Abstract
Introduction and objectives: Evidence of the long-term prognostic benefit of new generation drug-eluting stents (DES) is limited, especially in the context of primary percutaneous coronary interventions. The goal of this study was to compare the long-term prognostic impact of the implantation of DESs versus bare-metal stents (BMSs) in real-world patients undergoing primary percutaneous coronary interventions.
Methods: A cohort study was conducted with 1499 consecutive patients diagnosed with ST-segment elevation myocardial infarction who underwent percutaneous coronary interventions between January 2008 and December 2015. A total of 24.9% of the patients received a DES. A matched propensity score analysis yielded 2 groups of 262 matched patients depending on whether they were treated with a DES or a BMS.
Results: During follow-up (median 1015 days), the patients who received DES had a lower all-cause mortality rate (6.5% vs 12.2%; P = .049) a lower composite endpoint of major adverse cardiac events (16.4% vs 25.2%; P = .049) and a lower patient-oriented composite endpoint of death from any cause, myocardial infarction and revascularization at follow-up (12.6% vs 22.5%; P = .017). No differences were seen in the definite stent thrombosis rate.
Conclusions: In our registry, in a real-world population of consecutive patients undergoing primary percutaneous coronary interventions, the use of DES versus BMS associated more survival and less clinically significant major adverse cardiac events and patient-oriented composite endpoints in a long-term follow-up, without any differences in stent thrombosis.
Keywords: Drug-eluting stent. Bare-metal stent. Primary PCI. ST-segment elevation myocardial infarction.
Resumen
Introducción y objetivos: La evidencia del beneficio en el pronóstico a largo plazo de los stents farmacoactivos (SFA) de nueva generación es limitada, en especial en los pacientes con angioplastia primaria. El objetivo de este trabajo fue comparar el impacto en el pronóstico a largo plazo de la implantación de SFA frente a stents metálicos (SM) en pacientes del mundo real tratados con angioplastia primaria.
Métodos: Estudio de cohortes en el que incluyeron 1.499 pacientes ingresados de forma consecutiva con diagnóstico de infarto agudo de miocardio con elevación del segmento ST y sometidos a angioplastia primaria entre enero de 2008 y diciembre de 2015. El 24,9% recibió un SFA. Mediante un análisis de emparejamiento por puntuación de propensión se obtuvieron 2 grupos de 262 pacientes emparejados según la implantación de SFA o SM.
Resultados: Durante el seguimiento (mediana de 1.015 días), los pacientes que recibieron SFA tuvieron tasas más bajas de mortalidad por todas las causas (6,5 frente a 12,2%; p = 0,049), así como en el objetivo combinado de eventos adversos mayores (16,4 frente a 25,2%; p = 0,049) y un objetivo combinado orientado al paciente que incluía muerte por cualquier causa, infarto de miocardio y revascularización en el seguimiento (12,6 frente a 22,5%; p = 0,017). No se observaron diferencias en cuanto a trombosis definitiva del stent.
Conclusiones: En nuestro registro, en una población del mundo real de pacientes consecutivos tratados con ICP primaria, la utilización de SFA frente a SM se asoció a una mayor supervivencia y una reducción de los eventos clínicos en el seguimiento a largo plazo, sin observar diferencias en la trombosis del stent.
Palabras clave: Stent farmacoactivo. Stent metálico. Angioplastia primaria. Infarto agudo de miocardio con elevación del segmento ST.
Abbreviations: BMS: bare-metal stent. DES: drug-eluting stent. MACE: major adverse cardiovascular event. PCI: percutaneous coronary intervention. STEMI: ST-segment elevation myocardial infarction.
Introduction
Percutaneous coronary intervention (PCI) is the treatment of choice for the management of ST-segment elevation myocardial infarction (STEMI). First-generation drug-eluting stents (DES) reduced restenosis and the need for reinterventions compared to bare-metal stents (BMS)1,2. However, the higher incidence rates of late thrombosis3, mortality and infarction4 ueled controversy over the implementation of these devices in patients with STEMI, a population with an identified increased risk of stent thrombosis5.
