ABSTRACT
Hypertension is the most prevalent cardiovascular risk factor. Despite pharmacological treatment, a high percentage of patients do not achieve an adequate blood pressure control. Renal sympathetic denervation is a minimally invasive intervention for the management of hypertension involving the interruption of the renal artery sympathetic nervous system using a catheter-based approach. The early studies showed promising results, but the controversial results coming from the SYMPLICITY HTN-3 trial sent this technique into oblivion. Over the last 3 years, new clinical trials have appeared including new devices used in different populations, which definitively proves the effectiveness of renal sympathetic denervation.
This joint position statement from the Spanish Society of Hypertension-Spanish League for Combating High Blood Pressure (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC) reviews the evidence available on the efficacy and safety profile of renal sympathetic denervation for the management of hypertension. Based on the results of clinical trials, recommendations have been established on what patients may be eligible for renal sympathetic denervation and under what circumstances.
Keywords: Hypertension. Renal sympathetic denervation. Blood pressure.
RESUMEN
La hipertensión arterial es el factor de riesgo cardiovascular más prevalente. A pesar del tratamiento farmacológico, un alto porcentaje de pacientes no consiguen un adecuado control. La denervación renal es una intervención mínimamente invasiva para el tratamiento de la hipertensión que implica la interrupción de los nervios simpáticos renales mediante un abordaje con catéter. Los estudios iniciales mostraron resultados prometedores, pero los controvertidos resultados del ensayo SYMPLICITY HTN-3 llevaron al abandono de la técnica. En los últimos 3 años han aparecido los resultados de nuevos ensayos clínicos, con nuevos dispositivos y en diferentes poblaciones, que demuestran definitivamente la eficacia de la denervación renal.
En este documento de posicionamiento conjunto de la Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) y la Asociación de Cardiología Intervencionista de la Sociedad Española de Cardiología (ACI-SEC) se revisa la evidencia disponible sobre la eficacia y la seguridad de la denervación renal en el tratamiento de la hipertensión. A partir de los resultados de los ensayos clínicos, se generan recomendaciones sobre qué pacientes y en qué condiciones podrían ser candidatos a una denervación renal.
Palabras clave: Hipertension arterial. Denervacion renal. Presion arterial.
Abbreviations ABPM: ambulatory blood pressure monitoring. BP: blood pressure. DBP: diastolic blood pressure. HMOD: hypertension-mediated organ damage. HTN: hypertension. RSD: renal sympathetic denervation. R-HTN: resistant hypertension. SBP: systolic blood pressure.
INTRODUCTION
The role of the sympathetic nervous system in the pathophysiology of hypertension (HTN) is well known. In 2007, to address the unmet need of patients with resistant HTN (R-HTN), the first percutaneous renal sympathetic denervation (RSD) procedures were performed. First observational studies showed positive results, and the use of RSD started in select centers around the world.1,2 However, in 2014, the publication of a study which did not demonstrate a greater efficacy of RSD vs a sham-control group to control blood pressure (BP)3 dramatically reduced the interest of the scientific community in this procedure, as well as in its clinical application. An increased knowledge of renal anatomy combined with the development of second-generation devices has led to new studies, in which the efficacy of RSD vs a sham-control group has been demonstrated.4-7 Although the road ahead is long, the new evidence provides a clear role for RSD in the management of patients with HTN.
The clinical practice guidelines on the management of hypertension published by the European Society of Cardiology and European Society of Hypertension (ESC/ESH) back in 2018 outlined the role of device-based approaches for the management of HTN in the context of clinical trials only.8 The practical effect of this is that it discouraged the use of RSD. Despite the short time that has gone by since the publication of these guidelines, the data provided by the new clinical trials would justify treating selected patients with RSD.
This document reviews the evidence available on RSD for the management of HTN, analyzes possible indications, and suggests strategies to identify potentially eligible patients, formulated from the opinion of a panel of experts selected by the Spanish Society of Arterial Hypertension-Spanish League for the fight against Arterial Hypertension (SEH-LELHA), and the Interventional Cardiology Association of the Spanish Society of Cardiology (ACI-SEC). The writing of the document was carried out by professionals proposed by these scientific societies undersigning this work following their experience in the management of patients treated with RSD. After the first draft, other experts with and without previous experience in RSD carried out a critical review of the document, and agreed on the changes that were deemed appropriate.
