ABSTRACT
Introduction and objectives: Pulmonary atresia with intact ventricular septum (PA/IVS) is a rare but serious cyanotic congenital heart disease. Depending on the patient’s anatomy, different therapeutic strategies—surgical or trancatheter—can be planned.
The objective of this study was to describe the results of transcatheter pulmonary valve perforation in patients with PA/IVS in a single tertiary center, and compare transjugular to transfemoral approach. The need for additional source of pulmonary flow (ductal stenting or systemic-to-pulmonary artery fistula) at follow-up was reviewed to identify possible risk factors associated with this reintervention.
Methods: patients with PA/IVS referred for transcatheter pulmonary valve perforation as first-line therapy from February 2004 through May 2022 were included. Technical procedural details, total procedural and fluoroscopy times, and demographic and echocardiographic data were studied.
Results: A total of 22 patients were included. Procedure was successful in 20 cases (91%). The rate of complications was 2/22 (9%). No deaths were reported. The transjugular and transfemoral approaches were equally safe and effective. The total median procedural (n = 20) and fluoroscopy times (n = 16), however, were shorter in the transjugular compared to the transfemoral approach (85 min vs 156 min, and 31 min vs 62 min, respectively), which reached statistical significance. At follow-up, 8/20 (40%) patients needed additional flow (4 ductal stenting, 4 systemic-to-pulmonary artery shunts). No significant risk factors regarding this reintervention were reported.
Conclusions: Transcatheter mechanical pulmonary valve perforation may be feasible in expert hands and properly selected patients being an attractive alternative to surgery. In our own experience, transjugular approach seems to simplify the procedure, and reduces procedural and fluoroscopy times.
Keywords: Congenital heart defect. Pulmonary atresia. Balloon valvuloplasty. Pulmonary valve. Newborn.
RESUMEN
Introducción y objetivos: La atresia pulmonar con septo íntegro (APSI) es una cardiopatía congénita cianosante infrecuente que por su gravedad requiere un tratamiento en época neonatal. En función de la anatomía del ventrículo derecho y de la circulación coronaria, se pueden plantear distintas estrategias. El objetivo fue describir los resultados de la perforación valvular pulmonar percutánea de los pacientes con diagnóstico de APSI en un centro terciario. Se comparó el abordaje transfemoral con el transyugular. Se revisó la necesidad de flujo adicional (fístula sistémico-pulmonar o stent ductal) en el seguimiento, procurando identificar posibles factores de riesgo asociados a esta reintervención.
Métodos: Se incluyeron todos los pacientes con APSI tratados con perforación percutánea de la válvula pulmonar como primera opción terapéutica desde febrero de 2004 hasta mayo de 2022. Se estudiaron los detalles técnicos del cateterismo, los tiempos de procedimiento y de escopia, y variables demográficas y ecocardiográficas.
Resultados: Se incluyeron 22 pacientes y el procedimiento fue exitoso en 20 (91%). Se presentaron complicaciones en 2 pacientes (9%). No hubo fallecimientos. Los abordajes transyugular y transfemoral mostraron una eficacia y una seguridad parecidas. Los tiempos medianos de procedimiento (n = 20) y de escopia (n = 16) fueron menores en los pacientes con acceso transyugular que en aquellos con acceso transfemoral (85 frente a 156 y 31 frente a 62 minutos, respectivamente), con significación estadística. En el seguimiento, 8 pacientes (40%) necesitaron flujo adicional (4 stent ductal y 4 fístula sistémico-pulmonar). No se encontró ningún factor asociado significativamente a esta reintervención.
Conclusiones: La perforación percutánea de la válvula pulmonar puede ser factible en manos expertas y en pacientes bien seleccionados, y representa una alternativa atractiva a la cirugía. En nuestra experiencia, el abordaje transyugular parece simplificar el procedimiento, reduciendo los tiempos de intervencionismo y de escopia.
Palabras clave: Cardiopatía congénita. Atresia pulmonar. Valvuloplastia con balón. Válvula pulmonar. Recién nacido.
Abbreviations
CTO: chronic total coronary occlusion. PA/IVS: pulmonary atresia with intact ventricular septum. PV: pulmonary valve. RV: right ventricle. TV: tricuspid valve.