Second-generation DES with thinner struts, biocompatible poly-mers, and thromboresistant properties proved to be safe and more effective than first-generation DES and traditional BMS, particularly with significant reductions in angiographic restenosis and unplanned revascularizations of the target injury or culprit artery.6. The actual clinical guidelines for the management of STEMI recommend the use of new-generation DES7.
In a combined analysis of the EXAMINATION and COMFORTABLEAMI clinical trials that compared new-generation DES versus BMS, the use of a DES was associated with increased safety and efficacy at 1 year8. In the 2-year follow-up of patients included in the COMFORTABLE-AMI trial, the use of DES was associated with a reduction in a composite of all-cause mortality, follow-up myocardial infarction, and new revascularizations9. The results of the 5-year follow-up of the EXAMINATION10, that compared an everolimus-eluting stent to a BMS showed that the new-generation DES was associated with more survival and less myocardial infarctions at follow-up6.
Our goal was to analyze the long-term prognostic impact of newgeneration DES in a real-world population of patients with STEMI.
Methods
Study population
This is a retrospective observational study that included (n = 1499) all consecutive patients admitted due to STEMI who underwent primary percutaneous interventions (PCI) at our center between January 2008 and December 2015. The patients who were not implanted with a stent during the percutaneous coronary intervention (PCI) (n = 131) and those implanted with a bioabsorbable scaffold (n = 11) were excluded. In 24.9% of patients (n = 374), the PCI was conducted with DES implantation in the infarctrelated artery.
The PCI was conducted following the guidelines from the European Society of Cardiology7,11 and the decision to implant a DES or a BMS was left to the attending interventional cardiologist clinical criteria. Antiplatelet therapy consisted of acetylsalicylic acid and a P2Y12 inhibitor (clopidogrel during the early years and ticagrelor, and prasugrel more recently).
Demographic, clinical, echocardiographic, coronary angiography and laboratory data were collected by cardiologists in a computerized database. Both the material used during the PCI and the characteristics of the procedure were included at the time of the PCI by the specialist in hemodynamics and the attending operator. The structured follow-up was conducted using the IANUS electronic health record system (the only one available and mandatory in Galicia, Spain). Events were independently adjudicated by 2 independent cardiologists and when they disagreed, by a third cardiologist.
Definitions
The diagnoses of STEMI and myocardial infarction were established based on the actual clinical guidelines7,12. Ischemia time was defined as the time elapsed between symptom onset and reperfusion (the passage of the guide wire through the culprit artery during PCI). Target vessel revascularization and target lesion revascularization were defined following the ARC (Academic Research Consortium) criteria13.
Major adverse cardiovascular events (MACE) included all-cause mortality, acute myocardial infarction, heart failure requiring hospitalization and new, unplanned revascularizations. Following the recommendations from the ARC for the study of stent prognosis, a composite goal of major patient-oriented composite endpoint (POCE) of death from any cause, any myocardial infarctions or new unplanned revascularization was included13. The deviceoriented composite endpoint (DOCE) included cardiac death, target vessel myocardial infarction and ischemia-driven target lesion revascularization. Definite stent thrombosis was considered as angiographically proven thrombosis.
Study objectives
The main objective of this study was to compare the long-term prognosis of revascularization with DES vs BMS in consecutive patients admitted due to STEMI who underwent PCI. The clinical outcomes were assessed based on all-cause mortality, a composite of MACE, POCE, and DOCE endpoints, and on each component separately. The median follow-up was 1015 days, and the interquartile range (IQR), 400-1800 days.
Statistical analysis
The differences in the descriptive analysis were assessed using the difference of means Student t test and the chi-square test of comparison of proportions, depending on whether the variable was continuous or categorical. To minimize the bias involved when studying the prognostic effect of the DES versus the BMS implant from an observational point of view, a propensity score matching analysis was performed. The variables included in the model were age, sex, body mass index, arterial hypertension, diabetes, dyslipidemia, smoking, ischemic heart disease, time of ischemia, infarct location, culprit artery involved in the infarction, use of glycoprotein IIb/IIIa inhibitors, number of diseased vessels, the glomerular filtration rate, the creatinine levels at admission, the peak troponin I levels, hemoglobin, glucose, heart rate, systolic blood pressure, Killip class, left ventricular ejection fraction, GRACE score, CRUSADE score, and year of inclusion in the analysis. An analysis of the variance inflation factor showed no issues of multicollinearity in the variables used (variance inflation factor 1.56 and no variable > 4). The caliper used was 0.25, and the sensitivity-specificity ratio obtained was high (75% area under the curve). No variable had a strong bias, being the average bias, 3.3%. After propensity score matching, no statistically significant differences were seen in any of the variables studied.
