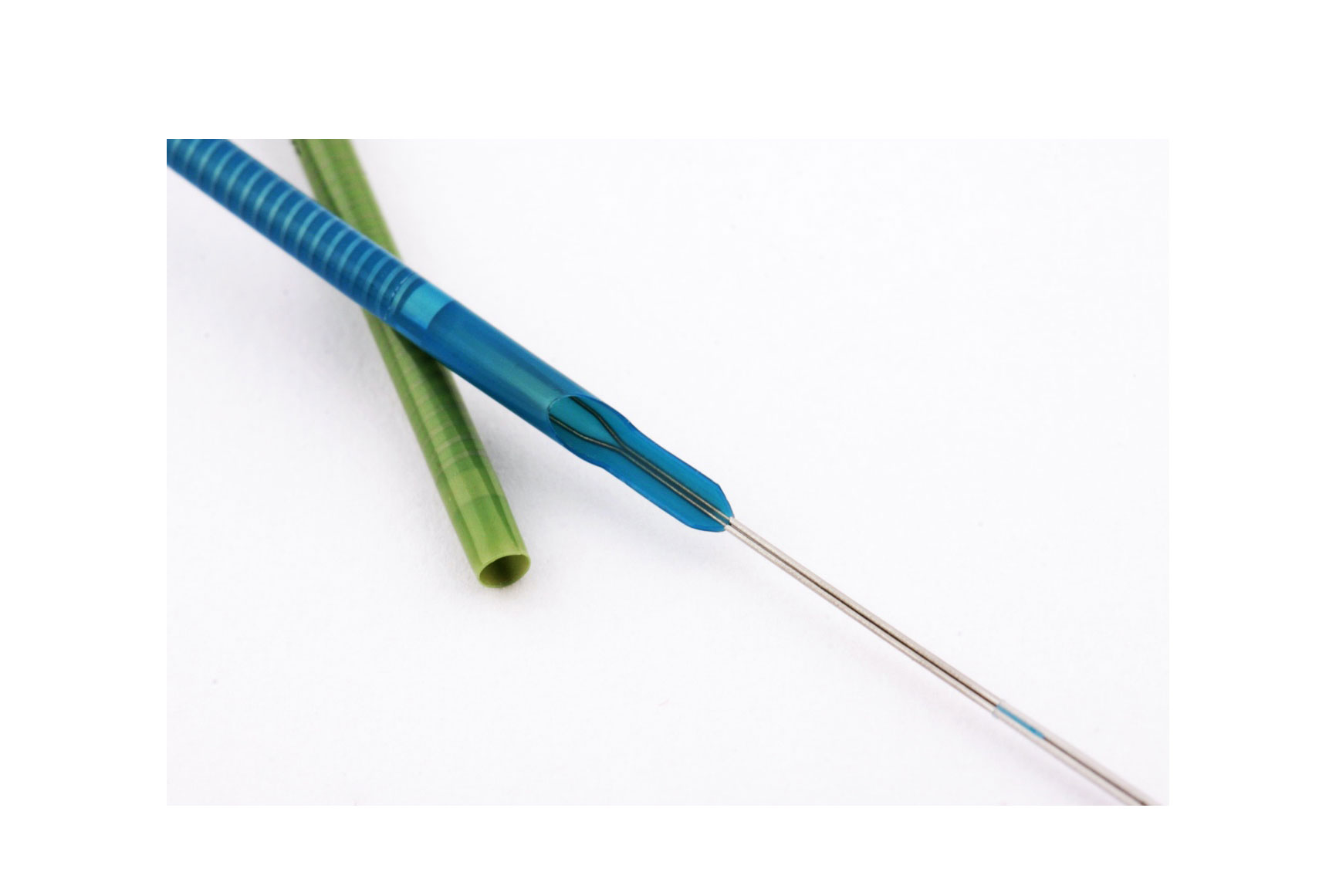
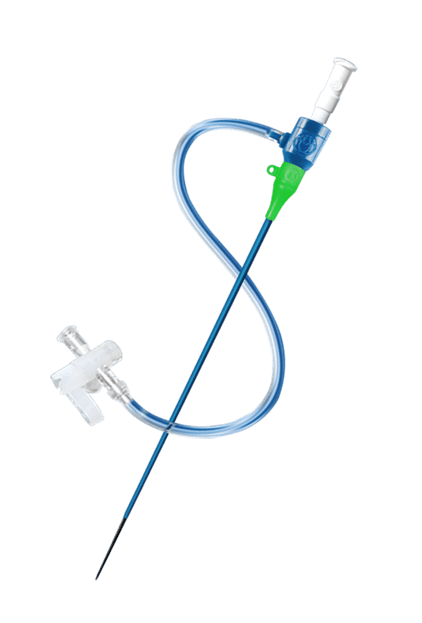
In radial-access interventions, innovation never stops. The latest development is a sheathless guide catheter from Medikit Co., Ltd (Japan). In minimally-invasive coronary intervention performed in patients with difficult vascular access, reliable devices are essential. Radial access is limited by the size and quality of the radial artery; at times it is impossible to pass a 6- or 7-French sheath. The Meito Masamune sheathless guiding catheter is available in two outer-diameter sizes: 5.3 French (1.79 mm) and 6.5 French (2.20 mm). By way of comparison, the 5.3-French system has an outer diameter close to that of a 3-French sheath (1.60 mm) and the 6.5-French system is smaller than a 5-French sheath (2.25 mm). There are several benefits to this device:
Los beneficios de este dispositivo son varios:
- It minimizes vascular trauma, reducing complications and facilitating compression
- It facilitates radial access is very small caliber arteries
- There is a universal guide catheter curve called BLK, which can be used to dilate any of the coronary arteries.
The device kit includes an introducer needle, a 0.025’’-0.035’’ guidewire (according to the size of the sheath), a knife, a predilator, a dilator with a radio-opaque tip, and the guiding catheter. The guiding catheter has a hydrophilic coating, which ensures its passage through complex anatomies.
Palabras clave: Catéter guía sin introductor. Keywords: SheathLess Guiding catheter.
Interventions performed via radial access are sometimes limited by the size of the guiding catheter that can fit in the coronary ostium. It is important to properly assess the characteristics of the coronary lesions to be treated, to choose the right therapeutic technique and ensure compatibility with the size of guiding catheter to be used.
