To the Editor,
Treatment of heavily calcified coronary artery disease (CAD) remains a technical challenge since a significant number of patients require some type or form of advanced plaque modification procedure in the cath lab. Therefore, interventional cardiologists should be aware of the complete array of plaque modification techniques available to prepare vessels to facilitate optimal stent deployment and expansion.1 In the presence of proximal calcified disease in tortuous vessels, orbital atherectomy can be used as an alternative to rotational atherectomy thanks to its greater stability with reverse ablation, improved ease of use, and convenience as a result of a single-size burr that can be used to treat a wide range of vessel profiles. In addition, it appears to have a similar safety profile compared to rotational atherectomy.2 We herein describe a case of 3-vessel proximal heavily calcified CAD where we demonstrate the feasibility of using orbital atherectomy to prepare all 3 epicardial vessels using a one-size burr guided by co-registered intravascular ultrasound (IVUS) and physiology prior to complete percutaneous revascularization in a single-staged procedure.
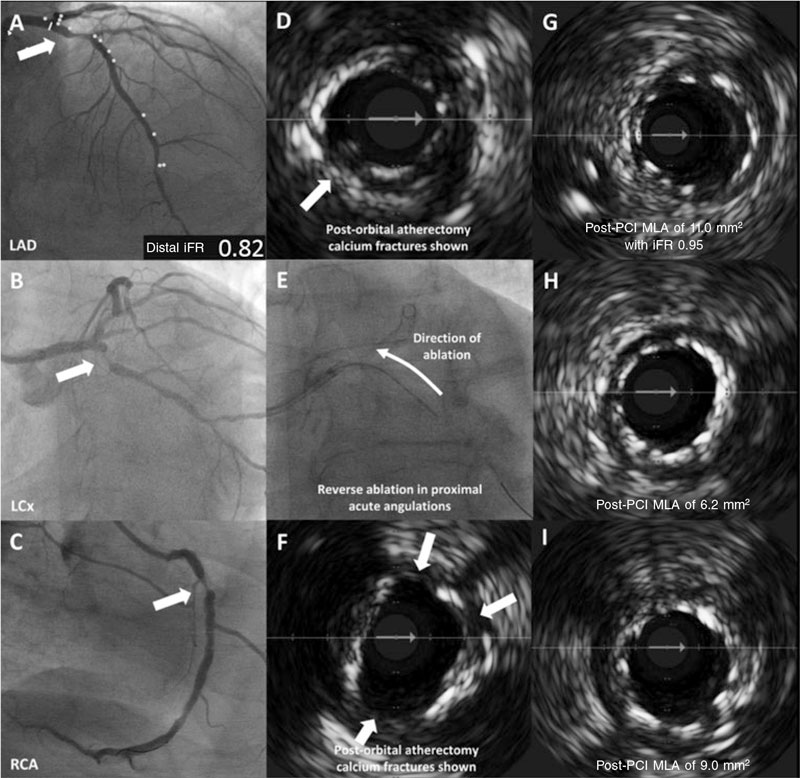
This is the case of a 73-year-old man with hypertension, type II diabetes mellitus, severe chronic obstructive pulmonary disease (forced expiratory volume in 1 second [FEV1] of 29%), and atrial fibrillation that presented with exertional chest tightness. Clinical examination was unremarkable, and the clinical hematology and biochemistry were normal except for an N-terminal pro-B-type natriuretic peptide of 1365 pg/mL (normal reference range, < 125 pg/mL) and troponin I levels of 351 ng/L [normal reference range, 3-58 ng/L]. The electrocardiogram confirmed the presence of atrial fibrillation. The transthoracic echocardiography identified an impaired left ventricular systolic function with an ejection fraction of 43% with inferior hypokinesia. After giving his informed consent, the patient underwent invasive coronary angiography that revealed the presence of heavily calcified proximal 3-vessel CAD with diffuse atheroma (left anterior descending coronary artery [LAD] figure 1A; left circumflex artery [LCx] figure 1B; right coronary artery [RCA] figure 1C; diseased segments are highlighted with arrows). Disease distribution was anatomically complex (SYNTAX score, 30) and considering the patient’s clinical status, the SYNTAX score II predicted a 4-year mortality rate associated with percutaneous coronary intervention (PCI) or coronary artery bypass graft of 8.3% and 17.7%, respectively. The patient eventually underwent a PCI as advised by the heart team.