The graphs figure 1 and figure 2 show the Nelson-Aalen estimate of the cumulative hazard function, and the differences were assessed using the log-rank test. The hazard ratio was calculated using the univariate Cox regression analaysis.
Statistical analysis was performed using the STATA 14 and SPSS 22.0 statistical packages.
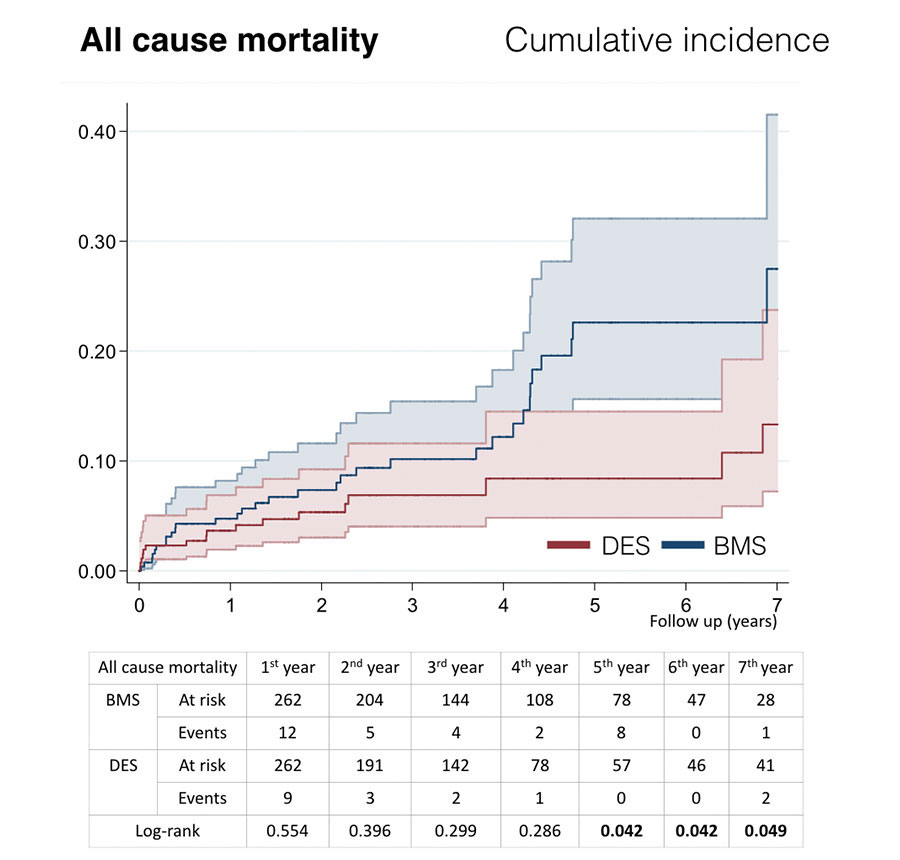
Figure 1. Cumulative incidence curves for survival. BMS, baremetal stent; DES, drug-eluting stent.