Usually, an introducer and a 6-French catheter are used, and if a larger size is needed, a 7-French may be used. In some patients, however, this is the limit for radial access, forcing the interventionalist to change to femoral access.
ATP Medical Inc. (Shenzhen, China) makes 6.5-French introducers with an outer diameter equal to that of a conventional 6-French introducer. This is made possible by reducing the thickness of the wall of the introducer without sacrificing performance or kink resistance. The sheath comes with a high-performance guiding catheter with an inner diameter of 6.5 French, 0.075’’ compared to the 0.071’’ of the conventional 6-French guiding catheter.
This characteristic can be important when performing certain coronary intervention techniques such as kissing stent or rotational atherectomy with a 1.75-mm burr, techniques that are not feasible with a 6 French guiding catheter.
These introducers and guiding catheters are also available in 5.5 French.
The following curved guiding catheter are available: Judkins, Amplatz, extra support, Williams, and multipurpose.
Palabras clave: introductor arterial, catéter guía. Keywords: introducer, guiding catheter.
Devices for treating coronary occlusions are under constant development; innovation in this field is very active.
In coronary intervention of such lesions, unknown coronary architecture represents a great problem that, even in the hands of experiences operators, can lead to the guidewire sticking in the subintimal space, unable to reach the true distal bed.