Figure 1. Baseline angiography with IVUS-guided orbital atherectomy showing the mechanisms of calcium modification with stent deployment and strut apposition. A: lesion in proximal LAD with iFR of 0.82. B: lesion in proximal LCx. C: lesion in proximal RCA. D: post-orbital IVUS-guided atherectomy with presence of calcium fractures (asterisks). E: reverse orbital atherectomy shown. F: post-orbital IVUS-guided atherectomy with presence of calcium fractures (asterisks). G: stent apposition in LAD with post-PCI MLA of 11.0 mm2 and iFR of 0.95. H: stent apposition in LCx with MLA of 6.2 mm2. I: stent apposition in RCA with MLA of 9.0 mm2. iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCx, left circumflex artery; MLA, minimum lumen area; PCI, percutaneous coronary intervention; RCA, right coronary artery.
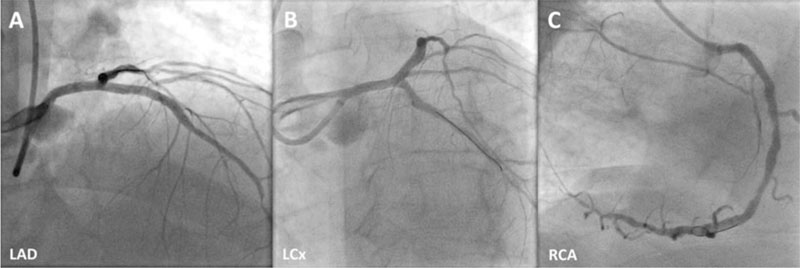
Via right radial artery, the pre-PCI instantaneous wave-free ratio (iFR) of the least angiographically severe lesion in the LAD was positive at 0.82. Longitudinal vessel analysis was performed using IVUS with co-registration to better characterize the extent of the calcified disease in the LAD and the RCA. The LCx stenosis was uncrossable with the IVUS catheter before calcium modification. Therefore, intracoronary imaging prior to the procedure was not performed in this vessel. Given that > 270º of heavy calcification was present in the target vessel landing zones in both the LAD and the RCA, upfront orbital atherectomy was performed in all 3 arteries using a 1.25 mm burr (Diamondback 360, CSI, United States). The use of orbital atherectomy produced significant calcium debulking and fractures (LAD, figure 1D; RCA, figure 1F; calcium fractures are shown with arrows). After additional treatment with non-compliant balloons, sirolimus-eluting stents were deployed both to the LAD and the LCx (3.5 mm × 35 mm and 3.0 mm × 15 mm, respectively; Osiro, Biotronik, Germany) and an everolimus-eluting stent was deployed to the RCA (3.5 mm × 33 mm; XIENCE, Abbott, United States). The post-PCI IVUS of all 3 vessels confirmed complete strut apposition, and good minimum stent areas and lesion coverage. In addition to the post-PCI physiology of the LAD that confirmed good functional outcomes with an iFR of 0.95 (LAD, figure 1G; LCx, figure 1H; RCA, figure 1I) the final angiographic outcomes of the 3 target vessels are shown on figure 2 (LAD, figure 2A; LCx, figure 2B; RCA, figure 2C). A summary of the devices used to perform the procedure is shown on table 1.
Figure 2. Final angiographic outcomes of LAD (A), LCx (B), and RCA (C) are shown. LAD, left anterior descending coronary artery; LCx left circumflex artery; RCA, right coronary artery.