INTRODUCTION
Pulmonary atresia with intact ventricular septum (PA/IVS) is a rare cyanotic congenital heart disease, that can exhibit a wide array of clinical presentations.1-8 It accounts for < 1% of all congenital heart defects, and its anatomical variability involves a complex decision-making process.9 Some procedures during the neonatal period are necessary to provide a reliable source of pulmonary blood flow.3,10,11 Patients with severe right ventricular (RV) hypoplasia or a RV-dependent coronary circulation (some ventricular areas are exclusively perfused from the RV cavity) should be eligible for univentricular heart physiology. In contrast, patients without RV-dependent coronary circulation, mild or moderate RV hypoplasia and membranous pulmonary atresia can be elilgible for transcatheter pulmonary valve (PV) perforation to achieve biventricular circulation.1,4,5,8,10-18 Overall, over the past 2 decades, we have been seeing a transition from surgical RV decompression to transcatheter approach, the latter being associated with a lower mortality rate.7 Nevertheless, thorough patient selection seems crucial to obtain good results.17,19,20
In the 90s, several techniques were described to perforate the atretic PV like laser, radiofrequency or puncture using the stiff end of a guidewire.2-4,10-12,14,15,17,21 Currently, however, laser has become obsolete due to its high cost, bulky equipment, and risk of retinal damage.12,22 Although radiofrequency seems like the method most commonly used, mechanical perforation with the stiff end of a coronary guidewire or a chronic total coronary occlusion (CTO) coronary guidewire can be a promising alternative when the former is not available or is too expensive.8,12,20
Whichever technique was used, complications were common in the early era. The growing experience and the continuous technical improvement, however, have allowed us to contemplate the percutaneous perforation of the PV as a first-line option for neonates with PA/IVS.4,5,11,12,16-18 Also, we should mention that establishing antegrade pulmonary flow early through the atretic valve may increase the chances of growing for the RV.1,3,8,17,23 Furthermore, the transcatheter procedure enables RV decompression, thus avoiding open heart surgery in the neonatal period.
The objective of this study is to describe the results of neonatal transcatheter PV perforation with PA/IVS in a single tertiary center, and compare both the transjugular and transfemoral approaches.
METHODS
This study was approved by Vall d’Hebron Hospital ethics committee (Barcelona) in compliance with the Declaration of Helsinki. Inform consent from the patients’ parents or legal guardians was obtained before publishing this manuscript.
Patients with PA/IVS treated with transcatheter perforation from February 2004 through May 2022 were included. Those with RV-dependent coronary circulation, unipartite RV, muscular pulmonary atresia, severe hypoplastic RV (z-score of tricuspid annulus < -5) or severe Ebstein’s disease were excluded.
Procedures were divided into 2 groups depending on which venous access—femoral or jugular—was used. Demographic, pre- and postoperative echocardiographic variables (PV and tricuspid valve [TV] z-score values, RV morphology), and procedural data were described and compared between the 2 groups. Technical procedural details and complications were also briefly described. We tried to identify risk factors associated with the need for an additional procedure to provide pulmonary blood flow (fistula, ductal stenting). The whole study was divided into 2 periods, from 2004 through 2011 (first period) and from 2012 through 2022 (second period) due to the existence of a new first operator and the selection of transjugular approach as the first option.
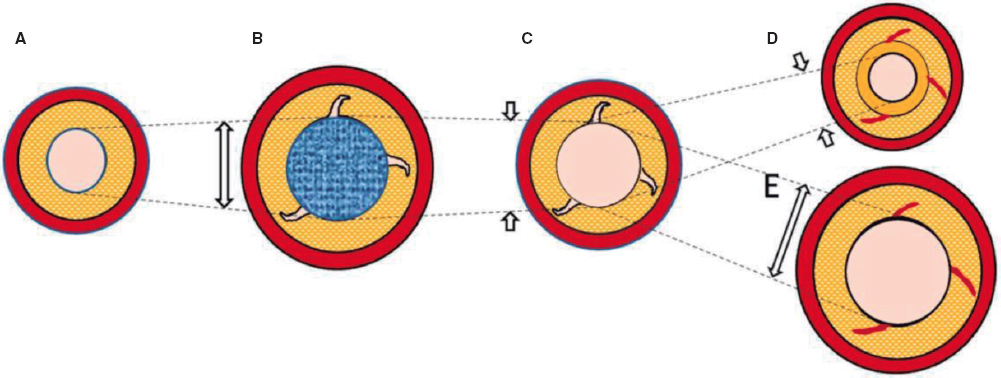
All procedures were performed under general anesthesia and mechanical ventilation. Absence of RV-dependent coronary circulation was assessed in all cases through right ventriculography (figure 1A). A 4F Judkins right catheter (via femoral access) or a C1/C3 Cobra catheter (via jugular access) were used and inserted into the right ventricle outflow tract below the atretic PV. Afterwards, using different 0.014 in CTO guidewires the PV was mechanically perforated. Thereafter, a 0.014 in microcatheter was advanced and an angiography performed in the pulmonary tree to confirm proper position (figure 1B). Unfrequently, the guidewire didn’t achieve the pulmonary trunk on the first attempt. Guidewire and microcatheter were removed uneventfully at this step of the procedure, and another attempt was made until the pulmonary trunk was reached. Once the proper location of the guidewire was confirmed, the CTO guidewire was exchanged for an BMW guidewire (Abbott Vascular, United States) followed by progressive balloon dilatations of the PV to improve pulmonary flow (figure 1C). Finally, a right ventriculography was performed to discard the presence of complications and proper flow across the PV (figure 1D).
