To the Editor,
Esophageal-pleural fistulas are a rare complication of thoracic surgery. They may occur as the direct result of trauma during surgery or use of a transesophageal device. The management of esophageal-pleural fistulas can be difficult, and they rarely heal spontaneously. Conservative management of esophageal-pleural fistulas are associated with high mortality rates. The traditional treatment of symptomatic patients is surgery. However, this type of surgery has high morbidity and mortality rates; for this reason, many patients are not eligible for surgery. The endoscopic treatment options to repair esophageal fistulas include the injection of glue, covered stents, and endoscopic suture or clipping. After treatment failure, we considered the use of an emerging therapeutic technique as an alternative treatment. In this context, we present a case of a iatrogenic esophageal fistula treated with an Amplazer device.
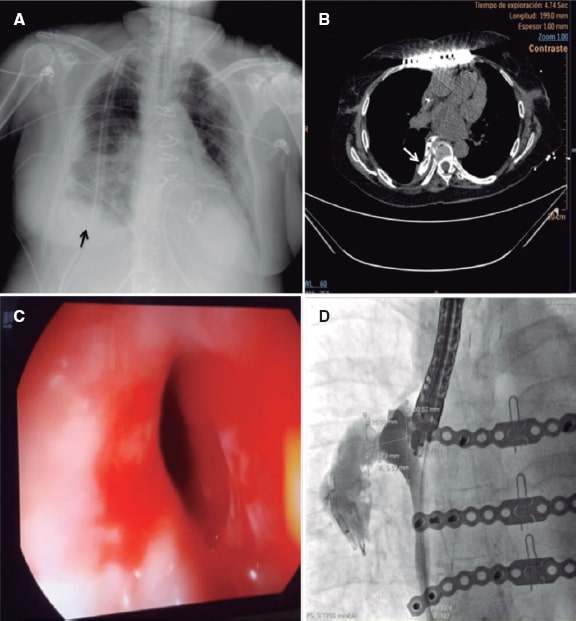
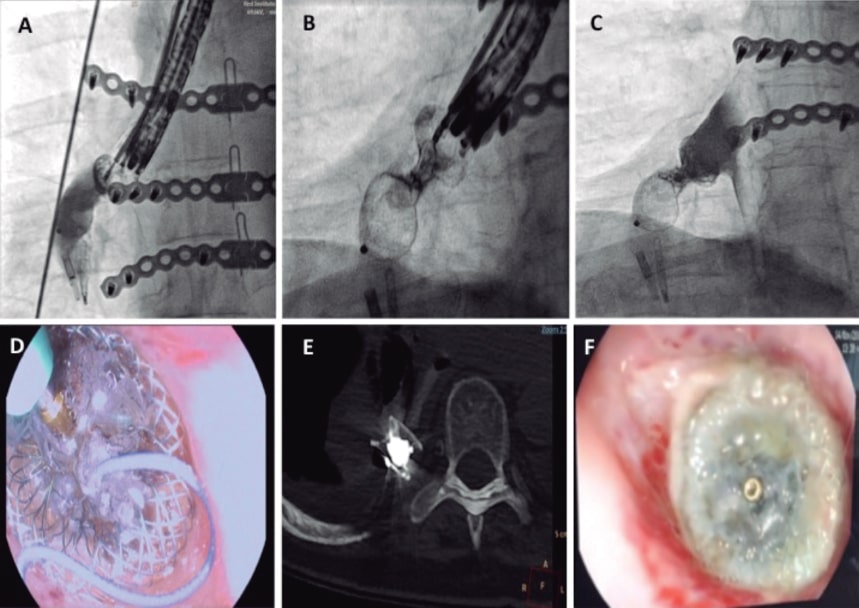
This is the case of a 72-year-old woman, former smoker with a past medical history of hypertension, chronic atrial fibrillation, and rheumatic mixed mitral valve disease who was admitted due to acute coronary syndrome. A thrombotic occlusion of the left circumflex artery was successfully treated with drug-eluting stent implantation. During her hospital stay, the patient developed bilateral arterial embolism in both lower limbs that was treated with an embolectomy. The transesophageal echocardiography performed confirmed the presence of severe mitral regurgitation, moderate mitral stenosis, left ventricular ejection fraction of 38%, pulmonary artery systolic pressure of 60 mmHg, and left atrial appendage thrombus. The patient underwent mitral valve replacement with a 25 mm ATS heart valve and transesophageal echocardiography guided surgical left atrial appendage closure. After the surgery, the patient developed mediastinitis, recurrent pleural effusions, and an empyema. The contrast computed tomography scan performed revealed the presence of an esophageal perforation that was confirmed by the endoscopy (figure 1A-C). Esophageal surgery was discarded and the endoscopic treatment of the perforation with hemoclips and a covered metal stent was attempted twice; however, both devices migrated to the stomach and had to be removed. A number of hemoclips migrated through the fistula tract into the pleura and were left in situ. A gastrostomy was performed to facilitate artificial enteral nutrition. After multiple infections and a 4-month stay at the intensive care unit, we were requested to close the perforation with an occluder device. To establish the size of the fistula, we injected a fluoroscopy guided contrast medium through the fistula and obtained a perpendicular view that correctly exposed the neck of the fistula for device selection purposes (figure 1D). The implantation was performed in the catheterization laboratory under general anesthesia with endoscopic and fluoroscopic guidance (figure 2A, F). A curved hydrophilic guidewire was introduced through the endoscope lumen to cross the fistula. Since the endoscope lumen was 4.2 mm and there was no space for further material inside, the endoscopic probe was retrieved followed by its parallel insertion to guide the procedure. A GLIDECATH catheter (Terumo Medical Corporation, NJ, United States) was advanced over the guidewire. A high support guidewire was introduced and a 7-Fr Destination Guiding Sheath (Terumo Medical Corporation, NJ, United States) was advanced. Contrast medium was injected to locate the fistula. A 14 mm Amplatzer Muscular VSD Occluder (Abbott, Illinois, United States) was advanced. The distal and proximal discs were released and a 0.010 in guidewire (Mirage microguidewire, Medtronic, Minnesota, United States) was used to penetrate the device proximal disc. Afterwards, a Marathon microcatheter (Medtronic, Minnesota, United States) was inserted and placed inside the neck of the device to infuse the Onyx liquid embolic system, a radiopaque copolymer that seals in 5 minutes. The administration of contrast confirmed the sealing and the successful final result. After the procedure, no recurrences of pleural effusion or infections were seen. In the subsequent endoscopies and CT scans performed (figure 2D, E), the effective sealing of the esophagus was confirmed and normal feeding was reinstated 1 month later. The 9-month clinical follow-up confirmed the patient was doing well with restored enteral feeding. Family signed a specific informed consent for this procedure.
