To the Editor,
The congenitally corrected transposition of the great arteries is a rare congenital defect characterized by atrioventricular and ventriculoarterial discordance. As a result, the tricuspid valve and the anatomical right ventricle sustain the systemic circulation. Typically, the patient remains asymptomatic at an early age, but the right ventricle and the tricuspid valve deteriorate with the passing of time. The only curative treatment for this condition is heart transplant. In this setting, percutaneous edge-to-edge tricuspid valve repair has been traditionally used to treat tricuspid regurgitation in patients who are ineligible for heart transplantation; however, to this date, the evidence available is scarce and based on case reporting in heterogeneous clinical settings.1-3
This is the case of a young male patient with congenitally corrected transposition of the great arteries, advanced heart failure, and torrential tricuspid regurgitation considered ineligible for heart transplantation due to irreversible severe pulmonary hypertension, but eligible for percutaneous edge-to-edge tricuspid valve repair. The patient signed an informed consent form authorizing the publication of his case that was eventually approved by our center ethics committee.
This is the case of a male diagnosed with congenitally corrected transposition of the great arteries and congenital atrioventricular block at the early age of 7 months. The patient remained asymptomatic until he was 29 years-old when he required a pacemaker due to presence of chronotropic incompetence. Afterwards, he was lost to follow-up until he was admitted to the intensive care unit with signs of pulmonary edema at the age of 35 when he was diagnosed with biventricular systolic dysfunction, severe systemic atrioventricular valve regurgitation, and pulmonary hypertension. Due to the occurrence of a cardiac arrest, an implantable cardioverter-defibrillator with resynchronization therapy was indicated followed by the optimal medical therapy.
Despite treatment, the patient remained symptomatic with New York Heart Association functional class III, and INTERMACS 4. The echocardiographic assessment revealed the presence of severe systemic ventricular systolic dysfunction (right ventricular ejection fraction = 35%) with severe regurgitation of the systemic atrioventricular valve. The valve showed an Ebstein-like anomaly (8.3 mm/m2), abnormal chordae structures, and thickened leaflets with restriction of motion causing a wide coaptation defect, mainly between the septal and posterior leaflets triggering torrential regurgitation (V/V) (figure 1). Cardiac catheterization revealed the presence of severe pulmonary hypertension (mean pulmonary artery pressure of 55 mmHg) with pre- and post-capillary components (transpulmonary gradient of 30 mmHg, and pulmonary vascular resistance of 6.4 WU). The vasodilator test with nitric oxide resulted in a maximum response, but without any significant changes. Considering all this information, the heart team decided that the patient remained ineligible for heart transplantation and suggested the percutaneous edge-to-edge tricuspid valve repair of the tricuspid valve with a MitraClip device (Abbott Vascular, United States) as palliative treatment.
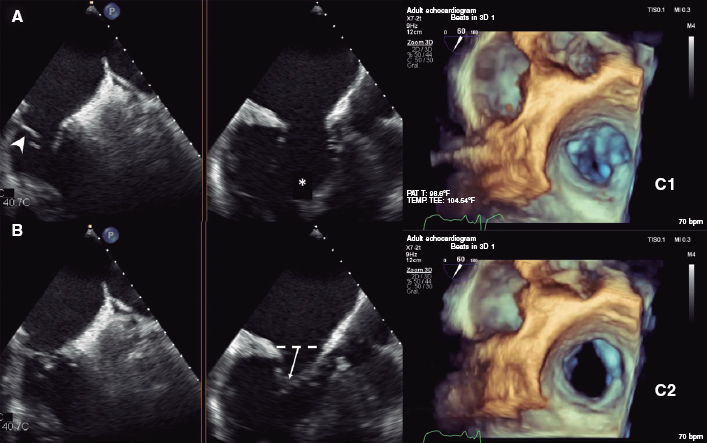
Figure 1. Detailed assessment of the anatomy of the tricuspid valve on a 2D and 3D transesophageal echocardiogram. X plane views in 55-degrees (left), and 145-degrees (middle) in end-diastole (A), and end-systole (B). Significantly dysplastic leaflets (arrowhead) with Ebstein-like apical displacement of the insertion, and anomalous chordae tendineae implantation (asterisk) causing tension and bulge (arrow). C: mitral valve assessment with 3D zoom showing a large coaptation gap predominantly between the septal and the posterior leaflets in end-systole (C1) and end-diastole (C2).
