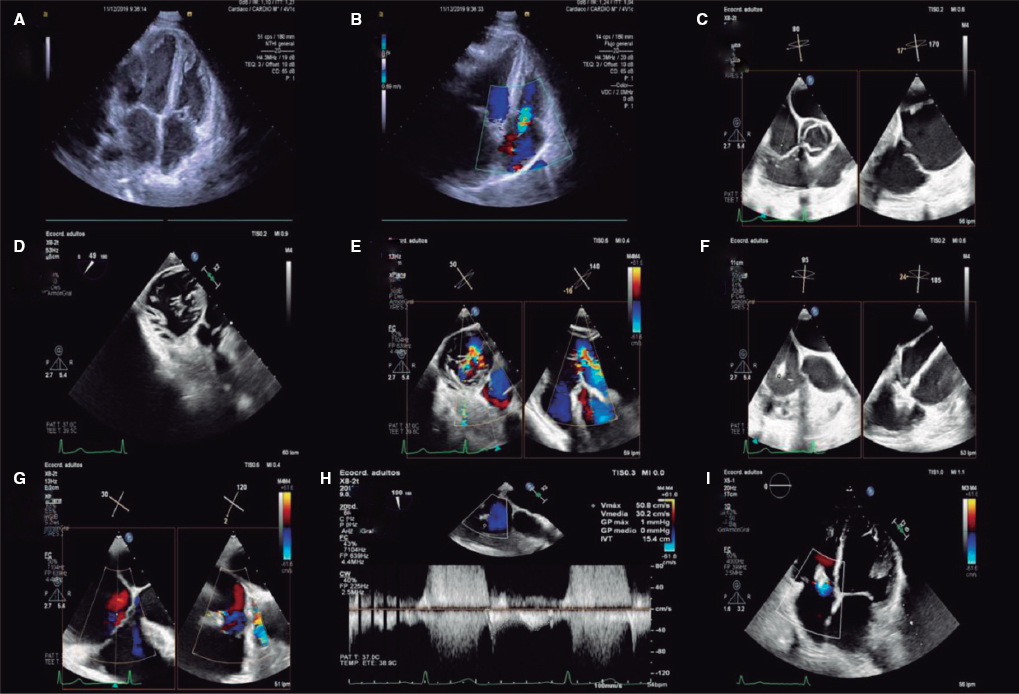
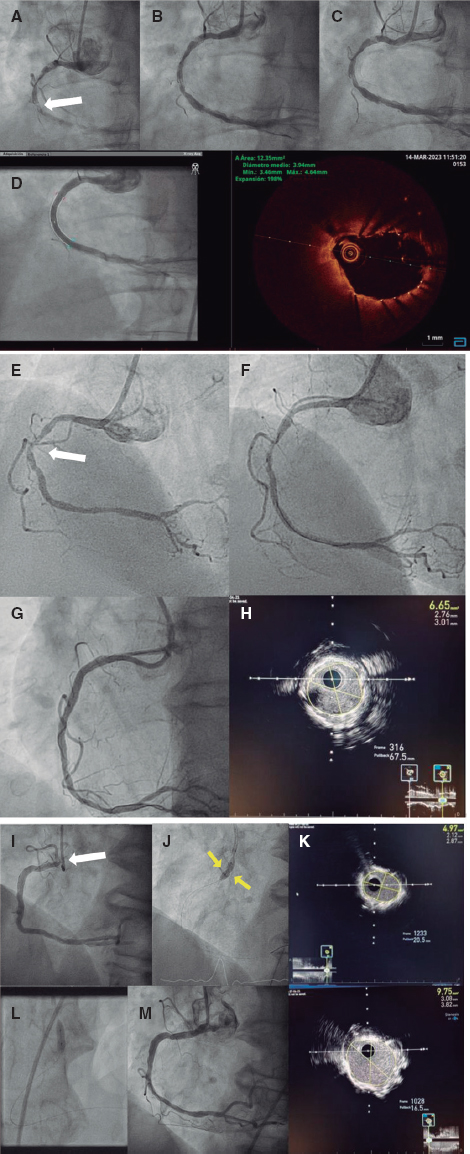
To the Editor,
Although the use of new stents has improved the results after coronary angioplasty, the development of in-stent restenosis (ISR) is still one of the leading problems following these interventions. ISR is defined as a stenosis > 50% developing in a segment or border of the stent (up to 5 mm). It is often due to progressive neointimal proliferation and has been reported in up to 30% of the patients with conventional stents and 10% of drug-eluting stent carriers.1-3
ISR can be due to several factors associated with the patient (diabetes, renal failure, acute coronary syndromes), the lesion (type B2-C complexity, length > 20 mm, diameter < 3 mm, chronic occlusions, ostial lesions, bifurcations, and coronary bridges), and the procedure (malapposition, insufficient expansion, luminal areas < 3 mm, multiple stents, stent fractures, border dissections and type of drug, polymer or stent structure).1,4,5
The most widely used system to describe ISRs is the Mehran angiographic classification. Although it was developed for conventional stents it is used in all types of stents. It is divided into 4 types: I: focal; II: diffuse; III: proliferative; IV: occlusive and it has prognostic value1. However, although there are studies on ISR following the implantation of multiple stents, its physiological mechanism after an angioplasty in the management of ST-segment elevation acute myocardial infarction (STEMI) is not fully understood. Also, it is a situation prone to the appearance of conditions that may favor the occurrence of ISR (insufficient stent expansion or malapposition, small stents for vessels constrained due to circulating catecholamines, thrombophilia, etc.).2,3
We conducted a study in our unit whose endpoint was the type of ISR (focal vs diffuse) and analyzed its correlation with the patient profile-procedure and type of stent in patients implanted with any type of stent in a primary angioplasty.