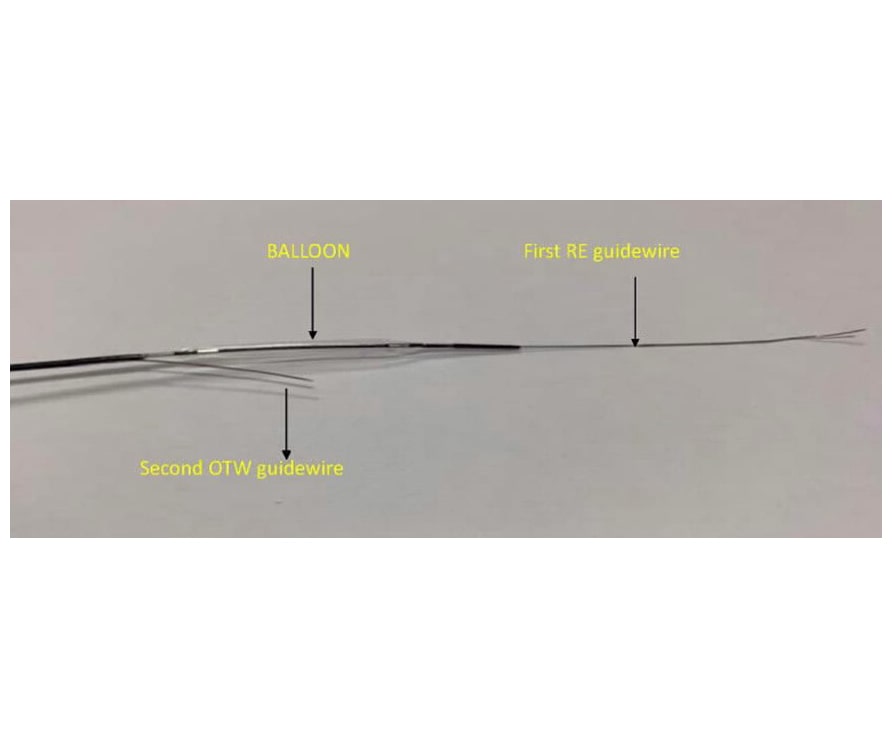
Complex re-entry techniques exist. Anoxia Inc. (USA), in collaboration with Dr Mauro Carlino, from Milan, have developed the coronary dilation Dual Guide Wire Balloon. The balloon is 20 mm long and comes in several diameters. It has a monorail distal port, for rapid exchange, and a coaxial port. When the guidewire reaches the subintimal space, the balloon is advanced (diameter selection according to vessel diameter) and is inflated in the subintimal space. Through the coaxial port, a polymeric guidewire of intermediate grammage is advanced and exits proximal to the balloon, the balloon is deflated, and several fenestrations are created in the tissue weakened by the dissection to facilitate passage to the distal true lumen.
This device is not yet commercially available to allow us to try it, although we have been performing such a technique for a while, using a conventional method without specific devices. Time and experience will tell if this is the solution for re-entry.
Palabras clave: fenestración anterógrada reentrada, oclusión coronaria crónica total. Keywords: antegrade fenestration re-entry, chronic total occlusion.

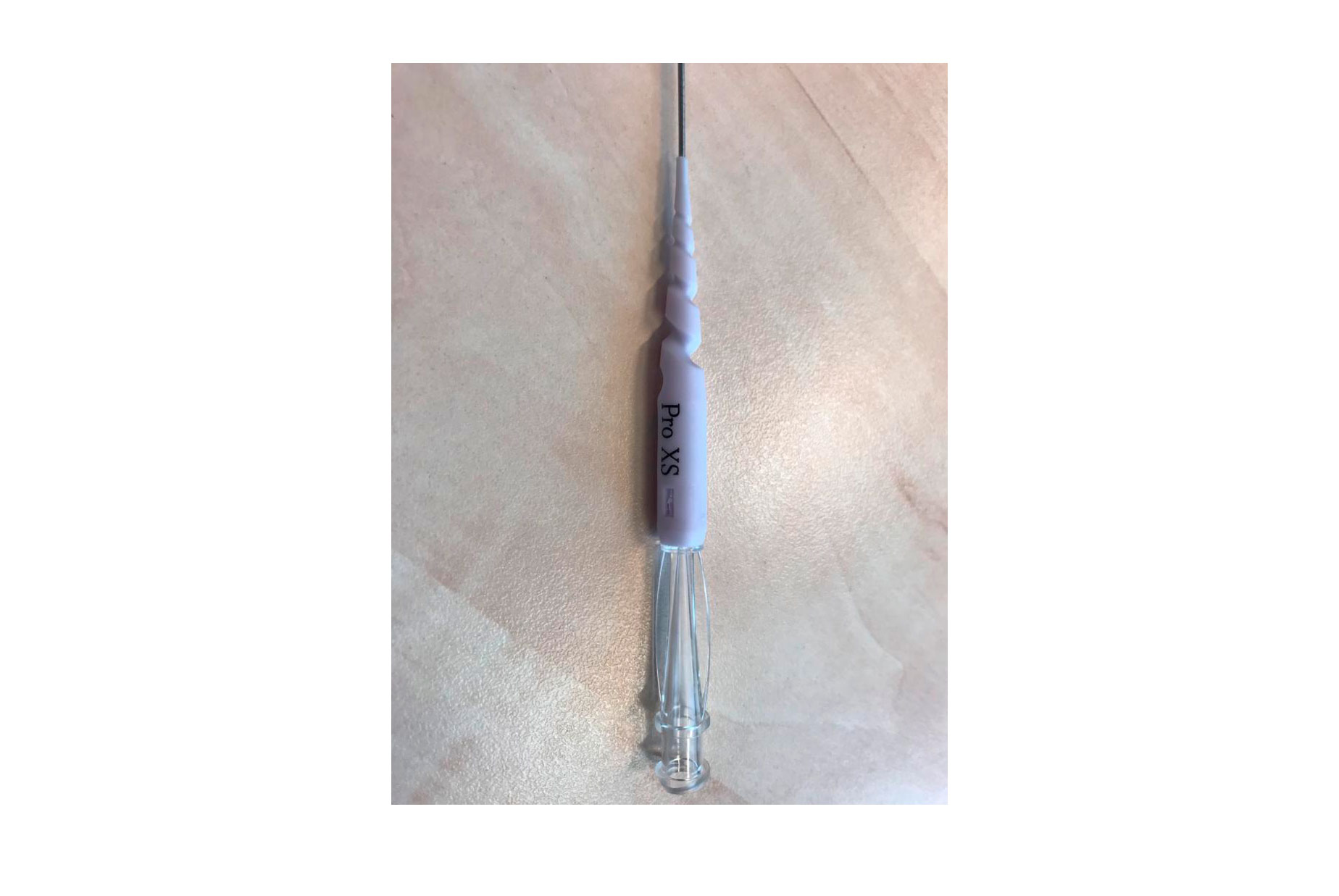
Corsair Pro XS is the new generation of microcatheter by Asahi Intecc Co., Ltd. (Japan). Its key characteristic is the SHINKA-Shaft: a new 14-wire braid that completely changes the rotational shaft design, increasing the performance of the device in