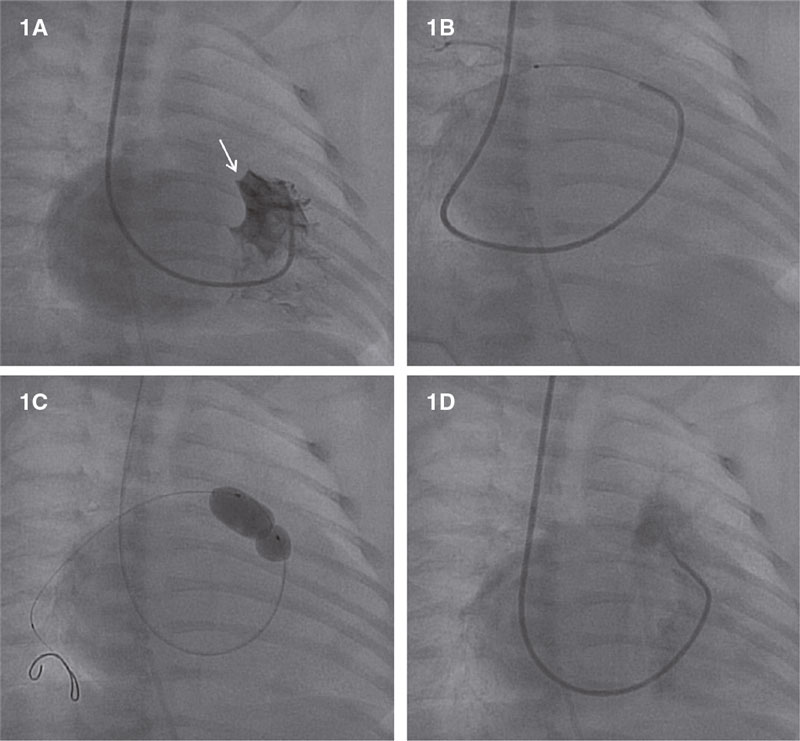
Figure 1. Transcatheter atretic pulmonary valve perforation. A: right ventriculography showing atretic pulmonary valve (white arrow) and no coronary sinusoids. B: microcatheter-based angiography showing proper positioning inside the right pulmonary artery. C: pulmonary valve valvuloplasty. D: right ventriculography showing flow towards the pulmonary artery without complications.
Indications for additional source of pulmonary blood flow (systemic-to-pulmonary artery fistula first—until 2011—and ductal stenting later) were failed prostaglandin E1 withdrawal with excessive cyanosis (hemoglobin saturation < 70%) 1 or 2 weeks after the procedure.
Statistical analysis
Descriptive analysis of data was expressed as median values and interquartile range (IQR) (25th-75th percentile) for qualitative, dichotomic or high dispersion variables, and as mean values with standard deviation (SD) for quantitative continuous variables (weight and gestational weeks). Fischer’s exact test was used to look for possible associations between the binary variables. P values < .05 were considered statistically significant
Statistical analysis was performed using SPSS (statistical package for social sciences) version 20.0 (Illinois, United States).
RESULTS
A total of 22 patients were included, 13 of whom were women (59%). The mean gestational age was 38.3 weeks (+/- 2.9), and the mean weight, 2.96 kg (+/- 0.62).
Before the procedure, the median PV and TV z-scores for the whole sample were -1.2, (n = 22; IQR, -2.01 - -0.25) and -2.0 (n = 21; IQR, -3- -0.65), respectively. All patients but 2 were categorized as tripartite RV and 2 as bipartite with a hypoplastic trabecular part.
Regarding the transcatheter venous access route, initially, the transjugular and transfemoral groups included 13 and 9 patients, respectively. In 3 transfemoral cases, however, the approach had to be changed for the transjugular one due to catheter instability or impossibility to perforate the valve. Therefore, the definitive transjugular and transfemoral groups included 16 and 6 patients, respectively. All transfemoral (n = 6) and 4/16 transjugular cases were performed within the first period while the remaining 12 transjugular cases were performed within the second period.
The median age of the whole sample when the procedure was performed was 1 day of life (1-3). The ratio between the maximum balloon diameter and pulmonary annulus went from 0.8 to 1.45. No peri- or postoperative deaths were reported. The overall procedural success rate was 91% (20/22) [15/16 (94%) and 5/6 (83%) for transjugular and transfemoral groups, respectively]. Data on all successful patients (n = 20), and the comparison between transjugular and transfemoral group are shown on Table 1.
