Innovation in interventional cardiology
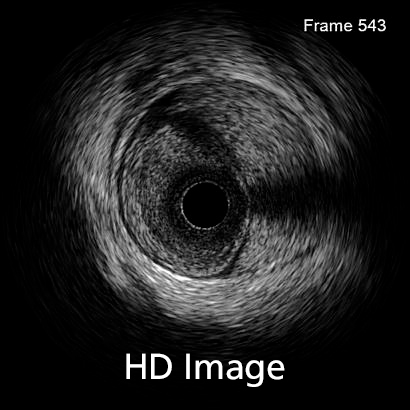
In 2016, 3672 diagnostic intravascular ultrasound (IVUS) studies were performed in Spain. Although the number of cases making use of this technique increased 1.6% compared with 2015, its use has decreased since 2011, when 5124 IVUS studies were performed, in favor of optical coherence tomography (OCT), the use of which has continued to increase.
The quality of intracoronary images acquired with IVUS is improving such that it will undoubtedly affect the choice of diagnostic imaging technique. Several systems provide intracoronary ultrasound images with piezoelectric transducers that range from 20 to 60 MHz. In recent years, there has been an improvement in the axial and lateral spatial resolution of intravascular ultrasound. While axial resolution was previously around 40 microns (even with 60 MHz detectors), with the new OptiCross HD system (Boston Scientific), IVUS resolution approaches that of OCT, which can reach 22 microns. The lateral resolution has also improved, from 80-200 to 50-140 microns, again, approaching that of OCT. This advance is due to a change in the design of the piezoelectric transducer of the catheter, which provides a more sensitive signal with less energy loss and a higher bandwidth, improving the resolution while maintaining the penetration of the IVUS.
This improved image quality makes diagnosis considerably easier and means other diagnostic techniques such as OCT, which require contrast to obtain images, are not required. This is highly relevant in certain patients, such as those with compromised renal function.
It remains to be seen how these changes will affect the rate of use of certain diagnostic imaging techniques over others.
Keywords: ultrasound, IVUS, intravascular ultrasound, HD, OCT, optical coherence tomography
The 2019 Mobile World Congress in Barcelona saw the presentation of an augmented reality system for cardiovascular intervention developed by Philips and Microsoft. The concept of augmented reality is based on the integration of real-time images with live clinical data and 3D medical images to guide procedures accurately and allow the user to concentrate fully on the patient.
This augmented reality system uses images from the new Azurion angiography platform by Philips and a headset with holographic glasses, called HoloLens 2, developed by Microsoft.
Using the HoloLens 2 glasses, Azurion can be controlled in three ways: by voice, eye tracking, or hand gestures. The system allows integration of X-ray and ultrasound images with other 2D and 3D images, meaning that it can be tailored more precisely to the intervention that is to be performed. In reality, HoloLens 2 is a self-contained computer with Wi-Fi connectivity, microphones, spatial audio, electromechanical systems and a power supply.
It is started up with eye movements using Windows Hello and has a holographic visual field that integrates several imaging systems (offline and in real-time) along with 3D clinical data and the controls of the Azurion platform. It safely incorporates Microsoft cloud and artificial intelligence services.
Currently-used systems have large screens showing live 2D images along with other sources of vital data. With this new system it will be possible to work in a 3D augmented reality environment with which doctors will have easy, intuitive and ergonomic control of all available data.
Keywords: Angiography, 3-dimensional imaging, 3D, innovation.
