Innovation in interventional cardiology
Nagare bidirectional steerable sheath
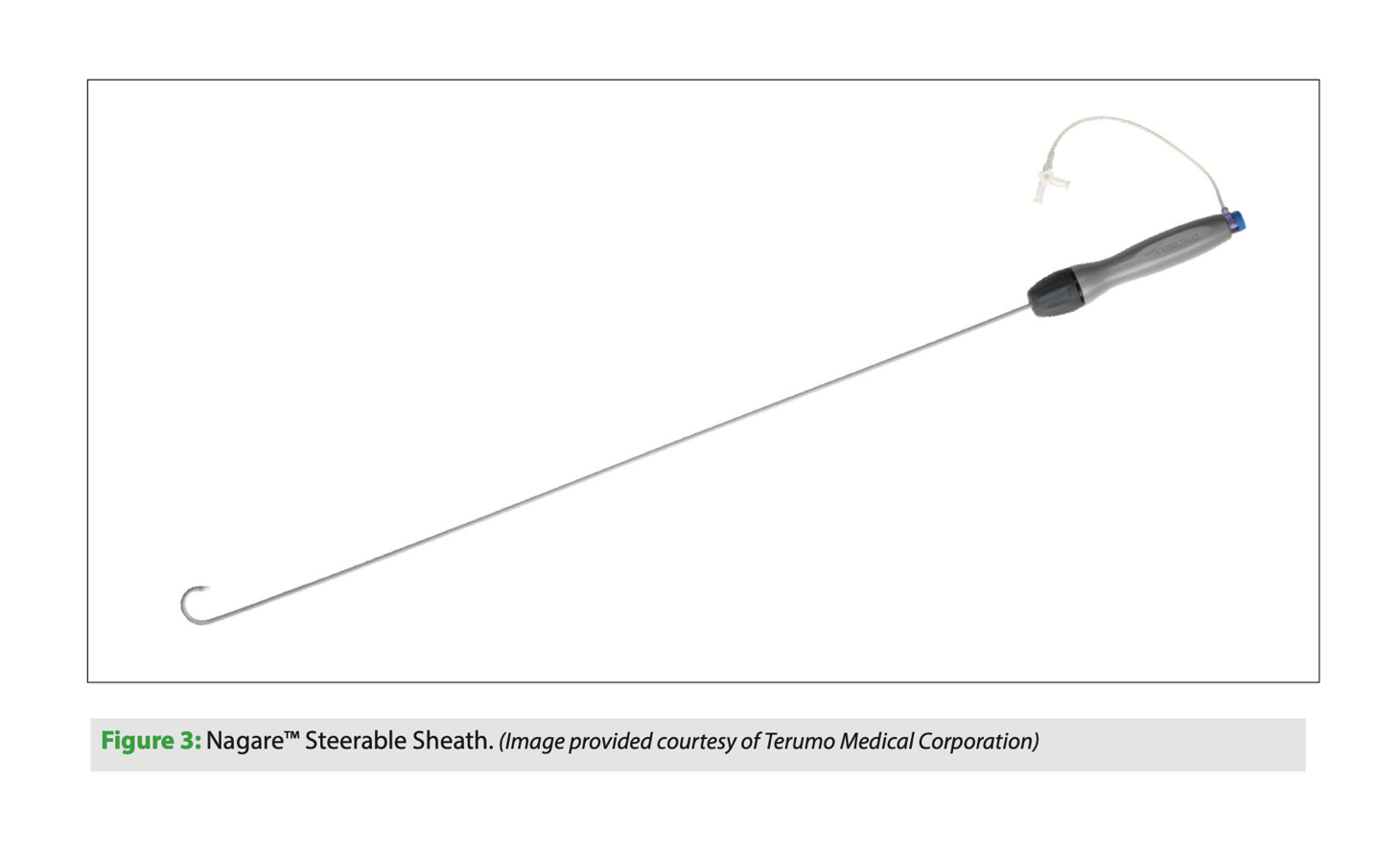
The Nagare bidirectional steerable sheath (Terumo, Japan) is a catheter that allows controlled navigation in all chambers of the heart. The sheath has bidirectional movement thanks to its TrueVector technology: the device is manufactured with a coaxial mesh in the shaft, unlike others that use pull wire systems to move the tip of the catheter. This mesh, which is Kevlar-coated, can move freely, and since it is pulled proximally along with the shaft, only the tip is deflected, with accuracy and stability. This prevents the classic whipping effect of other similar catheters in which deflection occurs along the length of the catheter rather than just the tip; this is an interesting improvement.