All patients diagnosed with angiographically significant ISR (> 50% visual stenosis) in a lesion previously treated with a stent angioplasty during a STEMI were retrospectively included between 2004 and 2014. Seventy-six consecutive patients were included. According to the Mehran angiographic classification, the type of ISR was divided into focal (type I, n = 42) or diffuse (II = 5, III = 17, and IV = 12) and both were analyzed together; n = 34). Regarding their position with respect to the stent, focal ISRs were located on the borders of 19 cases (45.2%). Most patients were male (82%) with a mean age of 61.5 years old. The cardiovascular risk factors were common; table 1 shows these stratified according to the type of restenosis. The right coronary artery (53%) was the most commonly compromised artery followed by the anterior descending artery (32%). The mean follow-up was 88 months (interquartile range, 37.2-111.0) and the mean time until the diagnosis of ISR was 8.7 months (interquartile range: 6.2-24.2). The comparisons between diffuse and focal patterns, and clinical profiles and procedures were similar (table 1). Focal ISRs were diagnosed earlier than diffuse ISRs after the STEMI (figure 1). Also, late ISRs were more common for the diffuse pattern (47.1% vs 21.4%. P = .018) and with higher degrees of angiographic stenosis (mean, 80.56% vs 70.86%. P = .02). Although overall there were no statistically significant differences on the type of restenosis based on the stent generation (P = .41), the focal patter was present in a higher percentage of bare-metal stents and first-generation drug-eluting stents. Also, the state-of-the-art second-generation drug-eluting stents showed a tendency towards a higher percentage of diffuse restenosis (figure 2). Being cautious about the size of the sample, it is suggested that this may have to do with lower doses of antiproliferative drugs, more homogeneous releases, and different polymers (some of them bioresorbable).
Table 1. Epidemiological and procedural data of patients analyzed based on their pattern of restenosis
| Characteristic | Diffuse pattern (Mehran II-IV) | Focal pattern (Mehran I) | P |
|---|---|---|---|
| Sex (male) | 26 (76.5%) | 36 (85.7%) | .30 |
| Age (years) | 62.6 ± 13.2 | 60.6 ± 11.9 | .50 |
| Size (cm) | 168.2 ± 6.3 | 167.5 ± 7.9 | .68 |
| Weight (kg) | 76.1 ± 10.5 | 78.8 ± 11.3 | .27 |
| Arterial hypertension | 21 (61.8%) | 24 (57.1%) | .68 |
| Diabetes mellitus | 9 (26.5%) | 14 (33.3%) | .51 |
| Dyslipidemia | 13 (38.2%) | 19 (45.2%) | .54 |
| Smoking | 20 (58.8%) | 31 (73.8%) | .16 |
| Alcohol | 1 (2.9%) | 1 (2.4%) | .87 |
| Family history of coronary artery disease | 2 (5.9%) | 0 | .11 |
| Peripheral vasculopathy | 1 (2.9%) | 2 (4.8%) | .68 |
| Chronic nephropathy | 0 | 1 (2.4%) | .36 |
| Prior angioplasty | 5 (14.7%) | 5 (11.9%) | .71 |
| Index procedure (primary angioplasty) | |||
| Type of stent: | .41 | ||
| Conventional stent | 25 (47.2%) | 28 (52.8%) | |
| First-generation drug-eluting stent | 5 (31.3%) | 11 (68.7%) | |
| Second-generation drug-eluting stent | 4 (57.1%) | 3 (42.9%) | |
| Maximum inflation pressure (atmospheres), mean (interquartile range) | 16 (14-18) | 14 (14-18) | .17 |
| Size: | .17 | ||
| Big (> 2.5 mm) | 27 (79.4%) | 38 (90.5%) | |
| Small (≤ 2.5 mm) | 7 (20.6%) | 4 (9.5%) | |
| Number of stents | 1.0 ± 0.4 | 1.1 ± 0.4 | .73 |
| Time to primary angioplasty, min mean (interquartile range) | 170 (120-375) | 180 (120-360) | .58 |
| Thromboaspiration | 13 (38.2%) | 12 (28.6%) | .37 |
| No-reflow | 2 (5.9%) | 2 (4.8%) | .92 |
| Culprit vessel: | .19 | ||
