QUESTION: How often do you see nonrevascularizable patients with refractory angina today?
ANSWER: The prevalence of this complex entity has gone up thanks to the better prognosis of patients with ischemic heart disease due to changes of lifestyle, drugs, and coronary interventions. Among these, surgical revascularization and, in particular, percutaneous coronary interventions increase survival in the acute coronary syndrome setting. For its diagnosis, reversible myocardial ischemia that cannot be controlled with a combination of medical therapy, angioplasty, and myocardial revascularization surgery needs to be documented first. Although it is often due to severe stenosis of epicardial coronary arteries, it can be seen in non-atherosclerotic coronary artery diseases (spasm, microvascular disease, etc.) and myocardial diseases within the heart-brain axis including myocardial metabolism, and coronary microcirculation. Also, in the perception of pain until neuromodulatory pathways that can also be therapeutic targets in this clinical condition. Therefore, obstructive coronary disease is only one of the possible phenotypes in patients (women in particular) with angina or myocardial ischemia. There is no clear evidence associated with a higher mortality rate; however, the quality of life of rehospitalized patients is worse and health costs are higher.1,2 Former studies1,3 describe a prevalence of refractory angina (RA) of 5% to 10% in patients with chronic coronary syndromes; these studies often focus on nonrevascularizable patients with angina on non-optimized antianginal drug therapy excluding patients without stenosis of epicardial coronary arteries. These studies do not report either on possible cases of RA due to coronary microvascular dysfunction that actually seem to be most of the patients with RA.
Q.: Please explain to us briefly the diagnostic approach used for screening etiologies different from ischemia due to epicardial coronary artery disease such as microvascular ischemia, vasospasm or even noncardiac causes.
A.: Beyond the traditional idea of ischemia-angina where imbalance between myocardial oxygen supply and demand is the main pathophysiological mechanism, we should admit that, in many cases, ischemia is not followed by angina and that many ischemic patients have a constellation of symptoms that may worsen their quality of life.
To improve quality of like is the primary endpoint in patients with angina in the chronic ischemic heart disease setting since its presence or myocardial ischemia do not seem to increase mortality. In this sense, rather than making us question the prognostic value of systematic myocardial revascularization in patients with angina in the chronic coronary syndrome setting, the recent results of the ISCHEMIA clinical trial4 should make us think of the need to conduct routine tests to detect ischemia. In this study, over 20% of the selected patients with angina/ischemia could not be randomized eventually for the lack of significant coronary stenoses. In this sense, it has been reported that nearly 40% of the patients with angina without coronary stenosis showed vasomotor response disturbances in coronary micro and macrocirculation. On the other hand, we should transcend the idea of hypoperfusion as the cause of angina and myocardial ischemia; the metabolic alterations of cardiomyocytes related to hypoxia can be due to peaks in the ischemia threshold as the adaptation of metabolism to hypoperfusion or decrease in metabolic situations of use of substrates of lower energy efficiency.1
The diagnosis of microvascular angina is often achieved after discarding significant epicardial coronary stenosis; since segmental contractility alterations are rare on these patients’ exercise echocardiography, we need specific techniques to assess coronary circulation/perfusion and confirm the diagnosis.
I’d like to add that angina, including RA, in the absence of significant coronary stenosis cannot be a diagnosis of exclusion. New protocols are needed to confirm not only the clinical signs and ischemia, but also the pathophysiological mechanisms involved. Only then the diagnostic and therapeutic strategy will be complete for each particular case.
Q.: Which are the best pharmacological strategies to treat refractory angina and how should drug escalation be done?
A.: I think that changing our management of nonrevascularizable patients with RA to improve their clinical situations is a priority. These patients often remain in some sort of diagnostic and therapeutic limbo. Disease exists beyond the possibilities of coronary dilatation. Therefore, only by knowing the pathophysiological substrate of angina in each particular case, we’ll be able to establish protocols including new therapeutic, pharmacological or other modalities.5
The standard of care for the management of patients with RA is to discard and treat secondary causes by optimizing medical therapy and rehabilitation. These patients should be treated with antianginal drug therapy, beta-blockers, dihydropyridine calcium channel blockers, and sustained-release nitrates. In cases of persistent angina, ivabradine can be used in patients who are in sinus rhythm with heart rates ≥ 70 beats/minute. Also, the possible combination of ranolazine, trimetazidine, and nicorandil is another antianginal therapeutic option. Patients with RA without obstructive coronary lesions benefit from a better control of their angina when therapy is based on every particular patient and the results of intracoronary functional tests. Patients with microvascular angina, reduced coronary flow reserve, increased resistances, and a negative acetylcholine testing respond positively to treatment with beta-blockers, angiotensin-converting enzyme inhibitors, and statins. They are also responsive to rehabilitation programs to control their risk factors, physical exercise, and weight loss. Patients with EKG changes and angina as a response to the acetylcholine testing but without epicardial coronary spasm should be treated as if they had vasospastic angina: dihydropyridine calcium channel blockers and sustained-release nitrates are the drugs of choice associated with changes in the patient’s lifestyle and control of their risk factors. Data available today show the efficacy of ranolazine plus traditional antianginal drugs in patients with microvascular angina; due to its mechanism of action, it should be considered a supplementary therapeutic option in this group of patients
Q.: Which non-invasive, non-pharmacological alternatives exist for these patients?
