To the Editor,
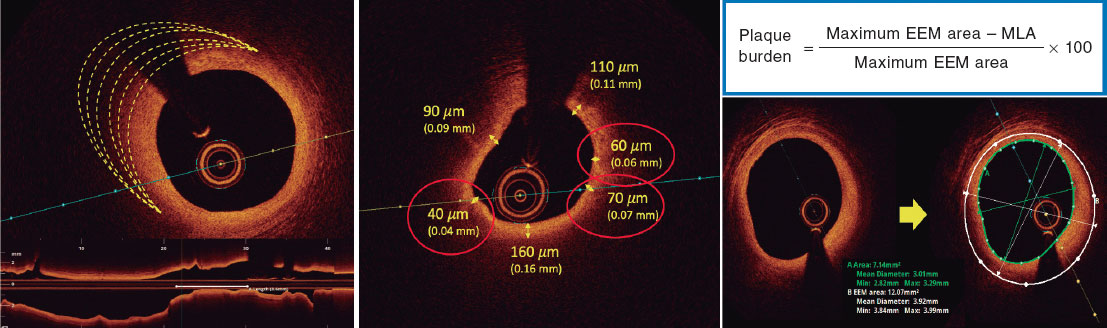
Drug-eluting stents (DES) can show mechanical failure at implantation. Diagnosis of stent underexpansion through intravascular ultrasound (IVUS) seems to be the main mechanism of thrombosis and restenosis.1 In the past, durable first-generation DES polymers have been associated with late adverse clinical events. The Firehawk stent (MicroPort Medical, China), is a cobalt-chrome structure with biodegradable sirolimus-containing polymer coating in abluminal grooves, designed to mitigate polymer load and to reduce drug concentrations in the vessel wall.2 This third generation DES device has been tested in various studies.3 The TARGET All Comers trial reported noninferiority in target lesion failure (TLF) at 1 year of follow-up with the Firehawk stent compared with SFA XIENCE (Abbott, United States) with durable polymer. Although the results have shown noninferiority, a 1.2% rate of definitive thrombosis was observed throughout the 12-month follow-up, which could be related to the lack of use of intravascular imaging to guide stent implantation. The use of this imaging modality leads to reductions in mortality, treated vessel-related myocardial infarction (MI) and clinically guided revascularization compared with procedures guided by angiography alone.
The aim of the present study was to assess the mid-term outcomes in real-world patients from a single center in Brazil who underwent Firehawk stent implantation guided by IVUS in nonselected coronary lesions.
This prospective, observational, nonrandomized, single arm pilot study included 100 patients with severe coronary artery disease treated with the Firehawk stent, guided by IVUS between May 2019 and December 2021 who were older than 18 years and had a wide range of clinical indications ranging from silent ischemia with positive functional tests and stable angina to acute coronary syndrome. Stent diameter and extension were selected based on IVUS data. Exclusion criteria were life expectancy less than 1 year, left ventricular ejection fraction < 40%, Firehawk stent implantation not guided by IVUS, and percutaneous coronary intervention (PCI) without at least 1 Firehawk stent. Following the consensus document of the European Association of Percutaneous Cardiovascular Interventions, stent expansion was defined as “the minimum stent cross sectional area either as an absolute measure (absolute expansion), or compared with the predefined reference area, which can be the proximal, distal, largest, or average reference area (relative expansion)”. Considering this reference, a relative stent expansion > 80% was used as a predefined criterion. All patients completed 12 months of clinical follow-up. The study protocol was approved by the research ethics committee (n. 59849822.2.0000.0098) and all patients signed an informed consent form.
The patients’ clinical characteristics and baseline angiographic lesions, procedural features and IVUS findings are shown in table 1. IVUS was used in all patients (100%), and 156 lesions were evaluated. IVUS diameter and the extension of these lesions were 2.88 ± 0.44 mm and 24.87 ± 7.21 mm, with these values being higher than those obtained through quantitative coronary angiography (2.45 ± 0.61 mm and 18.21 ± 7.14 mm, respectively). In total, 126 vessels (156 lesions) received 164 Firehawk DES (1.6/patient). The mean diameter and length of implanted DES were 3.0 ± 0.53 mm and 25.23 ± 8.35 mm, respectively.