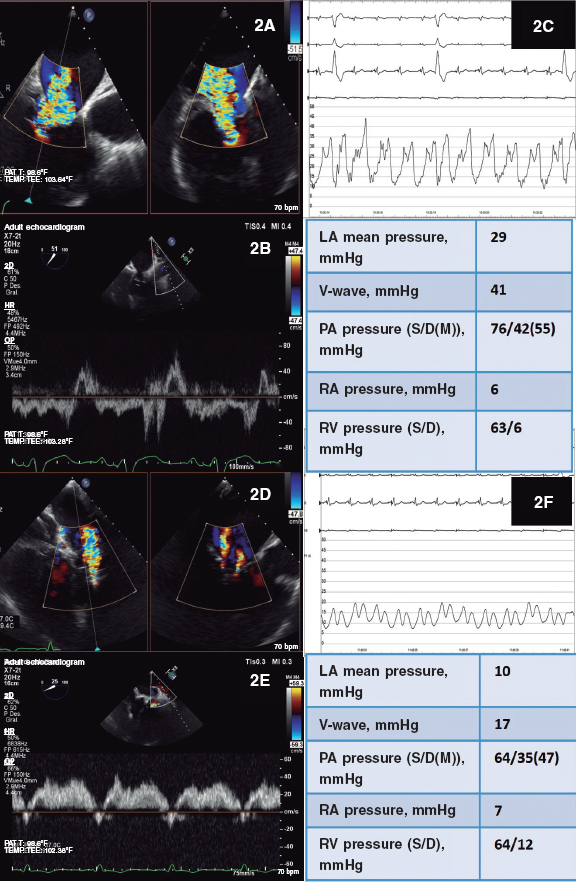
During the procedure the presence of torrential tricuspid regurgitation was confirmed (figure 2A,B) with consistent invasive hemodynamic findings (figure 2C). An early MitraClip XTR device was implanted at the origin of the regurgitant jet at the center of the septal-posterior coaptation line that was able to reduce regurgitation significantly. However, due to the presence of moderate-to-severe persistent regurgitation (II-III/V) without significant stenosis a second device had to be implanted between the anterior and septal valves for being the area with the greatest residual regurgitation. The outcome assessment confirmed the presence of mild-to-moderate residual tricuspid regurgitation (II/V) (figure 2D,E) without stenosis. This positive outcome was also confirmed on the invasive assessment (figure 2F), which is why the procedure was considered terminated.
Figure 2. Tricuspid regurgitation before and after the procedure. A, D: bi-plane color TEE. B, E: pulsed wave Doppler ultrasound of the left superior pulmonary vein. C, F: traces and values of the invasive hemodynamic assessment. LA, left atrium; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
Despite the slight worsening of tricuspid regurgitation at the 6-month follow-up (grade III/V), and the presence of systemic ventricular dysfunction (right ventricular ejection fraction = 35%) and severe pulmonary hypertension (pulmonary artery systolic pressure > 60 mmHg) the patient showed a maintained functional class improvement (New York Heart Association II). Also, the values of the amino-terminal fraction of B-type brain natriuretic propeptide dropped significantly (from 9787 pg/mL to 2083 pg/mL), and fewer diuretics were required. This translated into a significant improvement of the patient’s quality of life, a better functional capacity, and no rehospitalizations 1 year after the procedure.
This case reinforces the role of percutaneous edge-to-edge tricuspid valve repair even in such an adverse setting as the congenitally corrected transposition of the great arteries. Very few case reports have previously described this indication,1-3 and always in more favorable clinical situations. Our case has various technical limitations that make it extra interesting, especially the presence of a dysplastic valve with significant restriction of motion, and a large coaptation defect. Therefore, the largest device available was used to target the area with greater regurgitation (septal-posterior). Deep leaflet capture followed due to the significant bulge present even at the risk of inadvertently capturing the chordae tendineae. However, a second device was required to reduce tricuspid regurgitation significantly. Despite the inherent empiricism of the clinical situation and the unfavorable hemodynamic conditions described with severe pulmonary hypertension and severe systemic right ventricular failure, the patient improved significantly and consistently through time regardless of the lack of improvement reported in the numbers of pulmonary artery pressure and right ventricular ejection fraction. That is why we believe that the maintained reduction of tricuspid regurgitation had a clinical impact. In this sense, pulmonary hypertension1 has been reported to improve in 1 case only, which allowed to reassess the eligibility of heart transplantation. Therefore, percutaneous edge-to-edge tricuspid valve repair should be considered an effective option in patients with congenitally corrected transposition of the great arteries who remain ineligible for heart transplantation or surgery.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. Salinas Gallegos, and E. Pozo Osinalde: study design, and writing of the manuscript. L. Nombela-Franco, P. Jiménez Quevedo, and R. Estevez-Loureiro: management of the patient, and manuscript review. J.A. de Agustín: manuscript review.
CONFLICTS OF INTEREST
L. Nombela-Franco, and R. Estévez-Loureiro are consultors, and proctors, and have received speaking fees from Abbott Vascular, Edwards Lifesciences, and Boston Scientific.
REFERENCES
1. Gaydos SS, Capps CD, Judd RN, et al. Hemodynamic impact of MitraClip procedure for systemic tricuspid regurgitation in congenitally-corrected transposition of great arteries:A case report. Cardiovasc Revasc Med. 2020. https://doi.org/10.1016/j.carrev.2020.08.034.
2. Picard F, Tadros VX, Asgar AW. From tricuspid to double orifice morphology:Percutaneous tricuspid regurgitation repair with the MitraClip device in congenitally corrected-transposition of great arteries. Catheter Cardiovasc Interv. 2017;90:432-436.
3. Van Melle JP, Schurer R, Willemsen M, Hoendermis ES, van den Heuvel AF. Percutaneous tricuspid valve repair using MitraClip(R) for the treatment of severe tricuspid valve regurgitation in a patient with congenitally corrected transposition of the great arteries. Neth Heart J. 2016;24:696-697.