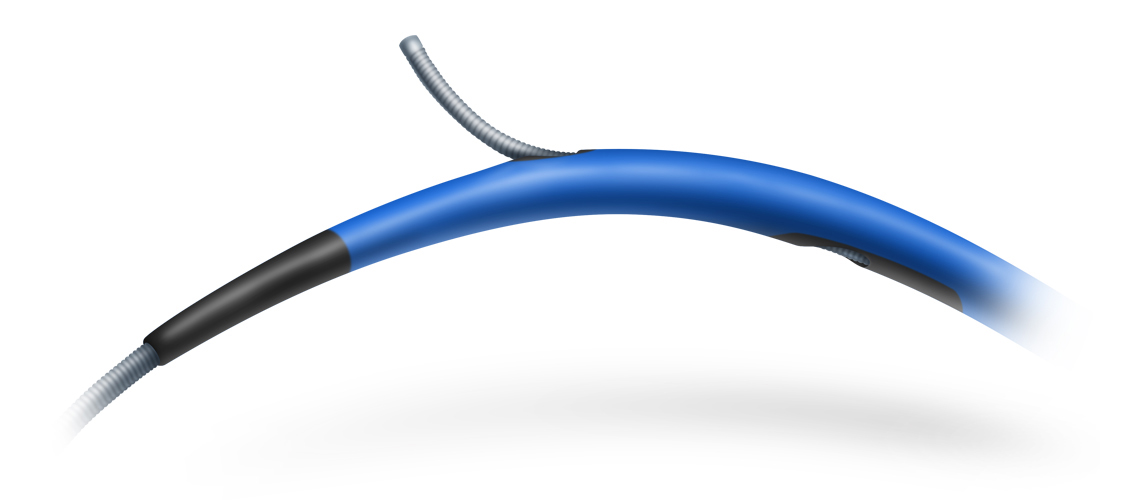
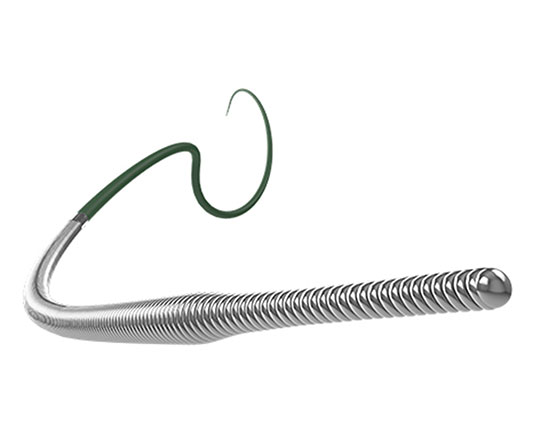
For a while now, several models of dual-lumen microcatheters have been available to interventional cardiology units to address common problems that occur in everyday practice. These devices can be used in certain bifurcations to access difficult-to-reach side branches with unfavorable angles, in complex coronary dissections, and, above all, in interventions for chronic total occlusion to facilitate the LAST (limited antegrade subintimal tracking) technique1. The dual lumen microcatheters that are currently available are characterised by a rapid exchange monorail lumen that runs to the tip of the catheter and a coaxial lumen with lateral exit port.
A different microcatheter is available, with two coaxial lumens (RECROSS; IMDS); one of these runs to the tip of the microcatheter and the other runs to two lateral exit ports at 8 and 12 mm from the tip, positioned 180° from each other. This modification solves one of the problems of conventional microcatheters when the lateral port is not directed toward the area of interest: the ostium of the side branch, the true lumen in a dissection, or intraplaque in an occluded segment. Having a second port facing the opposite direction allows the user to withdraw the coronary guidewire via the opposite side while still looking at the area of interest.
This microcatheter has a 1.5 Fr tip, hydrophilic coating and a stylet for increased support and access to more difficult areas.
Nonetheless, whenever using these devices, it is essential to have a good three-dimensional orientation of the architecture of the vessel we are working in, to obtain the maximum efficiency from the microcatheter.
References
1 . Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal Dissection/Re-entry Strategies in Coronary Chronic Total Occlusion Interventions. Circ Cardiovasc Interv. 2012;5:729–738.
Keywords Chronic total occlusions, microcatheter, dual-lumen microcatheter, rre-entry
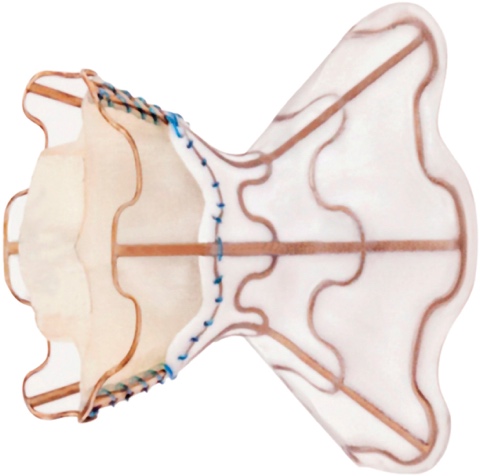
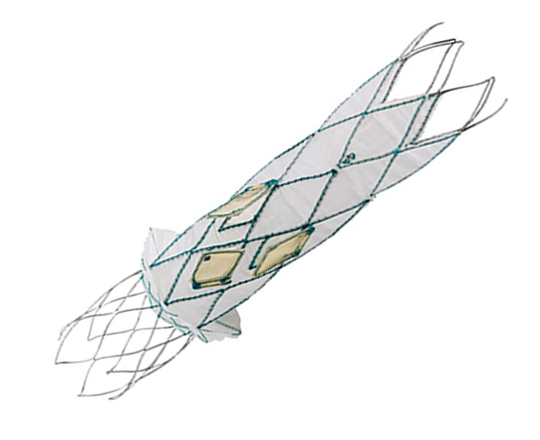
Recently, data were published on a device for the treatment of New York Heart Association (NYHA) class III-IV/IV heart failure in patients with impaired or preserved ventricular function1,2.