Table 1. Patients’ clinical characteristics and baseline angiographic lesions, procedural features, and IVUS findings
| Clinical, angiographic, and procedure findings | N = 100 | Clinical, angiographic, and procedure findings | N = 100 |
|---|---|---|---|
| Male sex | 71 (71) | Lesion classification* B2/C | 95 (61) |
| Diabetes mellitus | 53 (53) | Syntax score | 18.5 ± 9.34 |
| Previous PCI | 37 (37) | QCA, vessel reference diameter (mm) | 2.45 ± 0.61 |
| Previous CABG | 9 (9) | QCA, lesion extension (mm) | 18.21 ± 7.14 |
| Previous MI | 21 (21) | Predilation due to lesion | 134 (85) |
| Baseline clinical diagnostic | Lesion predilation with CB or PTCRA | 14 (10) | |
| Q-wave MI | 7 (7) | Procedural success | 100 (100) |
| Non-Q-wave MI | 16 (16) | Pre- and post-PCI IVUS | N = 156/164 |
| Unstable angina | 28 (28) | Evaluation based on pre-PCI IVUS | 156 (100) |
| Stable angina | 26 (26) | Fibrolipid plaque | 81 (53) |
| Atypical angina | 5 (5) | Calcified plaque | 38 (24) |
| Silent ischemia | 15 (15) | Fibrotic plaque | 26 (17) |
| LVEF | 62.8 ± 7.4 | Intrastent restenosis | 10 (6) |
| Multivessel disease | 67 (67) | Stenosis diameter | 80.82 ± 6.21 |
| Treated vessels | N = 126 (100) | Reference diameter, mm | 2.88 ± 0.44 |
| Left main coronary artery | 9 (5) | Lesion extension, mm | 24.87 ± 7.21 |
| Left anterior descending coronary artery | 67 (41) | Lesion with extension > 28 mm | 48 (31) |
| Left circumflex coronary artery | 34 (21) | Stents implanted per lesion | 1.6 ± 0.84 |
| Right coronary artery | 54 (33) | Diameter of implanted stent, mm | 3.0 ± 0.53 |
| Treated lesions | N = 156 (100) | Extension of implanted stent, mm | 25.23 ± 8.35 |
| De novo lesions | 146 (94) | Final evaluation through post-PCI IVUS | 164 (100) |
| Intrastent, restenosis | 10 (6) | Post-IVUS reintervention | 27 (16) |
| Total oclusion | 8 (5) | Mean stent expansion, (%) (distal reference) | 91.8 |
| Bifurcation | 55 (35) | ||
|
BP, blood pressure; CABG, coronary artery bypass graft surgery; CB, cutting balloon; IVUS, intracoronary ultrasound; LDL, low-density lipoproteins; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PTCRA, percutaneous transluminal rotational atherectomy; PCI, percutaneous coronary intervention; PCI, percutaneous coronary intervention; QCA, quantitative coronary analysis. * According to the ACC/AHA. Data are expressed as No. (%) or mean ± standard deviation |
|||
Among the 164 DES assessed through IVUS after satisfactory angiographic results, 27 (16%) required reintervention for the following reasons: a) acute malapposition in 12 (44.5%); b) underexpansion in 10 (37%); c) edge dissection in 3 (11%); and d) plaque protrusion in 2 (7.5%). Considering the stent expansion criteria, the analysis of this cohort showed a mean stent expansion of 91.8% regarding the distal reference (table 1).
Table 2 provides a detailed description of all clinical events at 12 months of follow-up, patient-oriented composite endpoints (PoCE), and device-oriented composite endpoints (DoCE)-TLF. PoCE were observed in 6% of patients (6 events in 5 patients), all-cause death in 1% (1 patient), MI in 1% (1 patient), and target vessel revascularization (TVR) in 4% (4 patients). DoCE-TLF were observed in 1% (1 stent with 3 events: non-Q-Wave MI in 1.00%, target vessel MI in 1% and ischemia-driven TLR in 1%), and cardiac death in 0%. The description of events is as follows: patient No. 1: TVR. A lesion was found on follow-up angiography when a new intervention was planned for second vessel disease. Patient No. 2: TVR and TLR. A lesion was found on follow-up angiography when a new intervention was planned for second vessel disease. Patient No. 3: NSTEMI, TVR and TLR. Patient No. 4: TVR with ischemic perfusion test. Patient No. 5: noncardiac death. There was no stent thrombosis. The relationship between IVUS final minimum sent area and clinical events is shown but the analysis is clearly underpowered.