Table 1. Comparison between transjugular and transfemoral group (successful cases)
| Approach | Global | Transjugular | Transfemoral | P |
|---|---|---|---|---|
| Succesful cases (n) | 20 | 15 | 5 | – |
| Procedural time (min)a | 105 (63-143.8) | 85 (53-130) | 156 (120.5-220) | |
| Duration < 140 min | 14 (70%) | 13 | 1 | .01 |
| Duration ≥ 140 min | 6 (30%) | 2 | 4 | |
| Fluoroscopy time (min); n = 16b | 35.6 (20.6-57.1) n = 16 | 31.2 (19-53.4) n = 11 | 62.5 (25.5-80) n = 5 | |
| Fluoroscopy < 60 min | 13/16 (81%) | 11 | 2 | .02 |
| Fluoroscopy ≥ 60 min | 3/16 (19%) | 0 | 3 | |
| Balloon/valve ratio | 1.24 (1.11-1.33) | 1.2 (1.1-1.28) | 1.35 (1.26-1.4) | |
| Invasive residual gradient ≤ 20 mmHg | 18 (90%) | 15 | 3 | .05 |
| Invasive residual gradient > 20 mmHg | 2 (10%) | 0 | 2 | |
| Complications; n = 20 | 1 (5%) | 1/15 | 0/5 | .75 |
| Echocardiographic assessment of PS < 25 mmHg; n = 19c | 11/19 (58%) | 8/15 | 3/4 | .43 |
| Echocardiographic assessment of PR (mod/sev); n = 19c | 15/19 (79%) | 11/15 | 4/4 | .35 |
| Need for AFS | 8 (40%) | 5/15 | 3/5 | .3 |
| DS | 4 | 4/5 | 0/3 | |
| Fistula | 4 | 1/5 | 3/3 | |
| Need of re-valvuloplasty | 4 (20%) | 3/15 | 1/5 | .72 |
| Biventricular circulation | 18 (90%) | 14/15 | 4/5 | .45 |
| Circulation 1 and 1/2 | 2 (10%) | 1/15 | 1/5 | |
AFS, additional flow source; DS, ductal stenting; min, minutes; PR, pulmonary regurgitation; PS, pulmonary stenosis. | ||||
Comparison between successful transfemoral and transjugular cases
The procedural duration of the 3 cases that crossed from one therapy to the other was considered from the moment transjugular approach was decided (and transfemoral dropped). Fluoroscopy time of these 3 cases was not estimated since we had no way of knowing what the exact fluoroscopy time of transjugular approach really was. There was also one missing piece of information in this variable. Therefore, the overall sample for fluoroscopy time was 16 (20 successful cases—3 cases crossed from one therapy to the other—1 missing data case). As shown on Table 1, the variables procedural duration and fluoroscopy time were significantly shorter in the transjugular group. A residual gradient ≤ 20 mmHg (n = 18) measured invasively prevailed in the transjugular group since 15 out of these 18 patients were from this group whereas patients with an invasive gradient > 20 mmHg (n = 2) were from the transfemoral group.
Complications and failed cases
The procedure failed in 2/22 cases (9%). One transfemoral procedure due to right ventricular outflow tract perforation with a significant cardiac hematoma (the patient was urgently treated with surgical valvotomy). In this patient, misjudging the CTO guidewire position—actually located outside the pulmonary tree—triggered balloon dilatation in an incorrect location, thus causing hemopericardium. The other failed case was transjugular due to the impossibility of perforating the valve for being very thick and hypoplastic (surgical valvulotomy and systemic-to-pulmonary artery shunt were performed 6 days later).
No deaths were reported, and the rate of complications was 2/22 (9%). Aside from the significant cardiac hematoma (first period), the other complication occurred in the transjugular approach [atrial flutter that required electrical cardioversion (second period)].
We did not find any factors (gestational week < 37, weight < 2.7kg, TV or PV annulus < -2.0, venous access) associated with complications.
Follow-up and reintervention of the successful cohort
Figure 2 shows a diagram on the management and reintervention rates of the entire cohort.