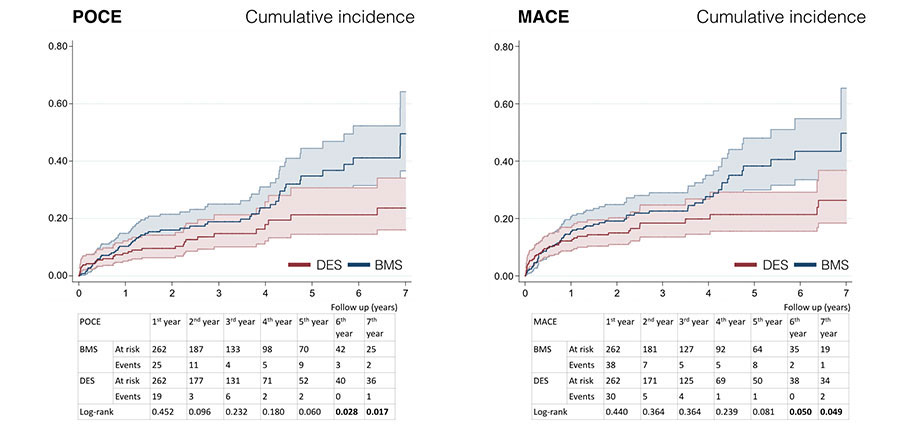
Figure 2. Cumulative incidence curves for POCE and MACE-free survival. BMS, bare-metal stent; DES, drug-eluting stent; MACE, major adverse cardiovascular events; POCE, patient-oriented combined endpoint.
Results
Baseline characteristics
The overall study cohort included 1357 patients; 983 patients received BMS and 374 received DES. The patients in the DES group were younger, more frequently males, with a higher body mass index and CRUSADE scores of higher hemorrhagic risk. The patients revascularized with BMS usually had anterior wall infarctions, lower hemoglobin levels, and poor renal function. The total length of the implanted stents was higher in the DES group, and the diameter of the stents was larger in patients with BMS. There were no significant differences in other cardiovascular risk factors, time of ischemia, peak troponin levels, hemodynamic status, Killip class at admission, left ventricular ejection fraction, GRACE score, number of lesions treated, number of stents used, or pharmacological treatment at discharge, with the exception of antiplatelet therapy (table 1).
The propensity score-matched cohort study consisted of 262 patients of each pair and showed no significant differences in any of the aforementioned variables (table 1).
Table 1. Baseline characteristics of the overall cohort and the propensity score matched cohort
| Overall cohort | Propensity score matched cohort | |||||
| BMS | DES | p | BMS | DES | p | |
| (n = 983) | (n = 374) | (n = 262) | (n = 262) | |||
| Demographics | ||||||
| Age (years) | 65 (14) | 62 (12) | <0,001 | 62 (14) | 63 (12) | 0,847 |
| Gender (male) | 76,5% | 81,6% | 0,037 | 80,9% | 81,3% | 0,911 |
| BMI (kg/m2) | 28 (4) | 29 (4) | 0,039 | 28 (4) | 29 (4) | 0,981 |
| Personal history | ||||||
| Hypertension | 48,3% | 49,5% | 0,707 | 50,8% | 49,6% | 0,794 |
| Diabetes mellitus | 19,9% | 28,6% | 0,001 | 26,0% | 24,8% | 0,764 |
| Dyslipidemia | 46,6% | 54,3% | 0,012 | 51,1% | 51,9% | 0,862 |
| Tobacco | 49,5% | 48,1% | 0,642 | 49,6% | 49,6% | 1 |
| Ischemic heart disease | 9,6% | 11,5% | 11,5% | 11,8% | 11,5% | 0,892 |
| PCI data | ||||||
| Ischemic time (min.) | 271 (202) | 289 (232) | 0,227 | 291 (215) | 279 (222) | 0,549 |
| Anterior wall location | 41,6% | 25,9% | <0,001 | 32,4% | 31,3% | 0,779 |
| Culprit artery in the infarction | 0,033 | 0,130 | ||||
| LAD | 40,5% | 42,8% | 40,8% | 38,2% | ||
| Cx | 15,4% | 18,5% | 17,2% | 20,2% | ||
| RCA | 43,2% | 36,4% | 42,0% | 39,3% | ||
| LM | 0,7% | 1,6% | - | 1,2% | ||
| Number of diseased vessels | 0,626 | 0,696 | ||||
| Two vessels | 28,1% | 27,0% | 28,2% | 25,2% | ||
| Three vessels | 14,8% | 16,8% | 13,4% | 14,9% | ||
| Number of lesions treated | 0,387 | 0,537 | ||||
| 1 | 93,9% | 92,8% | 95,0% | 93,5% | ||
| 2 | 5,3% | 6,2% | 5,0% | 5,7% | ||
| 3 | 0,8% | 0,8% | - | 0,4% | ||
| Pre-PCI TIMI flow | 0,380 | 0,982 | ||||
| 0 | 80,9% | 80,8% | 83,6% | 83,2% | ||
| 1 | 4,7% | 2,9% | 3,1% | 2,7% | ||
| 2 | 7,5% | 9,4% | 7,2% | 8,0% | ||
| 3 | 6,9% | 7,0% | 6,1% | 6,1% | ||
| Post-PCI TIMI flow | 0,262 | 0,917 | ||||