retrograde approach. This type of braided wire allows the microcatheter to be rotated many more times than the previous generations of Corsair, which is why it is indicated in cases requiring a microcatheter (antegrade or retrograde approach) with a high degree of rotation to cross the plaque. The tip entry profile is 1.3 Fr (0.44 mm), like the Corsair Pro, but with a distal shaft outer diameter of 2.1 Fr (0.71 mm), smaller than the 2.6 Fr (0.87 mm) of the Corsair pro. The soft tapered tip is shorter than other similar catheters, 6.5 mm vs 10 mm, which facilitates plaque penetration. It has a 70 cm hydrophilic coating (normally 60 cm) and a hypotube that is more flexible and resistant to mechanical fatigue, a common problem in long procedures.
This represents a great advance in device innovation for complex percutaneous procedures.
Palabras clave: microcatéter, oclusión coronaria crónica total. Keywords: microcatheter, chronic total occlusion.
When performing catheterisation via radial access, interventional cardiologists use a coated guidewire. If the arteries are excessively tortuous or severely calcified, it may be necessary to use a hydrophilic polymer-coated guide wire, which carries the risk of perforating secondary branches, as these guidewires slip easily, but provide limited tip control. In some cases, coronary angioplasty guidewires are needed to negotiate a highly-tortuous radial artery, but they lack the sufficient support to advance the catheter.