A.: As I already mentioned, patients with RA should join structured cardiac rehabilitation programs to change their lifestyle. Exercise adapted to each particular case has proven to improve ischemia and stimulate collateral circulation, coronary vascular function, and control the main cardiovascular risk factors. To control the symptoms, transcutaneous electrical nerve stimulation has been suggested. Extracorporeal shock waves can promote myocardial revascularization, and external counterpulsation generates diastolic retrograde aortic flow that increases the coronary diastolic and mean pressures. Also, retrograde flow improves myocardial perfusion after inducing flow-mediated coronary vasodilation and stimulates angiogenesis. There is not a single solid piece of evidence on the utility of these techniques in patients with RA. Actually, they should be used based on each particular case strictly as compassionate use and only when the remaining therapeutic options have failed.1
Q.: What evidence do we have today on the Reducer device?
A.: Several invasive techniques have been developed to improve angina including neural stimulation, stellate ganglion block, myocardial laser revascularization, angiogenic stimulation with plasmid DNA, and implantation of CD34+ and CD133+ cells. The results of a recent meta-analysis on the intramyocardial injection of CD34+ have confirmed that it improves angina, the patients’ functional capacity, and prognosis. However, for the time being these techniques are in research stage only.1
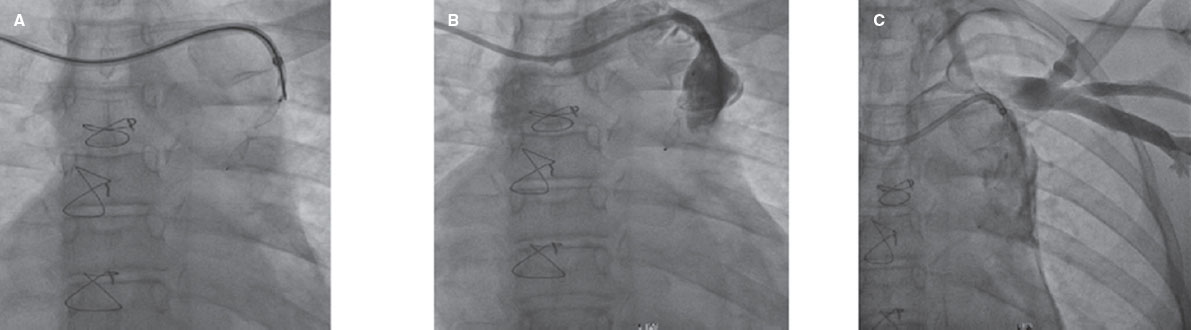
The description that an increased coronary sinus pressure induces myocardial flow redistribution in the ischemic regions of patients with RA is the basis of the Reducer device design (Neovasc Inc., Canada). It is a metal balloon-expandable percutaneous device that causes the focal the stenosis of coronary sinus rising its pressure and elevating it in the venules and capillaries too. This promotes the redistribution of flow bringing the endocardial/epicardial perfusion gradient back to normal.1 Several studies confirm the safety profile of this technique and its benefits regarding symptom improvement; the early study results of 15 patients confirmed its long-term safety profile with 12-year patency in 10 of these patients and symptom improvement. However, device migration and coronary sinus perforation and bleeding have been reported; these are complications that should be taken into consideration when giving this device a clinical use. From my own point of view, the only randomized clinical trial published to this date, the COSIRA (Coronary sinus reducer for treatment of refractory angina)6 offers modest efficacy results. A total of 104 patients with RA were randomized to receive the Reducer device or undergo a sham procedure. The patients who received the device improved their angina symptoms and quality of life. However, no significant changes were seen in the stability and frequency of angina or in the duration of exercise.
Several real-world registries confirm the efficacy and safety profile seen in the clinical trial with maximum clinical benefits reported after 4 months and maintained at the 2-year follow-up.7 Improvements in inducible ischemia, functional capacity in the cardiopulmonary exercise testing, myocardial perfusion, and cardiac function have been reported. A special comment should be made on symptom efficacy and myocardial perfusion with the Reducer device in 8 patients with microvascular angina.1
Considering the time elapsed since the arrival of the Reducer device and the scarce quality of scientific evidence with just one randomized clinical trial available and a very qualitative primary endpoint (the assessment of the patient’s self-reported angina), in my opinion, at least, 2 clinical trials should be conducted: the first one in patients with RA due to nonrevascularizable coronary lesions. The second one in patients with microvascular angina. The first study primary endpoint should include symptom improvement according to some objective test of overload and myocardial perfusion assessed through cardiac magnetic resonance imaging. The second study primary endpoint should also include these components plus the functional assessment of coronary microcirculation. In the meantime, I think it should only be used as compassionate use in quality high-volume PCI-capable centers for the management of patients with RA on an optimized therapeutic strategy and clinical follow-up including objective tests to assess ischemia and myocardial perfusion.
FUNDING
No funding was received for this work.
CONFLICTS OF INTEREST
The authors declare no conflicts related to this work.
REFERENCES
1. Gallone G, Baldetti L, Tzanis G, et al. Refractory angina. From pathophysiology to new therapeutic nonpharmacological technologies. JACC Cardiovasc Interv. 2020;13:1-19.
2. Lanza GA, Crea F, Kaski JC. Clinical outcomes in patients with primary stables microvascular angina:is the jury still out?Eur Heart J Qual Care Clin Outcomes. 2019;5:283-291.
3. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445-1453.
4. Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395-1407.
5. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
6. Verheye S, Jolicœur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372:519-527.
7. Giannini F, Baldetti L, Ponticelli F, et al. Coronary Sinus Reducer Implantation for the Treatment of Chronic Refractory Angina:A Single-Center Experience. JACC Cardiovasc Interv. 2018;11:784-792.
Corresponding author: Servicio de Cardiología, Hospital Clínico Universitario, Travesía de A Choupana s/n, 15706 Santiago de Compostela, A Coruña, Spain.
E-mail address: jose.ramon.gonzalez.juanatey@sergas.es (J.R. González-Juanatey).