| 0 | 0,9% | 0,8% | 1,2% | 1,2% | ||
| 1 | 0,9% | 0,5% | 0,8% | 0,8% | ||
| 2 | 2,8% | 1,1% | 1,9% | 1,2% | ||
| 3 | 98,4% | 97,6% | 96,2% | 97,5% | ||
| Use of glycoprotein IIb/IIIa inhibitors | ||||||
| Thrombectomy | 68,8% | 69,0% | 0,939 | 73,3% | 72,5% | 0,845 |
| Number of stents | 0,619 | 0,192 | ||||
| 1 | 71,4% | 67,9% | 70,6% | 69,1% | ||
| 2 | 22,6% | 24,3% | 21,8% | 24,4% | ||
| 3 | 4,6% | 6,4% | 5,0% | 5,7% | ||
| 4 | 1,0% | 0,8% | 2,7% | 0,4% | ||
| 5 | 0,3% | 0,5% | – | 0,4% | ||
| 6 | 0,1% | – | – | – | ||
| Laboratory parameters | ||||||
| GFR (mL/min) | 83 (37) | 97 (38) | < 0,001 | 96 (44) | 93 (35) | 0,378 |
| Creatinine levels (mg/dL) | 1,1 (0,6) | 0,9 (0,6) | 0,001 | 1,0 (0,6) | 1,0 (0,6) | 0,752 |
| Peak troponin I levels (ng/mL) | 107 (133) | 105 (113) | 0,747 | 111 (127) | 108 (113) | 0,769 |
| Hemoglobin (g/dL) | 14,3 (1,8) | 14,6 (2,9) | 0,018 | 12,4 (1,6) | 14,4 (1,7) | 0,860 |
| Glucose (mg/dL) | 170 (87) | 174 (115) | 0,552 | 169 (79) | 166 (81) | 0,662 |
| Clinical data | ||||||
| Heart rate (bpm) | 77 (21) | 76 (19) | 0,339 | 74 (19) | 75 (19) | 0,639 |
| SBP (mmHg) | 128 (29) | 130 (29) | 0,209 | 132 (25) | 129 (29) | 0,320 |
| Killip class | 0,379 | 0,731 | ||||
| Class I | 82,7% | 84,0% | 87,4% | 88,6% | ||
| Class II | 6,3% | 7,2% | 4,6% | 5,7% | ||
| Class III | 2,9% | 1,3% | 2,7% | 1,5% | ||
| Class IV | 8,1% | 7,5% | 5,3% | 6,1% | ||
| LVEF (%) | 51 (12) | 52 (11) | 0,200 | 52 (11) | 52 (10) | 0,699 |
| GRACE score | 162 (46) | 158 (78) | 0,432 | 152 (40) | 153 (41) | 0,785 |
| CRUSADE score | 27 (18) | 22 (14) | < 0,001 | 21 (14) | 22 (13) | 0,581 |
| Treatment at discharge | ||||||
| Acetylsalicylic acid | 99,0% | 99,5% | 0,401 | 99,6% | 99,2% | 0,563 |
| P2Y12 inhibitor | < 0,001 | 0,126 | ||||
| Clopidogrel | 88,2% | 59,6% | 69,1% | 73,3% | ||
| Prasugrel | 5,19% | 15,1% | 11,5% | 13,7% | ||
| Ticagrelor | 5,74% | 24,7% | 19,5% | 12,6% | ||
| Beta-blockers | 87,8% | 89,5% | 0,754 | 84,4% | 88,8% | 0,132 |
| ACE inhibitor | 81,0% | 84,3% | 0,247 | 80,9% | 83,8% | 0,381 |
| Statins | 97,4% | 97,8% | 0,282 | 98,1% | 96,9% | 0,514 |
|
ACE inhibitor, angiotensin-converting enzyme inhibitor; BMI, body mass index; BMS, bare-metal stent; Cx, circumflex artery; DES, drug-eluting stent; GFR, glomerular filtration rate; LAD, left anterior descending artery; LCA, left coronary artery; LM, left main; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction. |
||||||
Events at follow-up
The events at follow-up are shown on table 2. The overall mortality rate was 16.9% (n = 205). In the overall study cohort, the DES implant was closely associated with lower risk of death from any cause (6.9% vs 12.2%; log-rank test, P < .001); the combined MACE and POCE were also less common in patients treated with DES. No differences were seen in the DOCE, cardiovascular mortality, myocardial infarction, target vessel myocardial infarction, target vessel revascularization, target lesion revascularization or revascularization by another vessel. No differences were seen in definite stent thrombosis at follow-up either.