The Nagare bidirectional steerable sheath has an external sheath diameter of 12 Fr and an internal diameter of 8.8 Fr, with a usable catheter length of 73.7 cm. Its size allows other devices to be passed through it. It has a smooth transition to the dilator for a 0.032’’ guidewire. It can be deflected 90° or 180° on its longitudinal axis with a turning radius of 25 mm.
This device is indicated for controlled transseptal puncture. Structural interventional procedures that are performed on the left side of the heart often require a posterior puncture below the interatrial septum. A steerable sheath of this type allows better control of the puncture site.
Palabras clave: introductor deflectable, punción transeptal. Keywords: steereble sheath, transseptal puncture.
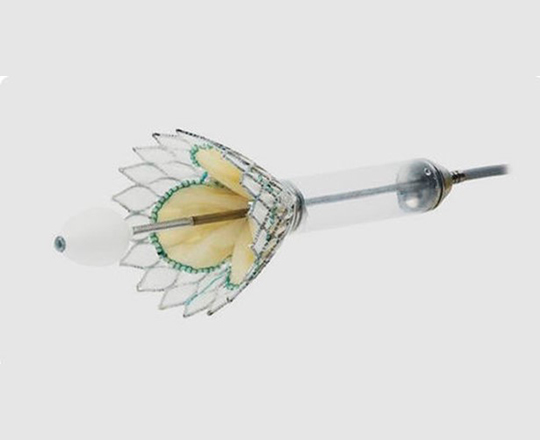
True Flow balloon valvuloplasty catheter
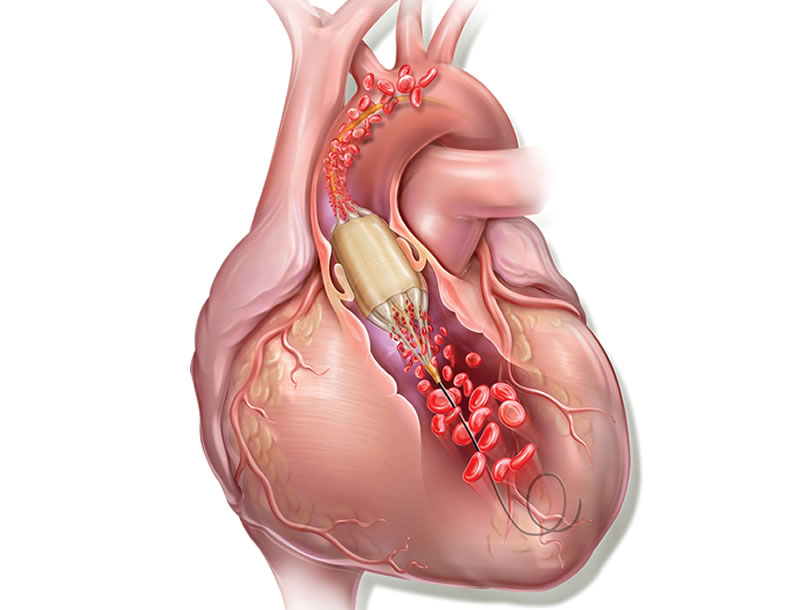
Aortic balloon valvuloplasty requires rapid cardiac pacing with a pacemaker to avoid the balloon moving while it is inflated and causing injury to the leaflets or even the annulus if it slips uncontrollably. In addition, during valvuloplasty, the inflated balloon (inflated for approximately 5 s) causes left ventricular outflow tract obstruction and ischemia, which in some patients may cause significant hemodynamic compromise.