| Left main coronary artery | 1 (2.9%) | 0 | |
| Left anterior descending coronary artery | 9 (26.5%) | 15 (35.7%) | |
| Left circumflex artery | 7 (20.6%) | 2 (4.8%) | |
| Right coronary artery | 16 (47.1%) | 24 (57.1%) | |
| Saphenous vein bridge | 1 (2.9%) | 1 (2.4%) | |
| LVEF | 53.0 ± 16.1 | 56.0 ± 11.5 | .38 |
| Peak creatine kinase levels, mean (interquartile range) | 988 (484-2715) | 1446 (480-3808) | .62 |
| Diagnosis of restenosis | |||
| Diagnosis for new catheterization: | .35 | ||
| Silent ischemia | 1 (2.9%) | 2 (4.8%) | |
| Asymptomatic* | 12 (35.3%) | 21 (50%) | |
| STEMI | 7 (20.6%) | 3 (7.1%) | |
| NSTEMI | 6 (17.6%) | 8 (19%) | |
| Unstable angina pectoris | 2 (5.9%) | 4 (9.5%) | |
| Stable angina pectoris | 3 (8.8%) | 0 | |
| Heart failure | 2 (5.9%) | 3 (7.1%) | |
| Ventricular tachycardia | 1 (2.9%) | 1 (2.4%) | |
| Time correlation: | .01 | ||
| Early ISR (< year) | 18 (52.9%) | 33 (78.6%) | |
| Late ISR (> year) | 16 (47.1%) | 9 (21.4%) | |
ISR, in-stent restenosis; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation acute myocardial infarction; STEMI, ST-segment elevation acute myocardial infarction. * Control catheterization is indicated by the treating physician (for academic purposes, clinical studies, preoperative or other reasons). | |||
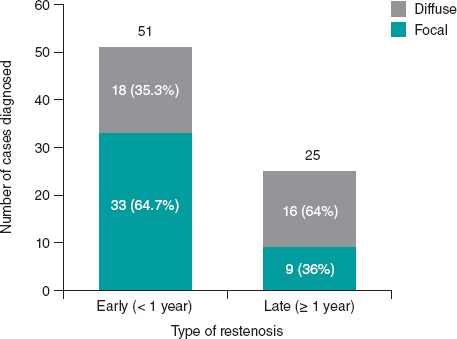
Figure 1. Number of diagnoses based on the time elapsed from primary angioplasty and stratified according to the type of stent restenosis (mean time to diagnosis in diffuse ISR, 29.5 months; in focal ISR, 14.0 months; P = .015). ISR, in-stent restenosis.
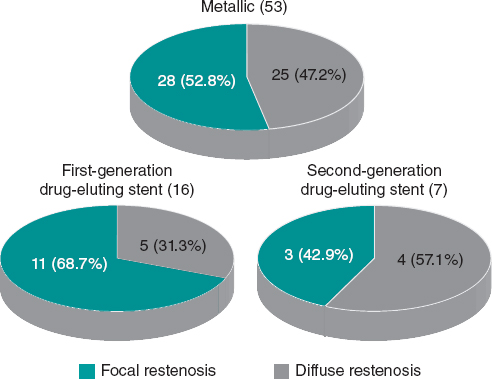
Figure 2. Type of de restenosis according to the Mehran angiographic classification based on the type of stent.
Small stents (≤ 2.5 mm) showed a non-significant tendency towards more diffuse disease (64% vs 37%, P = .17). No significant differences were found on the time elapsed until the diagnosis of ISR stratified according to the type of stent (conventional, firstor second-generation drug-eluting stents).
Given the characteristics of the study with a small number of cases, among other limitations, it was difficult to estimate the exact rate of ISR since no follow-up coronary angiography was performed in all the STEMIs treated in our center during the study period. No timeline of the exact moment when the ISRs developed was given either since they are often oligosymptomatic. However, the patients’ clinical characteristics and the behavior of several stents are consistent with data previously published on ISRs in patients in other clinical contexts1.
In conclusion, regarding ISR, both pattern and time may be influenced by the type of stent implanted after a STEMI.
REFERENCES
1. Her AY, Shin ES. Current Management of In-Stent Restenosis. Korean Circ J. 2018;48:337-349.
2. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659-2673.
3. Alfonso F, Rivero F. Network meta-analyses on in-stent restenosis treatment:dealing with complexity to clarify efficacy and safety. J Thorac Dis. 2015;7:1678-1683.
4. Diego A, Pérez de Prado A, Cuellas C, et al. Instent restenosis related to vessel injury score degree. Are current experimental models valid for drug-eluting stents analysis?Rev Esp Cardiol. 2011;64:745-751.
5. Goel SS, Dilip Gajulapalli R, Athappan G, et al. Management of drug eluting stent in-stent restenosis:a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;87:1080-1091.
Corresponding author: Hospital Clínico San Carlos, Calle Profesor Martín Lagos s/n, 28040 Madrid, Spain.
E-mail address: ibnsky@yahoo.es (I.J. Núñez-Gil).