Table 2. Adverse events during follow-up
| Overall cohort | Propensity score matched cohort | |||||
|---|---|---|---|---|---|---|
| BMS (n = 983) | DES (n = 374) | log-rank test P value | BMS (n = 262) | DES (n = 262) | log-rank test P value | |
| Death from any cause | 18.3% (180) | 6.7% (25) | < .001 | 12.2% (32) | 6.5% (17) | .049 |
| MACE | 33.2% (326) | 16.0% (60) | < .001 | 25.2% (66) | 16.4% (43) | .049 |
| POCE | 28.0% (275) | 13.1% (49) | .004 | 22.5% (59) | 12.6% (33) | .017 |
| DOCE | 10.0% (98) | 5.9% (22) | .706 | 10.6% (28) | 7.3% (19) | .764 |
| Cardiovascular mortality | 3.76% (37) | 1.87% (7) | .860 | 2.7% (7) | 3.8% (10) | .409 |
| MI at follow-up | 5.3% (52) | 2.1% (8) | .437 | 5.0% (13) | 2.7% (7) | .243 |
| Target MI at follow-up | 2.0% (20) | 0.8% (3) | .765 | 2,3% (6) | 1,1% (3) | .713 |
| Heart failure | 4.0% (39) | 3.5% (13) | .97 | 3.1% (8) | 3.4% (9) | .759 |
| TVR | 6.7% (66) | 4.0% (15) | .435 | 8.4% (22) | 4.6% (12) | .114 |
| TLR | 6.2% (61) | 4.0% (15) | .664 | 7.6% (20) | 4.6% (12) | .199 |
| Definite thrombosis | 3.7% (36) | 2.7% (10) | .973 | 2.7% (7) | 1.9% (5) | .686 |
|
BMS, bare-metal stent; DES, drug-eluting stent; DOCE, device-oriented composite endpoint; MACE, major adverse cardiovascular events; MI, myocardial infarction; POCE, patient-oriented combined endpoint; TLR, target lesion revascularization; TVR, target vessel revascularization. |
||||||
In the propensity score-matched cohort study, patients who received a DES had a significantly lower all-cause mortality rate (6.7% vs 18.3%; log-rank test, P < .001) and lower incidence rates of MACE and POCE at follow-up (16.8% vs 25.6%, log-rank test, P = .049; 12.6% vs 22.5%, log-rank test, P = .017, respectively). Target vessel revascularization (4.6% vs 8.4%) and target lesion revascularization (4.6% vs 7.6%) tended to drop but were not statistically significant. The DOCE was numerically lower in the DES group. No differences were seen in cardiovascular mortality, myocardial infarction, target myocardial infarction or revascularization by another vessel. Survival curves revealed that both groups diverged over time compared to the beginning of the follow-up, and the differences were significant after five years of follow-up(figure 1). The cumulative incidence curves for MACE and POCE (figure 2) show a similar pattern, although the differences were statistically significant after six years of follow-up in both of them. Finally, no significant differences were observed in the rate of definite stent thrombosis showing both groups low rates of 2.7% in the BMS group and 1.9% in the DES group (log-rank test, P = .686).