Figure 1. A: X-Ray image showing right pleural effusion (arrow). B: computed tomography scan with oral contrast, the axial section shows extravasation of contrast to the pleural space (white arrow). C: endoscopic image of the perforation after transesophageal echocardiography. D: fluoroscopy with oral contrast showing the morphology and measurements of the esophageal-pleural fistula.
Figure 2. A: fluoroscopic projection showing the release of a 14 mm Amplatzer muscular VSD occluder. B: administration of the Onyx Liquid inside the neck of the Amplatzer device using a microcatheter (note the device neck turned radiolucent). C: contrast injection into the esophagus confirming the immediate sealing of the device. D: endoscopic view of the microcatheter during the injection of the embolic sealing liquid. E: computed tomography scan showing the device with an embolic sealing agent inside. F: endoscopic image of the device after 1-month showing partial endothelization.
Endoscopic fistula closure with cardiovascular devices has been tried mainly for the management of bronchopleural fistulas with good clinical outcomes. The evidence for using these devices to close esophageal-pleural fistulas is scarce. To this day, only 3 case reports have been published. The first used an Amplatzer vascular plug, coils, and histoacryl glue. In this case additional coil packing was required for definitive closure.1 The second case used an Amplatzer atrial septal occluder without a sealing agent. The procedure was unsuccessful due to device migration.2 In the third case, an Amplatzer ventricular septal occluder was used together with a liquid copolymer leading to an immediate sealing and good clinical outcomes.3
Establishing the correct size of the fistula is a critical step, and the techniques used to do this have not been previously described in the medical literature. We think that the use of fluoroscopic measurement using contrast media is a beneficial option that allows the correct selection of the device and a real-time strategy. If the visualization of the defect is suboptimal, a sizing balloon often used to size cardiac defects may be used.
The use of the Onyx sealing agent proved to be one of the key elements that led to therapeutic success. The complete isolation of the pleural space is vital to prevent recurrent infections.3
FUNDING
No funding was received for this work.
AUTHORS' CONTRIBUTIONS
R. Vera-Urquiza: performance of the procedure, Critical review of data, drafting the manuscript. J. M. Esteban López-Jamar: performs the procedure, review the manuscript. L. Nombela-Franco: performs the procedure, review the manuscript. M. Vázquez Romero: performs the procedure, review the manuscript. C. Espejo: clinical follow-up, review the manuscript. P. Jiménez-Quevedo: plans and performs the procedure, Critical review of data, drafting the manuscript.
CONFLICTS OF INTEREST
L. Nombela-Franco is a proctor for Abbott and has participated in and received speaking fees from Edwards Lifesciences. The rest of authors have no conflicts of interest.
REFERENCES
1. Koo JH, Park KB, Choo SW, Kim K, Do YS. Embolization of postsurgical esophagopleural fistula with AMPLATZER vascular plug, coils, and Histoacryl glue. J Vasc Interv Radiol. 2010;21:1905-1910.
2. Kadlec J, Turner K, Van Leuven M. Attempted closure of a post-pneumonectomy esophagopleural fistula with an Amplatzer atrial septal occluder. Interact Cardiovasc Thorac Surg. 2013;6:538-540.
3. Lassaletta AD, Laham RJ, Pinto DS, et al. Successful closure of an esophagopleural fistula with an Amplatzer occluder sealed with liquid copolymer, with 3-year follow-up and review of literature. Pleura. 2016;3:1-6.