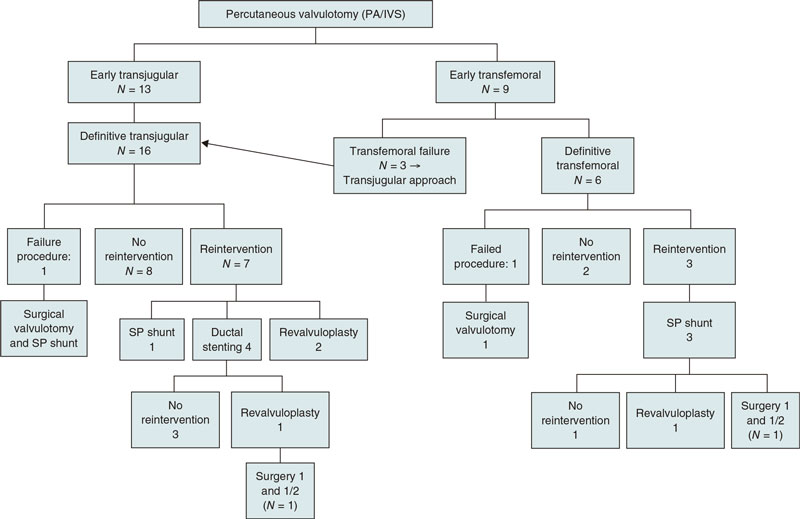
Figure 2. Management and reintervention of the whole sample. PA/IVS, pulmonary atresia with intact ventricular septum; SP, systemic-to-pulmonary artery.
The 2 failed cases sent to surgery were excluded from the follow-up.
The median follow-up was 9.39 years (3.97-11.94) [6.61 (3.67-10.29) and 13.9 (11.66-16.67) for the transjugular and transfemoral groups, respectively]. Of note, 10/20 patients (50%) were followed over 10 years. At follow-up, 10/20 patients (50%) needed some type of reintervention (4 ductal stenting, 4 systemic-to-pulmonary (SP) artery shunts, and 2 re-valvuloplasties) for a median of 12 days (5.2-30). The median time for using an additional flow source (4 SP artery shunts, 4 ductal stenting) was 7.5 days (from the procedure day), 3.0 days for SP fistula (0.87-13.75), and 13.5 days for ductal stenting (6.25-18.5). During the 2004-2011 period all patients who needed additional source intervention underwent a SP shunt (n = 4) whereas from 2012 through 2022 patients underwent a ductal stenting (n = 4) following the new hospital policy.
The median time of re-valvuloplasty as first reintervention (n = 2) was 4.4 months. Another 2 patients required re-valvuloplasty 3 and 9.5 months after a SP shunt and ductal stenting, respectively. Three of the 10 patients who required some reintervention were from the transfemoral group and 7 from the transjugular one. Table 2 compares patients who did not need (n = 12) or who did need (n = 8) an additional source of pulmonary blood flow. An association (P = .05) was seen between the RV/systemic pressure ratio < 150% before the procedure (n = 7) and the need for additional source intervention later since 5/7 patients (71%) with ratios < 150% and only 3/13 (23%) patients with ratios > 150% needed it.
Table 2. Comparison between successful cases requiring or not additional flow
| Additional flow source (AFS) | Global | No need for AFS | Need for AFS | P (Fischer’s exact test) |
|---|---|---|---|---|
| Global (n) | 20 | 12 | 8 | – |
| GW ≤ 37 | 5 (25%) | 14 | 1 | .31 |
| Weight < 2.7 kg | 4 (20%) | 12 | 2 | .53 |
| PV z-score pre-flow < -2.0 | 5 (25%) | 12 | 3 | .3 |
| TV z-score pre-flow < -2.0 | 10 (50%) | 16 | 4 | .68 |
| RVP/systemic ratio ≥ 150% | 13 (65%) | 10 | 3 | .05 |
| RVP/systemic ratio < 150% | 7 (35%) | 12 | 5 | |
| Residual echocardiographic gradient at discharge (≥ 25 mmHg); n = 19 | 8/19 (42%) | 15 | 3 | .66 |
| PR at discharge (mod/sev); n = 19 | 15/19 (79%) | 10 | 5 | .48 |
| PR at discharge (min-mild); n = 19 | 4/19 (21%) | 12 | 2 | |
AFS, additional flow source; GW, gestational week; kg, kilograms; PR, pulmonary regurgitation; min, minimum; mod, moderate; sev, severe; PV, pulmonary valve; RVP, right ventricular pressure; TV, tricuspid valve. | ||||
Overall, 18/20 (90%) patients achieved biventricular circulation, and 2/20 (10%) a 1.5 ventricle repair (bidirectional superior cavopulmonary anastomosis surgery for right ventricular unloading) at a median time of 21.7 months after the index transcatheter procedure. One of these 2 patients were from the transfemoral group and the other from the transjugular group. No study patient underwent univentricular circulation.
DISCUSSION
In patients with PA/IVS and proper anatomy, decompressing the RV with early transcatheter PV perforation may help promote the RV growth and functional development, and facilitate the growth of pulmonary arteries, thus enhancing the options to achieve a biventricular circulation.1,3,17,19,21,24 In this setting, the most frequently reported approach until now has been the transfemoral one.4,5,8,13,15,19,20,23-25
Although with a very modest sample (n = 22), this study is, as far as the authors know, the first to describe the use of transjugular access to perforate the PV in neonates with PA/IVS. Therefore, it is the first one to compare the transjugular and transfemoral approaches.