Discussion
The results of this study show that in a real-world population of consecutive patients with STEMI who underwent PCI, the use of new-generation DES was associated with a lower overall mortality rate and long-term MACE and POCE and no differences in the incidence rate of definite stent thrombosis. The protective effect of DES was maintained in analyses of the cohort grouped by propensity score matching, where both subgroups had similar distributions of covariates.
Our results indicate that the use of new-generation DES in PCI in patients with STEMI is associated with a prognostic benefit compared with BMS, indicative that they may be the first-choice approach in these patients, which is consistent with the actual recommendations of the clinical practice guidelines11.
In our study, we saw a reduction in all-cause mortality in the group of revascularized patients with DES, with no differences in cardiovascular mortality. When it comes to reducing the overall mortality rate the protective effect of DES cannot be established directly; however, these findings are consistent with the long-term results of former studies10. It is known that the luminal loss of BMS is greater than that of DES14. An explanation for this difference in the overall mortality rate may have to do with a higher rate of subclinical restenosis in patients with BMS that could be causing silent ischemia, a reduced ejection fraction and/or a lower coronary flow reserve, which in the event of intercurrent events such as infections, bleeding or cancer, among others, could lead to worse prognosis. The NORSTENT study15, a large multicenter trial of 9013 patients randomized to receive new-generation DES or BMS, showed no differences in the composite primary endpoint of allcause mortality or new nonfatal myocardial infarction after 6 years of follow-up. In this study, no differences were found in the overall mortality rate. The population had a lower risk profile compared to our registry: less than one-third of the patients were admitted due to STEMI, and patients with prior percutaneous revascularization, life expectancy below 5 years, on anticoagulant therapy and with bifurcation lesions were excluded. Despite the fact that no differences were found in the primary endpoint, the DES proved their effectiveness which was associated with a reduced need for new revascularizations (16.5% vs 19.8%; P < .001) and target lesion revascularizations (5.6% vs 10.2%; P < .001). Likely due to the small sample size of our study, we saw a statistically nonsignificant tendency towards less target lesion revascularizations and target vessel revascularizations in patients who received DES.
The reduction of POCE in our registry had a similar pattern to the one observed in the 5-year follow-up of the EXAMINATION trial10, where the differences favorable to the DES grew progressively bigger during follow-up, being statistically significant from the third year onwards. In the EXAMINATION trial, DES also lowered the follow-up DOCE, being the differences statistically significant after the 3-year follow-up10. In our registry the rate of DOCE was similar to that of the EXAMINATION trial at 2 years (≈ 9%); in any case, we only found a numerical reduction of the DOCE, probably due to the lack of statistical power.
The long-term evidence available of DES vs BMS is very limited; most clinical trials that compare BMS to first-generation DES conducted <2 year-follow-up studies16-22 yet usually they showed a greater efficacy of DES at the cost of less new revascularizations of the target lesion, with no differences in other clinical events or survival. Only 2 clinical trials, the EXAMINATION18 and the COMFORTABLE-AMI, have compared second-generation DESs vs BMS in patients with STEMI, and in both cases a 1-year follow-up was conducted: in the COMFORTABLE-AMI trial, the use of biolimus-eluting stents (BioMatrix; Biosensors Europe SA, Morges, Switzerland) was associated with less new infarctions based on the culprit vessel and ischemia-guided target vessel revascularizations.23. Similarly, in the EXAMINATION study, the use of an everolimus-eluting stent (Xience V; Abbott Vascular, Santa Clara, CA, United States) was associated with lower target vessel revascularizations and target lesion revascularization rates.18. In a combined analysis of both studies, the use of DES reduced the POCE which, in turn, led to less target lesion revascularization and a lower risk of infarct-related artery new infarctions.8. The late catch-up phenomenon (ie, thrombosis or restenosis 1 year after stent implantation) has been described for first-generation DES, which has raised concerns about their long-term efficacy and safety24,25. Compared to BMS, that show maximum intimal hyperplasia at 6 months26, first-generation DES show progressive luminal loss after 2 years of angiographic follow-up27. Some studies suggest that this effect is also present in new-generation DES28. Our results and those from the long-term EXAMINATION study support the hypothesis that the clinical effectiveness of newgeneration DES in terms of increased survival and decreased MACE and POCE is seen during long-term follow-up studies.