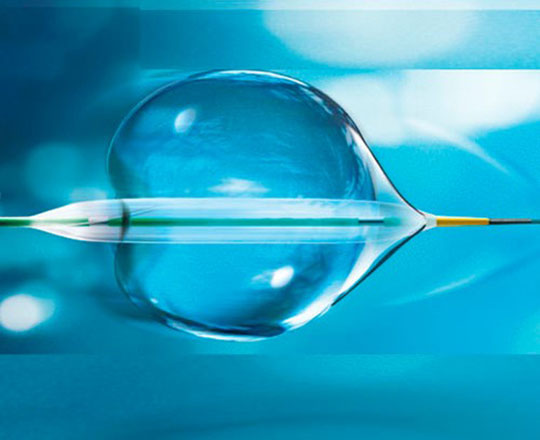
True Flow (Bard, USA) is a valvuloplasty balloon catheter that allows continued blood flow while it is inflated. It has an outer fiber-based shell that prevents the balloon from sliding when it is opened onto the valve leaflets, so a pacemaker is not required. This shell is made of high-molecular weight polyurethane, polyester and aramid fibers (a structural component of Kevlar) and has minimal stretch and high resistance to rupture. The inside of the balloon has 8 small balloons around the edge of the main balloon, with an inner lumen between them where blood can flow. The device measures 3.5 cm long and is available in six sizes, with diameters from 18 mm to 26 mm. The smaller models are compatible with an 11 Fr sheath and the largest, with a 16 Fr sheath.
This type of balloon catheter allows valvuloplasty to be performed for 60 s or more without rapid pacing, with a 1/3 reduction in the mean aortic pressure while the balloon is inflated, which means a 50% reduction in the aortic gradient. This could be especially useful in patients with severe dysfunction.
Palabras clave: valvuloplastia aórtica, balón de valvuloplastia. Keywords: aortic valvuloplasty, valvuloplasty balloon.
Capbuster
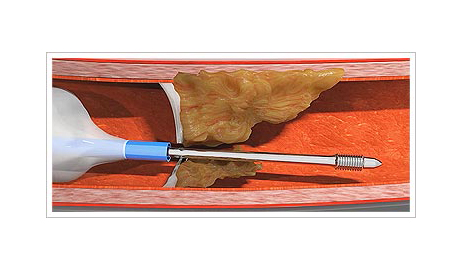
Several challenges may arise for the clinician performing an interventional procedure on coronary arteries with chronic total occlusion. One such challenge is that of an impenetrable plaque. Possible solutions to this problem include active and passive support techniques, as well as devices such as the Tornus (Asahi Intech, Japan) and the Turnpike Gold (Teleflex, USA). However, these devices require high passive support and they are not always effective.
The CapBuster (Capsos Medical, Ireland) is a balloon catheter combined with a special guide wire designed to perforate impenetrable plaques. The microcatheter balloon is inflated inside the coronary artery, proximal to the plaque. This balloon provides, within the coronary artery, the active support required to allow the guidewire to be advanced through the plaque. Another of its benefits is that, once inflated, the tip of the microcatheter centers in the coronary artery to ensure an excellent central, coaxial alignment with the plaque.
One the balloon is inflated, the guidewire is advanced as if it were a lag screw: it is turned clockwise to advance it and, one the plaque has been crossed, it is turned anticlockwise to remove it. Once the plaque has been penetrated, a standard guidewire for chronic total occlusion can be used as per the interventionist’s choice.
It is estimated that the CapBuster will obtain the CE mark in 2021. Until then, we will have to wait be this device that, in theory, will help solve these important problems.
Palabras clave: placa coronaria impenetrable, oclusión coronaria crónica. Keywords: uncrossable coronary plaque, chronic total occlusion.