Compared to other case series where the method to perforate the valve was mixed,1,2,4,11,25 the method of this study was always mechanical perforation using a CTO coronary guidewire.
The rates of success associated with performing mechanical PV perforation with CTO or other coronary guidewires are between 73% and 100%.5,10,19 Overall, the success rate of our cohort was 91% (20/22). The failed transfemoral case was due to perforation and dilatation in an incorrect place of the right ventricular outflow tract whereas the transjugular one was probably due to improper case selection since the anatomy seen in the cath lab was worse compared to the one expected in the echocardiography. The annulus and subvalvular area were more hypoplastic, and the membrane was thick at PV level. These findings rendered catheter maneuvers cumbersome. Based on this case and other studies, proper case selection is key for successful procedures.1,2,14,15,19,20,24
Percutaneous PV perforation involves significant risks including RV or pulmonary trunk perforation.16 Some authors consider mechanical perforation with a coronary guidewire has a higher risk compared to using laser or radiofrequency procedures.10,12,19,20 Nevertheless, the smaller diameter of the CTO guidewire and its microcatheter would add an advantage in relation to larger diameters of the RF guidewire (0.016 in) and microcatheter (0.024 in), thus causing a smaller injury usually without significant consequences if the guidewire is not in the correct place.12 Moreover, CTO guidewires seem to provide increased catheter stability, and advanced torquability and maneuverability to allow more accurate and controlled perforations.12,19,20
As far as the authors know, ours is one of the largest series of neonates with PA/IVS using exclusively mechanical PV perforation with CTO coronary guidewire. Overall, we reported 1/22 (4,5%) cases of significant right ventricular outflow tract perforation due to balloon dilatation. When radiofrequency is unavailable or unsuccessful, the mechanical PV perforation using CTO coronary guidewires may be an attractive alternative in view of its penetration capabilities and reduced cost although it can be technically challenging.8,12,19,20
Comparison between the transfemoral and transjugular groups
As far as the authors know, there is no previous comparative studies between the 2 different approaches in the medical literature currently available. Therefore, according to our observations of the overall procedural time, transjugular procedures were faster compared to transfemoral ones (85 min vs 156 min). This was associated with shorter fluoroscopy times in the transjugular group (31 min vs 62 min). Based on our own experience, the transjugular approach with a Cobra catheter seems to bring good stability, allow better positioning under PV leaflets, and greater support to perforate the atretic valve. Aside from the cited reasons, time differences between the 2 groups can also be due to the learning curve since all transfemoral cases (n = 6) were performed within the first period while 12/16 transjugular cases were performed within the second one. Also, the systematic use of the echography to canalize the central venous access since 2018 (n = 5) can also have contributed, in part, to the procedural time differences seen.
Need for additional flow source, re-valvuloplasty, and follow-up
The rate of reinterventions reported in several case series is still noticeable (from 30% to 72%). They seem to be mainly associated with the increased pulmonary blood flow.4,17,18,25 In our study and among the successful procedures, 8 out of 20 patients (40%) needed additional flow source interventions, a percentage slightly lower compared to the one reported by Kim YH, Cho MJ or Hasan.5,10,11 A certain trend—that almost reached statistical significance (P = .05)—was seen between the RV/systemic pressure ratio < 150% before the procedure (n = 7) and the need for further additional flow source intervention since 5/7 (71%) patients with ratios < 150% needed an extra flow source compared to only 3/13 (23%) patients with ratios > 150%. This observation could show that these right ventricles that were more dysfunctional and less capable of generating pressure could not provide an adequate antegrade pulmonary flow after the procedure. No other variables were associated with the need for an additional flow source intervention. In contrast, other studies reported lower tricuspid z-score values as a risk factor for additional flow source interventions.11,13,15,18,23 Also, Wang et al. reported smaller PV diameters (median z-score of -2.11) and higher echocardiographic gradients 1 month after the procedure as risk factors for reintervention.6
Noteworthy, the median follow-up of our patients was 9.39 years (3.97-11.94). At follow-up, 10/20 patients (50%) did not require any type of reintervention, a higher number compared to the one published by Hasan (7/26, 27%), Schwartz (6/21, 29%) or Chen (7/36, 19%).11,13,25
Regarding the successful group (n = 20) all patients but 2 achieved biventricular circulation (90%), a percentage similar to the data published by Schwartz (18/19, 95%) or Chen (26/31 84%) series.13,25 Our 2 cases with non-biventricular circulation had a TV z-score of -3 but a normal PV z-score (-1.3 and +1.5, respectively). Chubb et al. reported that patients with early smaller TV and PV z-scores were less likely to achieve biventricular circulation.4 Yoldas¸ reported PV z-score values > -1.7 and TV z-core values > -1 as good predictors of biventricular circulation in 31 neonates with PA/IVS treated with radiofrequency PV perforation.18
Limitations
The small sample of patients with PA/IVS from a single center limits the power to draw conclusions. Also, given the study retrospective nature, the definitive 2 groups (transjugular and transfemoral) were not balanced (16 vs 6 patients) since the transfemoral approach failed in 3 of these patients and had to be changed. This imbalance limits the inter-group comparison and prevents drawing more solid conclusions.