CLINICAL EVIDENCE ON THE ROLE OF RENAL SYMPATHIC DENERVATION IN THE MANAGEMENT OF HYPERTENSION
The supplementary data shows the epidemiological characteristics of HTN (section 1), as well as the role of sympathetic nervous system in the management of HTN (section 2), thus improving our understanding of clinical trials. Table 1 of the supplementary data shows the main studies in which the efficacy of RSD has been assessed.
Table 1. Studies prior to renal sympathetic denervation in patients with uncontrolled hypertension
| Evaluation of pharmacological treatment |
| Type and number of drugs |
| Drug adequate dosage |
| Assess use of aldosterone antagonist |
| Assess lack of therapy compliance |
| Assess intolerance to drug therapy |
| 24-hour ABPM study |
| Rule out pseudo-resistant hypertension or white coat effect |
| Confirm uncontrolled hypertension (SBP > 130 mmHg/DBP > 80 mmHg at the 24-hour levels or SBP > 135/DBP > 85 in the day’s levels) |
| Rule out secondary causes of hypertension (table 2) |
| Cardiovascular risk assessment |
| Coexistence of other cardiovascular risk factors such as dyslipidemia, diabetes or smoking |
| Presence of HMOD |
| Presence of established cardiovascular or kidney disease |
| Imaging of the renal anatomy by computerized tomography or nuclear magnetic resonance imaging (assessment of occlusive stenosis, accessory branches, arterial diameter) |
| Complementary tests recommended: |
| Hemogram, renal function parameters, liver and lipid profiles, and urine sediment tests to detect the presence of microalbuminuria |
| Specific analytical determinations: |
| Baseline plasma aldosterone-to-renin ratio |
| Thyroid hormones |
| Calcium-phosphorus metabolism with parathyroid hormone levels |
| Cortisol (basal and 24-hour urine ratios) |
| Catecholamines with 24-hour urinary metanephrines ratio |
| Polysomnography |
ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure, HMOD, hypertension-mediated organ damage; SBP, systolic blood pressure. |
Back in 2009, the first study on RSD in patients with R-HTN, the SYMPLICITY HTN-1 trial, was published. This study suggested a high efficacy profile of RSD, and a lower office systolic blood pressure (SBP) down to 27 mmHg at 12 months, without any significant complications being reported.1
The SYMPLICITY HTN-3 trial was the first one to include a control group with a sham procedure and a 24-hour BP endpoint. No differences between the 2 treatment groups in terms of the efficacy profile of BP control in patients with R-HTN were reported at the 6-month follow-up.3 The disagreement between the results of this study and the previous ones, as well as the identification of several confounding factors9 brings up the need for designing new studies specifically aimed at solving these questions.
Definitive evidence on the efficacy of RSD has come from the SPYRAL HTN and RADIANCE-HTN studies. The SPYRAL HTN-ON MED trial enrolled patients with uncontrolled HTN treated with 1 to 3 antihypertensive drugs, randomized to receive RSD or a sham procedure. The 24-hour ambulatory SBP and diastolic BP (DBP) levels, as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 6-month follow-up.4 With a similar design, the SPYRAL HTN-OFF MED Pivotal trial enrolled uncontrolled hypertensive patients with office SBP levels between 150 mmHg and 180 mmHg in the absence of antihypertensive treatment. The 24-hour SBP and office SBP levels were reported at the 3-month follow-up.5 The RADIANCE-HTN SOLO trial enrolled patients with HTN and ambulatory blood pressure monitoring (ABPM) levels ≥ 135/85 mmHg and ≤ 170/105 mmHg without pharmacological treatment. The 24-hour ambulatory SBP and DBP levels as well as the office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.6 The RADIANCE-HTN TRIO trial enrolled patients with R-HTN on a fixed-dose, single-pill combination of a calcium channel blocker, an angiotensin receptor blocker, plus a thiazide diuretic, randomized to receive ultrasound catheter-based RSD or a sham procedure. The 24-hour ambulatory SBP and DBP levels, as well as office SBP and DBP levels dropped significantly in the RSD group compared to the sham control at the 2-month follow-up.7
Real-life registries have enrolled more than 3500 patients treated with RSD showing lower office BP and ABPM levels. Some registries have demonstrated that the reduction of BP is not associated with the medication burden or with an increased number of antihypertensive drugs. RSD has proven to be safe and has a low rate of complications associated with the procedure.10 The GLOBAL SYMPLICITY registry, with over 2900 patients is the largest and longest duration analysis to this date of renal sympathetic denervation to show the efficacy and safety profile of RSD in a real-life scenario.10 Table 2 of the supplementary data shows a summary of different registries on RSD.