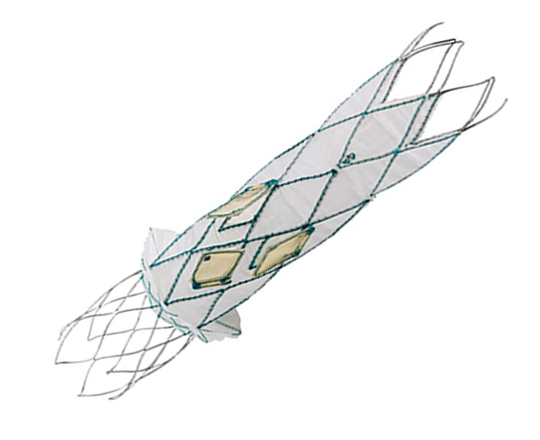
Forma tricuspid repair system
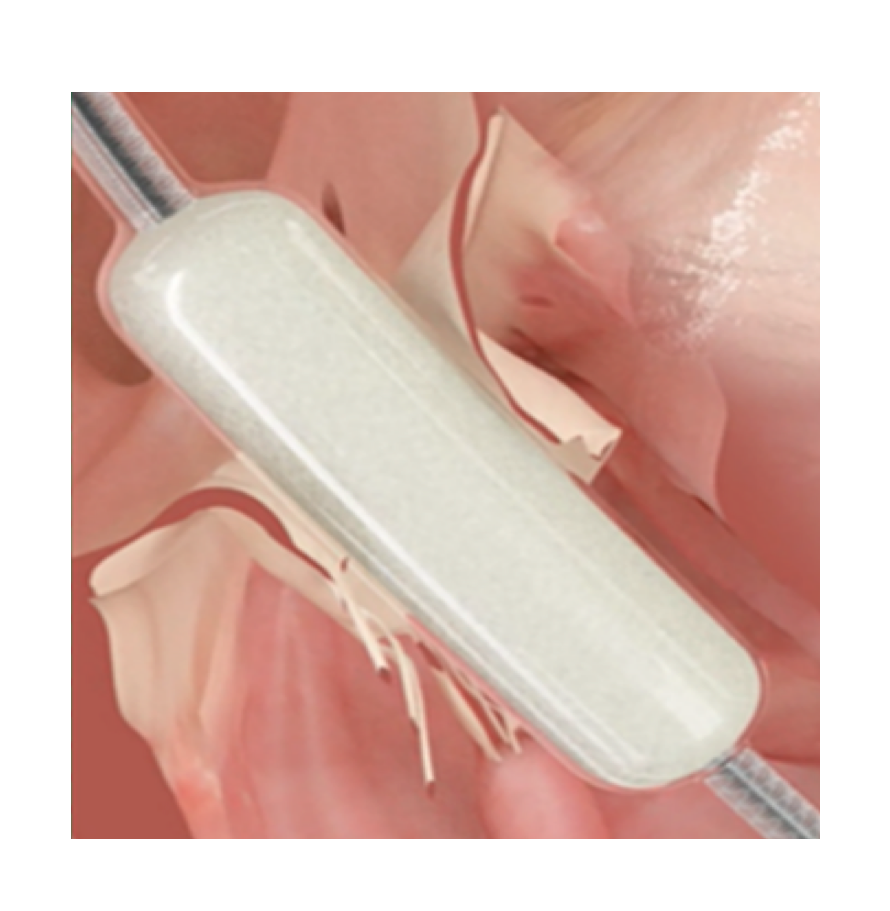
Tricuspid regurgitation is a significant clinical problem, with complex palliative solutions that carry a high surgical risk. Industry continues to dedicate substantial resources to the development of devices to allow percutaneous treatment.
The Forma system (Edwards, USA) has been under research for several years. It has been modified following the results from a clinical trial involving an initial compassionate use experience in patients with tricuspid regurgitation who are unsuitable for surgery.1
The Forma system is inserted via axillary access with a 24 French sheath. Through this sheath, a balloon catheter is advanced to the right ventricular apex, where the guiding rail is anchored to the ventricular wall. Using this rail, a 15 mm or 18 mm cylindrical spacer is positioned perpendicular to the tricuspid valve in the gap between the leaflets. The leaflets coapt onto this cylindrical spacer, substantially reducing the regurgitant volume from the right ventricle. One the position is confirmed by visualization on 3D echocardiography, the rail is pulled taught and fixed in the subcutaneous space next to the insertion zone in the subclavian vein.
Four-year results are now available from the first clinical trial,2 which show an improvement in quality of life, functional status based on NYHA (New York Heart Association) classification, and 6-minute walk test.
Two other clinical trials are currently underway, one of which involves 75 patients in Canada and Europe (EU/Canada SPACER trial NCT02787408).
REFERENCES
1. Rodés-Cabau J. Transcatheter Tricuspid Valve Therapies 3: FORMA. Description, Results and a Case. En: Transcatheter Cardiovascular Therapeutics 2016; 2016 November 1, 2016; Washington, USA. Available at: https://www.tctmd.com/slide/transcatheter-tricuspid-valve-therapies-3-forma-description-results-and-case. Accessed 03 Abr 2020.