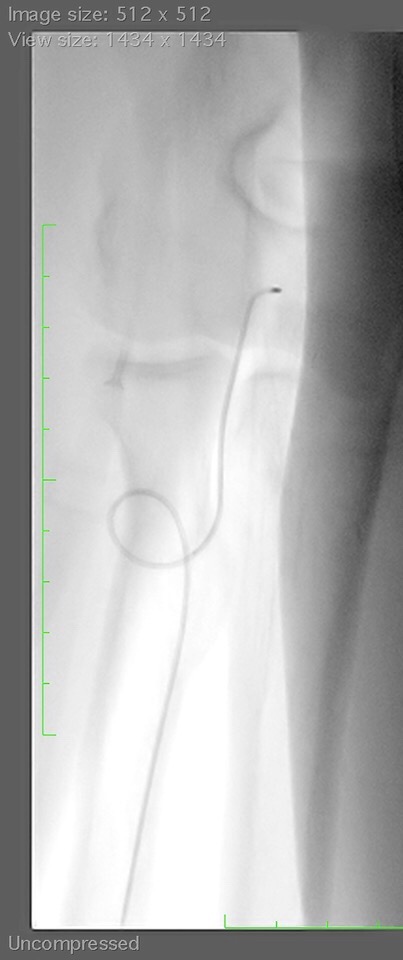
The Silverway guidewire (Asahi Intecc Co, LTD., Japan) represents a great innovation in endovascular guidewires: it facilitates access in these difficult situations and has substantially better performance and safety than other commonly-used guidewires. It is a 0.035’’ metal guidewire, with a double coil structure at the distal tip and a multi-wire coil with ACT ONE technology in the proximal shaft. This structure is the same as that of angioplasty guidewires. It has a hybrid coating along its entire length. The distal 15 cm have a silicone coating that provides tactile feedback from the tip to the hand. This silicone coating improves safety, as it prevents the guidewire from perforating the vessel. Proximal to these 15 cm, the guidewire has a hydrophilic coating that facilitates smooth delivery in the arteries. The proximal shaft is silicone-coated, to provide better control of the guidewire and 1 to 1 torque transmission to the tip.
Among the available lengths, of note is the 180 cm version, which allows exchange of catheters in the ascending aorta while maintaining the desired position of the guidewire and the 1.5 mm J shape, which is easily straightened manually. The tip of the guidewire is radio-opaque, allowing it to be visualised at all times. This guidewire can be used for any access that is difficult to navigate due to tortuosity or calcification.
In my opinion, this guidewire represents innovation in a field in which I thought nothing remained to be invented.
Palabras clave: guía endovascular. Keywords: endovascular guidewire.

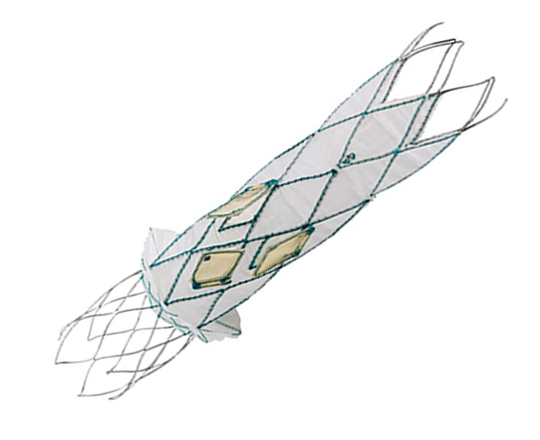
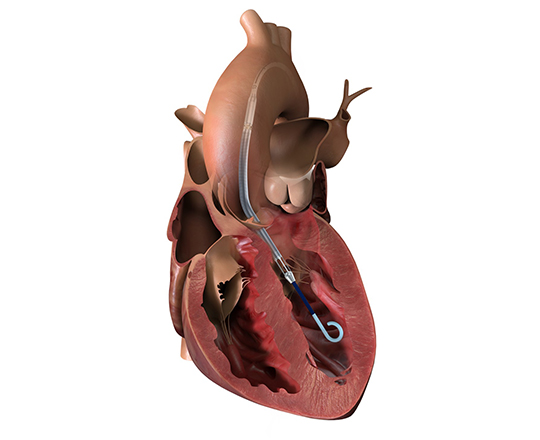
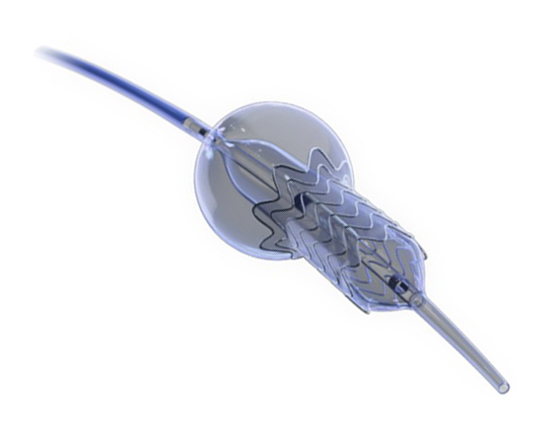
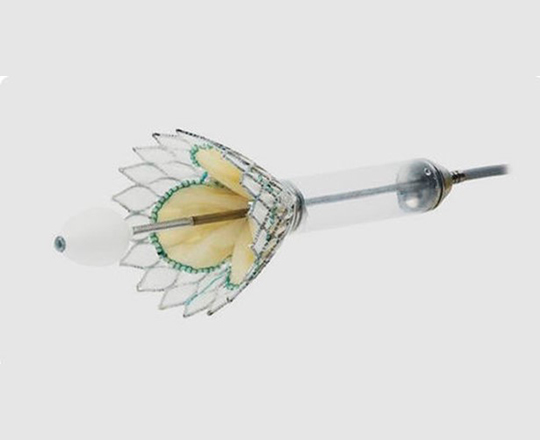
New Valve Technology (Switzerland) recently received CE mark approval for the treatment indication of percutaneous implantation of the ALLEGRA aortic valve in a degenerated bioprosthesis.
The ALLEGRA valve has two main characteristics: firstly, it is a self-expanding supra-annular valve with flexible commissures, and secondly, it has a low frame height that minimizes potential occlusion of the coronary ostium.
This approval came after extensive in vitro research on the behaviour of the ALLEGRA valve when implanted in the more commonly-used bioprostheses,1 followed by some very promising clinical results from the VIVALL study.2