Table 2. Causes of secondary hypertension
| Renal parenchymal diseases | Glomerulopathies |
| Renovascular diseases | Fibrodysplasia |
| Suprarrenal diseases | Primary hyperaldosteronism |
| Vasculares diseases | Aortic coarctation |
| Endocrine-metabolic | Thyroid dysfunction |
| Neurological diseases | Dysautonomiay |
| Toxic-pharmacological diseases | Corticosteroids |
| Genetic diseases | Monogenic forms |
RSD has been confirmed as a safe intervention. The incidence rate of both immediate complications associated with the procedure and renal and vascular complications in the short- and mid-term (6-12 months) is very low and is mainly associated with local problems at the puncture site; serious renal complications (renal artery dissection or stenosis) are anecdotal. Table 3 of the supplementary data summarizes the safety data from the main randomized clinical trials that often have a short-term clinical follow-up.
Table 3. Precautions and contraindications to renal sympathetic denervation
| • Renal sympathetic denervation has not been evaluated in patients who are pregnant, nursing, intend to become pregnant or in patients with type I diabetes mellitus, previous renal angioplasty, indwelling ureteral stents, aortic grafts or abnormal renal anatomy |
| • Subjects in whom a reduction of blood pressure would be considered hazardous (such as those with hemodynamically significant valvular heart disease) |
| • Implantable pacemakers and implantable cardioverter/defibrillators may be adversely affected by radiofrequency ablation. Consider deactivating implantable cardioverter/defibrillators during ablation, have temporary external sources |
| of pacing and defibrillation available during ablation, and perform a complete analysis of the functionality of the device implanted after ablation |
| • Avoid treating arteries with diameters < 3 mm or > 8 mm |
| • Avoid treating arteries with significant disease or flow-limiting obstructions |
POSSIBLE INDICATIONS FOR RENAL SYMPATHETIC DENERVATION WITH DATA FROM THE LATEST CLINICAL TRIALS
Data from both randomized clinical trials and registries prove that the RSD procedure is safe and effective reducing BP, which is consistent across different populations including high-risk subgroups, and with different devices. Section 3 of the supplementary data reviews various consensus documents and recommendations previously published by different scientific societies prior to the publication of the SPYRAL HTN and RANDIANCE-HTN clinical trials.
RSD can be considered in patients with resistant HTN (BP > 140/90 mmHg despite lifestyle changes treated with ≥ 3 antihypertensive drugs at optimal doses, one of them being a diuretic or HTN controlled with ≥ 4 drugs),8 and also in patients with uncontrolled HTN (BP > 140/90 mmHg in patients with poor therapeutic compliance), and high cardiovascular risk.
Renal sympathetic denervation in patients with resistant hypertension
Patients with R-HTN were the first group in whom the role of RSD was assessed. The SYMPLICITY HTN-3 trial failed to demonstrate the increased efficacy of RSD vs sham control in patients with R-HTN.3 However, subsequent analysis revealed design and execution limitations that cast doubts on the reliability of the results.9 In the recently published RADIANCE-HTN TRIO trial, patients with R-HTN treated with a standardized triple combination pill experienced a drop in their BP levels 2 months after RSD compared to a sham procedure.7 If the BP lowering effect and the safety of RSD are maintained in the long term, RSD might be an alternative to the addition of more antihypertensive medications in patients with R-HTN.