2. Muntane-Carol M, Del Val D, Bedard E et al. Transcatheter innovations in tricuspid regurgitation: FORMA device. Prog Cardiovasc Dis. 2019;62:496-499.
Palabras clave: Insuficiencia tricuspídea, intervencionismo percutáneo estructural. Keywords: tricuspid regurgitation, percutaneous structural interventions.
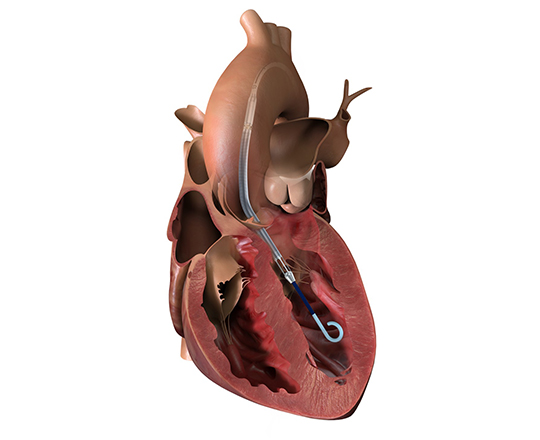
Occlutech AFR (atrial flow regulator)

The Occlutech AFR (atrial flow regulator) (Occlutech AG, Switzerland) is a nitinol device similar to the occluders implanted in the percutaneous treatment of atrial septal defects or patent foramen ovale. It has a double-disc shape with a waist in the center that allows it to anchor into the septal wall. The device has a central fenestration that allows the shunting of blood between the two atria. On the right atrial side of the device there is a welded ball that serves as an anchor and connection hub to the release system.
Implantation of the device requires transseptal access, and the interatrial septum is dilated with a 6-mm balloon. The device’s central fenestration measures between 8 and 10 mm.
A pilot study was performed to determine the safety and the potential for Occlutech AFR implantation. The study was called AFR-PREVIELE1 and was a phase II, prospective, multicenter, nonrandomized trial in symptomatic heart failure patients (New York Heart Association [NYHA] functional class 3-4) with a preserved or reduced ejection fraction and pulmonary capillary pressure over 15 mmHg at rest or over 25 mmHg with exercise. It was possible to implant the device in 100% of the interventions and the shunt remained patent in all cases at 3-month follow-up. 8.3% of the patients had re-admission with acute heart failure and the rest improved clinically in terms of symptoms and surrogate outcomes: NYHA functional class, quality of life questionnaires, and 6-minute walking test.
The Occlutech atrial flow regulator is indicated for cases of pulmonary arterial hypertension, congenital defects, and heart failure.
REFERENCES
1. C Paitazoglou, R Özdemir, R Pfister, et al. The AFR-PRELIEVE trial: a prospective, non-randomised, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention 2019; 15: 403-410.
Palabras clave: Insuficiencia cardíaca, nuevos dispositivos, fenestración septo auricular. Keywords: Heart failure, new devices, atrial septum fenestration.
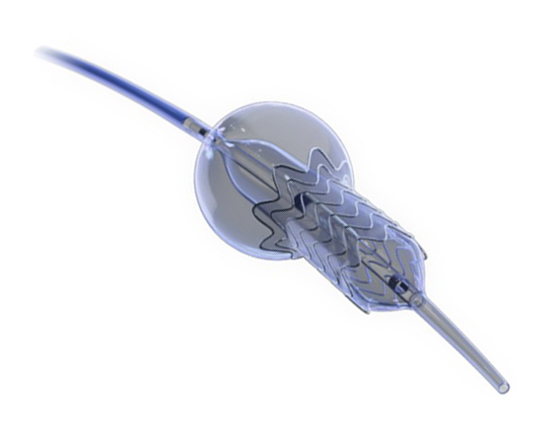
The Conformal left atrial appendage seal (CLAAS)

The Conformal device (Conformal Medical, USA) is designed to adapt to each patient’s left atrial appendage anatomy and reduce the need for transesophageal echocardiography and general anesthetic during implantation.