In addition, all transfemoral cases were performed within the first period, thus implying a potential “period time” bias also associated with the learning curve and the effect of evolving improvements in procedural materials and technique like the systematic echography-guided vessel puncture from 2018 or or the change of first option for additional flow source (ductal stenting instead of Blalock Taussig shunt) from 2012 through 2022.
CONCLUSIONS
Transcatheter mechanical PV perforation in patients with PA/IVS may be feasible in expert hands and is a reasonable alternative to heart surgery. Proper case selection appears to be key for transcatheter success. In our own experience, compared to the transfemoral approach, the transjugular one seems to simplify the procedure by reducing procedural and fluoroscopy times. However, new and larger prospective studies would be necessary to corroborate these findings.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
M. Figueras Coll: study design and idea, data curation, image acquisition, statistical analysis, drafting of the manuscript, and final approval of the version that will be published eventually. He takes full responsibility for all aspects of this manuscript. A. Fidalgo García: study designa an idea, statistical analysis, collaboration drafting the manuscript, and final approval of the version that will be published eventually. He takes full responsibility for all aspects of this manuscript. G. Martí Aguasca: study design, image provision, collaboration drafting the manuscript, critical review of its content and intellectual interest, and final approval of the version that will be published eventually. He takes full responsibility for all aspects of this manuscript. P. Betrián Blasco: study idea and design, image provision, collaboration drafting the manuscript, critical review of its content and intellectual interest, and final approval of the version that will be published eventually. He takes full responsibility for all aspects of this manuscript.
CONFLICTS OF INTEREST
None reported.
WHAT IS KNOWN ABOUT THE TOPIC?
- Pulmonary atresia with intact ventricular septum (PA/IVS) is a rare but serious cyanotic congenital heart disease. Over the past 2 decades, we have seen a move away from surgical right ventricular decompression to a more transcatheter-centered approach that has reduced mortality. However, not all cases with PA/IVS are eligible for transcatheter perforation. Therefore, proper case selection remains key for procedural success. In patients with no right ventricular dependent coronary circulation, mild or moderate RV hypoplasia and membranous (not muscular) pulmonary atresia, transcatheter PV perforation may be a valuable therapeutic option to achieve biventricular circulation.
WHAT DOES THIS STUDY ADD?
- As far as the authors know, this study is the first one to describe transjugular access to perforate the pulmonary valve in neonates with PA/IVS, and the proper anatomy. Also, it is the first to compare the results of transfemoral vs transjugular approach. Based on our own experience, the transjugular approach has proven safe and effective, simplifies the procedure, and reduces interventional and fluoroscopy times.
REFERENCES
1. Humpl T, Söderberg B, McCrindle BW, et al. Percutaneuos balloon valvotomy in pulmonary atresia with intact ventricular septum: impact on patient care. Circulation. 2003;108:826-832.
2. Agnoletti G, Piechaud JF, Bonhoeffer P, et al. Perforation of the atretic pulmonary valve. Long-term follow-up. J Am Coll Cardiol. 2003;41:1399-1403.
3. Qureshi SA. Catheterization in neonates with pulmonary atresia with intact ventricular septum. Catheter Cardiovasc Interv. 2006;67:924-931.
4. Chubb H, Pesonen E, Sivasubramanian S, et al. Long-term outcome following catheter vavlotomy for pulmonary atresia with intact ventricular septum. J Am Coll Cardiol. 2012;59:1468-1476.
5. Kim YH. Pulmonary valvotomy with echocardiographic guidance in neonates with pulmonary atresia and intact ventricular septum. Catheter Cardiovasc Interv. 2015;85:E123-8.
6. Wang Q, Wu YR, Zhang LN, et al. Evaluating the risk factors of reintervention of neonates with PA/IVS and CP/IVS after PBPV as initial intervention method. J Cardiol. 2016;68:190-195.
7. Wright LK, Knight JH, Thomas AS, Oster ME, St Louis JD, Kochilas LK. Long-term outcomes after intervention for pulmonary atresia with intact ventricular septum. Heart. 2019;105:1007-1013.