Renal sympathetic denervation in patients with uncontrolled hypertension
The new evidence available introduces a paradigm shift for a technique that was initially conceived for the management of R-HTN when all other therapeutic options fail, and is currently an option that should be taken into consideration in patients with persistent BP > 140/90 mmHg despite drug treatment.
The concept of uncontrolled HTN includes a high percentage of hypertensive patients (maybe even > 60%) with highly heterogenous clinical characteristics and cardiovascular risk. Given the invasive nature of the RSD procedure, and until more information becomes available on the reduction of cardiovascular events in more specific subgroups of patients, there are some high-risk situations in which BP control is essential to reduce the risk of cardiovascular events:
a) Patients with frequent hypertensive crises. Hypertensive crises with SBP levels > 180 mmHg and/or DBP levels > 110 mmHg can cause brain, cardiac or microvascular damage. Emergency visits for hypertensive crises exceed 4% of all visits to the emergency room.11 Even in the absence of hypertension-mediated organ damage (HMOD), episodes of hypertensive crisis can have long-term implications to the extent that these patients may have a 50% higher risk of suffering cardiovascular events compared to controlled hypertensive patients. Nonetheless, outside the crisis setting they show similar BP levels.12
b) Patients with low compliance to pharmacological treatment. Pharmacological treatment of HTN is generally a long-term option and in most cases, for life. Poor compliance is a common problem to the extent that almost one third of all hypertensive patients do not start a new prescription of antihypertensive drugs,13 and around 50% become non-compliant within the first year after starting treatment.13 In the SPYRAL HTN trials, the 24-hour ABPM levels showed decreased BP levels throughout the entire 24-hour period in patients treated with RSD compared to no changes in the control group in the absence of drugs or incomplete control in the presence of drugs.4,5 Furthermore, in the SPYRAL HTN OFF-MED trial, the treatment group experienced an average reduction of 9.2 mmHg in office SBP levels.5 A meta-analysis of 123 studies including 613 815 patients showed that a drop of office SBP levels of 10 mmHg was associated with a significantly lower risk of cardiovascular events.14 Poor compliance is a serious problem of public health since these patients in whom an adequate BP control is not achieved, even due to poor therapeutic compliance, have a high cardiovascular risk.15 However, we should stress that RSD alone cannot bring BP levels down enough to achieve BP control in most patients. In the RADIANCE-HTN SOLO trial, the 24-h ABPM only 25% of the patients treated with RSD reached values < 130/80 mmHg.6 In these non-compliant patients, the main strength of RSD is the “always on” effect regardless of pharmacokinetics and compliance to drugs.
c) Patients with hypertension-mediated organ damage. The presence of HMOD identifies a group of patients with high cardiovascular risk in whom conventional treatment has failed to prevent the progression of the disease.16 Achieving the BP levels recommended is especially important in these patients because, in the early stages of the disease, some types of HMOD can be reversed; in more advanced stages, HMOD is irreversible despite adequate BP control. But this is important since it slows its progression while reducing the cardiovascular risk of these high-risk patients.17 A meta-analysis including 698 patients treated with RSD revealed an independent effect of RSD on HMOD, which advocates for the use of RSD in this group of high-risk patients.18
d) Patients at high cardiovascular risk. The European guidelines on the management of HTN establish the factors that influence cardiovascular risk in hypertensive patients including clinical characteristics, analytical characteristics, presence of HMOD or established cardiovascular or kidney disease. All these factors establish a 10-year cardiovascular risk that is categorized into 4 groups: low, moderate, high or very high risk to the extent that, for example, in high-risk patients the estimated cardiovascular mortality is 5% and in very high-risk patients > 10%.8 The assessment of cardiovascular risk should play an important role in the decision-making process to the extent that the higher the risk, the greater the benefits expected with better BP control. Therefore patients at high or very high-risk would be eligible for RSD whenever BP control is not adequate.