It has a nitinol skeleton with tw––o rows of 20 anchors that attach to the wall of the left atrial appendage, with a plug-shaped foam matrix coating. The upper portion of this, the part which is exposed to blood, is coated with an expanded polytetrafluoroethylene (ePTFE) layer to prevent device thrombosis1.
The foam matrix allows more effective sealing of potential leaks that occur with other devices, and the two available sizes can adapt to most anatomical variations of the left atrial appendage. The great advantage of the ePTFE coating is that it substantially reduces clot formation on the device, particularly when anticoagulation or anti-platelet agents are contraindicated.
A prospective, multicenter, single-arm clinical trial (ClinicalTrials.gov identifier NCT03616028) is currently underway to assess the device’s safety (mortality, ischemic stroke, systemic thromboembolism, or any device or procedural complication requiring cardiac surgery) and efficacy (complete closure on transesophageal echocardiography at 45 days).
REFERENCES
1. William Gray. Second-generation LAA occlusion: Single-size and conforming to a broad range of anatomies. En: Transcatheter Cardiovascular Therapeutics (TCT) 2019; 2019 September 25-29; San Francisco, EE.UU. Disponible en: https://www.tctmd.com/slide/second-generation-laa-occlusion-single-size-and-conforming-broad-range-anatomies-conformal Consultado 08/01/2020.
Palabras clave: cierre de orejuela izquierda, fibrilación auricular, hemorragia. Keywords: left atrial appendage closure, atrial fibrilation, anticoagulation, bleeding.
Sion Black Pre-shape angioplasty guidewire

Over the years, the retrograde access methods for the percutaneous treatment of chronic total coronary occlusion have undergone multiple adaptations. One of the most important changes is the use of angioplasty guidewires depending on the anatomy of the collateral branches.
The Sion guidewire (Asahi Intecc, Japan) has become the guidewire of choice for crossing these channels when they are invisible, have a highly tortuous anatomy, or have lateral branches arising from curved sections. In such cases, when the Sion guidewire cannot cross the collaterals, a Fileder XT-R guidewire or a Suoh03 guidewire (both made by Asahi Intecc, Japan) are used. At the moment, the third-line option in most cases is the Sion Black (Asahi Intecc, Japan). This guidewire (0.8 g tip load) has been on the market for a while, in two forms: straight or J-shaped. The J-shaped curve is too large and is not suitable for crossing collaterals. In contrast, the straight version, which is often used, is difficult to shape manually due to the rigidity of its polymer coating.
The new version that will be on the market is the Sion Black Pre-shape with an angled tip for crossing collaterals during retrograde access. This curve is much smaller than the current J-shaped version, which means that it does not need to be manually shaped and can be used directly to cross collaterals. The tip shape is designed to provide greater durability throughout the procedure and, even if the angle does require modification, it is easier than with the straight guidewire, and a greater precision and durability are guaranteed.
Palabras clave: guía de angioplastia coronaria, oclusión coronaria crónica total, acceso retrógado. Keywords: coronary angioplasty wire, chronic total occlusion, retrograde access.
Pachyderm guiding catheter custom-shaped for BASILICA procedure
The BASILICA procedure (Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction) is the intentional splitting of the left cusp of the degenerated bioprostheses in the aortic position when percutaneous vale-in-valve implantation presents a risk of coronary artery occlusion.
To locate the point at which to traverse the valve leaflet, a guiding catheter such as Amplatz left (AL) with varying curvatures or Extra Back Up (EBU) is used, sometimes telescoped with a mammary artery catheter inside them. The Pachyderm catheters1 are curved in a way that reproduces the shape of the Alplatz left and mammary artery catheters together.
The Pachyderm guiding catheters, which will soon have the CE mark, will save a substantial amount of time, reducing manipulation and improving the efficacy of the procedure.
REFERENCES
1.. JC Lisko, VC Babaliaros, RJ Lederman, et al. Pachyderm-Shape Gudiding Catheters to Simplify BASILICA Leaflet Traversal. Cardiovasc Revasc Med 2019; 20: 782-785.
Palabras clave: implante de válvula aórtica percutánea, BASILICA, prótesis aórtica degenerada, válcula en válvula. Keywords Percutaneous aortic valve implantation, BASILICA, degenerated aortic prosthesis, valve in valve.