Table 2. Clinical outcomes at 12 months of follow-up in 100 patients
| N = 100 | ||
|---|---|---|
| Primary endpoints | ||
| PoCE | 6 (6) | |
| All-cause death | 1 (1) | |
| All MI | 1 (1) | |
| All revascularization | 4 (4) | |
| TVR | 4 (4) | |
| Secondary endpoints | ||
| DoCE (TLF) | 1 (1) | |
| Cardiac death | 0 (0) | |
| Q-wave MI | 0 (0) | |
| Non-Q-wave MI | 1 (1) | |
| Target vessel-related MI | 1 (1) | |
| Ischemia-drivenTLR | 1 (1) | |
| Definitive/probable thrombosis (acute or late) | 0 (0) | |
| Clinical events analysis considering IVUS final luminal area | ≤ 5.5 mm² (51 stents) | > 5.5 mm² (113 stents) |
| DoCE (TLF) | 0 (0) | 1 (0.88) |
| Cardiac death | 0 (0) | 0 (0) |
| Q-wave MI | 0 (0) | 0 (0) |
| Non-Q-wave MI | 0 (0) | 1 (0.88) |
| Target vessel-related MI | 0 (0) | 1 (0.88) |
| TLR | 1 (1.96) | 1 (0.88) |
| Ischemia-driven TLR | 0 (0) | 0 (0) |
| Definitive/probable thrombosis (acute or late) | 0 (0) | 0 (0) |
|
DoCE, device-oriented composite endpoints (secondary endpoints), composite of cardiac death, target vessel myocardial infarction, ischemia-driven target lesion revascularization, and definite or probable (acute or late) thrombosis; MI, myocardial infarction; n, number; PoCE, patient-oriented composite endpoints (primary endpoints), composite of all-cause death, any myocardial infarction, and any target vessel revascularization; TLF, target lesion failure; TLR, target lesion revascularization; TVR, target vessel revascularization. Data are expressed as No. (%). |
||
The present study reports our initial experience of using the Firehawk stent with routine use of IVUS before and after PCI. Safety and efficacy were demonstrated by the low PoCE and DoCE at 12 months of clinical follow-up, highlighting the absence of stent thrombosis. Some studies have identified stent underexpansion, geographical miss, and dissection of stent edges as independent causes of intrastent thrombosis.4 All these predictors can be detected and properly treated through IVUS. According to the final IVUS analysis, this study showed that reintervention for optimization was required in 16% of the cases. All patients in this database received dual antiplatelet therapy for at least 12 months. These 2 factors can be closely linked to lack of thrombotic events in the assessed population.
The main limitations of this prospective study are its population size, due to its observational and nonrandomized nature. However, its value lies in the fact that it represents one of the main clinical experiences in Brazil with Firehawk stent implantation guided by IVUS, at all procedure stages, showing favorable performance after 12 months of follow-up. These findings and the data available in the literature, provide clinical support for the use of the fully biodegradable sirolimus-containing polymer-coated Firehawk stent.
FUNDING
No funding sources.
ETHICAL CONSIDERATIONS
The study protocol was approved by the research ethics committee (n. 59849822.2.0000.0098). All patients signed the informed consent form.
Considering the small size of the group of patients analyzed and that the percentages of both genders reflect those observed in our daily practice, the authors believe that there was no reason to carry out a sex/gender analysis.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCE
The authors confirm that artificial intelligence was not used in the preparation of this work.
AUTHORS’ CONTRIBUTIONS
C.R. Costantini, M.A. Denk, S.G. Tarbine: study idea, and data mining and analysis. C.O. Costantini, V. Shibata, R.M. De Macedo: manuscript review and edition.
CONFLICTS OF INTEREST
The authors declare no conflict of interest related to the present manuscript.
REFERENCES
1. Mintz GS. Features and parameters of drug-eluting stent deployment discoverable by intravascular ultrasound. Am J Cardiol. 2007;100(8B):26M-35M.
2. Gao RL, Xu B, Lansky AJ, et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent:clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013;9:75-83.
3. Li C, Guan C, Zhang R, et al. Safety and efficacy of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent for the treatment of de novo coronary lesions:Final five-year results of the patient-level pooled analysis from the TARGET I and TARGET II trials. Catheter Cardiovasc Interv. 2019;93:818-24.
4. Liu X, Tsujita K, Maehara A, et al. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv.2009;2:997-1004.
* Corresponding author.
E-mail address: sgtarbine@gmail.com (C.R. Frack Costantini)