Empowering the hypertensive patient in the setting of a shared decision-making process
Over the last few years, shared decision-making process has emerged as the go-to model in the management of different conditions. In the field of RSD, a recent survey revealed that 38% of hypertensive patients who still don’t take antihypertensive medication would prefer RSD to lifelong drug therapy even knowing that it would probably not replace medication in many cases. Just this already reduces BP significantly.19 With the evidence provided in recent trials, RSD could be a valid treatment option in patients with uncontrolled HTN and high to very-high cardiovascular risk in whom, in a shared decision-making process context, consensus with the patient can be reached. In any case, we should mention that the treatment of HTN always requires the adoption of healthy lifestyle habits, and the recommendation to patients should include drug treatment as the first option.
STUDY PRIOR TO RENAL SYMPATHETIC DENERVATION
Patients should be examined in a unit specialized in HTN and vascular risk 3 months prior to the procedure in a center with proven experience.20 Table 1 summarizes the studies to be conducted in patients eligible for RSD.
Uncontrolled HTN should be confirmed through 24-hour ABPM.21 After confirming the presence of uncontrolled HTN, the clinical situations that increase BP levels such as obesity or obstructive sleep apnea should be identified and corrected. Also, substances such as salt or certain drugs that may also lead to HTN should be suspended or minimized. Non-compliance to treatment, which is very common and not always identified by the patient, if not rigorously investigated, should be ruled out.22 It is essential to rule out secondary HTN (table 2) or, if diagnosed, treat it effectively. Still, it is not an absolute contraindication to RSD.23
RENAL SYMPATHETIC DENERVATION PROCEDURE WITH RADIOFREQUENCY DEVICES
Section 4 of the supplementary data shows more in-depth technical aspects of RSD. Figure 1 of the supplementary data summarizes the RSD procedure.
A better knowledge of the anatomy of renal nerves24 and the development of new ablation devices have optimized the treatment technique,5,6 which is based on 3 main objectives:

Figure 1. Detail of renal sympathetic innervation. Renal nerves are usually arranged in large bundles and only form a true plexus when they are close to entering the kidney. Some nerves bypass the main renal artery and join distally to the different arterial divisions of the main renal artery (late arrival nerves). In this case, a late arrival nerve is seen joining the proximal third of the anterior division of the main renal artery (blue asterisk). It can also be seen how the proximal main renal artery is occupied by fused ganglia of the solar plexus (GM), and by the lumbar sympathetic chain (LSC). Both provide innervation to the kidneys, but also to other abdominal and pelvic organs, which can be accidentally denervated if the proximal third of the main renal artery is treated. The image also shows that the maximum proximity of nerve fibers to the arterial wall mainly occurs at branch level, but also at main trunk level. This is the target area of treatment, always avoiding the application of radiofrequency at renal pelvis level. AG, adrenal gland; CT, celiac trunk; GM, ganglionic mass made up of the aorticorenal and celiac ganglia; LSC, lumbar sympathetic chain; RK, right kidney; SMA, superior mesenteric artery; blue asterisk, late arrival nerve. In red, arterial structures. In yellow, nervous tissue.
Management of the renal artery main trunk and branches
It is common for the renal nerves to reach the kidney after bypassing the main renal artery.24 In animal models, it has also been confirmed that the application of combined radiofrequency in the renal artery main trunk and branches reduced the content of norepinephrine in the renal tissue even more, and in the cortical axonal density, both associated with the response to RSD.25
In patients treated with RSD, the presence of untreated accessory arteries leads to a lower hypotensive response.26 Their identification and treatment is essential and, if they are amenable to treatment thanks to their diameter (minimum diameters of 3 mm), the treatment of accessory arteries is advised.
Last but not least, the perivascular space around the ostium and the proximal third of the main renal artery is often occupied by ganglia of solar plexus and by the lumbar sympathetic chain (figure 1). Both carry innervation to the kidneys, but also to other abdominal and pelvic organs, and they could be accidentally denervated if the treatment is applied to the ostium and proximal third of the main renal artery. Therefore, until more information becomes available, it seems reasonable to be cautious when treating the most ostial portion of renal arteries.24
Treatment of the 4 quadrants of the renal artery
The distribution of nerve fibers around the renal artery follows a variable pattern across different individuals.27 Preclinical studies in a porcine model have shown that the application of radiofrequency in one point produces effects on approximately 25% of the arterial circumference,27 and procedures that use multiple helically staggered ablations in the 4 quadrants are more effective reducing the norepinephrine content into the renal tissue.28
Application of the maximum possible number of ablation points
A post-hoc analysis of the SYMPLICITY HTN-3 trial confirmed that patients with a greater number of radiofrequency applications reduced their BP levels even more without any associated adverse events.9 We recommend applying the maximum number of ablation points possible, always respecting a distance of 5 mm among them with a 4-quadrant distribution.