Finally, the safety of new-generation DES when it comes to their low rate of definite stent thrombosis, with no differences from BMS being reported, is consistent with what some clinical trials have published on new DES in patients with STEMI8,10,15. On the timing of stent thrombosis, it is remarkable that there was no very late stent thrombosis among patients who received DES.
Limitations
This was a retrospective observational and nonrandomized study with consecutive inclusion of patients conducted in a single center. Thus, it is the limitations inherent to this type of study that need to be taken into consideration.
To avoid bias and to control the effects of possible confounding factors, propensity score adjustment was conducted; however, the effects of the confounding factors that were not analyzed cannot be precluded. Due to the lack of data on treatment modifications during follow-up, we cannot rule out the possibility that the observed differences may be influenced, at least partially, by treatment. Finally, the existence of the effect of heterogeneity among the different types of DES cannot be precluded either.
Conclusions
According to our registry, in a real-world population of patients, the implementation of new-generation DES compared to BMS was associated with increased survival rates at long-term follow-up, reductions of MACE and POCE and no differences in definite stent thrombosis.
Funding
This research has been funded by The MAPFRE Foundation.
Conflicts of interests
The authors declared no conflicts of interest whatsoever.
What is known about the topic?
- Despite recommendations from the actual guidelines, the evidence on the long-term outcomes of new drugeluting stents in the management of STEMI is limited and mostly based on clinical trials.
What does this study add?
- The population of this study reflects the management of a real-world STEMI cohort.
- Our results confirm the long-term efficacy and safety of new-generation drug-eluting stents in an all-comers registry.
References
1. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drugeluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-948.
2. Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030-1039.
3. Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123: 2819-2828, 6 p following 2828.
4. Camenzind E, Steg PG, and Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440-1455; discussion 1455.
5. Kukreja N, Onuma Y, Garcia-Garcia HM, et al. The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2009;2:534-541.
6. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873-2891.
7. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177.
8. Sabate M, Raber L, Heg D, et al. Comparison of newer-generation drugeluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (Clinical Evaluation of the Xience-V stent in Acute Myocardial Infarction) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55-63.
9. Raber L, Kelbaek H, Taniwaki M, et al. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: two-year clinical results of the COMFORTABLE AMI trial. Circ Cardiovasc Interv. 2014;7:355-364.
10. Sabate M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387:357-366.
11. Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517-592.
12. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020-2035.
13. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351.
14. Tsai ML, Chen CC, Chen DY, et al. Review: The outcomes of different vessel diameter in patients receiving coronary artery stenting. Int J Cardiol. 2016;224:317-322.
15. Bonaa KH, Mannsverk J, Wiseth R, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242-1252.
16. Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (Paclitaxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) Randomized Trial. JACC Cardiovasc Interv. 2009;2:515-523.
17. Menichelli M, Parma A, Pucci E, et al. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49:1924-1930.
18. Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482-1490.
19. Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093-1104.
20. Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360: 1946-1959.
21. Valgimigli M, Campo G, Arcozzi C, et al. Two-year clinical follow-up after sirolimus-eluting versus bare-metal stent implantation assisted by systematic glycoprotein IIb/IIIa inhibitor infusion in patients with myocardial infarction: results from the STRATEGY study. J Am Coll Cardiol. 2007;50: 138-145.
22. van der Hoeven BL, Liem SS, Jukema JW, et al. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention Study. J Am Coll Cardiol. 2008;51:618-626.
23. Raber L, Kelbaek H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787.
24. Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007; 356:1009-1019.
25. Nakagawa Y, Kimura T, Morimoto T, et al. Incidence and risk factors of late target lesion revascularization after sirolimus-eluting stent implantation (3-year follow-up of the j-Cypher registry). Am J Cardiol. 2010;106:329-336.
26. Kimura T, Yokoi H, Nakagawa Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996;334:561-566.
27. Byrne RA, Iijima R, Mehilli J, et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc Interv. 2009;2:291-299.
28. Iijima R, Araki T, Nagashima Y, et al. Incidence and predictors of the late catch-up phenomenon after drug-eluting stent implantation. Int J Cardiol. 2013;168:2588-2592.