8. Kamalı H, Tanıdır I·C, Erdem A, Sarıtas¸ T, Güzeltas¸ A. The Use of Chronic Total Occlusion (CTO) Wires for Perforation of Atretic Pulmonary Valve; Two Centers Experience. Pediatr Cardiol. 2021;42:1041-1048.
9. Li QZ, Cao H, Chen Q, Zhang GC, Chen LW, Chen DZ. Balloon valvuloplasty through the right ventricle: another treatment of pulmonary atresia with intact ventricular septum. Ann Thorac Surg. 2013;95:1670-1674.
10. Cho MJ, Ban KH, Kim MJ, Park JA, Lee HD. Catheter – based treatment in patients with critical pulmonary stenosis or pulmonary atresia with intact ventricular septum: a single institute experience with comparison between patients with and without additional procedure for pulmonary flow. Congenit Heart Dis. 2013;8:440-449.
11. Hasan BS, Bautista-Hernandez V, Mc Elhinney DB, et al. Outcomes of transcatheter approach for initial treatment of pulmonary atresia with intact ventricular septum. Catheter Cardiovasc Interv. 2013; 81:111-118.
12. Alwi M, Budi RR, Mood MC, Leong MC, Samion H. Pulmonary atresia with intact septum: the use of Conquest Pro coronary guidewire for perforation of atretic valve and subsequent interventions. Cardiol Young. 2013;23:197-202.
13. Schwartz MC, Glatz AC, Dori Y, Rome JJ, Gillespie MJ. Outcomes and predictors of reintervention in paitents with pulmonary atresia and intact ventricular septum treated with radiofrequency perforation and ballon pulmonary valvuloplasty. Pediatr Cardiol. 2014;35:22-29.
14. El Shedoudy S, El-Doklah E. Transcatheter perforation of atretic pulmonary valve by the stiff end of a coronary wire in neonates with pulmonary atresia with intact ventricular septum: A solution in developing countries. J Saudi Heart Assoc. 2018;30:222-232.
15. Hascoët S, Borrhomée S, Tahhan N, et al. Transcatheter pulmonary valvulplasty in neoantes with pulmonary atresia and intact ventricular septum. Arch Cardiovasc Dis. 2019;112:323-333.
16. Asnes JD, Fahey JT. Novel catheter positioning technique for atretic pulmonary valve perforation. Catheter Cardiovasc Interv. 2008;71:850-852
17. Morgan GJ, Narayan SA, Goreczny S, et al. A low threshold for neonatal intervention yields a high rate of biventricular outcomes in pulmonary atresia with intact ventricular septum. Cardiol Young. 2020;30:649-655.
18. Yoldas¸ T, Örün UA, Dog˘an V, et al. Transcatheter radiofrequency pulmonary valve perforation in newborns with pulmonary atresia/intact ventricular septum: Echocardiographic predictors of biventricular circulation. Echocardiography. 2020;37:1258-1264.
19. Haddad RN, Saliba Z. Optimal management of pulmonary atresia with intact ventricular septum in a developing country: the art of pulmonary valve mechanical perforation in the era of CTO hardware. Am J Cardiovasc Dis. 2021;11:21-28.
20. Bakhru S, Marathe S, Saxena M, et al. Transcatheter pulmonary valve perforation using chronic total occlusion wire in pulmonary atresia with intact ventricular septum. Ann Pediatr Cardiol. 2017;10:5-10.
21. Haddad RN, Hanna N, Charbel R, Daou L, Chehab G, Saliba Z. Ductal stenting to improve pulmonary blood flow in pulmonary atresia with intact ventricular septum and critical pulmonary stenosis after balloon valvuloplasty. Cardiol Young. 2019;29:492-498.
22. Rosenthal E, Qureshi SA, Chan KC, et al. Radiofrequency-assisted balloon dilatation in patients with pulmonary valve atresia and an intact ventricular septum. Br Heart J. 1993;69:347-351.
23. Marasini M, Gorrieri PF, Tuo G, et al. Long-term results of cathter-based treatment of pulmonary atresia and intact ventricular septum. Heart. 2009;95:1520-1524.
24. Rathgeber S, Auld B, Duncombe S, Hosking MC, Harris KC. Outcomes of radiofrequency perforation for pulmonary atresia and intact ventricular septum: A single-centre experience. Pediatr Cardiol. 2017;38:170-175.
25. Chen RHS, K T Chau A, Chow PC, Yung TC, Cheung YF, Lun KS. Achieving biventricular circulation in patients with moderate hypoplastic right ventricle in pulmonary atresia intact ventricular septum after transcatheter pulmonary valve perforation. Congenit Heart Dis. 2018;13:884-891.