Section 4 of the supplementary data shows how to perform a RSD procedure using a tetrapolar radiofrequency catheter. Table 3 shows the precautions and contraindications regarding RSD.
Care after renal sympathetic denervation procedure
Once the procedure is finished, it is important to ensure adequate hemostasis in the femoral puncture. Usually, in the absence of complications, patients can be discharged after 24 to 48 hours with the same antihypertensive treatment they had before the procedure or with treatment adjustments in cases that show an immediate response, but still with adjustment appointments within 5 to 7 days. Of note, the effects of the intervention can take weeks to materialize.29
CLINICAL MANAGEMENT AFTER RENAL SYMPATHETIC DENERVATION
The main objective of the follow-up should be to confirm the safety of the intervention and the absence of complications in the short-, mid-, and long-term follow-up, as well as to monitor the evolution of BP levels and the adjustment of drug treatment.
At the clinical follow-up, it is important to maintain a multidisciplinary team same as during the selection of candidates. Table 4 shows the clinical management after RSD.
Table 4. Clinical management after renal sympathetic denervation*
| Blood pressure control |
| Home self-measurement of blood pressure is recommended to assess blood pressure decrease |
| Patient education to detect symptoms of hypotension |
| Pharmacological de-escalation, when appropriate |
| 24-hour ambulatory blood pressure monitoring levels at 3-6 months to assess response to RSD |
| 24-hour ambulatory blood pressure monitoring levels to assess long-term durability of renal sympathetic denervation |
| Renal function: in patients at risk of contrast nephropathy, control should follow after 7-10 days (individualize based on the clinical criteria) |
| Routine renal imaging modalities (echocardiography, computed tomography scan, magnetic resonance imaging) are ill-advised |
* Control after renal sympathetic denervation should be performed in a hypertension- specific unit as part of a regulated renal sympathetic denervation program. |
REQUIREMENTS OF A RENAL SYMPATHETIC DENERVATION PROGRAM
The success of a RSD program is based on the existence of a multidisciplinary team that performs a comprehensive assessment of the patient from the selection of candidates through their assessment prior to the intervention, the RSD procedure, and subsequent follow-up. This process should be carried out at specific units specialized in the management of HTN in collaboration with interventional cardiology units. Figure 2 shows the selection process of eligible patients.
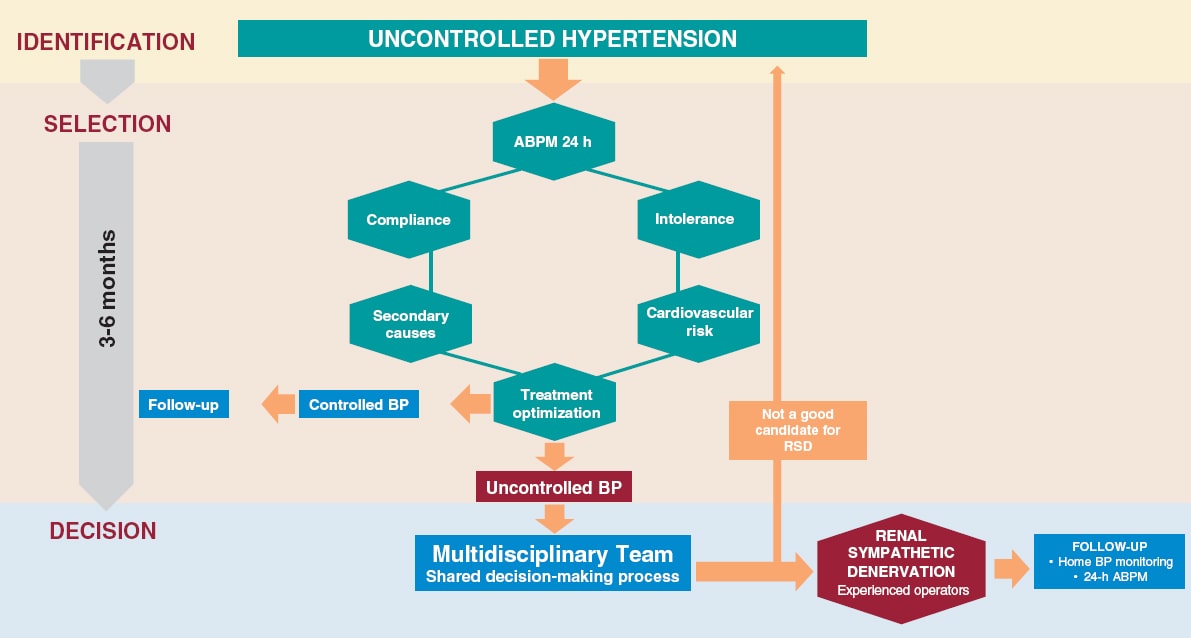
Figure 2. Identification process, patient selection and decision on RDN. Patients with uncontrolled HTN (BP > 140/90 mmHg despite treatment) should be evaluated in an HTN unit. The lack of control should be confirmed by ABPM, assess adherence/intolerance to drugs, rule out secondary causes and cardiovascular risk. If, after optimizing the treatment, the lack of control persists, in patients at high or very high risk, and in a shared-decision process with the patient, RDN may be indicated. Adherence is defined as the extent to which a person’s behavior —taking medication, following a diet, and/or executing lifestyle changes— corresponds with the agreed recommendations from a healthcare provider. Drug intolerance refers to an inability to tolerate the adverse effects of a medication, generally at therapeutic or subtherapeutic doses. Treatment optimization refers to lifestyle changes and pharmacological recommendations, including target doses, recommended by clinical practice guidelines.8 ABPM, ambulatory blood pressure monitoring; BP, blood pressure; RSD: renal sympathetic denervation.