Table 1. Devices used to perform PCIs
| Vessel | Device |
|---|---|
| LAD | 3.5 mm 7-Fr EBU guide catheter |
| ViperWire coronary wire (Cardiovascular Systems Inc., United States) | |
| Sion Blue coronary wire (ASAHI Intecc Inc., Japan) | |
| OmniWire physiology and coronary guidewire (Philips, the Netherlands) | |
| FineCross microcatheter (Terumo Corporation, Japan) | |
| Diamondback 360 coronary orbital atherectomy system with 1.25 mm burr (Cardiovascular Systems Inc., United States of America) | |
| Eagle Eye IVUS catheter (Philips, the Netherlands) | |
| 2.5 mm x 15 mm Trek balloon (Abbott, United States) | |
| NC Xperience 3.5 mm x 10 mm balloon (iVascular, Spain) | |
| 3.5 mm x 35 mm Orsiro drug-eluting stent (Biotronik, Germany) | |
| LCx | ViperWire coronary wire (Cardiovascular Systems Inc., United States) |
| Sion Blue coronary wire (ASAHI Intecc Inc., Japan) | |
| Diamondback 360 coronary orbital atherectomy system with 1.25 mm burr (Cardiovascular Systems Inc., United States) | |
| Eagle Eye IVUS catheter (Philips, the Netherlands) | |
| 2.5 mm x 15mm Trek balloon (Abbott, United States) | |
| 3.0 mm x 15 mm NC Xperience balloon (iVascular, Spain) | |
| 3.0 mm x 15 mm Orsiro drug-eluting stent (Biotronik, Germany) | |
| RCA | ViperWire coronary wire (Cardiovascular Systems Inc., United States) |
| Sion Blue coronary wire (ASAHI Intecc Inc., Japan) | |
| Diamondback 360 coronary orbital atherectomy system with 1.25mm burr (Cardiovascular Systems Inc., United States) | |
| Eagle Eye IVUS catheter (Philips, the Netherlands) | |
| 3.0 mm x 12 mm Trek balloon (Abbott, United States) | |
| 3.5 mm x 10 mm NC Xperience balloon (iVascular, Spain) | |
| 3.5 mm x 33 mm XIENCE Skypoint drug-eluting stent (Abbott, United States) | |
|
EBU, extra-back up; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LCx, left circumflex artery; NC, non-compliant; PCI, percutaneous coronary intervention; RCA, right coronary artery. |
|
This case is an example of the feasibility of plaque modification with orbital atherectomy to multiple vessels in a patient with calcified CAD through intravascular imaging and physiology guidance, calcified plaque preparation with orbital atherectomy, and post-PCI assessments. These technologies are key to guarantee durable results and, collectively, add prognostic value.3 Atherectomy in all 3 coronary vessels was justified given the extent of calcification. In addition, the use of orbital atherectomy (rather than rotational atherectomy) was carefully considered due to its unique features, specifically, reverse ablation (figure 1E) and variable debulking diameter thereby allowing the management of vessels of different diameters with the use of a one-size burr.4,5 In selected cases, these features of orbital atherectomy can be particularly useful in ostial and/or angulated vessels particularly in the LCx as in this case where tortuosity may prevent adequate plaque modification of heavily calcified lesions, be subject to burr bias or limited by solely antegrade ablation, thus impacting guide catheter support and the overall procedural success.
Herein we highlighted the feasibility of performing co-registration assisted 3-vessel orbital atherectomy in heavily calcified proximal lesions, and demonstrated the performance of this technique in the management of complex disease with effective calcium ablation and fracture.
Informed and written consent from the patient were deemed necessary for publication purposes.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A. Shabbir, and D. Chipayo both drafted the manuscript and prepared the images. A. Jerónimo conducted a critical review of the manuscript intellectual content and contributed substantially to it. A. Travieso also conducted a critical review of the manuscript intellectual content and contributed substantially to it. N. Gonzalo, and J. Escaned conceptualized the manuscript and conducted its critical review. All co-authors gave their final approval to the version that would eventually be published, and all take full responsibility for all aspects of the manuscript.
CONFLICTS OF INTEREST
A. Shabbir declared having received speaker fees and honoraria from Philips. A. Travieso declared having received an unrestricted research training grant from Philips. J. Escaned is supported by the Intensification of Research Activity project INT22/00088 from Spanish Instituto de Salud Carlos III. He also declares serving as speaker and advisory board of Philips. A. Jerónimo, D. Chipayo, and N. Gonzalo declared no conflicts of interest whatsoever.
REFERENCES
1. Riley RF, Henry TD, Mahmud E, et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. 2020;96:346-362.
2. Khan AA, Murtaza G, Khalid MF, et al. Outcomes of rotational atherectomy versus orbital atherectomy for the treatment of heavily calcified coronary stenosis: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2021;98:884-892.
3. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J. 2017;38:3124-3134.
4. Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510-518.
5. Lee M, Genereux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18:261-264.