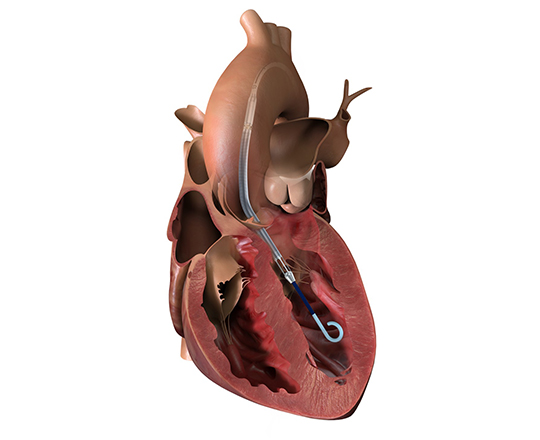
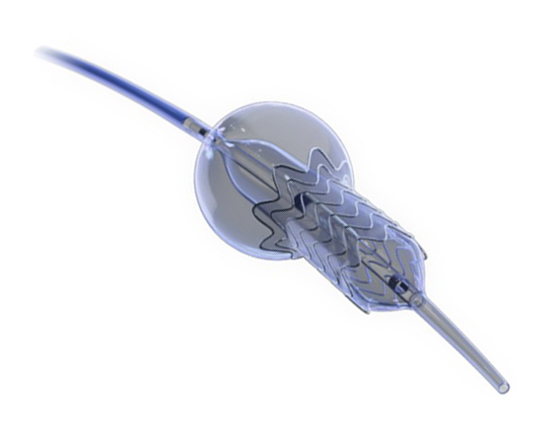
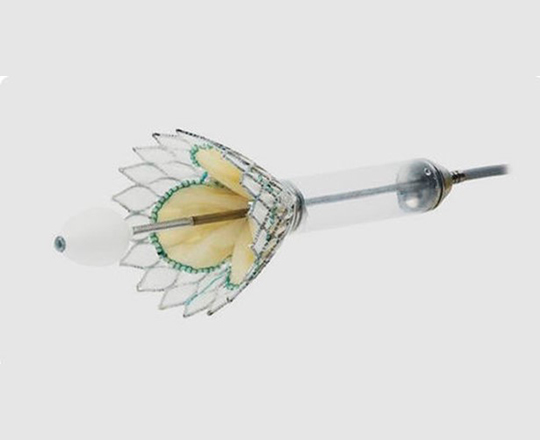
The medical treatment of heart failure can be complemented with cardiac resynchronization devices, ventricular assistance, and transplantation. It is now possible to add a new device: a one-way, hourglass-shaped bioprosthesis that is implanted in the interatrial septum via transseptal puncture. The aim of this treatment is to create a left-to-right shunt in patients with a high left atrial pressure or very restrictive left atrium. This decongestion reduces the pulmonary capillary pressure at rest and during exercise, with a small reduction in cardiac output. The created shunt is small, with a Qp/Qs of approximately 1.27. The volume overload on the right ventricle is low, so the probability of causing pulmonary hypertension is low.
The risk of complications from the procedure is very low: there is a risk of cardiac tamponade, essentially due to the transseptal access (2.6%). However, the improvement in functional class and exercise capacity is significant, as is the reduction in hospitalization, transplantation, ventricular assistance and pulmonary capillary pressure. Nonetheless, some complications have been reported at 12 months’ follow-up, including valve closure in 14% of patients and valve stenosis in 36%.
The technological innovation in this field is promising for the improvement of symptoms in patients with heart failure; in the coming years we will see advances to avoid stenosis in these devices with new randomized trials.
References
1 . Rodés-Cabau J, Bernier M, Amat-Santos IJ, et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave system. J Am Coll Cardiol Intv. 2018; 11, 2300-2310. Epub ahead of print.
2 . Sorajja P, Samara M, Eckman P. Atrial shunting for heart failure: where do we need to go? J Am Coll Cardiol Intv. 2018;11,2311-2313. Epub ahead of print.
Keywords: interatrial shunt, heart failure