We strongly discourage isolated procedures outside this controlled environment. RSD should not be performed in centers with volumes < 10 cases/year. Centers without a structured RSD program, but with eligible patients, should refer them to an experienced center rather than performing isolated procedures.
RSD procedures should be performed by operators experienced in the management of endovascular treatment. The SYMPLICITY HTN-3 trial post-hoc analysis showed the importance of an experienced interventional specialist given one of the factors influencing the results of the study was the operator’s lack of experience.9 Therefore, we recommend that procedures should be performed at centers with proven experience only and that, in centers that lack this experience, the possibility of monitoring should be available including assistance during the patient selection process and supervision of the procedure until enough experience is gained to ensure optimal results.
CONCLUSIONS
This expert consensus document has reviewed the information available regarding RSD in the management of patients with HTN. Also, it has established, for the first time, the indication for RSD in cases of uncontrolled HTN, especially in patients at high cardiovascular risk with HMOD or cardiovascular disease while taking the patient’s opinion into consideration as part of a shared decision-making process, and as long as it is evaluated by a multidisciplinary team and performed by experienced operators.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
Study concept and design: O. Rodríguez-Leor, and J.A. García-Donaire; manuscript writing: O. Rodríguez-Leor, F. Jaén-Águila, J. Segura, I. J. Nuñez-Gil, A. García-Touchard, E. Rubio, M. Troya, J. Diego-Mediavilla, and J.A. García-Donaire; critical review: O. Rodríguez-Leor, Á. Cequier, R. Moreno, N. Martell, P. Beltrán, and E. Molina.
CONFLICTS OF INTEREST
O. Rodríguez-Leor, and J. A. García-Donaire have received personal fees from Medtronic, outside the submitted work. A. García-Touchard reports having received grants from Medtronic, and personal fees from Medtronic, also outside the submitted work. Á. Cequier reports having received grants and personal fees from Abbott Vascular, grants, and personal fees from Biosensors, grants from Boston Scientific, grants, and personal fees from Medtronic, grants from Biomenco, Cordis, Orbus Neich, and from the Spain Society of Cardiology, personal fees from Ferrer International, Terumo, Astra Zeneca, and from Biotronik outside the submitted work. R. Moreno is associate editor of REC: Interventional Cardiology. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed. F. Jaén-Águila, J. Segura, I. J. Núñez-Gil, E. Rubio, M. Troya, J. Diego-Mediavilla, R. Moreno, N. Martell, P. Beltrán, and E. Molina declared no relationship whatsoever relevant to the contents of this paper worthy of disclosure.