Internationally, close to 100 cases of ALLEGRA valve implantation in degenerated surgical bioprostheses have been performed with very favourable outcomes in terms of residual gradient, paravalvular leak, and pacemaker implantation. In the VIVALL study,2 a reduction in transvalvular gradient (invasive measurement) from 37.1 ± 13.3 mmHg to 11.6 ± 3.7 mmHg was observed, with a 30-day mortality of 0%, and no paravalvular leaks or pacemaker implantations; the effective orifice area increased from 1.18 ± 0.58 cm2 to 1.4 ± 0.52 cm2.
Currently, patients of this type are being recruited for the post-marketing clinical study FOLLOW; when complete, this data will expand the clinical evidence on this percutaneous valve.
REFERENCES
1. Sedaghat A, Sinning JM, Werner N et al. In vitro hydrodynamic and acute clinical performance of a novel self-expanding transcatheter heart valve in various surgical bioprostheses. EuroIntervention. 2018;13:2014-2017.
2. Schaefer U, Butter C, Landt M et al. Thirty-day outcomes of a novel transcatheter heart valve to treat degenerated surgical valves - The “VIVALL” multi-center, single-arm, pilot study. EuroIntervention. 2019;15:e757-e763.
Palabras clave: prótesis valvular percutánea. Keywords: percutaneous valve prothesis.
The guide extension catheter has become an essential device for treating stenotic lesions in complex coronary interventional procedures. When the guide catheter has limited support, reaching a target lesion for balloon dilatation and stent implantation can become mission impossible. Guide extension catheters, an extension of the guide catheter, facilitate safe device passage. It is amazing to see how they are able to reach lesions that balloons or stents would not even get near.
A new guide catheter extension has been released, called Boosting (QX Medical, USA), which has some new features that others do not have. The catheter is hydrophilic along its entire length, with a rounded tip to avoid a razor-blade effect on the atheroma plaque.
At the transition zone, a unique characteristic is worthy of note: the proximal part of the catheter is very wide, and its lumen accommodates the guide catheter without leaving a space between the two. This feature prevents flow between the guide catheter and the extension, so that, if fluid or contrast is given, it goes straight to the tip of the guide catheter extension. Also, if we want to advance a guidewire it can be introduced directly into the extension, without the risk of passing outside it. The portion that is used to push is made of two soldered wires with great flexibility and pushability that are integrated directly with the shaft of the catheter.
Palabras clave: Extensor de catéter guía. Keywords: Guide extension catheter.