REFERENCES
1. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension:a multicentre safety and proof-of-concept cohort study. Lancet 2009;373:1275-1281.
2. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial):a randomised controlled trial. Lancet. 2010;376:1903-1909.
3. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401.
4. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs:6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346-2355.
5. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal):a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444-1451.
6. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE HTN-SOLO):a multicentre, international, single-blind, randomised, sham controlled trial. Lancet. 2018;391:2335-2345.
7. Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO):a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476-2486.
8. Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology:ESH/ESC Task Force for the Management of Arterial Hypertension. Eur Heart J. 2018;39:3021-3104.
9. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the Symplicity HTN-3 trial. Eur Heart J. 2015;36:219-227.
10. Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes:3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–3482.
11. Janke AT, McNaughton CD, Brody AM, Welch RD, Levy PD. Trends in the incidence of hypertensive emergencies in US emergency departments from 2006 to 2013. J Am Heart Assoc. 2016;5:e004511.
12. Vleck M, Bur A, Woisetschläger C, Herkner H, Laggner AN, Hirschl MM. Association between hypertensive urgencies and subsequent cardiovascular events in patients with hypertension. J Hypertens. 2008;26:657-662.
13. Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence:analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284-290.
14. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death:a systematic review and meta-analysis. Lancet. 2016;387:957-967.
15. Gupta P, Patel P, Strauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113-1120.
16. Cordero A, Morillas P, Bertomeu-Gonzalez V, et al. Prevalence of Peripheral Arterial Disease in Patients with Acute Coronary Syndrome Investigators. Clustering of target organ damage increases mortality after acute coronary syndromes in patients with arterial hypertension. J Hum Hypertens. 2011;25:600-607.
17. Lonnebakken MT, Izzo R, Mancusi C, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6:e004152.
18. Kordalis A, Tsiachris D, Pietri P, et al. Regression of organ damage following renal denervation in resistant hypertension:a meta-analysis. J Hypertens. 2018;36:1614-1621.
19. Schmeider RE, Hogerl K, Jung S, Bramlage P, Veelken R, Ott C. Patient preference for therapies in hypertension:a cross-sectional survey of German patients. Clin Res Cardiol. 2019;108:1331-1342.
20. Schmieder RE, Redon J, Grassi G, et al. ESH Position Paper:Renal denervation - an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837-841.
21. Banegas JR, Messerli FH, Waeber B, et al. Discrepancies between office and ambulatory blood pressure:clinical implications. Am J Med. 2009;122:1136-1141.
22. Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218-225.
23. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension:when, who, and how to screen?Eur Heart J. 2014;35:1245-1254.
24. García-Touchard A, Maranillo E, Mompeo B, Sañudo JR. Microdissection of the Human Renal Nervous System:Implications for Performing Renal Denervation Procedures. Hypertension. 2020;76:1240-1246.
25. Mahfoud F, Tunev S, Ewen S, et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766-1775.
26. Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation?JACC Cardiovasc Interv. 2013;6:1085-1091.
27. Tzafriri AR, Mahfoud F, Keating JH, et al. Procedural and anatomical determinants of multi-electrode renal denervation efficacy –Insights from preclinical models. Hypertension. 2019;74:546-554.
28. Tzafriri AR, Mahfoud F, Keating JH, et al Innervations patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079-1087.
29. Fink G, Phelps JT. Can we predict the blood pressure response to renal denervation?Auton Neurosci. 2017;204:112-118.
* Corresponding author: Hospital Universitari Germans Trias i Pujol, Carretera de Canyet s/n, 08916 Badalona, Barcelona, Spain.
E-mail address: oriolrodriguez@gmail.com (O. Rodríguez-Leor).